Abstract
Background
Animal studies suggest a role of inflammation in the pathophysiology of anxiety, but human studies of inflammatory markers and anxiety disorders are scarce. We report a study of serum C-reactive protein (CRP) and generalised anxiety disorder (GAD) from the general population-based ALSPAC birth cohort.
Methods
DSM-IV diagnosis of GAD was obtained from 5365 cohort members during face-to-face clinical assessment at age 16 years, of which 3392 also provided data on serum high sensitivity CRP levels. Logistic regression calculated odds ratio (OR) for GAD among individuals in top and middle thirds of CRP distribution compared with the bottom third. Effect of comorbid depression was assessed. Age, sex, body mass, ethnicity, social class, maternal education, maternal age at delivery, and family history of inflammatory conditions were included as potential confounders.
Results
Forty participants met DSM-IV criteria for GAD (0.74%). CRP levels were higher in GAD cases compared with the rest of the cohort (P = 0.005). After adjusting for potential confounders, participants in the top third of CRP values compared with the bottom third were more likely to have GAD; adjusted OR 5.06 (95% CI, 1.31–19.59). The association between CRP and GAD was consistent with a linear dose-response relationship. The pattern of association between CRP and GAD remained unchanged after excluding cases with co-morbid depression.
Conclusions
The findings are consistent with a role of inflammation in anxiety disorders. Longitudinal studies of inflammatory markers, subsequent anxiety taking into account current and past psychological stress are required to understand this association further.
Keywords: Biological markers, C-reactive protein, Systemic inflammation, Generalized anxiety disorder, Birth cohort study
Abbreviations: CRP, C-reactive protein; GAD, generalized anxiety disorder; ALSPAC, Avon Longitudinal Study of Parents and Children; DSM-IV, diagnostic and statistical manual of mental disorders, fourth edition; ICV, intra-cerebroventricular; CNS, central nervous system; DAWBA, Development and Well-being Assessment; BMI, body mass index; IQR, interquartile range; OR, odds ratio; CI, confidence internal
1. Introduction
Emerging evidence indicates an important role of inflammation in the pathophysiology of mood and anxiety disorders where inflammatory cytokines are thought to play a key role (Khandaker et al., 2014, Dantzer et al., 2008, Hodes et al., 2014). In healthy volunteers, simulated bacterial infection with the injection of an immune activating agent, lipopolysaccharide (LPS, a bacterial cell wall endotoxin), has been reported to produce anxiety and low mood as well as increased serum levels of interleukin 6 (IL-6, an inflammatory cytokine) (Reichenberg et al., 2001). Similarly, in mice immune activation is associated with anxiety-like behaviour as well as increased proinflammatory cytokines both in peripheral circulation and the brain (Gibney et al., 2013, Rossi et al., 2012). Moreover, anxiety inducing effects of social stress could be blocked by intra-cerebroventricular (ICV) administration of IL-1β (a proinflammatory cytokine) receptor antagonist immediately after stress exposure (Rossi et al., 2012). The results indicate inflammatory cytokines are important mediators of the relationship between psychological stress and anxiety in the central nervous system (CNS). However, despite these intriguing findings from preclinical research human studies of inflammation and anxiety are scarce. Rarer still are general population-based studies that are less prone to bias arising from inappropriate control selection. Existing studies are limited in number and have mixed findings; some suggest an association between anxiety symptoms and circulating inflammatory markers, such as C-reactive protein (CRP) (Liukkonen et al., 2011, Pitsavos et al., 2006), while others do not (Copeland et al., 2012, Baune et al., 2012). Methodological issues, such as study design, physical comorbidity, methods for measuring CRP and anxiety, might account for some of the discrepancy in findings in existing studies (see discussion). A clearer understanding of the association between anxiety and inflammation would come from studies that are based on general population samples, include robust assessment of inflammation, anxiety disorder as well as relevant socio-demographic, physical and clinical parameters, thus reducing chances of bias and confounding.
Using data from the general population-based Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort, we report a study of generalised anxiety disorder (GAD), diagnosed according Diagnostic and Statistical Manual of Mental Disorders fourth edition (DSM-IV) criteria, and serum CRP levels in adolescence at age 16 years. We have used a clinical diagnosis of GAD as the outcome rather than all anxiety disorders or anxiety symptoms, because anxiety disorders include a range of conditions with varied presentations that might have different pathophysiology and aetiology, whilst anxiety symptoms are non-specific and may accompany many physical and mental illnesses.
2. Methods and materials
2.1. Sample
The ALSPAC birth cohort comprises 14,062 live births from pregnant women resident in county Avon, a geographically defined region in southwest of England, with expected dates of delivery between April 1991 and December 1992 (http://www.bristol.ac.uk/alspac/) (Boyd et al., 2013, Fraser et al., 2013). Avon included both urban and rural areas, and the population was broadly representative of all children in the UK. The parents completed regular postal questionnaires about all aspects of their child's health and development since birth. Since the age of 7 years the entire cohort attended an annual assessment clinic during which they participated in a range of face-to-face interviews and physical tests.
The current study is based on 5365 cohort members who took part in psychiatric assessment at age 16 years, of which 3392 also provided data on serum CRP levels. Analysis of the association between CRP and GAD was based on this sample of 3392. We subsequently repeated the analysis after imputation of missing CRP data (N = 5365).
Ethical approval for the study was obtained from ALSPAC Ethics and Law Committee and the Local Research Ethics Committees.
2.2. Assessment of GAD
Diagnosis of GAD according to DSM-IV criteria was obtained using computerised child version of the Development and Well-being Assessment (DAWBA), which was administered on 5365 cohort members during face-to-face clinical assessment at age 16 years (Goodman et al., 2000, APA, 1995). DAWBA assessed symptoms of GAD occurring in the six-month period preceding the assessment. In addition, it included questions on symptom severity, duration, and their effect on life and development. All individuals with no psychiatric diagnosis or a diagnosis other than GAD were included in the comparison group.
2.3. Measurement of CRP
Blood samples were collected from participants who gave consent for venepuncture during clinical assessment at age 16 years. Participants fasted overnight before attending the clinic if seen in the morning, or at least for 6 h if seen in the afternoon. Blood samples were immediately spun, frozen and stored at −80 °C, which were analysed within 3–9 months of blood sampling with no freeze-thaw cycles in between. High sensitivity CRP was measured by automated particle-enhanced immunoturbidimetric assay (Roche UK, Welwyn Garden City, UK). A valid measure of serum CRP was obtained from 3490 participants in total, which ranged from 0.07 to 72.55 mg/L (60 subjects over 10 mg/L). No other inflammatory makers were measured.
The sample was divided into three groups based on the tertiles of CRP distribution in all 3490 subjects regardless of their case or non-case status at follow-up. Values for the 33rd and 66th percentiles of the CRP distribution were 0.26 mg/L and 0.64 mg/L respectively. Thus, CRP levels for participants in the bottom, middle and top thirds of the distribution were 0.07–0.25 mg/L, 0.26–0.63 mg/L, and 0.64–72.55 mg/L respectively.
2.4. Assessment of potential confounders
We included a number of socio-demographic, physical and clinical parameters that are relevant to CRP levels and psychiatric illnesses. The data were collected during face-to-face clinical assessments or by postal questionnaires completed by parents. We included age at the time of assessment of GAD (in weeks), sex, body mass index (BMI) at the time of assessment of CRP, ethnicity, father's social class, maternal age at delivery and educational level. As per the UK Office of National Statistics classification system, father's social class was recorded in six categories: I, II, III non-manual, III manual, IV, V (in descending order with professionals and higher managerial workers representing social class I). Maternal age at delivery was grouped into six categories (age in years: <20, 20–24, 25–29, 30–34, 35–40, >40). Maternal highest educational achievement was recorded in four groups (secondary school, vocational qualification, O level, A level, degree). In addition, we included family history of chronic inflammatory conditions: arthritis and rheumatism in mothers and maternal grandparents. Finally, we examined the effect of concurrent infection, and separately, depression (assessed by DAWBA) on the association between GAD and CRP (see below). We did not include the use of anti-inflammatory or psychotropic medications as potential confounders as the numbers of adolescent subjects taking these drugs are likely to be small.
2.5. Statistical analysis
2.5.1. Baseline comparison
Baseline characteristics between cases of GAD and the rest of the cohort were compared using the Chi-square test and independent sample t-test for categorical and continuous variables, respectively.
2.5.2. Comparison of serum CRP levels between GAD cases and non-cases
Values of serum CRP level were not normally distributed. Therefore, we used median and interquartile range (IQR) for descriptive results. Independent sample Kruskal–Wallis test was used to compare distribution of CRP values between groups.
2.5.3. Association between serum CRP and GAD
Binary logistic regression was used to calculate odds ratio (OR) and 95% confidence internal (CI) for GAD for participants in the middle and top thirds of CRP distribution, compared with those in the bottom third. The use of logistic regression was appropriate because GAD is a binary variable. Linearity of association was examined by inspection of OR across the thirds of CRP. Regression models were controlled for sex, age at the time of assessment of GAD, BMI at the time of assessment of CRP, ethnicity, father's social class, maternal highest educational level, maternal age at delivery, and family history of chronic inflammatory conditions.
2.5.4. Effect of depression and infection
Effect of depression on the CRP-GAD association was examined by excluding GAD cases with comorbid depression. Serum CRP levels increase sharply during infection. Because we were interested in the association between GAD and CRP in healthy individuals, we re-ran the analyses after excluding individuals with serum CRP levels >10 mg/L as such levels are highly suggestive of a current infection (Copeland et al., 2012).
2.5.5. Imputation for missing CRP data
We employed a fully conditional specification using the multiple imputation by Markov chain Monte-Carlo algorithm in SPSS to predict missing CRP data across 25 imputed datasets (Muller et al., 2004). Each imputation model included 45 variables including past and current anthropometric measures, cardiometabolic and other blood biomarkers, socio-demographic indicators, and psychiatric measurements to predict missing CRP data (full list available on request). The association between CRP and GAD was analysed after imputation across 25 imputed datasets.
3. Results
3.1. Baseline characteristics
Out of total 5365 participates assessed at age 16 years, 40 met DSM-IV criteria for a diagnosis of GAD; 0.74% (95% CI, 0.55–1.02). About 90% of GAD cases were female (Table 1).
Table 1.
Baseline characteristics of DSM-IV Generalised Anxiety Disorder (GAD) cases and non-cases at age 16 years in the ALSPAC cohort.
| Characteristics | GAD | No GAD | χ2; p-value |
|---|---|---|---|
| Number | 40 | 5325 | – |
| Age in years, mean (SD) | 15.56 (0.24) | 15.53 (0.31) | 0.603; 0.54a |
| BMI, mean (SD) | 22.55 (3.52) | 21.40 (3.63) | 2.158; 0.03a |
| Female (%) | 90 | 52.3 | 22.63; <0.0001 |
| British White ethnicity (%) | 91.7 | 98.1 | 23.68; 0.003 |
| Father's social class (%) | 3.24; 0.77 | ||
| I | 5.9 | 14.0 | |
| II | 44.1 | 37.3 | |
| III non manual | 8.8 | 12.4 | |
| III manual | 32.4 | 26.3 | |
| IV | 5.9 | 8.0 | |
| V | 2.9 | 2.0 | |
| Maternal highest education (%) | 3.47; 0.48 | ||
| Secondary school | 16.7 | 11.2 | |
| Vocational | 5.6 | 7.8 | |
| O level | 41.7 | 34.7 | |
| A Level | 16.7 | 28.4 | |
| Degree | 19.4 | 17.9 | |
| Maternal age at delivery (%) | 15.13; 0.01 | ||
| <20 | 2.6 | 1.7 | |
| 20–24 | 30.8 | 12.9 | |
| 25–29 | 43.6 | 38.7 | |
| 30–34 | 20.5 | 33.9 | |
| 35–39 | 2.6 | 11.3 | |
| >40 | 0 | 1.5 | |
| History of arthritis in mothers (%) | 8.1 | 3.4 | 2.37; 0.12 |
| History of rheumatism in mothers (%) | 5.4 | 3.9 | 0.21; 0.65 |
| History of arthritis in maternal grandparents (%) | 10.8 | 30.9 | 7.18; 0.06 |
| History of rheumatism in maternal grandparents (%) | 16.2 | 19.5 | 1.26; 0.73 |
Independent sample t-test statistic and p-value.
3.2. Serum CRP levels in GAD cases and non-cases at age 16 years
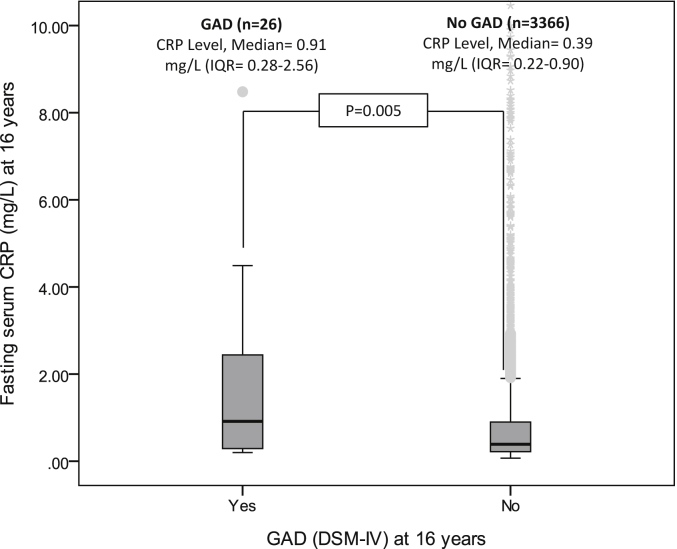
Data on both serum CRP and GAD were available on 3392 individuals; this analytic sample included 26 cases of GAD (65% of all cases of GAD at age 16 years). Median values of serum CRP were significantly higher in the GAD group compared with the rest of the cohort (Fig. 1).
Fig. 1.
Serum CRP levels (mg/L) in DSM-IV GAD cases and non-cases at age 16 years in the ALSPAC cohort.
3.3. Odds ratio for GAD at 16 years
Fig. 2 presents proportion of GAD cases at age 16 years by thirds of CRP. Individuals in the top third of CRP distribution were nearly five times more likely to have a diagnosis of GAD compared with those in the bottom third, which was significant after adjusting for potential confounders. The association between CRP and GAD at age 16 years was consistent with a linear dose-response relationship (Table 2).
Fig. 2.
DSM-IV GAD at age 16 years by tertiles of CRP levels in the ALSPAC cohort.
Table 2.
Odds ratio for DSM-IV GAD for serum CRP levels at age 16 years in the ALSPAC cohort.
| Groupsa |
N |
GAD cases, n (%) |
Odds ratio (95% confidence interval) |
||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted for age, sex, BMI | Adjusted for age, sex, BMI, social class, ethnicity | Adjusted for age, sex, BMI, social class, ethnicity, maternal education and age at delivery | Additional adjustment for family history of chronic inflammatory conditionsb | |||
| Bottom third | 1093 | 3 (0.3) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Middle third | 1151 | 9 (0.8) | 2.86 (0.77–10.60) | 2.92 (0.78–10.92) | 2.39 (0.60–9.47) | 2.42 (0.60–9.75) | 2.25 (0.55–9.20) |
| Top third | 1148 | 14 (1.2) | 4.48 (1.28–15.65) | 4.67 (1.28–16.97) | 4.69 (1.25–17.51) | 4.88 (1.28–18.54) | 5.06 (1.31–19.59) |
| Linear trend | 3392 | 26 (0.76) | 1.95 (1.14–3.32) | 1.99 (1.14–3.47) | 2.11 (1.16–3.85) | 2.16 (1.17–3.98) | 2.25 (1.19–4.23) |
The group at the bottom third of CRP distribution was used as reference category. Cut off values for the top and bottom thirds of CRP distribution in the entire sample were 0.64 and 0.26 mg/L, respectively.
Includes history of arthritis and rheumatism in mother and maternal grandparents.
3.4. Effect of comorbid depression
Out of 26 cases of GAD included in analyses, eight had comorbid depression (30.77%). Median (IQR) of CRP values was not significantly different between GAD cases with and without comorbid depression: 0.69 mg/L (0.25–1.72) and 1.04 mg/L (0.30–3.48), respectively. Out of 18 GAD cases without comorbid depression, ten were in the top third of CRP distribution while seven were in the middle and one in the bottom third. These results clearly suggest that observed associations between CRP and GAD are primarily due to higher levels of CRP in GAD cases rather than an artefact of comorbid depression.
3.5. Results after excluding concurrent infection
In total, 60 participants had serum CRP levels >10 mg/L, which is suggestive of a current infection. We re-ran the regression analyses reported in Table 2 after excluding these individuals (N = 3332, including 25 cases of GAD). All effect estimates remained virtually unchanged (see online supplementary Table 1).
3.6. Results after imputation of missing CRP data
The pattern of results remained similar, i.e. higher CRP levels were associated with increased risk of GAD. Out of total 40 GAD cases, 20 (0.96%) were in the top third of CRP distribution, 12 (0.82%) were in the middle, and 8 (0.44%) in the bottom third. This equated to a two-fold increased risk of GAD for participants in the top compared with the bottom third of CRP distribution, although the associations fell short of conventional statistical significance (see online supplementary Table 2).
4. Discussion
The findings suggest an association between higher serum CRP levels at age 16 years and DSM-IV GAD in the general population-based ALSPAC birth cohort, which is independent of a number of potential confounders including sex, body mass, socio-economic status and family history of inflammatory conditions. Sensitivity analysis after excluding participants with a suspected infection around CRP assay did not alter the results. Comparison between cases of GAD with and without comorbid depression showed the association was not driven by higher levels of CRP in cases with comorbid depression. The use of a general population birth cohort, robust assessment of outcome based on DSM-IV criteria, and examination of a number of important confounders are some of the strengths of this study. To our knowledge this is one of the first general population-based studies to report an association between GAD in adolescence and a circulating inflammatory biomarker.
General population-based studies of inflammatory markers and anxiety disorder are scarce. However, the findings are consistent with previous studies reporting an association between symptoms of anxiety and higher serum levels of CRP (Liukkonen et al., 2011, Pitsavos et al., 2006, Bullmore and Lynall, 2014, Danese et al., 2008, Laan et al., 2007, Bankier et al., 2009, Gegenava et al., 2011). One study based on 31 year follow up of the 1966 Northern Finland birth cohort reported an association between anxiety symptoms and serum CRP in men (Liukkonen et al., 2011). Another study based on a general population sample of healthy adults reported associations between several inflammatory markers including CRP and anxiety symptoms in both genders (Pitsavos et al., 2006). However, a third population-based study did not find an association between inflammatory markers and anxiety symptoms (Baune et al., 2012), which could be due to sample characteristics as it focused exclusively on older adults aged between 70 and 90 years. With regards to GAD specifically, one study reported an association between GAD and CRP, which was based on 120 stable coronary heart disease outpatient attendees (Bankier et al., 2008). While individuals with chronic physical conditions may not be representative of people with anxiety disorders in general, the use of GAD diagnosis as outcome is a strength of this study. We found only one general population-based study on this topic but it did not find an association between GAD and CRP (measured in dried blood spots) (Copeland et al., 2012). The discrepant results might be attributed to study methodology. There is evidence that CRP levels in dried blood spots decline over time making it more difficult to detect (Brindle et al., 2010).
Limitations of the current study includes relatively small number of GAD cases, which led to reduced statistical power as reflected by large confidence interval around effect estimates both in the observed and imputed datasets. Studies with a larger number of GAD cases are required. However, the prevalence of GAD during past six months diagnosed according to DSM-IV criteria at 16 years in the ALSPAC sample was 0.74%, which is similar to other general population-based studies of similar age groups. One year prevalence of DSM-IV GAD was reported to be 0.3% in a study of 3042 children aged 8–15 years from the USA (Laan et al., 2010). According to a national survey, the prevalence of GAD defined according to ICD-10 criteria was 0.9% in children age 11–15 years in the UK (Statistics OfN, 1999). Another issue is missing data. Out of 5365 individuals assessed for GAD at age 16 years, about a third had no data on CRP. However, the proportion of GAD cases was similar between the analytic sample and the group with missing CRP data. No significant differences between these groups were observed in terms of distribution of potential confounders such as age, sex, social class and ethnicity. Thus, any significant bias arising from missing data is unlikely. Data regarding inflammatory markers included only serum CRP. In the future, examination of a range of inflammatory markers, such as IL-6 and other cytokines would be useful. CRP levels were appropriate for the age of the subjects.
GAD is characterised by persistent, excessive and uncontrollable worry (APA, 1995). A cross-sectional association between levels of CRP and GAD might reflect increased levels of stress perceived by individuals with this illness. Both physical and psychological stress can provoke transient increase in proinflammatory cytokines. Rats exposed to electric foot-shock, physical restraint, or a conditioned aversive stimulus have increased levels of plasma IL-6 (Zhou et al., 1993). Similarly, in humans acute or chronic stress is associated with increase in levels of circulating proinflammatory cytokines (Maes et al., 1998, Kiecolt-Glaser et al., 2003). The current findings are consistent with previous studies reporting increased CRP and other inflammatory markers in depression and post-traumatic stress disorder (von Kanel et al., 2007, Howren et al., 2009, Haapakoski et al., 2015). Recently a population-based longitudinal study has reported a longitudinal association between serum IL-6 in childhood and risks of depression and psychosis in young adult life (Khandaker et al., 2014); these findings along with other longitudinal studies (Gimeno et al., 2009, Wium-Andersen et al., 2014) indicate a potentially causal role of inflammation in depression (Khandaker et al., 2014, Gimeno et al., 2009) and schizophrenia (Khandaker et al., 2015, Khandaker and Dantzer, 2015). In future longitudinal studies of inflammatory markers and GAD are needed for a clearer understanding of the direction of this association.
The relationship between systemic inflammation, mood and anxiety is complex (Messay et al., 2012). There is evidence that prior stress exposure have a priming effect on inflammatory cytokine response, as reflected by a larger or more rapid induction of these molecules following immune activation (Johnson et al., 2002). Thus, increased inflammatory markers in GAD might reflect previous exposure to stressful events, a known risk factor for anxiety disorders (Heim and Nemeroff, 2001). In the future, studies should include measures of current psychological stress, past trauma and maltreatment as well as stress-related biomarkers such as cortisol in order to elucidate the effect of stress on the association between inflammation and anxiety.
Animal studies provide useful insights into potential mechanisms underlying the association between systemic inflammation and anxiety. Peripheral cytokines can communicate with the brain in a number of ways to leading to neuropsychiatric symptoms relevant for anxiety, mood and psychotic disorders; for reviews see (Dantzer et al., 2008, Khandaker and Dantzer, 2015, Stolk et al., 2007, D'Mello and Swain, 2014, Quan and Banks, 2007). Possible routes for peripheral immune to brain communication include (i) leaky regions in the blood–brain barrier, such as circumventricular organs, (ii) active transport via soluble transport molecules, (iii) activation of endothelial cells and macrophages in the lining of cerebral vasculature (which then produce cytokines and facilitate transmigration of inflammatory cells in the brain), and (iv) retrograde axonal transport through peripheral afferent nerve fibres (e.g. the vagus nerve). Once the cytokine signal reaches the brain, the CNS cytokine network (made up of neurons and glial cells) not only produce cytokines and cytokine receptors within brain tissue but also amplify the signal (Dantzer, 2004). This, in turn, leads to a number of changes relevant for neuropsychiatric symptoms seen in anxiety and depression. The changes include (i) increased metabolism and reuptake of serotonin and other mood-relevant neurotransmitters, (ii) stimulation of the hypothalamic-pituitary-adrenal axis and release of corticotrophin releasing hormone in hypothalamus and amygdala, (iii) increasing oxidative stress and thus reducing synaptic plasticity (Dantzer et al., 2008, Miller et al., 2009). Crucial evidence linking proinflammatory cytokines, anxiety and depression comes from recent animal studies. Rossi and colleagues reported that administration of the cytokine IL-1β induces anxiety in mice, and anxiety inducing effects of social defeat could be blocked by ICV administration of an IL-1β receptor antagonist immediately after stress exposure. Similarly, in mice inhibiting IL-6 by monoclonal antibody injection prevents stress-induced depression-like behaviour (Hodes et al., 2014). The results suggest that inflammatory cytokines may be important mediators of the link between stress and anxiety.
In summary, we report a general population-based study of an association between higher serum levels of CRP and clinical diagnosis of GAD according to DSM-IV criteria in adolescence. These findings need replication. Current epidemiological evidence regarding anxiety and inflammatory markers is limited, although population-based studies indicate an association between higher serum CRP levels and symptoms of anxiety. Longitudinal studies with measures of inflammation, subsequent diagnosis of anxiety taking into account current and past exposure to psychological stress are required to further elucidate the association between anxiety and inflammation.
Funding
Dr Khandaker is supported by a Clinical Lecturer Starter Grant from the UK Academy of Medical Sciences (Grant no. 80354) and a Gosling Fellowship from the Royal College of Psychiatrists. Prof Jones acknowledges grant support from the Wellcome Trust (095844/Z/11/Z & 088869/Z/09/Z) and NIHR (RP-PG-0606-1335). The UK Medical Research Council (Grant ref: 74882), the Wellcome Trust (Grant ref: 092731) and the University of Bristol provide core support for ALSPAC. The funding bodies had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Declaration of interest
The authors have no conflicts of interest to disclose. PBJ received an honorarium, that he donated to his department, from Roche for taking part in an advisory board to advise on education about schizophrenia for psychiatrists.
Authors' contributions
Golam Khandaker designed the study, analysed data and wrote the first draft. Stanley Zammit, Glyn Lewis and Peter Jones contributed to study design, analysis and in the revision of the manuscript.
Acknowledgement
We are grateful to all families who took part in this study, midwives for their help in recruitment, and the whole ALSPAC team, including interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ynstr.2016.02.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- APA . fourth ed. American Psychiatric Association; Washitngton D.C: 1995. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. [Google Scholar]
- Bankier B., Barajas J., Martinez-Rumayor A., Januzzi J.L. Association between C-reactive protein and generalized anxiety disorder in stable coronary heart disease patients. Eur. Heart J. 2008;29(18):2212–2217. doi: 10.1093/eurheartj/ehn326. [DOI] [PubMed] [Google Scholar]
- Bankier B., Barajas J., Martinez-Rumayor A., Januzzi J.L. Association between anxiety and C-reactive protein levels in stable coronary heart disease patients. Psychosomatics. 2009;50(4):347–353. doi: 10.1176/appi.psy.50.4.347. [DOI] [PubMed] [Google Scholar]
- Baune B.T., Smith E., Reppermund S., Air T., Samaras K., Lux O. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: the prospective Sydney Memory and Aging Study. Psychoneuroendocrinology. 2012;37(9):1521–1530. doi: 10.1016/j.psyneuen.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Boyd A., Golding J., Macleod J., Lawlor D.A., Fraser A., Henderson J. Cohort profile: the ‘Children of the 90s'–the index offspring of the Avon longitudinal study of parents and children. Int. J. Epidemiol. 2013;42(1):111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle E., Fujita M., Shofer J., O'Connor K.A. Serum, plasma, and dried blood spot high-sensitivity C-reactive protein enzyme immunoassay for population research. J. Immunol. Methods. 2010;362(1–2):112–120. doi: 10.1016/j.jim.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E.T., Lynall M.E. Immunologic therapeutics and psychotic disorders. Biol. Psychiatry. 2014;75(4):260–261. doi: 10.1016/j.biopsych.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Copeland W.E., Shanahan L., Worthman C., Angold A., Costello E.J. Generalized anxiety and C-reactive protein levels: a prospective, longitudinal analysis. Psychol. Med. 2012;42(12):2641–2650. doi: 10.1017/S0033291712000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello C., Swain M.G. Liver-brain interactions in inflammatory liver diseases: implications for fatigue and mood disorders. Brain Behav. Immun. 2014;35:9–20. doi: 10.1016/j.bbi.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Danese A., Moffitt T.E., Pariante C.M., Ambler A., Poulton R., Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. General Psychiatry. 2008;65(4):409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 2004;500(1–3):399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A., Macdonald-Wallis C., Tilling K., Boyd A., Golding J., Davey Smith G. Cohort profile: the Avon longitudinal study of parents and children: ALSPAC mothers cohort. Int. J. Epidemiol. 2013;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenava T., Gegenava M., Kavtaradze G. C-reactive protein level correlation with depression and anxiety among patients with coronary artery disease. Georgian Med. News. 2011;194:34–37. [PubMed] [Google Scholar]
- Gibney S.M., McGuinness B., Prendergast C., Harkin A., Connor T.J. Poly I: C-induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain Behav. Immun. 2013;28:170–181. doi: 10.1016/j.bbi.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Gimeno D., Kivimaki M., Brunner E.J., Elovainio M., De Vogli R., Steptoe A. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med. 2009;39(3):413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R., Ford T., Richards H., Gatward R., Meltzer H. The development and well-being assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J. Child Psychol. Psychiatry Allied Discip. 2000;41(5):645–655. [PubMed] [Google Scholar]
- Haapakoski R., Mathieu J., Ebmeier K.P., Alenius H., Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Nemeroff C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hodes G.E., Pfau M.L., Leboeuf M., Golden S.A., Christoffel D.J., Bregman D. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. U. S. A. 2014;111(45):16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Johnson J.D., O'Connor K.A., Deak T., Stark M., Watkins L.R., Maier S.F. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav. Immun. 2002;16(4):461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- von Kanel R., Hepp U., Kraemer B., Traber R., Keel M., Mica L. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J. Psychiatr. Res. 2007;41(9):744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Khandaker G.M., Dantzer R. Is there a role for immune-to-brain communication in schizophrenia? Psychopharmacology. 2015 doi: 10.1007/s00213-015-3975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Pearson R.M., Zammit S., Lewis G., Jones P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. J. Am. Med. Assoc. Psychiatry. 2014;71(10):1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Cousins L., Deakin J., Lennox B.R., Yolken R., Jones P.B. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2(3):258–270. doi: 10.1016/S2215-0366(14)00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J.K., Preacher K.J., MacCallum R.C., Atkinson C., Malarkey W.B., Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl. Acad. Sci. U. S. A. 2003;100(15):9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan W., Selten J.P., Grobbee D.E., Smeets H., Kahn R.S., Burger H. Non-steroidal anti-inflammatory drugs and the risk of psychosis. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2007;17(4):309–311. doi: 10.1016/j.euroneuro.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Laan W., Grobbee D.E., Selten J.P., Heijnen C.J., Kahn R.S., Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry. 2010;71(5):520–527. doi: 10.4088/JCP.09m05117yel. [DOI] [PubMed] [Google Scholar]
- Liukkonen T., Rasanen P., Jokelainen J., Leinonen M., Jarvelin M.R., Meyer-Rochow V.B. The association between anxiety and C-reactive protein (CRP) levels: results from the Northern Finland 1966 birth cohort study. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2011;26(6):363–369. doi: 10.1016/j.eurpsy.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Maes M., Song C., Lin A., De Jongh R., Van Gastel A., Kenis G. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10(4):313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- Messay B., Lim A., Marsland A.L. Current understanding of the bi-directional relationship of major depression with inflammation. Biol. Mood Anxiety Disord. 2012;2(1):4. doi: 10.1186/2045-5380-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N., Ulmschneider M., Scheppach C., Schwarz M.J., Ackenheil M., Moller H.J. COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy. Eur. Arch. Psychiatry Clin. Neurosci. 2004;254(1):14–22. doi: 10.1007/s00406-004-0478-1. [DOI] [PubMed] [Google Scholar]
- Pitsavos C., Panagiotakos D.B., Papageorgiou C., Tsetsekou E., Soldatos C., Stefanadis C. Anxiety in relation to inflammation and coagulation markers, among healthy adults: the ATTICA study. Atherosclerosis. 2006;185(2):320–326. doi: 10.1016/j.atherosclerosis.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Quan N., Banks W.A. Brain-immune communication pathways. Brain Behav. Immun. 2007;21(6):727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Reichenberg A., Yirmiya R., Schuld A., Kraus T., Haack M., Morag A. Cytokine-associated emotional and cognitive disturbances in humans. Arch. General Psychiatry. 2001;58(5):445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Rossi S., Sacchetti L., Napolitano F., De Chiara V., Motta C., Studer V. Interleukin-1beta causes anxiety by interacting with the endocannabinoid system. J. Neurosci. Off. J. Soc. Neurosci. 2012;32(40):13896–13905. doi: 10.1523/JNEUROSCI.1515-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics OfN . Department of Health, the Scottish Health Executive and the National Assembly for Wales; London: 1999. The Mental Health of Children and Adolescents in Great Britain. [Google Scholar]
- Stolk P., Souverein P.C., Leufkens H.G., Weil J.G., Egberts A.C., Heerdink E.R. The association between exposure to COX-2 inhibitors and schizophrenia deterioration. A nested case-control study. Pharmacopsychiatry. 2007;40(3):111–115. doi: 10.1055/s-2007-977714. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen M.K., Orsted D.D., Nordestgaard B.G. Elevated C-reactive protein associated with late- and very-late-onset schizophrenia in the general population: a prospective study. Schizophr. Bull. 2014;40(5):1117–1127. doi: 10.1093/schbul/sbt120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Kusnecov A.W., Shurin M.R., DePaoli M., Rabin B.S. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133(6):2523–2530. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.