Abstract
Atherosclerosis is a chronic inflammatory vascular wall disease, and endothelial cell dysfunction plays an important role in its development and progression. Under the influence of laminar shear stress, however, the endothelium releases homeostatic factors such as nitric oxide and expresses of vasoprotective microRNAs that are resistant to atherosclerosis. Adhesion molecules such as E-selectin, exhibited on the endothelial surface, recruit monocytes that enter the vessel wall to form foam cells. Accumulation of these foam cells form fatty streaks that may progress to atherosclerotic plaques in the blood vessel wall. Interestingly, E-selectin may also serve as an affinity moiety for targeted drug delivery against atherosclerosis. We have recently developed an E-selectin-targeted platform that enriches therapeutic microRNAs in the inflamed endothelium to inhibit formation of vulnerable atherosclerotic plaques.
Keywords: atherosclerosis, vulnerable plaque, endothelial cell, microRNA, nanoparticle
Introduction
Atherosclerosis is a chronic inflammatory cardiovascular disease in which plaque forms in the blood vessel and may progress to impede blood flow.1 Rupture of an atherosclerotic plaque with associated thrombosis may cause an acute blockage that can precipitate a stroke, heart attack, and even death. Multiple factors can increase the risk of atherosclerosis, including a family history of the disease, high blood levels of low-density lipoprotein cholesterol (LDL-C) or triglycerides, low levels of high density lipoprotein cholesterol (HDL-C), hypertension, smoking, gender, race, diabetes, obesity, a high-fat diet, and a sedentary lifestyle.1,2 Each of these risk factors may contribute to alterations in endothelial cells that can promote an inflammatory response.3 For example, LDL-C can be trapped by proteoglycans in the subendothelial space and oxidized. The oxidized LDL-C is then recognized by macrophages, which accumulate the lipid and become foam cells. These foam cells release cytokines and chemokines that attract more monocytes and trigger smooth muscle cell (SMC) proliferation, causing the lesion to progress. An inflamed plaque can become unstable due to thinning of the fibrous cap, making the lesion susceptible to rupture, which in turn can cause an acute thrombosis and occlusion of the vessel and/or embolism, with occlusion of smaller vessels downstream.4 When the endothelium is exposed to steady laminar shear stress, however, it undergoes cellular responses that release homeostatic factors such as nitric oxide and vasoprotective microRNAs that make it resistant to atherosclerosis.
One arena of research activity in cardiovascular medicine involves the design and development of multifunctional devices to target, diagnose, and treat atherosclerosis. In this review, we discuss the potential of targeting the inflammatory endothelium with shear stress-inducible microRNAs to reverse atherosclerosis and vascular complications associated with metabolic syndrome.
Endothelial Dysfunction and Atherosclerosis
Endothelial cells (ECs) produce several bioactive substances, including nitric oxide (NO), prostaglandins, reactive oxygen species, and vasoactive peptides such as angiotensin II. Imbalance of these factors promotes vasoconstriction, inflammatory cell adhesion and migration, platelet aggregation, and vascular SMC proliferation that eventually lead to atherogenesis. Metabolic alterations associated with diabetes (e.g., hyperglycemia, dyslipidemia, and insulin resistance) impair EC function by initiating processes that lead to plaque formation and worsen the outcome of atherosclerosis-related cardiovascular events.
Atherosclerosis is a chronic and progressive inflammatory disease of the arterial wall that is mostly symptomatic when it affects the carotid arteries, coronary arteries, or aortoiliac and infrainguinal arteries.5 The atherosclerotic plaque contains varying degrees of lipid, extracellular matrix, calcification, and cell debris as well as immune cells and vascular SMCs. These lesions typically form in the intima of large- and medium-sized arteries.6 Accumulating evidence suggests that endothelial inflammation plays a major role in the growth and progression of atherosclerosis.7 Inflamed ECs express adhesion molecules that mediate recruitment of immune cells, such as monocytes and lymphocytes, to promote the initiation and evolution of atheroma.8 At advanced stages of atherosclerosis, endothelial inflammation could also destabilize the plaque.9,10 Vulnerable plaques are prone to rupture, resulting in thrombosis and acute cardiovascular events.11 To date, pharmacotherapies to prevent the progression of atherosclerosis have been largely directed toward management of risk factors, e.g., lowering LDL-C levels. While this approach has been effective, cardiovascular disease remains a major public health problem. Therefore, it is of great importance to develop new technologies and therapeutic approaches that can dramatically hinder or even reverse the progression of atherosclerosis to further reduce cardiovascular morbidity and mortality.
Shear Stress and Atherosclerosis
Blood vessels are constantly subjected to hemodynamic forces, including cyclic stretch, hydrostatic pressure, and fluid shear stress. Vascular ECs, the monolayer directly in contact with blood flow, are constantly exposed to the tractive force of fluid flow, which is termed shear stress. Steady laminar shear stress, which typically occurs in the thoracic or abdominal aorta, causes ECs to release autocrine and paracrine factors that regulate inflammatory responses, thrombosis/homeostasis, vascular remodeling, and vascular SMC phenotype.20–22 However, atherosclerotic plaque tends to form in arterial branches and curvatures where there is disturbed blood flow (e.g., recirculation eddies, flow separation and reattachment, and reciprocating flow).23–27 Activated endothelium recruits circulating monocytes that eventually differentiate into macrophages and accumulate in the lesion, suggesting that EC dysfunction initiates atherosclerosis.12–15 In fact, several endothelial surface molecules and receptors have been shown to participate in the initiation of inflammation and plaque progression.16–19
Low, reciprocating, and oscillatory shear stress induces a number of atherogenic genes in ECs. These include adhesion molecules, chemokines, oxidative enzymes, vasoconstrictors, and other genes involved in SMC proliferation and migration, extracellular matrix remodeling, lipid synthesis, and inflammation—all of which lead to the development of atherosclerotic lesions. These changes are accompanied by a decrease in antioxidant enzymes or molecules, such as heme oxygenase 1, glutathione, NO, prostacyclin, and growth-arrest hormones.28–30 Conversely, laminar and pulsatile flow produce an opposing effect on gene regulation in ECs and SMCs.31–33
MicroRNA and Shear Stress
MicroRNAs are highly conserved, non-coding RNAs that regulate gene expression post-transcriptionally by binding to the sequence located in the 3'-untranslated region of the target gene mRNA, thus inhibiting protein translation and reducing mRNA stability. The emerging role of microRNAs has been implicated in coronary heart disease, heart failure, atherosclerosis, and hypertension. Notably, several microRNAs have been identified as being regulated by shear stress, including miR-92a, miR-21, miR-663, miR-19, miR-126, and the miR-143/145 cluster.34–40 However, the effect of shear stress on microRNA expression and function in the vasculature during the development of atherosclerotic plaques is poorly understood.
MicroRNA arrays have given us significant insight in identifying some of the important microRNAs that are altered under laminar shear stress conditions. We have further confirmed a decrease of miR-146a and miR-181b in the aortic arch of type 2 diabetic db/db mice, although these microRNAs were upregulated after treadmill exercise. Both microRNAs were also upregulated by laminar flow in mouse aortic ECs. These data prompted us to further explore the contribution of microRNAs in mediating the atheroprotective effect of shear stress. One of the challenges, however, is that RNA molecules are unstable in circulation and cellular microenvironments in vivo. Overexpression of microRNAs by viral vectors leads to unwanted genomic integration of viral DNA. Therefore, it is necessary to develop a robust and stable delivery system, such as a nanoparticle platform, that can enable efficient delivery of functional microRNAs.
Nanotechnology in Targeted Drug Delivery
Nanotechnology has played a significant role in the development of therapeutic interventions.41,42 MicroRNAs are synthetic nucleic acids that are vulnerable to degradation by plasma and tissue nucleases. Their negative charge under physiological conditions also prohibits effective cell entry. Consequently, multiple forms of nanovectors have been developed to deliver nucleic acid-based therapeutics, including microRNAs.43,44 The multistage vector (MSV) platform was designed to overcome sequential biological barriers for drug delivery.45 It is one of the most powerful delivery vehicles within this group. We have applied MSVs to effectively deliver small interfering RNA (siRNA) and microRNA into primary and metastatic tumors and demonstrated potent inhibition of gene expression and subsequent inhibition of tumor growth.46–48 We recently identified a thioaptamer that binds specifically to E-selectin (ESTA) with a high affinity and conjugated ESTA onto the surface of a MSV to generate a delivery system that specifically targets the inflamed vasculature.49 The feasibility of this ESTA-MSV delivery system has been successfully tested in mouse xenograft models of metastatic breast cancer and leukemia and has demonstrated drug enrichment in the disease lesions.49,50
Treating Atherosclerosis through Targeted Delivery of MicroRNAs to Inflamed Vasculature
In a recent study, we used an ESTA-MSV to deliver miR-146a and miR-181b for the treatment of atherosclerosis, taking advantage of overexpressed E-selectin levels in the inflamed endothelium.51 These two shear force-regulated microRNAs function by suppressing CCL2 expression, which eliminates the chemokine essential for recruiting monocytes to the inflammatory vasculature. The miR-146a- and miR-181b-loaded microparticles bind the over-expressed endothelial surface protein E-selectin at the disease lesion and release the cargo on site. The nanoformulated microRNAs are then internalized by the endothelial cells, where they exert their biological functions (Figure 1).
Figure 1.
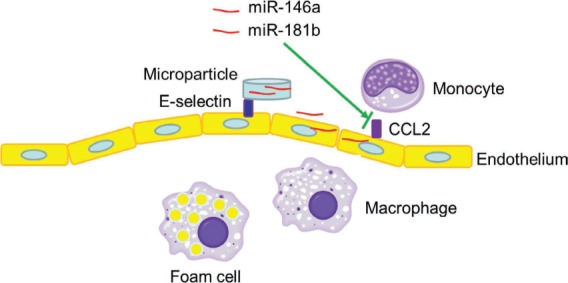
Schematic view of miR-146a and miR-181b microRNAs delivered by E-selectin thioaptamer-multistage vector for treatment of atherosclerosis. CCL2: monocyte chemoattractant protein-1
In our study, microRNAs were packaged into polyethylene glycol-polyethyleneimine (PEG-PEI) polyplex nanoparticles and loaded into the 45- to 80-nm nanopores of ESTA-MSV microparticles. We treated high-fat diet-challenged ApoE knockout mice with PEG-PEI/microRNA in their free nanoparticle forms or in ESTA-MSV and examined plaque formation. In the groups treated with PEG/PEI/miRNAs, there was a moderate decrease of plaque size compared to the vehicle-treated group; however, both microRNAs packaged in ESTA-MSV microparticles significantly decreased the plaque size (Figure 2).51 This result demonstrates the power of targeted delivery of therapeutics to the disease lesion for effective treatment.
Figure 2.
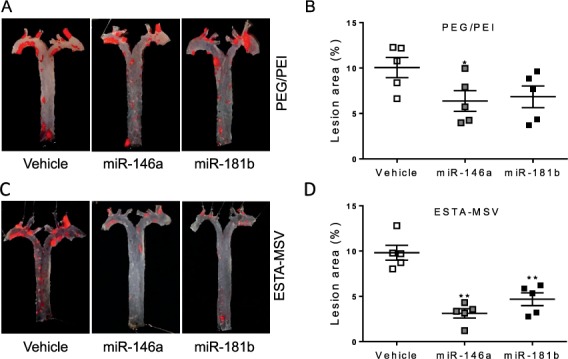
Effects of PEG/PEI/micro-RNAs and ESTA-MSV/microRNAs on the aortic atherogenesis of apolipoprotein E-deficient (ApoE−/−) mice. (A) Representative en face Oil Red O-stained aortic arches and thoracic aortae of ApoE−/− mice intravenously injected with vehicle, miR-146a, and miR-181b (15 μg) loaded in PEG/PEI nanoparticles or (C) ESTA-MSV microparticles biweekly for 12 weeks. (B, D) Quantification of en face aortic plaque size. * P < 0.05, **P < 0.01 vs vehicle group. Adapted from Ma et al.51 ESTA-MSV: E-selectin thioaptamer-multistage vector; PEG-PEI: polyethylene glycol-polyethyleneimine
Conclusion
Recent advances in science and technology have provided us with unprecedented opportunities to develop novel treatments for cardiovascular diseases. Our research has demonstrated the power of disease lesion-targeted delivery of therapeutic microRNAs. This approach may be applicable to any disease with an inflammatory vasculature.
Key Points
The inflammatory vasculature in atherosclerotic lesions displays a unique expression pattern of surface proteins.
The overexpressed vascular endothelial surface protein E-selectin serves as a docking site for targeted delivery of therapeutics.
Enrichment of shear stress-inducible microRNAs prevents the formation of vulnerable atherosclerotic lesions.
Footnotes
Conflict of Interest Disclosure: Drs. Shen, Wong, and Ma are supported by two grants from the George and Angelina Kostas Research Center.
References
- 1. Libby P, Ridker PM, Maseri A.. Inflammation and atherosclerosis. Circulation. 2002. March 5; 105( 9): 1135– 43. [DOI] [PubMed] [Google Scholar]
- 2. Beckman JA, Creager MA, Libby P.. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002. May 15; 287( 19): 2570– 81. [DOI] [PubMed] [Google Scholar]
- 3. Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008. January; 54( 1): 24– 38. [DOI] [PubMed] [Google Scholar]
- 4. Casscells W, Naghavi M, Willerson JT.. Vulnerable atherosclerotic plaque: a multifocal disease. Circulation. 2003. April 29; 107( 16): 2072– 5. [DOI] [PubMed] [Google Scholar]
- 5. Libby P, Ridker PM, Hansson GK.. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011. May 19; 473( 7347): 317– 25. [DOI] [PubMed] [Google Scholar]
- 6. Libby P, Ridker PM, Hansson GK; Leducq Transatlantic Network on Atherothrombosis. . Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009. December 1; 54( 23): 2129– 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jaipersad AS, Lip GY, Silverman S, Shantsila E.. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol. 2014. January 7–14; 63( 1): 1– 11. [DOI] [PubMed] [Google Scholar]
- 8. Zernecke A, Weber C. Chemokines in atherosclerosis: proceedings resumed. Arterioscler Thromb Vasc Biol. 2014. April; 34( 4): 742– 50. [DOI] [PubMed] [Google Scholar]
- 9. Shah PK. Inflammation and plaque vulnerability. Cardiovasc Drugs Ther. 2009. February; 23( 1): 31– 40. [DOI] [PubMed] [Google Scholar]
- 10. Camici PG, Rimoldi OE, Gaemperli O, Libby P.. Non-invasive anatomic and functional imaging of vascular inflammation and unstable plaque. Eur Heart J. 2012. June; 33( 11): 1309– 17. [DOI] [PubMed] [Google Scholar]
- 11. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013. May 23; 368( 21): 2004– 13. [DOI] [PubMed] [Google Scholar]
- 12. Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011. January; 91( 1): 327– 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moore KJ, Sheedy FJ, Fisher EA.. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013. October; 13( 10): 709– 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flammer AJ, Anderson T, Celermajer DS, . et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012. August 7; 126( 6): 753– 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Libby P, Lichtman AH, Hansson GK.. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013. June 27; 38( 6): 1092– 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoon MH, Reriani M, Mario G, . et al. Long-term endothelin receptor antagonism attenuates coronary plaque progression in patients with early atherosclerosis. Int J Cardiol. 2013. September 30; 168( 2): 1316– 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmitt MM, Megens RT, Zernecke A, . et al. Endothelial junctional adhesion molecule-a guides monocytes into flow-dependent predilection sites of atherosclerosis. Circulation. 2014. January 7; 129( 1): 66– 76. [DOI] [PubMed] [Google Scholar]
- 18. Funk SD, Yurdagul A Jr, Albert P, . et al. EphA2 activation promotes the endothelial cell inflammatory response: a potential role in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012. March; 32( 3): 686– 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Gils JM, Ramkhelawon B, Fernandes L, . et al. Endothelial expression of guidance cues in vessel wall homeostasis dysregulation under proatherosclerotic conditions. Arterioscler Thromb Vasc Biol. 2013. May; 33( 5): 911– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007. March; 292( 3): H1209– 24. [DOI] [PubMed] [Google Scholar]
- 21. Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995. July; 75( 3): 519– 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li YS, Haga JH, Chien S.. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005. October; 38( 10): 1949– 71. [DOI] [PubMed] [Google Scholar]
- 23. Kilner PJ, Yang GZ, Mohiaddin RH, Firmin DN, Longmore DB.. Helical and retrograde secondary flow patterns in the aortic arch studied by three-directional magnetic resonance velocity mapping. Circulation. 1993. November; 88( 5 Pt 1): 2235– 47. [DOI] [PubMed] [Google Scholar]
- 24. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005. June 28; 111( 25): 3481– 8. [DOI] [PubMed] [Google Scholar]
- 25. Stone PH, Coskun AU, Yeghiazarians Y, . et al. Prediction of sites of coronary atherosclerosis progression: In vivo profiling of endothelial shear stress, lumen, and outer vessel wall characteristics to predict vascular behavior. Curr Opin Cardiol. 2003. November; 18( 6): 458– 70. [DOI] [PubMed] [Google Scholar]
- 26. VanderLaan PA, Reardon CA, Getz GS.. Site specificity of atherosclerosis: Site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004. January; 24( 1): 12– 22. [DOI] [PubMed] [Google Scholar]
- 27. Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res. 1990. April 1; 66: 1045– 66 [DOI] [PubMed] [Google Scholar]
- 28. Hsiai TK, Cho SK, Wong PK, . et al. Monocyte recruitment to endothelial cells in response to oscillatory shear stress. FASEB J. 2003. September; 17( 12): 1648– 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shyy YJ, Hsieh HJ, Usami S, Chien S.. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci U S A. 1994. May 24; 91( 11): 4678– 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hwang J, Ing MH, Salazar A, Lassègue B, Griendling K, Navab M, Sevanian A, Hsiai TK.. Pulsatile versus oscillatory shear stress regulates nadph oxidase subunit expression: Implication for native ldl oxidation. Circ Res. 2003. December 12; 93( 12): 1225– 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malek AM, Gibbons GH, Dzau VJ, Izumo S.. Fluid shear stress differentially modulates expression of genes encoding basic fibro-blast growth factor and platelet-derived growth factor B chain in vascular endothelium. J Clin Invest. 1993. October; 92( 4): 2013– 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998. May; 18( 5): 677– 85. [DOI] [PubMed] [Google Scholar]
- 33. Berk BC. Atheroprotective signaling mechanisms activated by steady laminar flow in endothelial cells. Circulation. 2008. February 26; 117( 8): 1082– 9. [DOI] [PubMed] [Google Scholar]
- 34. Schober A, Nazari-Jahantigh M, Wei Y, . et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014. April; 20( 4): 368– 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Son DJ, Kumar S, Takabe W, . et al. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat Commun. 2013; 4: 3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hergenreider E, Heydt S, Tréguer K, . et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012. February 12; 14( 3): 249– 56. [DOI] [PubMed] [Google Scholar]
- 37. Zhou J, Wang KC, Wu W, . et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. 2011. June 21; 108( 25): 10355– 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ni CW, Qiu H, Jo H.. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol. 2011. May; 300( 5): H1762– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qin X, Wang X, Wang Y, . et al. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2010. February 16; 107( 7): 3240– 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Loyer X, Potteaux S, Vion AC, . et al. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res. 2014. January 31; 114( 3): 434– 43. [DOI] [PubMed] [Google Scholar]
- 41. Blanco E, Shen H, Ferrari M.. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015. September; 33( 9): 941– 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferrari M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotechnol. 2010. April; 28( 4): 181– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shen H, Mittal V, Ferrari M, Chang J.. Delivery of gene silencing agents for breast cancer therapy. Breast Cancer Res. 2013. May 8; 15( 3): 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shen H, Sun T, Ferrari M.. Nanovector delivery of siRNA for cancer therapy. Cancer Gene Ther. 2012. June; 19( 6): 367– 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tasciotti E, Liu X, Bhavane R, . et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol. 2008. March; 3( 3): 151– 7. [DOI] [PubMed] [Google Scholar]
- 46. Shen H, Rodriguez-Aguayo C, Xu R, . et al. Enhancing chemotherapy response with sustained EphA2 silencing using multistage vector delivery. Clin Cancer Res. 2013. April 1; 19( 7): 1806– 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu R, Huang Y, Mai J, . et al. Multistage vectored siRNA targeting ataxia-telangiectasia mutated for breast cancer therapy. Small. 2013. May 27; 9( 9–10): 1799– 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dave B, Granados-Principal S, Zhu R, . et al. Targeting RPL39 and MLF2 reduces tumor initiation and metastasis in breast cancer by inhibiting nitric oxide synthase signaling. Proc Natl Acad Sci U S A. 2014. June 17; 111( 24): 8838– 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mai J, Huang Y, Mu C, . et al. Bone marrow endothelium-targeted therapeutics for metastatic breast cancer. J Control Release. 2014. August 10; 187: 22– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zong H, Sen S, Zhang G, . et al. In vivo targeting of leukemia stem cells by directing parthenolide-loaded nanoparticles to the bone marrow niche. Leukemia. 2015. December 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ma S, Tian XY, Zhang Y, . et al. E-selectin-targeting delivery of microRNAs by microparticles ameliorates endothelial inflammation and atherosclerosis. Sci Rep. 2016. March 9; 6: 22910. [DOI] [PMC free article] [PubMed] [Google Scholar]


