Abstract
Purpose
To evaluate the safety, efficacy, and contralateral eye comparison of topography-guided myopic LASIK with two different refraction treatment strategies.
Setting
Private clinical ophthalmology practice.
Patients and methods
A total of 100 eyes (50 patients) in consecutive cases of myopic topography-guided LASIK procedures with the same refractive platform (FS200 femtosecond and EX500 excimer lasers) were randomized for treatment as follows: one eye with the standard clinical refraction (group A) and the contralateral eye with the topographic astigmatic power and axis (topography-modified treatment refraction; group B). All cases were evaluated pre- and post-operatively for the following parameters: refractive error, best corrected distance visual acuity (CDVA), uncorrected distance visual acuity (UDVA), topography (Placido-disk based) and tomography (Scheimpflug-image based), wavefront analysis, pupillometry, and contrast sensitivity. Follow-up visits were conducted for at least 12 months.
Results
Mean refractive error was −5.5 D of myopia and −1.75 D of astigmatism. In group A versus group B, respectively, the average UDVA improved from 20/200 to 20/20 versus 20/16; post-operative CDVA was 20/20 and 20/13.5; 1 line of vision gained was 27.8% and 55.6%; and 2 lines of vision gained was 5.6% and 11.1%. In group A, 27.8% of eyes had over −0.50 diopters of residual refractive astigmatism, in comparison to 11.7% in group B (P<0.01). The residual percentages in both groups were measured with refractive astigmatism of more than −0.5 diopters.
Conclusion
Topography-modified refraction (TMR): topographic adjustment of the amount and axis of astigmatism treated, when different from the clinical refraction, may offer superior outcomes in topography-guided myopic LASIK. These findings may change the current clinical paradigm of the optimal subjective refraction utilized in laser vision correction.
Keywords: TMR, topography-modified refraction, myopic LASIK, femtosecond laser, FS200, EX500 excimer laser, long-term stability, regression, astigmatism correction, post-LASIK refraction
Introduction
Laser vision correction has been established over the last 2 decades as a safe and effective intervention, with Laser-assisted in situ keratomileusis (LASIK) being one of the main techniques practiced globally.1,2
Femtosecond laser-assisted LASIK has become a popularized modification over the last decade and over the standard LASIK technique utilizing mechanical microkeratomes.3,4
Excimer lasers, used in the second step of a LASIK procedure to correct refractive error (RE), have been evolving as well, with most platforms currently utilizing flying spot technology of very high repetition rates and advanced tracking mechanisms to track intra-operatively the pupillary aperture and some even to provide cyclorotation adjustment.5–7 Aspherical ablation profiles have been employed to reduce spherical aberration associated with myopic corrections, so most LASIK procedures are performed currently as “wavefront optimized”.8 Further customization of the ablation pattern has been practiced by pairing wavefront eye data to the refractive data chosen to be corrected, as wavefront-guided treatments in pursue of improving visual function in aberrated eyes, or pre-empting wavefront aberrations created by the standard procedures.9,10 Since most inherent and acquired eye aberrations lie in the cornea – the premiere refractive medium of the eye – topography-guided treatments have been additionally employed as a means of ablation customization in pursue of additionally correcting corneal irregularities affecting visual function and/or for angle kappa.11,12
LASIK outcomes appear to have improved in safety and efficacy with the evolutions described earlier1,13 in both refractive outcomes and induced high-order aberrations.5,14,15
Further customization of the ablation pattern by utilizing topography data has been reported to provide excellent refractive data with regard to uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA; WaveLight Allegretto Wave® Eye-Q Excimer Laser16).
The clinical refraction utilized in most laser vision correction treatments has been based on the subjective interaction of clinician and patient and ranging from the dry manifest to the wet manifest or a clinician-preferred medium of the two.
The author has had extensive experience in treating very irregular corneas, learning that refractive data sometimes are different from topographic data with regard to the amount and axis of the astigmatism. Thus, the adjustment of the clinical refraction with topographic data: topography-modified refraction (TMR) was introduced. The purpose of this study was to validate this novel measurement by comparing the visual outcomes when the TMR was used in myopic LASIK to using the standard clinical refraction.
Patients and methods
This prospective, randomized, interventional study received approval from the ethics committee of the LaserVision Clinical and Research Institute, adherent to the tenets of the Declaration of Helsinki. Written informed consent was obtained from each subject at the time of the intervention. The study was conducted in the author’s clinical practice on patients during scheduled pre- and post-operative procedure visits.
The 50 consecutive patients enrolled in the study underwent uncomplicated primary bilateral topography-guided LASIK by the same surgeon (AJK) on the same refractive surgery platform (FS200 femtosecond and EX500 excimer lasers).
Pre-operative spherical equivalent was between 0.00 and −8.00 D and up to 6 D of cylinder RE (expressed in minus value).
Exclusion criteria for the LASIK operation were systemic or ocular diseases, eyes with a history of corneal dystrophy or herpetic eye disease, topographic evidence of keratoconus (as evidenced by Placido topography) or warpage from contact lenses, corneal scaring, glaucoma, severe dry eye, and collagen vascular diseases. Programmed planned flap thickness was 110 µm and diameter was 8 mm.
All eyes were evaluated pre-operatively for distance best CDVA and post-operatively for UDVA. Pre-operative evaluations included wavefront analysis, pupillometry, and contrast sensitivity utilizing the functional vision analyzer (Stereo Optical, Chicago, IL, USA). Post-operative examination included manifest and dilated refraction, slit-lamp microscopy, tonometry, and keratometry, by means of corneal topography utilizing the Vario (WaveLight, Erlagen, Germany) and Scheimpflug tomography assessment utilizing a Pentacam-based device, the Oculyzer II (WaveLight).
The treatment used for the eyes randomized in group A was the subjective clinical refraction, entered into the topography-guided software as the surgeon override data, and is the standard operation in these treatments.
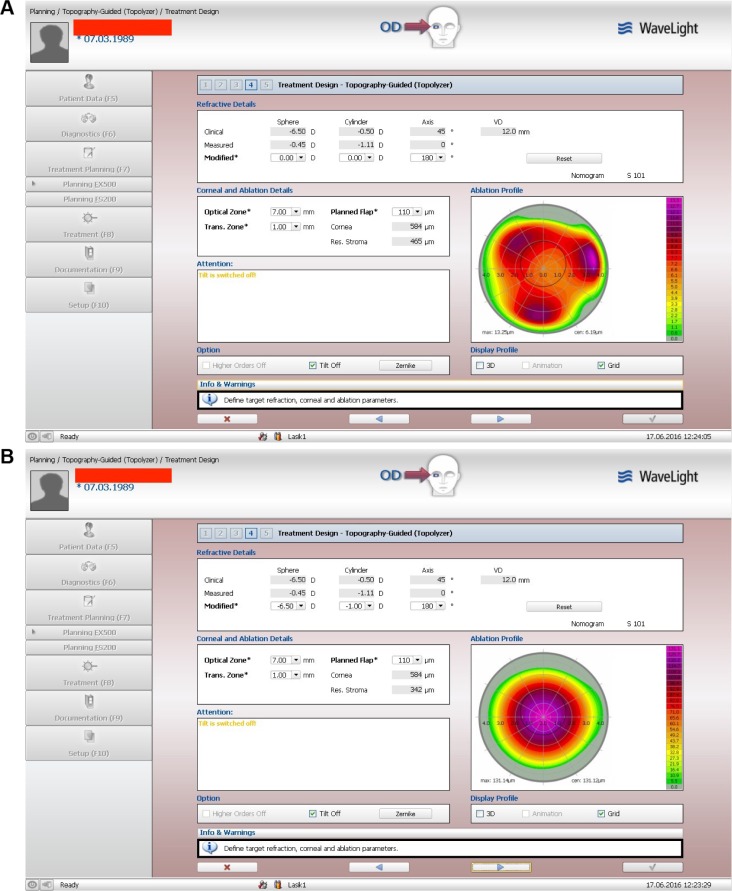
The clinical refraction data are the first line of data on the treatment planning software window shown in Figure 1, both top and bottom. The specific patient example shows the clinical refraction on the top, of −6.50 D of sphere and −0.50 D of cylinder at 45°. The middle line of refraction in both top and bottom windows of Figure 1 shows the topography software-recommended cylindrical refraction. In this particular case, the sphere is −0.45 D (the topography software has no axial length data and cannot calculate myopia), and the cylinder that derives from several consistent Vario, topolyzer (Alcon, Fort Worth, TX, USA) topographies shows −1.11 D at 0°. The lower third refraction in both top and bottom images in Figure 1 is modified by the surgeon. In the top, all refraction data are set on purpose to 0°. In this way, the topography-guided software reveals the correction it will attempt in order to normalize this cornea with regard to the corneal vertex. Thus, the trefoil-like ablation image on the right of the top image in Figure 1. The trefoil-like image is also presented here in correlation with the pupillary aperture, also captured by the Vario topographer, and in this top window it shows clearly decentration to the trefoil-like normalization ablation, revealing angle-kappa compensation by the topography software. This ablation pattern will induce some myopia and it was calculated that in order to keep it neutral there is a need to add −0.25 D of myopia to the clinical refraction sphere, so the total sphere should be −6.75 D. The cylinder suggested by the software (middle refraction) is much higher than the clinical by 0.5 D and of different axis: 0° instead of 45°. The topography suggested cylinder is then entered into the third lower “modified” refraction to read −1.0 D at 0° or 180° in the bottom version of Figure 1. This would further increase the spherical equivalent by −0.25 D of sphere; hence the final adjustment made to the modified refraction on the bottom window of this same treatment image is to reduce the sphere from −6.75 D, as noted earlier, to −6.50 D so as to keep the same spherical equivalent. Therefore, the TMR in this case is −6.50 D to 1.00 D at 180°, instead of the −6.50 D to 0.50 D at 45° of the standard clinical refraction.
Figure 1.
Image A shows the topography-guided treatment when refractive error is adjusted by the user to zero sphere and zero cylinder. This image allows the user to visualize the corrections made by the software and added to the excimer correction in order to normalize the anterior cornea curvature to the cornea vertex. Image B illustrates the topography treatment pattern after the refraction has been adjusted by the user to the desired sphere and cylinder and includes the changes noted in image A.
Notes: “Clinical” is the clinical refraction dialed by the user into the system; the “measured” refraction is the cylindrical amount and axis calculated by the topography software, to be corrected so the anterior cornea can me maximally normalized in regard to the cornea vertex; “modified” represents the surgeon/user adjusted data.
Abbreviations: 3D, three-dimensional; max, maximum, cen, center; trans, transition zone; res, residual; VD, vertex distance.
The treatment used in the contralateral eyes of group B was modified, as noted earlier, to reflect the topography-guided suggested cylinder power and axis calculated by the software based on the Vario topographies uploaded, as seen in the middle “measured” refraction of both top and bottom images of Figure 1. In most cases, the cylinder was increased and the sphere decreased accordingly, but there were some cases that the actual clinical refraction cylinder was reduced and the sphere increased accordingly. In the TMR cases of group B, the cylinder axis was always that suggested by the topography processing as noted in the middle measured refraction line.
Post-operative follow-up examinations were conducted at 1 week, 1, 3, and 6 months, and at 1 year. Data processed in this study represent the 3-, 6-, and 12-month visits.
Data were loaded in and processed by the web-based ophthalmic outcome analysis software application known as “Internet-based refractive analysis” (IBRA; Zubisoft GmbH, Oberhasli, Switzerland).17
Results
Of the 50 patients, 32 were female and 18 were male. Mean age at the time of the operation was 29.6±7.8 years, range 18–52 years.
The mean pre-operative UDVA for group A eyes were: UDVA mean in decimal scale and standard deviation (SD): 0.04±0.007, UDVA range: 0.001–0.8; spherical equivalent (SE) mean and SD: −5.19±2.15 D, SE range −8.00 D to −0.50 D; cylinder mean: −1.15±1.15 D and cylinder range: −4.25 D to 0 D.
The respective pre-operative data for the randomized group B contralateral eyes were: UDVA: 0.04±0.05, UDVA range: 0.002–0.8, SE mean: −4.75±2.49 D, SE range: −7.50 D to −0.75 D, cylinder mean: −1.12 D±0.95 D, and cylinder range: −3.75 D to −0.50 D. These data are also shown in Table 1.
Table 1.
Pre-operative parameters
| Pre-operative parameters | Group A | Group B |
|---|---|---|
| UDVA mean/SD | 0.04±0.07 | 0.04±0.05 |
| UDVA range | 0.001–0.8 | 0.002–0.8 |
| SE mean/SD | −5.19±2.15 | −4.75±2.49 |
| SE range | −8.00 to −0.50 | −7.50 to −0.75 |
| Cyl mean/SD | −1.15±1.15 | −1.12±0.95 |
| Cyl range | −4.25 to 0 | −3.75 to −0.50 |
Notes: Group A: one eye with the standard clinical refraction; Group B: the contralateral eye with the topographic astigmatic power and axis (topography-modified treatment refraction). Units of measure: SE units = diopters; UDVA units = decimal scale; Cyl = cylinder in diopter units.
Abbreviations: UDVA, uncorrected distance visual acuity; SD, standard deviation; SE, spherical equivalent; Cyl, cylinder.
Post-operative average RE for the standard group A was −0.27±0.39 D and for the topography adjusted group B was +0.15±0.19 D at 3 months. At the 12-month visit, the residual refractive error for group A was −0.47±0.55 D and for group B (the topography modified refraction group) −0.19±0.08 D. These data were statistically significantly different by P<0.02. This compares to the pre-operative average RE of −5.29±2.39 D (range −8.00 D to −0.50 D) for all eyes.
Post-operative refractive astigmatism was −0.54±0.24 D for the standard group A and −0.15±0.14 for the topography adjusted group B at 3 months and −0.76±0.44 and −0.18±0.24 for the 1-year visit, respectively. This compares to the pre-operative refractive astigmatism of −1.07±0.91 D (range −4.25 D to 0 D) as an average for all eyes.
Corrected and uncorrected visual acuity outcome and stability
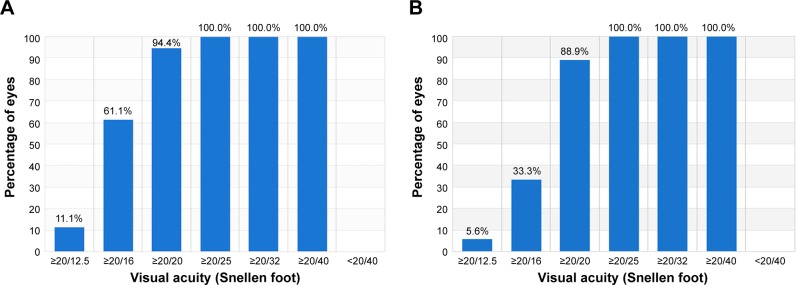
The corrected visual acuity (distance monocular; CDVA) outcome at 3 months (Figure 2) shows that 88.9% of the eyes in group A had post-operatively CDVA better than 1.0 (decimal) after 3 months, which was maintained at the 1 year visit. Figure 2 also depicts the best-corrected preoperative distance visual acuity (CDVA) for group B: 94.4%. CDVA of 20/16 or better was measured for 33.3% of group A eyes, compared to 61.1% of group B eyes (statistically significant at P<0.001).
Figure 2.
Corrected distance visual acuity at 3 months: (A) group A and (B) group B.
Notes: Group A: one eye with the standard clinical refraction; Group B: the contralateral eye with the topographic astigmatic power and axis (topography-modified treatment refraction).
UDVA of 20/20 or better was measured at 3 months (Figure 3) in 61.1% of eyes in group A and 83.3% of eyes in group B (P<0.002). UDVA of 20/16 or better was measured in 22.2% of eyes in group A and 50% of eyes in group B, a difference that was also statistically significant (P<0.001).
Figure 3.
Post-operative uncorrected distance visual acuity at 3-month visit: (A) group A and (B) group B.
Notes: Group A: one eye with the standard clinical refraction; Group B: the contralateral eye with the topographic astigmatic power and axis (topography-modified treatment refraction).
Safety of corrected visual acuity
As shown in Figure 4, the safety distance visual acuity graph at 3-month visit comparison of pre-operative best CDVA and post-operative UDVA indicates that 66.7% of the eyes were unchanged, while 27.8% of the eyes gained one Snellen line and 5.6% gained two or more Snellen lines in group A. In group B, 33.3% of the eyes were unchanged, while 55.6% of the eyes gained one Snellen line and 11.1% gained two or more Snellen lines. All differences were statistically significant at P<0.2. Residual RE grouped in 3 categories of +0.01 to +0.5 D, −0.5 to 0 D, and −1 to −0.5 D, at the 3 month visit for groups A and B is illustrated in Figure 5.
Figure 4.
Safety distance visual acuity graph (percentage of eyes with gain/loss in Snellen lines), at 3-month visit: (A) group A and (B) group B.
Notes: Group A: one eye with the standard clinical refraction; Group B: the contralateral eye with the topographic astigmatic power and axis (topography-modified treatment refraction).
Figure 5.
Defocus equivalent results at 3-month visit: (A) group A and (B) group B.
Notes: Group A: one eye with the standard clinical refraction; Group B: the contralateral eye with the topographic astigmatic power and axis (topography-modified treatment refraction).
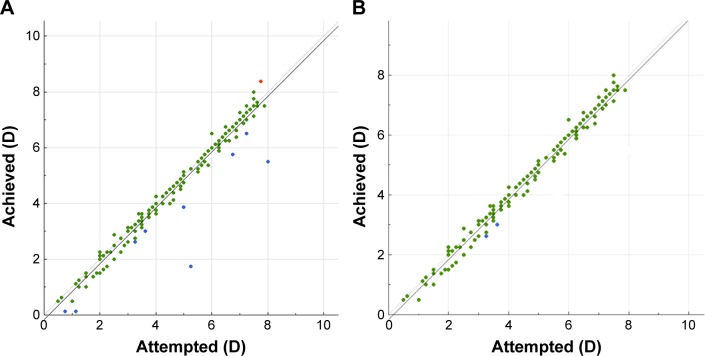
Refractive predictability
Predictability is demonstrated in Figure 6, where the achieved SE versus attempted SE (in D) is plotted, for gate =0.5 D for group A and group B.
Figure 6.
Predictability of spherical equivalent (SE) correction, showing achieved SE versus attempted SE: (A) group A and (B) group B.
Notes: Group A: one eye with the standard clinical refraction; Group B: the contralateral eye with the topographic astigmatic power and axis (topography-modified treatment refraction). Green marks show outcomes within 0.25 diopters of attempted vs achieved; blue marks show attempted vs achieved of under 0.25 diopters (under-corrections); red marks show attempted vs achieved of over 0.25 diopters (over-corrections).
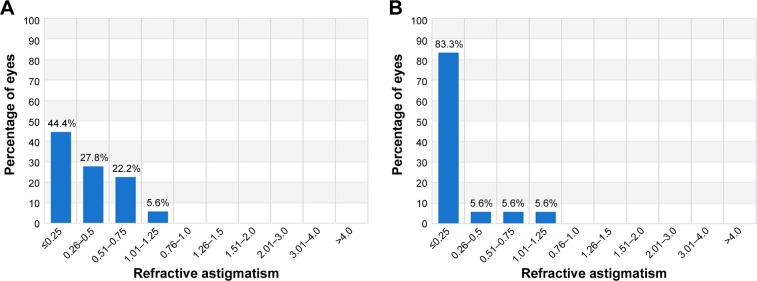
Post-operative RE data for the two groups are noted in Figure 5. Refractive astigmatism is noted in Figure 7 with again statistically significant differences with group B presenting less refractive astigmatism.
Figure 7.
Refractive astigmatism, pre-operatively and at 3-month post-operative visits.
Notes: Percentage of eyes (vertical axis) versus refractive astigmatism (D), horizontal axis: (A) group A and (B) group B. Group A: one eye with the standard clinical refraction; Group B: the contralateral eye with the topographic astigmatic power and axis (topography-modified treatment refraction).
Contrast sensitivity
Figure 8 illustrates the contrast sensitivity results in special frequencies of 1.5–18 cycles per degree for all pre-operative, group A, and group B.
Figure 8.
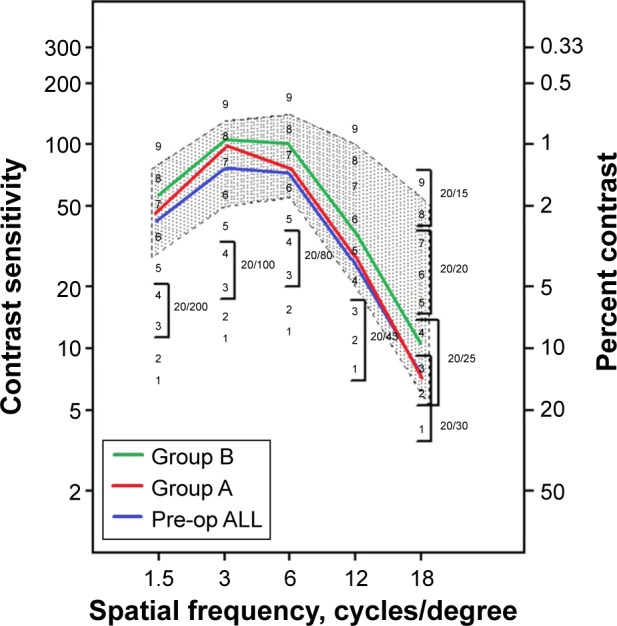
Contrast sensitivity comparison at 3-month visit.
Notes: As pre-operative data were not statistically significant in groups A and B, they are noted together as “Pre-op ALL”. The average contrast sensitivity data is indicated with the green, red, and blue lines. Group A: one eye with the standard clinical refraction; Group B: the contralateral eye with the topographic astigmatic power and axis (topography-modified treatment refraction).
High-order aberrations and coma, as measured with the Allegro Wavefront analyzer (WaveLight): at 3 months average high order aberrations (RMSH) were for group A: 0.85 µm and for group B: 0.025 µm; coma measurements were 0.65 µm and 0.45 µm, respectively.
Discussion
The combined Alcon/WaveLight refractive surgery laser platform comprising the FS200 femtosecond laser,18 the EX500 excimer laser, and a series of diagnostic networked devices that constitute the Refractive Suite® (including Vario Placido topography, Oculyzer II Scheimpflug topometry imaging, and Tscherning Wavefront system) were used clinically.19
Clinical results and features of the previous generations of the WaveLight excimer series (200 Hz Allegretto and 400 Hz Eye Q) have been reported by the author’s team.12,20
The EX500 laser, the latest evolution of the aforementioned excimer lasers, employs a 1,050 Hz multidimensional active tracker with estimated response time (latency) of 2 ms, able to track pupil size from 1.5 mm to 8 mm.21
The Refractive Suite operates on its own ethernet network and allows the import of diagnostic data from networked screening devices into the planning software tools of both lasers. The platform offers the ability to import into the laser treatment planning mode the topographic data from the Placido-disk topographer (Vario, WaveLight) and topometry data from a Scheimpflug-based device (Oculyzer II-WaveLight), and accordingly customize the excimer treatment to the cornea (eg, for topography-guided treatment).12 This study and previous studies have reported extensively the applications of topography-guided treatments with these platforms in irregular and normal eyes.22–29
The long-term clinical results with the systems described in the “Patients and methods” section show impressive refractive outcome, predictability, and stability with both refraction treatments. The modification of the clinical refraction with topography-guided data with regard to the amount and axis of astigmatism appears to offer superior visual function with regard to UDVA, CDVA, residual RE in terms of spherical equivalent and residual astigmatism, total high-order aberrations, coma, and functional contrast sensitivity. Specifically, the percentage of eyes achieving a post-operative spherical equivalent refraction within 0.5 D of the target and the total range of RE, which are considered the best markers of the quality of a refractive procedure. The better refractive results obtained in terms of spherical equivalent refraction were also confirmed by the defocus equivalent results achieved from the first 3 months with the refraction adjusted with topography data (group B). This parameter gives a better understanding of the accuracy of a procedure for correcting the entire RE.
It was theorized that this treatment adjustment pre-emptively bypasses the bias of lenticular astigmatism that is probably active in young myopic eyes in their physiological state and may compensate for some amount of cornea astigmatism and cornea coma generated by angle kappa. This theoretical lenticular astigmatism appears to be the factor that distorts the objective cylindrical refractive data, resulting in wavefront refractive data similar to the subjective refraction, which appears to subside within months following the refractive surgery intervention. The author has adopted this concept in modern refractive cataract surgery, assuming that the post-operative cylindrical refraction will depend mainly on the cornea astigmatism, but have not adopted this concept yet in laser vision correction techniques applied on the cornea. The data in this contralateral eye study are compelling in studying further this novel principle that may increase accuracy in refractions used for laser vision correction.
Conclusion
Topographic adjustment of the amount and axis of astigmatism treated: TMR when different from the clinical refraction may offer superior outcomes in topography-guided myopic LASIK. These findings may change the current clinical paradigm of the optimal subjective refraction utilized in laser vision correction. Further studies by additional investigators may further validate this data.
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Kugler LJ, Wang MX. Lasers in refractive surgery: history, present, and future. Appl Opt. 2010;49(25):F1–F9. doi: 10.1364/AO.49.0000F1. [DOI] [PubMed] [Google Scholar]
- 2.Lukenda A, Martinović ZK, Kalauz M. Excimer laser correction of hyperopia, hyperopic and mixed astigmatism: past, present, and future. Acta Clin Croat. 2012;51(2):299–304. [PubMed] [Google Scholar]
- 3.Reggiani-Mello G, Krueger RR. Comparison of commercially available femtosecond lasers in refractive surgery. Expert Rev Opthalmol. 2011;6(1):55–56. [Google Scholar]
- 4.Salomão MQ, Wilson SE. Femtosecond laser in laser in situ keratomileusis. J Cataract Refract Surg. 2010;36(6):1024–1032. doi: 10.1016/j.jcrs.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vega-Estrada A, Alió JL, Arba Mosquera S, Moreno LJ. Corneal higher order aberrations after LASIK for high myopia with a fast repetition rate excimer laser, optimized ablation profile, and femtosecond laser-assisted flap. J Refract Surg. 2012;28(10):689–696. doi: 10.3928/1081597X-20120921-03. [DOI] [PubMed] [Google Scholar]
- 6.Winkler von Mohrenfels C, Khoramnia R, Lohmann CP. Comparison of different excimer laser ablation frequencies (50, 200, and 500 Hz) Graefes Arch Clin Exp Ophthalmol. 2009;247(11):1539–1545. doi: 10.1007/s00417-009-1102-x. [DOI] [PubMed] [Google Scholar]
- 7.Iseli HP, Mrochen M, Hafezi F, Seller T. Clinical photoablation with a 500-Hz scanning spot excimer laser. J Refract Surg. 2004;20(6):831–834. doi: 10.3928/1081-597X-20041101-12. [DOI] [PubMed] [Google Scholar]
- 8.de Ortueta D, Magnago T, Triefenbach N, Arba Mosquera S, Sauer U, Brunsmann U. In vivo measurements of thermal load during ablation in high-speed laser corneal refractive surgery. J Refract Surg. 2012;28(1):53–58. doi: 10.3928/1081597X-20110906-01. [DOI] [PubMed] [Google Scholar]
- 9.Aslanides IM, Kolli S, Padron S, Arba Mosquera S. Stability of therapeutic retreatment of corneal wavefront customized ablation with the SCHWIND CAM: 4-year data. J Refract Surg. 2012;28(5):347–352. doi: 10.3928/1081597X-20120410-01. [DOI] [PubMed] [Google Scholar]
- 10.Smadja D, Reggiani-Mello G, Santhiago MR, Krueger RR. Wavefront ablation profiles in refractive surgery: description, results, and limitations. J Refract Surg. 2012;28(3):224–232. doi: 10.3928/1081597X-20120217-01. [DOI] [PubMed] [Google Scholar]
- 11.Kanellopoulos AJ. Topography-guided custom retreatments in 27 symptomatic eyes. J Refract Surg. 2005;21(5):S513–S518. doi: 10.3928/1081-597X-20050901-19. [DOI] [PubMed] [Google Scholar]
- 12.Kanellopoulos AJ. Topography-guided hyperopic and hyperopic astigmatism femtosecond laser-assisted LASIK: long-term experience with the 400 Hz eye-Q excimer platform. Clin Ophthalmol. 2012;6:895–901. doi: 10.2147/OPTH.S23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng H, Song LW. Visual quality of Q-value-guided LASIK in the treatment of high myopia. Yan Ke Xue Bao. 2011;26(4):208–210. doi: 10.3969/j.issn.1000-4432.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Alio JL, Vega-Estrada A, Piñero DP. Laser-assisted in situ keratomileusis in high levels of myopia with the amaris excimer laser using optimized aspherical profiles. Am J Ophthalmol. 2011;152(6):954–963. doi: 10.1016/j.ajo.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 15.El Awady HE, Ghanem AA, Saleh SM. Wavefront-optimized ablation versus topography-guided customized ablation in myopic LASIK: comparative study of higher order aberrations. Ophthalmic Surg Lasers Imaging. 2011;42(4):314–320. doi: 10.3928/15428877-20110421-01. [DOI] [PubMed] [Google Scholar]
- 16.WaveLight Allegretto Wave® Eye-Q Excimer Laser – P020050/S012 Summary of Safety and Effectiveness Data [SSED] [Accessed August 27, 2016]. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf2/P020050S012b.pdf.
- 17.Zuberbühler B, Galloway P, Reddy A, Saldana M, Gale R. A web-based information system for management and analysis of patient data after refractive eye surgery. Comput Methods Programs Biomed. 2007;88(3):210–216. doi: 10.1016/j.cmpb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Mrochen M, Wüllner C, Krause J, Klafke M, Donitzky C, Seiler T. Technical aspects of the WaveLight FS200 femtosecond laser. J Refract Surg. 2010;26(10):S833–S840. doi: 10.3928/1081597X-20100921-12. [DOI] [PubMed] [Google Scholar]
- 19.Kanellopoulos AJ, Asimellis G. Correlation between central corneal thickness, anterior chamber depth, and corneal keratometry as measured by oculyzer II and WaveLight OB820 in preoperative cataract surgery patients. J Refract Surg. 2012;22:1–6. doi: 10.3928/1081597X-20121005-07. [DOI] [PubMed] [Google Scholar]
- 20.Kanellopoulos AJ. Long term results of a prospective randomized bilateral eye comparison trial of higher fluence, shorter duration ultraviolet A radiation, and riboflavin collagen cross linking for progressive keratoconus. Clin Ophthalmol. 2012;6:97–101. doi: 10.2147/OPTH.S27170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuura T, Ikeda H, Idota N, Motokawa R, Hara Y, Annaka M. Anisotropic swelling behavior of the cornea. J Phys Chem B. 2009;113(51):16314–16322. doi: 10.1021/jp907232h. [DOI] [PubMed] [Google Scholar]
- 22.Han DC, Chen J, Htoon HM, Tan DT, Mehta JS. Comparison of outcomes of conventional WaveLight(®) Allegretto Wave(®) and Technolas(®) excimer lasers in myopic laser in situ keratomileusis. Clin Ophthalmol. 2012;6:1159–1168. doi: 10.2147/OPTH.S29660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celik HU, Alagöz N, Yildirim Y, et al. Accelerated corneal crosslinking concurrent with laser in situ keratomileusis. J Cataract Refract Surg. 2012;38(8):1424–1431. doi: 10.1016/j.jcrs.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 24.Kanellopoulos AJ. Long-term safety and efficacy follow-up of prophylactic higher fluence collagen cross-linking in high myopic laser-assisted in situ keratomileusis. Clin Ophthalmol. 2012;6:1125–1130. doi: 10.2147/OPTH.S31256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanellopoulos AJ, Asimellis G. Refractive and keratometric stability in high myopia LASIK with high-frequency femtosecond and excimer lasers. J Refract Surg. 2013;29(12):832–837. doi: 10.3928/1081597X-20130924-02. [DOI] [PubMed] [Google Scholar]
- 26.Kanellopoulos AJ, Asimellis G. Keratoconus management: long-term stability of topography-guided normalization combined with high-fluence CXL stabilization (the Athens Protocol) J Refract Surg. 2014;30(2):88–92. doi: 10.3928/1081597X-20140120-03. [DOI] [PubMed] [Google Scholar]
- 27.Kanellopoulos AJ, Asimellis G. Corneal refractive power and symmetry changes following normalization of ectasias treated with partial topography-guided PTK combined with higher-fluence CXL (the Athens Protocol) J Refract Surg. 2014;30(5):342–346. doi: 10.3928/1081597X-20140416-03. [DOI] [PubMed] [Google Scholar]
- 28.Kanellopoulos AJ, Asimellis G. LASIK ablation centration: an objective digitized assessment and comparison between two generations of an excimer laser. J Refract Surg. 2015;31(3):164–169. doi: 10.3928/1081597X-20150225-01. [DOI] [PubMed] [Google Scholar]
- 29.Kanellopoulos AJ, Asimellis G. Novel placido-derived topography-guided excimer corneal normalization with cyclorotation adjustment: enhanced athens protocol for keratoconus. J Refract Surg. 2015;31(11):768–773. doi: 10.3928/1081597X-20151021-06. [DOI] [PubMed] [Google Scholar]