Abstract
Although many acute and chronic diseases are managed via pharmacological means, challenges remain regarding appropriate drug targeting and maintenance of therapeutic levels within target tissues. Advances in nanotechnology will overcome these challenges through the development of lipidic particles, including liposomes, lipoproteins, and reconstituted high-density lipoproteins (rHDL) that are potential carriers of water-soluble, hydrophobic, and amphiphilic molecules. Herein we summarize the properties of human plasma lipoproteins and rHDL, identify the physicochemical determinants of lipid transfer between phospholipid surfaces, and discuss strategies for increasing the plasma half-life of lipoprotein- and liposome-associated molecules.
Keywords: liposomes, reconstituted HDL, lipid metabolism, apolipoproteins, membrane dynamics, lipoprotein structure
Introduction: Nanotechnology in Medicine
Advances in nanotechnology have revealed its great potential in the diagnosis and treatment of major diseases, including atherosclerosis.1 However, there are many challenges in the development of new therapeutics, such as nonspecific distribution to and inadequate accumulation within target tissues. Some argue that site-specific delivery of therapeutics is a distant reality due to the biological barriers that a particle encounters following intravenous administration. These barriers include: (A) opsonization and subsequent sequestration by the phagocytosis, (B) nonspecific distribution, (C) hemorheological/blood vessel flow limitations, (D) pressure gradients, (E) cellular internalization, (F) escape from endosomal and lysosomal compartments, and (G) drug efflux pumps.2,3 Some of these challenges might be overcome by using nanoparticles with natural in vivo compatibility. Liposomes have already been recognized as potential carriers of bioactive materials,4 and the extension of this nanoparticle method to lipoproteins is the logical next step.
A Review of the Plasma Lipoproteins
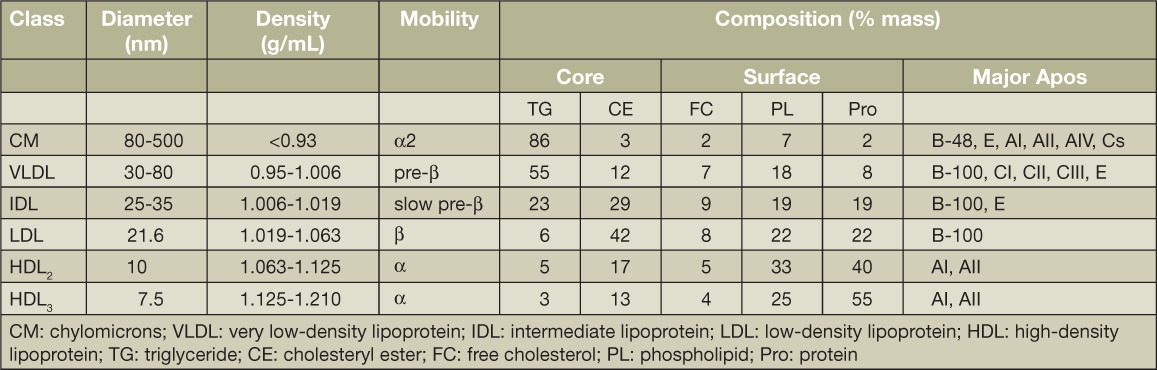
Plasma lipoproteins are lipid-protein complexes that transport lipids between various tissue sites for utilization as energy or for steroidogenic hormone production and storage.5 They are composed of lipids, cholesterol, cholesteryl esters, triglycerides, and phospholipids, mainly phosphatidylcholine plus specialized proteins called apolipoproteins (apos). Lipoproteins are classified according to the densities at which they are isolated: very low (VLDL), low (LDL), intermediate (IDL), and high-density lipoproteins (HDL). Following a fat-containing meal, the intestine secretes triglyceride (TG)-rich lipoproteins called chylomicrons, which are even smaller than VLDLs. The density of lipoproteins is inversely related to their size, with HDL being the smallest yet most dense lipoprotein. The classification of lipoproteins according to their densities is biologically arbitrary but widely accepted. Lipoproteins are not discrete particles, and each class extends over a range of sizes and density subclasses. For example, HDL occurs at two different density ranges: designated HDL3 and HDL2, the larger and usually less-abundant subclass. Figure 1 depicts simple models of the major plasma lipoproteins as oily neutral lipid cores of cholesteryl esters and triglycerides surrounded by a surface of phospholipids and apos. Lipoprotein size is determined by neutral lipid content: chylomicrons and VLDLs are triglyceride-rich, LDLs are cholesteryl ester-rich, and HDLs are rich in protein and phospholipids. The relevant properties and compositions of the human plasma lipoproteins are summarized in Table 1. Nearly all plasma lipids are found on each of the lipoproteins, but their stoichiometries differ across classes and subclasses. The table does not include minor components that have been recently discovered using a proteomics approach.6
Figure 1.
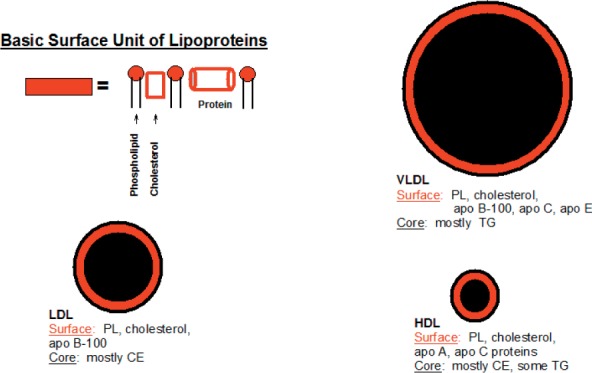
Oil drop model of plasma lipoproteins. Each lipoprotein comprises a neutral lipid core surrounded by a surface monolayer of proteins and phospholipids. LDL: low-density lipoprotein; HDL: high-density lipoprotein; VLDL: very low-density lipoprotein; PL: phospholipid; CE: cholesteryl ester
Table 1.
Properties of human plasma lipoproteins. From Pownell et al.5
The secretion and digestion activities of lipoproteins differ by type. HDLs and VLDLs are secreted by the liver but chylomicrons are secreted by the intestine, and all three are remodeled by plasma activities. Chylomicron and VLDL triglycerides are hydrolyzed by lipoprotein lipase, an enzyme that is activated by apo CII. This activity converts chylomicrons into remnants that are hepatically removed. VLDLs are converted to IDLs that are then converted to LDLs by hepatic lipase activity and finally removed by liver hepatocytes bearing LDL receptors.
After entering the plasma, early forms of HDL are remodeled by three activities. First, cholesterol is esterified by lecithin:cholesterol acyltransferase, which is activated by apo AI, converting the discoidal nascent HDL to its mature spherical form. Second, the cholesteryl ester transfer protein exchanges the HDL cholesteryl esters for VLDL and chylomicron triglycerides, which may be hydrolyzed by hepatic lipase. Finally, the phospholipid transfer protein exchanges phospholipids among HDL subfractions. The mature forms of all native lipoproteins are essentially spherical.
The Apolipoproteins
The apolipoproteins comprise apo B-100, apo B-48, and the exchangeable apos that include apos AI, AII, AIV, AV, CI, CII, CIII, and E. Apo B-100 is hepatically secreted with VLDL as a 500-kDa protein. Apo B-48, which contains 48% of the amino terminus of apo B-100, is secreted by the intestine with chylomicrons. Apo B-100 contains a ligand sequence that attracts LDL—but not VLDL—to the hepatic LDL receptor, where it undergoes endocytosis and degradation. Apo B-48 lacks the LDL receptor ligand sequence; therefore, the disposal of chylomicrons involves lipolysis followed by apo E-mediated uptake of the remnants. The genes for the exchangeable apos, which belong to the same gene family,7 comprise four exons, the first three of which share homology. The members of this gene family are distinguished by exon IV, which codes for sequences that have unique structures and attendant activities. Activities of apos include lecithin:cholesterol acyltransferase activation (apos AI and CI),8 lipoprotein lipase activation (apo CII),9 and binding to the LDL receptor (apo E).10 Apo E occurs as three isoforms—E2, E3, and E4—with E2 being the normal-functioning isoform. E3 and E4 are associated with dyslipidemias and premature cardiovascular disease, and E4 is also a risk factor for Alzheimer's disease.
Drug Delivery
The therapeutic efficacy of drugs is a function of two factors: the local biological effect on cells and cellular targeting and an acceptable safety and risk/benefit profile. Physicochemically, drugs fall into three broad classes—hydrophobic/water-insoluble, polar/water-soluble, and amphiphilic (i.e., drugs that bind to a surface-water interface). Water-soluble drugs are by nature practically systemic; tissue sites penetrated by plasma are readily accessible to water-soluble drugs. Although this does not ensure that the drugs are taken up by cells in all tissues, such an effect is highly likely.
Lipoproteins are potential vehicles for the delivery of hydrophobic and amphiphilic drugs that are more challenging to deliver systemically. These types of drug molecules are expected to associate with the core (hydrophobic) and surface (amphiphilic) of plasma lipoproteins. As natural components of plasma, all lipoproteins can cloak exogenous molecules, permitting a drug to evade an immunological response. In addition, lipoproteins are easily isolated in large quantities by flotation.11 HDL is especially attractive because of its longer plasma half-life compared to LDL and VLDL (~5 days versus ~3 days and ~5 hours, respectively). Reconstituted HDL (rHDL) is readily made in vitro by spontaneous association12 or by detergent-removal methods,13 and drugs can be incorporated into rHDL with the lipid components using either method.
The Hydrophobic Effect and Lipid Bioavailability
The lipophilicity of molecules is a major determinant of their ability to cross biological membranes and other lipid surfaces, including lipoproteins. This has been confirmed with studies of lipids and proteins in which their lipophilicity was altered by the covalent attachment of acyl chains of varying lengths. Studies of fatty acids, their methyl esters, alcohols, and alkanes showed that the rate of transfer for each of these four lipid classes is a predictable function of their hydrophobicity as determined by the number of methylene-methyl groups within each molecule.14 In contrast, the addition of double bonds increases the water solubility of amphiphiles and reduces the free energy of activation for transfer. Moreover, according to chemical kinetics and absolute rate theory, each methylene-methyl moiety contributes ~700 cal/mol to the free energy of activation for transfer between lipid surfaces, a value that varies only slightly among lipids with different functional groups. Increasing the acyl chain length by two methylene units decreases the transfer rate by a factor of eight, whereas each added double bond increases the rate by a factor of four.15 Similar data collected on pyrene-labeled phospholipids showed the same trend, with the specific type of polar head group making only a small difference in the transfer rates.16 The rate-limiting step for molecular transfer between lipid surfaces is desorption of the lipid from the membrane or lipoprotein surface; this process follows Kelvin's law stating that the rate of evaporation of a rain drop is inversely related to the radius of the rain drop. Thus, molecular desorption from surfaces (i.e., lipid transfer and evaporation) are controlled by similar forces.
The principles developed from studies with pyrene-labeled lipids were validated with natural lipids. It was determined that the addition of more methylene units to a molecule increases its transfer time between lipid surfaces in a predictable way, whereas increasing the number of double bonds has the opposite effect.17 A qualitative perspective of this is given in Table 2. Of note, human plasma contains transfer proteins that transport phospholipids and cholesteryl esters between lipoproteins. Similarly, the rates of transfer of natural phospholipids follow Kelvin's law. The transfer time of lipids from VLDL, the largest plasma lipoprotein, is five times longer than that from HDL, the smallest lipoprotein.
Table 2.
Lipid transfer half-times.
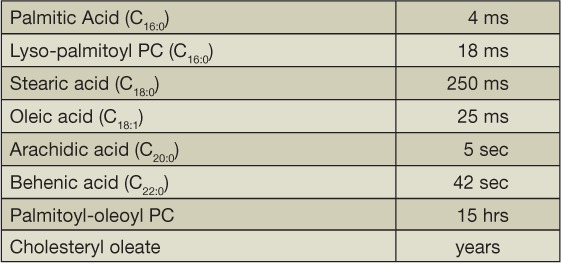
Similar to liposomes, plasma lipoproteins have variable plasma half-lives dependent on the hydrophobicity of their major protein components. For example, free cholesterol binds to LDL but transfers to other lipoproteins on a time scale of minutes, which is orders of magnitude shorter than that of apo B-100 with a plasma half-life of approximately3 days. The cholesteryl ester transfer protein, which transfers cholesteryl esters between lipoproteins, can reduce its plasma half-life by transfer from HDL to VLDL. Interestingly, mice do not express a cholesteryl ester transfer protein.
The transfer mechanisms of phospholipids differ from cholesteryl esters in some ways. Human plasma contains lipid transfer proteins for both cholesteryl esters and phospholipids. The cholesteryl ester transfer protein transfers HDL- and LDL-cholesteryl ester to VLDL in exchange for triglyceride,18 while the phospholipid transfer protein disproportionates HDL into larger and smaller particles.19,20 Both types of transfer proteins likely accelerate the turnover of their target lipids. However, the greatest threat to the life of a plasma phospholipid is lipolysis by hepatic lipase, endothelial lipase, lipoprotein lipase, phospholipases, and lecithin:cholesterol acyltransferase, all of which have phospholipase activities.21,22 Thus, the integrity and survival of a lipoprotein or liposome in plasma depends on its resistance to phospholipolytic activity and a long lipid transfer time. Increasing the transfer time is readily accomplished by increasing the number of methylene units attached to the phospholipid. This can be calculated from log τ1/2 = 0.234n − 0.189m – 5.32, where τ 1/2 is the transfer half-life in minutes, n is the number of methylene + methyl moieties in the acyl chains, and m is the number of double bonds. For example, the calculated τ 1/2 of 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC) from HDL is 280 min, which is relatively short compared to the lifetimes of HDL and LDL. However, replacing the palmitoyl chain of POPC with a behenoyl, which has 20 carbons and no double bonds, increases the transfer time to ~50 hours.
Another challenge to increasing transfer time is that plasma phospholipases rapidly convert a phosphatidylcholine to a fatty acid and a lysophosphatidylcholine, both of which transfer in a matter of milliseconds. One solution is to formulate liposomes and rHDL from apos and phospholipids in which the ester linkages are replaced by ether bonds that are phospholipase-resistant. Ether phospholipids have physical properties similar to those of their ester analogs but resist phospholipolytic activity.23,24 These findings led to studies of phospholipid turnover in rats, which revealed the effects of phospholipid chain length and substitution of ether on the plasma half-life of ester phospholipids.25 Phosphatidylcholine saturated with 28 (dimyristoylphosphatidylcholine, DMPC), 32 (dipalmitoylphosphatidylcholine, DPPC), and 36 acyl carbons (desaturated phosphatidylcholine, DSPC) along with monounsaturated phosphatidylcholine with 34 acyl carbons (POPC) were compared. The phosphatidylcholine esters have affinities that are similar to their ether analogs. In vitro, the lecithin:cholesterol acyltransferase reactivity of these lipids increased: DMPC > DPPC > POPC > DSPC. Following injection into rats, the plasma half-lives of the ester and ether phosphatidylcholines increased: DMPC < DPPC < DSPC < POPC. However, the respective plasma half-lives of the ether phosphatidylcholines were 80%, 60%, 85%, and 110% longer than their ester analogs. The plasma half-life of a phosphatidylcholine increases with increasing acyl chain length and by replacing the acyl ester bonds with ether bonds.
To summarize, an ideal lipid for liposome-based therapies would (1) be resistant to phospholipases, (2) contain two long acyl chains, making it very lipophilic, and (3) be saturated so that it could not be readily oxidized. Diphytanoyl phosphatidylcholine contains two 20-carbon branched acyl chains and satisfies these criteria.26
Figure 2.
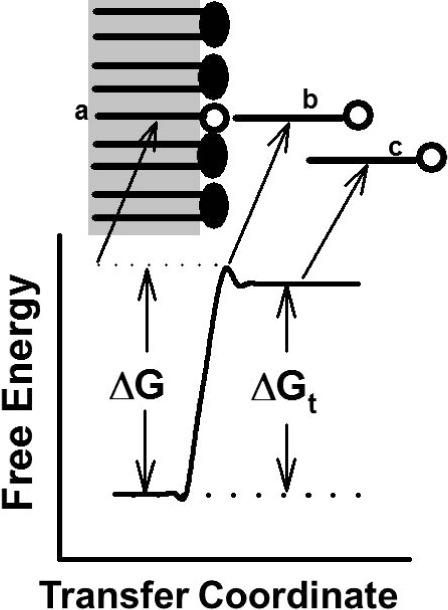
Lipid transfer reaction coordinate. (A) A monoacyl lipid resides in the outer phospholipid monolayer of a membrane or lipoprotein. (B) With sufficient free energy, the lipid escapes the membrane but remains noncovalently tethered, thereby reducing its rotational freedom. (C) As it diffuses into the surrounding aqueous phase, the lipid reaches a lower free energy.
Increasing the Plasma Lifetimes of Proteins
Ponsin et al. tested whether the plasma half-life of an apo could be controlled by the covalent attachment of acyl chains of varying lengths. This was tested comparing a small, synthetic, lipid-associating peptide apo analog (LAP-20; 2 kDa; 20 amino acid residues) with apo AI (28 kDa). Similar to apo AI, LAP-20 binds HDL and activates lecithin:cholesterol acyltransferase.27 Moreover, increasing the length of the acyl chain attached to the LAP-20 amino terminus increased binding to rHDL by three orders of magnitude.28 The plasma half-lives of the various acylated LAP-20, measured in rats, increased as the acyl chain length was increased from 0 to 16 and the site of LAP-20 degradation shifted from the kidneys to the liver.29
Comparison of in vitro and in vivo data suggested that the plasma half-life of a protein can be controlled by graded acylation and that the plasma half-life of LAP-20 could be greatly extended by hyperacylation. This was tested with a diacyl LAP-20 that was shown to be nontransferable and a valid biomarker for HDL.30 Interestingly, the rate of clearance of diLAP-labeled HDL was slower than that of apo AI. A fraction of native human apo AI is cleared by the kidneys, whereas the liver was the preferred site for diLAP-labeled uptake.
Reconstituted HDL
Reconstituted HDL (rHDL) therapy for atherosclerosis and other disorders has been the focus of numerous studies, some of which showed that apo AI and phospholipids self-assemble into rHDL,31–33 enhance cholesterol efflux from cells,33,34 and have purported antiatherosclerotic effects.35 For example, rHDL treatment reverses atherosclerotic plaques36–38 and also improves endothelial function in patients with isolated low HDL cholesterol.39
Figure 3.
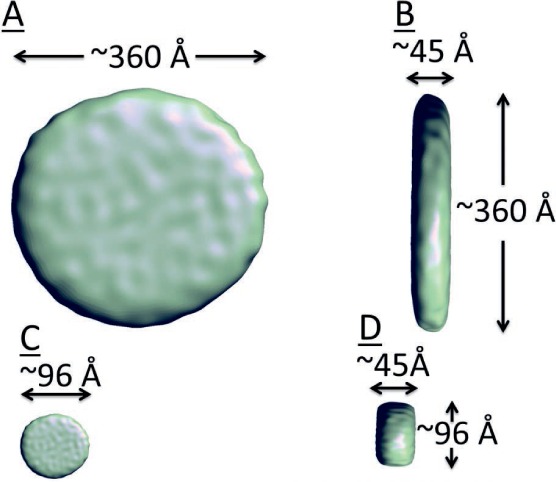
Electron cryomicroscopy reconstruction of reconstituted HDL (rHDL). (A) Isosurface representation of rHDL viewed in an en face orientation. The diameter of the particle is ~360 Å. (B) Isosurface representation of the rHDL particle viewed laterally; the thickness is ~45 Å, the same as that of a single dimyristoylphosphatidylcholine bilayer. (C, D) Isosurface representation of rHDL scaled to diameter = 96 Å, but preserving thickness viewed in en face and lateral orientations assuming the same thickness but different density.
Reconstituted HDL has been studied by Raman spectroscopy, 13C NMR, calorimetry, chemical cross-linking, molecular dynamics, hydrogen-deuterium exchange, and electron microscopy. Studies have shown that increasing rHDL's free cholesterol content up to 20 mol% increases its size from ~10 to ~40 nm.40 An early model of rHDL was a bilayer disc circumscribed by two antiparallel apo AI molecules in a head-to-tail configuration.41 An alternative structure, the superhelical model, was based on small-angle neutron scattering and molecular dynamics simulations.42,43 However, electron cryomicroscopy and image reconstruction of rHDL containing 15 mol% cholesterol and 8 apo AI molecules per particle40 shows rHDL as a 360 Å disc with a thickness of ~45 Å, which corresponds to the expected dimensions of a phospholipid bilayer.44 Although negative stain electron microscopy studies support a discoidal model, this approach can introduce artifacts, especially in lipidated molecules. In addition, molecules do not always remain in their native conformations when using native stain electron microscopy. However, with the electron cryomicroscopy method, the rapid freezing of the sample locks the particle in its native conformation.
Conclusion
Native and reconstituted lipoproteins bind to a myriad of hydrophobic and amphiphilic molecules with an affinity that is determined by the number of methylene and methyl groups. Reconstituted HDL are particularly attractive vehicles for drugs because they can be easily prepared and can accommodate a variety of lipophilic molecules. Preparing these and other carriers with ether phospholipids, which allow lipoproteins to cloak exogenous molecules, is likely to greatly increase their time in the plasma and, therefore, their therapeutic potential.
Key Points
The plasma lifetime of molecules that associate with liposomes or lipoproteins can be controlled in a predictable way by the covalent attachment of acyl chains of varying lengths. This approach can be used to make nontransferable analogs of lipids and proteins.
The in vivo plasma lifetime of a phospholipid increases with its hydrophobicity and can be further increased by replacing ester bonds with nonhydrolyzable ether bonds.
The sizes of rHDL prepared from apolipoprotein AI and phospholipids can be increased by the addition of free cholesterol; electron cryomicroscopy shows that rHDL, which are potential nanoparticle carriers of hydrophobic drugs, are discoidal.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1. Buxton DB, Lee SC, Wickline SA, Ferrari M; National Heart, Lung, Blood Institute Nanotechnology Working Group. . Recommendations of the National Heart, Lung, and Blood Institute. Nanotechnology Working Group. Circulation. 2003. December 2; 108( 22): 2737– 42. [DOI] [PubMed] [Google Scholar]
- 2. Blanco E, Shen H, Ferrari M.. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015. September; 33( 9): 941– 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrari M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotechnol. April 2010; 28( 4): 181– 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lasic DD, Papahadjopoulos D. Liposomes revisited. Science. 1995. March 3; 267( 5202): 1275– 6. [DOI] [PubMed] [Google Scholar]
- 5. Pownall H, Gillard BK, Moone JE, Gotto AM.. Human plasma lipoprotein metabolism. : Ballantyne C, . Clinical Lipidology. Philadelphia, PA: Elsevier; 2015; p 10. [Google Scholar]
- 6. Davidson WS, Silva RA, Chantepie S, Lagor WR, Chapman MJ, Kontush A.. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler Thromb Vasc Biol. 2009. June; 29( 6): 870– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li WH, Tanimura M, Luo CC, Datta S, Chan L.. The apolipoprotein multigene family: biosynthesis, structure, structure-function relationships, and evolution. J Lipid Res. 1988. March; 29( 3): 245– 71. [PubMed] [Google Scholar]
- 8. Fielding CJ, Shore VG, Fielding PE.. A protein cofactor of lecithin:cholesterol acyltransferase. Biochem Biophys Res Commun. 1972. February 25; 46( 4): 1493– 8. [DOI] [PubMed] [Google Scholar]
- 9. Ekman R, Nilsson-Ehle P. Effects of apolipoproteins on lipoprotein lipase activity of human adipose tissue. Clin Chim Acta. 1975. August 18; 63( 1): 29– 35. [DOI] [PubMed] [Google Scholar]
- 10. Innerarity TL, Mahley RW. Enhanced binding by cultured human fibroblasts of apo-E-containing lipoproteins as compared with low density lipoproteins. Biochemistry (Mosc). 1978. April 18; 17( 8): 1440– 7. [DOI] [PubMed] [Google Scholar]
- 11. Havel RJ, Eder HA, Bragdon JH.. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955. September; 34( 9): 1345– 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pownall HJ, Massey JB, Kusserow SK, Gotto AM Jr.. Kinetics of lipid--protein interactions: interaction of apolipoprotein A-I from human plasma high density lipoproteins with phosphatidylcholines. Biochemistry (Mosc). 1978. April 4; 17( 7): 1183– 8. [DOI] [PubMed] [Google Scholar]
- 13. Pownall HJ, Van Winkle WB, Pao Q, Rohde M, Gotto AM Jr.. Action of lecithin:cholesterol acyltransferase on model lipoproteins. Preparation and characterization of model nascent high density lipoprotein. Biochim Biophys Acta. 1982. December 13; 713( 3): 494– 503. [DOI] [PubMed] [Google Scholar]
- 14. Pownall H, Hickson DL, Smith LC.. Transport of biological lipophiles: Effect of lipophile structure. J Amer Chem Soc. 1983; 105: 2440– 5. [Google Scholar]
- 15. Massey JB, Bick DH, Pownall HJ.. Spontaneous transfer of monoacyl amphiphiles between lipid and protein surfaces. Biophys J. 1997. April; 72( 4): 1732– 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Massey JB, Gotto AM Jr, Pownall HJ.. Kinetics and mechanism of the spontaneous transfer of fluorescent phospholipids between apolipoprotein-phospholipid recombinants. Effect of the polar headgroup. J Biol Chem. 1982. May 25; 257( 10): 5444– 8. [PubMed] [Google Scholar]
- 17. Pownall HJ, Bick DL, Massey JB.. Spontaneous phospholipid transfer: development of a quantitative model. Biochemistry. 1991. June 11; 30( 23): 5696– 700. [DOI] [PubMed] [Google Scholar]
- 18. Barter PJ, Brewer HB Jr, Chapman MJ, Hennekens CH, Rader DJ, Tall AR.. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003. February 1; 23( 2): 160– 7. [DOI] [PubMed] [Google Scholar]
- 19. Rao R, Albers JJ, Wolfbauer G, Pownall HJ.. Molecular and macromolecular specificity of human plasma phospholipid transfer protein. Biochemistry. 1997. March 25; 36( 12): 3645– 53. [DOI] [PubMed] [Google Scholar]
- 20. Settasatian N, Duong M, Curtiss LK, . et al. The mechanism of the remodeling of high density lipoproteins by phospholipid transfer protein. J Biol Chem. 2001. July 20; 276( 29): 26898– 905. [DOI] [PubMed] [Google Scholar]
- 21. Wong H, Schotz MC. The lipase gene family. J Lipid Res. 2002. July; 43( 7): 993– 9. [DOI] [PubMed] [Google Scholar]
- 22. Aron L, Jones S, Fielding CJ.. Human plasma lecithin-cholesterol acyltransferase. Characterization of cofactor-dependent phospholipase activity. J Biol Chem. 1978. October 25; 253( 20): 7220– 6. [PubMed] [Google Scholar]
- 23. Pownall HJ, Pao Q, Massey JB.. Acyl chain and headgroup specificity of human plasma lecithin:cholesterol acyltransferase. Separation of matrix and molecular specificities. J Biol Chem. 1985. February 25; 260( 4): 2146– 52. [PubMed] [Google Scholar]
- 24. Massey JB, Pao Q, Van Winkle WB, Pownall HJ.. Interaction of human plasma lecithin:cholesterol acyltransferase and venom phospholipase A2 with apolipoprotein A-I recombinants containing nonhydrolyzable diether phosphatidylcholines. J Biol Chem. 1985. September 25; 260( 21): 11719– 23. [PubMed] [Google Scholar]
- 25. Pownall HJ, Hickson-Bick D, Massey JB.. Effects of hydrophobicity on turnover of plasma high density lipoproteins labeled with phosphatidylcholine ethers in the rat. J Lipid Res. 1991. May; 32( 5): 793– 800. [PubMed] [Google Scholar]
- 26. Pownall HJ, Pao Q, Brockman HL, Massey JB.. Inhibition of lecithin-cholesterol acyltransferase by diphytanoyl phosphatidylcholine. J Biol Chem. 1987. July 5; 262( 19): 9033– 6. [PubMed] [Google Scholar]
- 27. Pownall HJ, Hu A, Gotto AM Jr, Albers JJ, Sparrow JT.. Activation of lecithin:cholesterol acyltransferase by a synthetic model lipid-associating peptide. Proc Natl Acad Sci U S A. 1980. June; 77( 6): 3154– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ponsin G, Strong K, Gotto AM Jr, Sparrow JT, Pownall HJ.. In vitro binding of synthetic acylated lipid-associating peptides to high-density lipoproteins: effect of hydrophobicity. Biochemistry. 1984. October 23; 23( 22): 5337– 42. [DOI] [PubMed] [Google Scholar]
- 29. Ponsin G, Sparrow JT, Gotto AM Jr, Pownall HJ.. In vivo interaction of synthetic acylated apopeptides with high density lipoproteins in rat. J Clin Invest. 1986. February; 77( 2): 559– 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ponsin G, Pulcini T, Sparrow JT, Gotto AM Jr, Pownall HJ.. High density lipoprotein interconversions in rat and man as assessed with a novel nontransferable apolipopeptide. J Biol Chem. 1993. February 15; 268( 5): 3114– 9. [PubMed] [Google Scholar]
- 31. Forte TM, Nichols AV, Gong EL, Levy RI, Lux S.. Electron microscopic study on reassembly of plasma high density apoprotein with various lipids. Biochim Biophys Acta. 1971. November 5; 248( 2): 381– 6. [DOI] [PubMed] [Google Scholar]
- 32. Hirz R, Scanu AM. Reassembly in vitro of a serum high-density lipoprotein. Biochim Biophys Acta. 1970. May 26; 207( 2): 364– 7. [DOI] [PubMed] [Google Scholar]
- 33. Jackson RL, Gotto AM, Stein O, Stein Y.. A comparative study on the removal of cellular lipids from Landschütz ascites cells by human plasma apolipoproteins. J Biol Chem. 1975. September 25; 250( 18): 7204– 9. [PubMed] [Google Scholar]
- 34. Bates SR, Rothblat GH. Regulation of cellular sterol flux and synthesis by human serum lipoproteins. Biochim Biophys Acta. 1974. July 26; 360( 1): 38– 55. [DOI] [PubMed] [Google Scholar]
- 35. Orekhov AN, Misharin A, Tertov VV, . et al. Artificial HDL as an anti-atherosclerotic drug. Lancet. 1984. November 17; 2( 8412): 1149– 50. [DOI] [PubMed] [Google Scholar]
- 36. Nissen SE, Tsunoda T, Tuzcu EM, . et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003. November 5; 290( 17): 2292– 300. [DOI] [PubMed] [Google Scholar]
- 37. Nicholls SJ, Tuzcu EM, Sipahi I, . et al. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A-I Milano. J Am Coll Cardiol. 2006. March 7; 47( 5): 992– 7. [DOI] [PubMed] [Google Scholar]
- 38. Shaw JA, Bobik A, Murphy A, . et al. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008. November 7; 103( 10): 1084– 91. [DOI] [PubMed] [Google Scholar]
- 39. Bisoendial RJ, Hovingh GK, Levels JH, . et al. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation. 2003. June 17; 107( 23): 2944– 8. [DOI] [PubMed] [Google Scholar]
- 40. Massey JB, Pownall HJ. Cholesterol is a determinant of the structures of discoidal high density lipoproteins formed by the solubilization of phospholipid membranes by apolipoprotein A-I. Biochim Biophys Acta. 2008. May; 1781( 5): 245– 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Davidson WS, Silva RA. Apolipoprotein structural organization in high density lipoproteins: belts, bundles, hinges and hairpins. Curr Opin Lipidol. 2005. June; 16( 3): 295– 300. [DOI] [PubMed] [Google Scholar]
- 42. Gogonea V, Wu Z, Lee X, . et al. Congruency between biophysical data from multiple platforms and molecular dynamics simulation of the double-super helix model of nascent high-density lipoprotein. Biochemistry. 2010. August 31; 49( 34): 7323– 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu Z, Gogonea V, Lee X, . et al. Double superhelix model of high density lipoprotein. J Biol Chem. 2009. December 25; 284( 52): 36605– 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murray SC, Gillard BK, Ludtke SJ, Pownall HJ.. Direct Measurement of the Structure of Reconstituted High-Density Lipoproteins by Cryo-EM. Biophys J. 2016. February 23; 110( 4): 810– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]


