Abstract
Many of the newly developed drugs for cancer, and some of those for cardiovascular disease, are poorly soluble in water and cannot be taken orally. This can be overcome by employing a new and effective delivery system utilizing nanotechnology. We present a new method for oral preparation of poorly soluble drugs that entails assembling (printing) drug-loaded polymeric micelles into sub-100 nm orally acceptable nanorods (NRs). Due to their small size, these NRs will have a high permeability through cells and thus should transport through the intestine to allow for drug delivery in the blood. These NRs drugs are expected to penetrate tumors more efficiently and much faster than individual nanoparticles and may also be useful for drug delivery to atherosclerotic plaque. This should lead to better bioavailability of the drug with reduced toxicity and side effects. Currently used micellar formulations are administered intravenously, which is invasive and could be toxic due to high doses and interaction with normal healthy tissues. Oral drug administration is the easiest and most desirable way to deliver most drugs, including those that are poorly soluble.
Keywords: drug-loaded micelles, nanorods, poorly soluble drugs, nanoparticles
Introduction
Many new drugs developed in the pharmaceutical industry are poorly soluble in water and therefore are administered intravenously with the help of solubilizing agents.1 However, intravenous (IV) administration is difficult for chronic therapies. The placement of an IV line for the chronic administration of a drug requires specialized techniques and technology that increase costs. Furthermore, indwelling lines represent a nidus for infection and/or thrombosis that further complicates care and increases cost.
Accordingly, a methodology for permitting poorly soluble drugs to be administered orally would be a significant advance. Such a methodology would provide a more convenient delivery system for the patient, reduce costs, and decrease morbidity from IV infusions of existing poorly soluble drugs. In addition, the ability to orally deliver poorly soluble drugs could expand the palette of the pharmaceutical industry, which may otherwise abandon a promising but poorly soluble agent.
Incorporating poorly soluble drugs into nanosized drug carriers offers many therapeutic advantages. One advantage is the ability to passively target the carrier's cargo to the site of action, such as a tumor or atherosclerotic plaque, through enhanced permeability and retention (EPR) of the drug preferentially at the diseased site. Lower amounts of drug could be delivered to preferentially accumulate at the site of disease, thus reducing the risk of systemic toxicity.2–5 If stealth nanocarriers were used, circulation time and bioavailability of chemotherapeutics would significantly increase since they would bypass rapid elimination by the liver and kidneys.
Currently, one of the most-used poorly soluble anticancer drugs is paclitaxel. This agent is solubilized and formulated in a mixture of Cremophor® EL and ethanol and marketed as Taxol®. However, it is highly toxic for many patients due to acute hypersensitivity reactions and neutropenia. Thus, an oral form of delivery that could compensate for paclitaxel's poor solubility would be beneficial. In terms of cardiovascular therapeutics, carvedilol is useful in treating hypertension, congestive heart failure, arrhythmias, and angina pectoris. However, its poor aqueous solubility and high first-pass metabolism give this agent a bioavailability of only 25% to 30%.6 Although it is already delivered in an oral formulation, an enhanced form of oral delivery that increases absorption would improve the predictability of drug levels and drug response. Another example is the use of adenosine to treat narrow-QRS-complex tachycardia. Although adenosine is generally given by rapid IV to slow electrical impulse conduction through the AV node,7 a method for rapid oral absorption may avoid the need for IV delivery.
One nanotechnological approach for improving the delivery of poorly soluble drugs is to incorporate them in micelles, which are colloidal particles between 5 and 10 nm in size. Micelles consist of amphiphilic molecules composed of a hydrophilic head group and a hydrophobic tail. At low concentrations in aqueous environments, they exist as monomers. But once the concentration increases above their critical micelle concentration (CMC), they self-assemble into micelles. Their structure allows poorly soluble drugs to be entrapped in the hydrophobic core, while their hydrophilic shell provides steric stability and—in case of polyethylene glycol (PEG)—also helps them to avoid uptake by the mononuclear phagocytic system. Although the current state of preclinical research related to micelles covers a wide range of drugs and polymer systems,8–11 it is important to note that many of these drugs are administered intravenously.
In this paper, we present an entirely new drug manufacturing process that will enable oral preparation of a wide range of poorly soluble drugs by forming drug-loaded polymeric micelles into orally acceptable NRs with sizes down to 25 nm.
Effect of Drug Nanorod Shape and Dimensions
Currently, spherical nanocarriers dominate preclinical research in the nanomedicine field. However, translation of these preclinical developments into clinical advances has been slow. New approaches to rational nanoparticle design may improve absorption, enhance circulation time, avoid immune surveillance mechanisms, and promote delivery to the site of disease.
Recent advances in nanotechnology have increased the flexibility of design and enhanced quality control of nanoparticles (NPs) such as nanotubes, NRs, nanowires, nanocages and nanodisks. Among these, NRs attract more attention due to their favorable properties over spherical particles. Ghandehari et al. demonstrated that circulation time of PEGylated NRs is higher than PEGylated gold nanospheres.12 Moreover, NRs were taken up by macrophages to a lesser extent than spherical NPs, which explains their increased circulation time and lower accumulation in the liver compared to spherical particles. Another important finding is that the serum proteins interacted strongly with spherical-shaped particles but not with NRs, which lead to less opsonization and up to 2-fold more tumor accumulation of NRs over spheres. Chauhan et al. compared PEGylated quantum-dot structures of nanospheres and NRs with equal hydrodynamic size and found that both could diffuse through 5-μm pores at the same rate. However, when the pore size decreased to the 100 nm to 400 nm range (the range of pores in tumor vasculature or the neovessels of atherosclerotic plaque), NRs diffused through the pores up to an order of magnitude faster than the nanospheres. Chauhan et al. also showed in vivo that NRs penetrated into the tumors four times as rapidly as the nanospheres.13 More recently, Dasgupta et al. reported a systematic approach to understanding how the shape of an NP affects its cellular internalization after finding that rod-like particles assumed stable endocytotic states.14 Their summarized data suggests that rod-shaped nanotherapeutics may be far more effective for cancer therapy than currently approved spherical approaches. (In this regard, one wonders if similar biophysical properties facilitate the enhanced internalization of many rod-like bacteria in nonphagocytic cells.)
Similarly, studies of the internalization into HeLa cells of monodispersed hydrogel particles fabricated by PRINT (particle replication in non-wetting templates) revealed that particles with a diameter of 150 nm and height of 450 nm were internalized 4 times faster than their spherical counterparts.15 However, size limitation of PRINT prevents the production of smaller-sized NRs (< 100 nm), which may be necessary for further enhancing cellular internalization. In order to achieve an effective intracellular entry that potentially enables oral delivery of various poorly soluble drugs, a nanomanufacturing process needs to be developed that can fabricate sub-100 nm drugs with controllable size, shape, and composition.
Dielectrophoretic Assembly of Nanoparticles
We have recently created and patented a water-based, room-temperature, and pressure nanomanufacturing process that uses electric field-assisted directed assembly of particles to fabricate 3-dimensional (3D) nanostructures featuring sizes as small as 25 nm.16 Although bottom-up directed assembly of NPs has been previously shown to fabricate functional and novel nanostructures,17–21 current directed assembly techniques have not been able to generate solid nanostructures with nanoscale precision. This requires a fundamental understanding of the forces driving the assembly of NPs into solid nanostructures and precise control of these forces to enable the assembly of more than one type of particle. We demonstrated that colloidal NPs can be precisely assembled and simultaneously fused into 3D solid nanostructures in a single step using an externally applied electric field (Figure 1a).22 By understanding the influence of various assembly parameters, we showed the fabrication of 3D structures made of various conducting, semiconducting, or insulating materials with complex geometries such as NRs, nanoboxes, and nanorings.16 The fabrication process is versatile, fast, and scalable to fabricate billions of NRs in minutes (Figure 1b).
Figure 1.
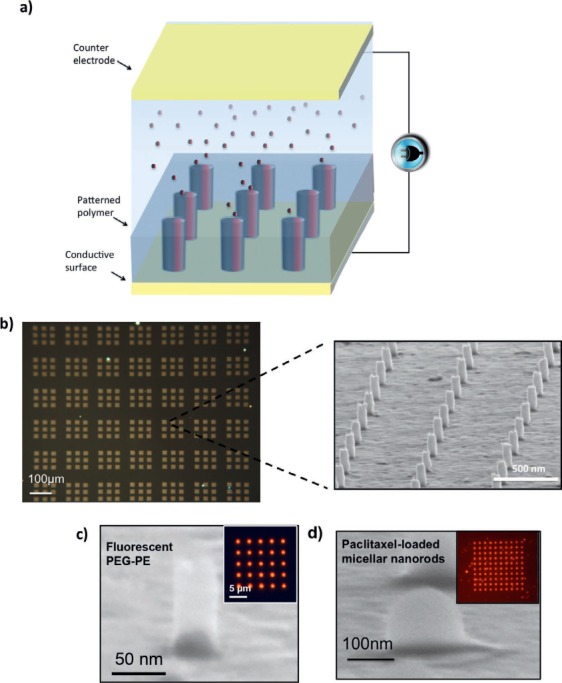
Fabrication of 3D nanorods (NRs) fabricated by fast and scalable directed assembly method. (a) Schematic of fabrication process. (b) Billions of NRs fabricated on a substrate. (c) 30-nm diameter NR fabricated from PEG-PE micelles. (d) 100-nm diameter NR fabricated from paclitaxel drug-loaded PEG-PE. PEG-PE: polyethylene glycol-phosphatidylethanolamine
This is an entirely new drug manufacturing process that will enable oral preparation of a wide range of poorly soluble drugs. It does so by forming drug-loaded polymeric micelles into an orally acceptable form of NRs using electric field directed assembly as shown in Figure 1a. Drug-loaded micelles suspended in deionized water are assembled into 3D NRs in a patterned thin polymer. This method provides several advantages compared to currently used drug delivery systems. First, as stated above, drug-loaded polymeric micelles are printed into orally acceptable 3D NRs with well-defined size and shape, providing a sufficiently high and fixed drug dosage in the blood to get a desired therapeutic response.23–25 Second, the 3D drug-loaded NRs are embedded into a unique polymer that can only dissolve in a basic pH. Thus, these drugs can be released in the intestine after passing through the stomach, which eliminates the stability issue due to pH variation in the gastrointestinal track. Third, due to their small size, these NRs will have a high permeability, thus enabling effective transport through the intestinal wall.26–28 Finally, compared to spheres or other shapes, the NR drugs are expected to penetrate tumors or atherosclerotic plaque more efficiently and much faster than individual NPs. This should lead to better bioavailability of the drug as well as reduced toxicity and side effects.29
Development of a Novel Nanoprinting Process for Paclitaxel
Paclitaxel is a useful drug for both oncotherapy and preventing restenosis after angioplasty. Accordingly, we have developed a novel nanoprinting process that enhances oral delivery of paclitaxel and is anticipated to allow greater access to diseased sites, such as tumor mass or vascular lesions. In preliminary work, we have prepared polymeric micelles containing 3% paclitaxel and fabricated these micelles into 3D NRs using our unique directed-assembly approach (Figure 1). Polyethylene glycol-phosphatidylethanolamine (PEG-PE) micelles were prepared using the thin film hydration method (Figure 2a),30 whereby the organic solvents were removed by rotary evaporation to form a thin film of homogeneous drug/polymer mixture. This film was further dried under high vacuum to remove any organic solvent traces. The film was then hydrated with deionized water, phosphate-buffered saline, or HEPES-buffered saline with a 7.4 pH. Unincorporated paclitaxel was removed by filtration through 0.2-μm filters. For microscopic characterization, 5 mol% of the fluorescent probe, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (Rh-PE), was also added to micelle-forming materials during the preparation. The size and zeta potential of the micelles were measured using dynamic light scattering. The average size of the micelles was 7.1 nm and the zeta potential was −23.2 mV.
Figure 2.
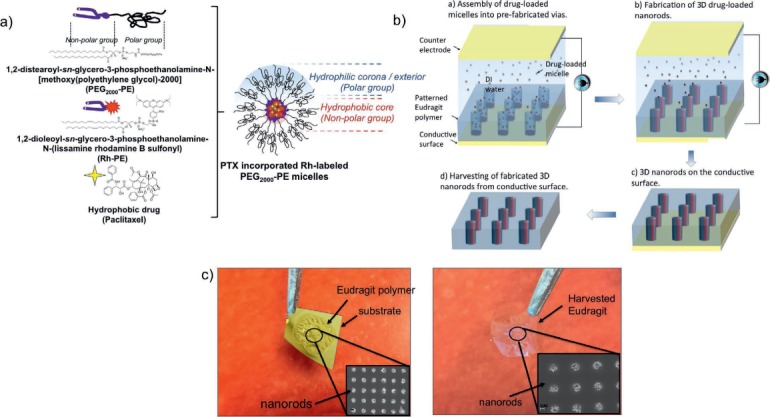
(a) Preparation of a paclitaxel drug-loaded tagged micellar system. (b) Fabrication and harvesting of orally acceptable 3-dimensional drug-loaded micellar nanorods (NRs) using electric field directed assembly. Red and black colors of the NRs indicate the dye and the drug incorporated into micelles, respectively. (c) Optical and scanning electron microscopy images of the drug-loaded micelles embedded in a water-soluble polymer before and after harvested from the substrate.
Figure 2b summarizes fabrication of the template with via patterns (cylindrical cavities made by lithography), assembly of drug-loaded micelles into vias, and separation of drug-loaded NRs from the substrate. We used conventional micro/nano fabrication processes to make the template with nanoscale patterns in which the prepared micelles will be assembled into 3D NRs.22 The template was a substrate comprising a conductive film coated by Eudragit® (Evonik Degussa Corp, Lake Forest, CA), an anionic copolymer based on methacrylic acid and methyl methacrylate. Eudragit S100 dissolves in solutions with a pH higher than 7.0 to allow delivery in the intestine.31 The nanoscale via geometries on the polymer can be fabricated using conventional electron beam lithography32 or nanoimprint lithography.33 The size of the vias (the diameter and the aspect ratio) determines the dimensions of the drug-loaded 3D NRs, which will be delivered directly into the blood via the intestinal epithelial cells. The diameter of the vias can be as small as 25 nm or lower with various aspect ratios ranging from 2 to 6.
To assemble the polymeric paclitaxel-containing micelles, the pH of the deionized water was adjusted below 7 to prevent the early dissolution of the Eudragit polymer. An AC electric field (dielectrophoresis)34 was applied to the fabricated template with via geometries and a counter electrode. This allowed assembly of the drug-loaded micelles into NRs in the prefabricated vias (Figure 2b). In our prior work, we identified the governing assembly parameters for successful NR fabrication in the vias.16,22 Optimization of the assembly conditions—such as voltages, frequency, particle concentration, and time—enabled successful fabrication of approximately 100-nm diameter and 150-nm tall paclitaxel-loaded NRs (Figure 1d). Figure 2c shows the optical image of the encapsulating Eudragit polymer before and after it is harvested from the substrate. The scanning electron microscope inset images clearly demonstrate the drug-loaded NRs embedded into the polymer. In total, around 1 million NRs were fabricated over a 1 × 1 mm area.
Product Concept
The approach we have taken is to leverage the electric field-directed assembly technique to develop a novel drug printing process that will generate an oral formulation of poorly soluble drugs.16 Figure 3 shows the scale-up of the technology and concept for the product. The electric field directed assembly process is fast and scalable to allow fabrication of billions of drug-loaded NRs in a few minutes. The NRs will be encapsulated by a biocompatible polymer Eudragit S100®, which will only dissolve at a pH > 7. Following the fabrication of NRs, the resulting Eudragit/NR mixture can be cut into small pieces and loaded into a gelatin or HPMC-based capsule to be used in oral delivery (Figure 3 II). After oral administration, the capsule will be dissolved in the acid stomach pH and will free the Eudragit/NR mixture. The Eudragit polymer will dissolve in the higher pH of the small intestine and release the NRs (Figure 3 III). We believe that released NRs will be absorbed through the intestinal tract in their intact form, stay longer in circulation because of surface-accessible PEG chains, accumulate in targeted tissues due to their small size and enhanced permeability and retention effect, and be internalized by the targeted cells to a greater extent than micellar and free paclitaxel. Thus, lower doses of drug will achieve effective concentrations at the site of pathobiology with less risk of systemic toxicity (Figure 3 IV).
Figure 3.
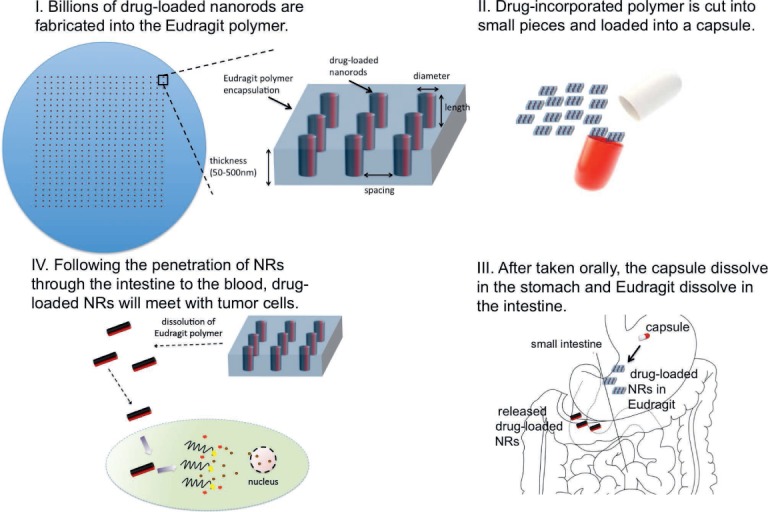
Product concept from the production of the drug-loaded nanorods to delivery to the tumor cells.
Conclusions and Outlook
We have described a new drug manufacturing technology that will enable oral administration of poorly soluble drugs. In this technology, drug-loaded polymeric micelles are integrated into an orally acceptable form of NR that will transport through the intestinal wall, allowing the drug to be preferentially targeted to tissues with more permeable endothelium (e.g., tumor or atherosclerotic plaque). The drug-loaded micelles are suspended in deionized water and assembled (printed) into lithography patterned via holes on a template by electric field directed assembly. Following the formation of micellar NRs in via holes, the drug-loaded NRs are separated from the template while being embedded in a water-soluble Eudragid polymer for oral administration. This approach is capable of producing drug-loaded NRs with controlled size and shape down to 25 nm in diameter, leading to high permeability and thus enabling effective transport through the intestinal wall.
Successful development of this technique will provide a new avenue for the oral administration of promising but poorly soluble drug candidates. This technology can lead to the reclassification of many Biopharmaceutical Classification System (BCS) Class IV drugs to Class II (or Class I according to the solubility enhancement) for biowaiver requirements. In addition, we predict that oral administration of the developed drugs will increase patient compliance and decrease the time and the cost involved in therapy. Due to the nanoscale size and larger surface area of the drug-loaded micellar NRs, the therapeutically effective dose of the formulation is expected to be lower compared to the IV dose. This could significantly reduce the overall bioburden of the drug and prevent undesirable side effects such as embolism, which is caused by the degradation of poorly soluble drugs when given intravenously.35 Compared to other conventional oral drugs, the drug-loaded NRs would require minimum or no excipients, which in turn could lead to better absorption, distribution, metabolism, and excretion properties.
Key points:
This technology will enable oral administration of various promising drug candidates that are currently given intravenously, which would be a significant breakthrough for the pharmaceutical industry.
The outcome of the technology is expected to greatly benefit both patients and clinicians.
The new drug delivery systems would likely increase patient compliance and decrease the time and cost involved in therapy while enhancing drug safety from the development-to-patient process.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1. Savjani KT, Gajjar AK, Savjani JK.. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012; 2012: 195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986. December; 46( 12 Pt 1): 6387– 92. [PubMed] [Google Scholar]
- 3. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K.. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000. March 1; 65( 1–2): 271– 84. [DOI] [PubMed] [Google Scholar]
- 4. Maeda H, Sawa T, Konno T.. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release. 2001. July 6; 74( 1–3): 47– 61. [DOI] [PubMed] [Google Scholar]
- 5. Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001; 41: 189– 207. [DOI] [PubMed] [Google Scholar]
- 6. Hirlekar RS, Kadam VJ. Design of buccal drug delivery system for a poorly soluble drug. Asian J Pharm Clin Res. 2009. Jul-Sep; 2( 3): 49– 53. [Google Scholar]
- 7. Bradamante S, Barenghi L, Villa A.. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004. Fall; 22( 3): 169– 88. [DOI] [PubMed] [Google Scholar]
- 8. Hamaguchi T, Matsumura Y, Suzuki M, . et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br J Cancer. 2005. April 11; 92( 7): 1240– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim TY, Kim DW, Chung JY, . et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004. June 1; 10( 11): 3708– 16. [DOI] [PubMed] [Google Scholar]
- 10. Kim SC, Kim DW, Shim YH, . et al. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release. 2001. May 14; 72( 1–3): 191– 202. [DOI] [PubMed] [Google Scholar]
- 11. Werner ME, Cummings ND, Sethi M, . et al. Preclinical evaluation of Genexol-PM, a nanoparticle formulation of paclitaxel, as a novel radiosensitizer for the treatment of non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013. July 1; 86( 3): 463– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arnida, Janát-Amsbury MM, Ray A, Peterson CM, Ghandehari H.. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur J Pharm Biopharm. 2011. April; 77( 3): 417– 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chauhan VP, Popović Z, Chen O, . et al. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew Chem Int Ed Engl. 2011. November 25; 50( 48): 11417– 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dasgupta S, Auth T, Gompper G.. Shape and orientation matter for the cellular uptake of nonspherical particles. Nano Lett. 2014. February 12; 14( 2): 687– 93. [DOI] [PubMed] [Google Scholar]
- 15. Gratton SE, Ropp PA, Pohlhaus PD, . et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008. August 19; 105( 33): 11613– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yilmaz C, Cetin AE, Goutzamanidis G, . et al. Three-dimensional crystalline and homogeneous metallic nanostructures using directed assembly of nanoparticles. ACS Nano. 2014. May 27; 8( 5): 4547– 58. [DOI] [PubMed] [Google Scholar]
- 17. Grzelczak M, Vermant J, Furst EM, Liz-Marzán LM.. Directed self-assembly of nanoparticles. ACS Nano. 2010. July 27; 4( 7): 3591– 605. [DOI] [PubMed] [Google Scholar]
- 18. Velev OD, Gupta S. Materials fabricated by micro- and nanoparticle assembly – the challenging path from science to engineering. Adv Mater. 2009; 21( 19): 1897– 1905. [Google Scholar]
- 19. Hermanson KD, Lumsdon SO, Williams JP, Kaler EW, Velev OD.. Dielectrophoretic assembly of electrically functional microwires from nanoparticle suspensions. Science. 2001. November 2; 294( 5544): 1082– 6. [DOI] [PubMed] [Google Scholar]
- 20. Liberman V, Yilmaz C, Bloomstein TM, . et al. A nanoparticle convective directed assembly process for the fabrication of periodic surface enhanced Raman spectroscopy substrates. Adv Mater. 2010. October 8; 22( 38): 4298– 302. [DOI] [PubMed] [Google Scholar]
- 21. Erb RM, Son HS, Samanta B, Rotello VM, Yellen BB.. Magnetic assembly of colloidal superstructures with multipole symmetry. Nature. 2009. February 19; 457( 7232): 999– 1002. [DOI] [PubMed] [Google Scholar]
- 22. Yilmaz C, Kim TH, Somu S, Busnaina AA.. Large-scale nanorods nanomanufacturing by electric-field-directed assembly for nanoscale device applications. IEEE T Nanotechnol. 2010; 9( 5): 653– 8. [Google Scholar]
- 23. Kam KR, Desai TA. Nano- and microfabrication for overcoming drug delivery challenges. J Mater Chem B Mater Biol Med. 2013; 1( 14): 1878– 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang P, Cheetham AG, Lock LL, Cui H.. Cellular uptake and cytotoxicity of drug-peptide conjugates regulated by conjugation site. Bioconjug Chem. 2013. April 17; 24( 4): 604– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li XQ, Wen HY, Dong HQ, . et al. Self-assembling nanomicelles of a novel camptothecin prodrug engineered with a redox-responsive release mechanism. Chem Commun (Camb). 2011. August 14; 47( 30): 8647– 9. [DOI] [PubMed] [Google Scholar]
- 26. Mathot F, van Beijsterveldt L, Préat V, Brewster M, Ariën A.. Intestinal uptake and biodistribution of novel polymeric micelles after oral administration. J Control Release. 2006. March 10; 111( 1–2): 47– 55. [DOI] [PubMed] [Google Scholar]
- 27. Gaucher G, Satturwar P, Jones MC, Furtos A, Leroux JC.. Polymeric micelles for oral drug delivery. Eur J Pharm Biopharm. 2010. October; 76( 2): 147– 58. [DOI] [PubMed] [Google Scholar]
- 28. des Rieux A, Ragnarsson EG, Gullberg E, Préat V, Schneider YJ, Artursson P.. Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium. Eur J Pharm Sci. 2005. Jul-Aug; 25( 4–5): 455– 65. [DOI] [PubMed] [Google Scholar]
- 29. Zhang J, Chen XG, Peng WB, Liu CS.. Uptake of oleoyl-chitosan nanoparticles by A549 cells. Nanomedicine. 2008. September; 4( 3): 208– 14. [DOI] [PubMed] [Google Scholar]
- 30. Elbayoumi TA, Pabba S, Roby A, Torchilin VP.. Antinucleosome antibody-modified liposomes and lipid-core micelles for tumor-targeted delivery of therapeutic and diagnostic agents. J Liposome Res. 2007; 17( 1): 1– 14. [DOI] [PubMed] [Google Scholar]
- 31. Khan MZ, Prebeg Z, Kurjaković N.. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation of drug release using Eudragit L100-55 and Eudragit S100 combinations. J Control Release. 1999. March 29; 58( 2): 215– 22. [DOI] [PubMed] [Google Scholar]
- 32. Rai-Choudhury P. Handbook of microlithography, micromachining and microfabrication (Book 1). Bellingham, WA: SPIE—Society of Photo-Optical Instrumentation Engineers; 1997. 765 p. [Google Scholar]
- 33. Jung GY, Johnston-Halperin E, Wu W, . et al. Circuit fabrication at 17 nm half-pitch by nanoimprint lithography. Nano Lett. 2006. March; 6( 3): 351– 4. [DOI] [PubMed] [Google Scholar]
- 34. Pohl HA. Dielectrophoresis: the behavior of neutral matter in nonuniform electric fields. New York, NY: Cambridge University Press; 1978. [Google Scholar]
- 35. Torchilin VP1, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B.. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci U S A. 2003. May 13; 100( 10): 6039– 44. [DOI] [PMC free article] [PubMed] [Google Scholar]


