Significance
Changes in the environment or in physiology can induce an animal cell to secrete signaling molecules that move through circulation to regulate distant cells. Extracellular RNAs can accumulate in circulation in humans, can change during disease, and can potentially act as signaling molecules, but their source and destination are typically unclear. When extracellular RNAs enter cells, they can regulate genes of matching sequence. We show that double-stranded RNA introduced into the circulation of the animal Caenorhabditis elegans can be transported to the next generation through oocytes and can silence matching genes in progeny. These results demonstrate that extracellular RNA can carry gene regulatory information between generations. Such intergenerational messages could transmit effects of ancestral experience to descendants in animals.
Keywords: circulating RNA, parental RNAi, epigenetics, transgenerational inheritance, endocytosis
Abstract
Experiences during the lifetime of an animal have been proposed to have consequences for subsequent generations. Although it is unclear how such intergenerational transfer of information occurs, RNAs found extracellularly in animals are candidate molecules that can transfer gene-specific regulatory information from one generation to the next because they can enter cells and regulate gene expression. In support of this idea, when double-stranded RNA (dsRNA) is introduced into some animals, the dsRNA can silence genes of matching sequence and the silencing can persist in progeny. Such persistent gene silencing is thought to result from sequence-specific interaction of the RNA within parents to generate chromatin modifications, DNA methylation, and/or secondary RNAs, which are then inherited by progeny. Here, we show that dsRNA can be directly transferred between generations in the worm Caenorhabditis elegans. Intergenerational transfer of dsRNA occurs even in animals that lack any DNA of matching sequence, and dsRNA that reaches progeny can spread between cells to cause gene silencing. Surprisingly, extracellular dsRNA can also reach progeny without entry into the cytosol, presumably within intracellular vesicles. Fluorescently labeled dsRNA is imported from extracellular space into oocytes along with yolk and accumulates in punctate structures within embryos. Subsequent entry into the cytosol of early embryos causes gene silencing in progeny. These results demonstrate the transport of extracellular RNA from one generation to the next to regulate gene expression in an animal and thus suggest a mechanism for the transmission of experience-dependent effects between generations.
The impact of ancestral experiences on descendants in animals has been evaluated and reevaluated for more than a century. Recent studies in animals have focused on changes in diet and stress as triggers in ancestors and found that such experiences correlate with changes in descendants (reviewed in refs. 1–4). Changes in diet, for example, are correlated with mortality of grandprogeny in humans (5), altered metabolism of progeny in mice (6), and longevity of descendants in the worm Caenorhabditis elegans (7). Maternal separation (8), social defeat (9), and chronic variable stress (10) are correlated with hypersensitivity to similar stresses in descendants in mice. Molecules that transmit gene regulatory information from one generation to the next generation in response to somatic cells that experience the effects of diet or stress could provide a mechanistic explanation for the observed correlations. Extracellular RNAs are candidates for transmitting gene-specific information from somatic cells to the germline and thus to the next generation because they can be detected in circulation (e.g., ref. 11), their composition is altered in disease states (e.g., ref. 12), and they can enter cells to regulate genes of matching sequence (e.g., ref. 13) (reviewed in ref. 14).
Studies in the worm C. elegans have provided some of the clearest evidence for RNA acting as a carrier of gene-specific information from somatic cells to germ cells in an animal. Expression of double-stranded RNA (dsRNA) in C. elegans neurons generates mobile RNAs that can silence a gene of matching sequence through RNA interference (RNAi) within the germline, and this silencing can persist for more than 25 generations (15). Similar persistent silencing also occurs when dsRNA is delivered into worms by injection (16), by soaking (17), or through expression within bacteria that worms ingest as food (18). Silencing of somatic genes typically persists for one generation, but silencing of germline genes can persist for many more generations (see Fig. S1 for a summary of previous studies). Silencing by extracellular dsRNA requires entry into the cytosol, which is the aqueous component of the cytoplasm within which various organelles and particles are suspended. In all cases, entry of extracellular dsRNA into the cytosol of C. elegans cells requires the dsRNA-selective importer SID-1 (19–21). Upon entry into the cytosol, dsRNA is processed to generate small RNAs that are used as guides to identify mRNA of matching sequence. The target mRNA is then used as a template to generate numerous secondary small RNAs that can direct the deposition of repressive chromatin marks (reviewed in ref. 22). Although secondary small RNAs and chromatin marks have been detected in progeny upon parental exposure to dsRNA (23, 24), it is unknown where extracellular dsRNA needs to interact with intracellular RNA or DNA to cause gene silencing in progeny.
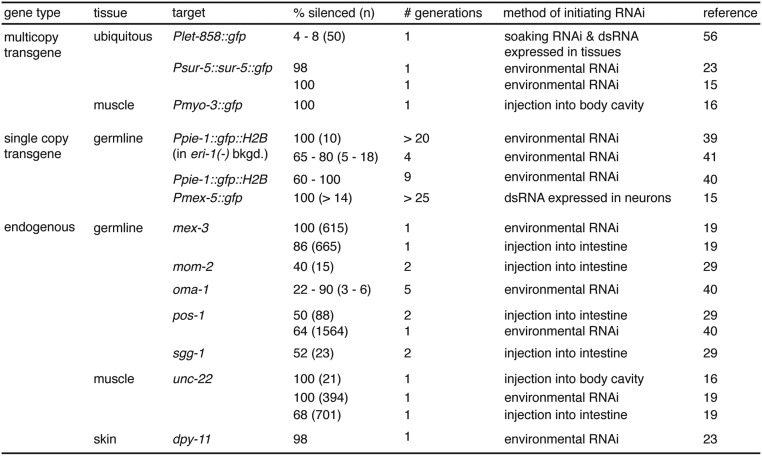
Fig. S1.
Published cases of silencing observed in self progeny when dsRNA against multicopy transgenes, single-copy transgenes, or endogenous genes was introduced outside the germline in hermaphrodites.
Here, we show that extracellular dsRNA can be transported to progeny without entry into any cytosol in the parent and that, upon entry into the cytosol in embryos, it can silence genes of matching sequence. Processing of ingested dsRNA within the parental germline or in early development of progeny generates additional forms of dsRNA that spread between cells in progeny to cause potent gene silencing. Use of fluorescently labeled RNA reveals that the dsRNA is imported into oocytes via the yolk endocytosis pathway.
Results
Silencing Signals Are Transported to Progeny Through Oocytes.
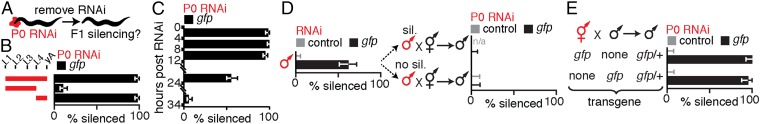
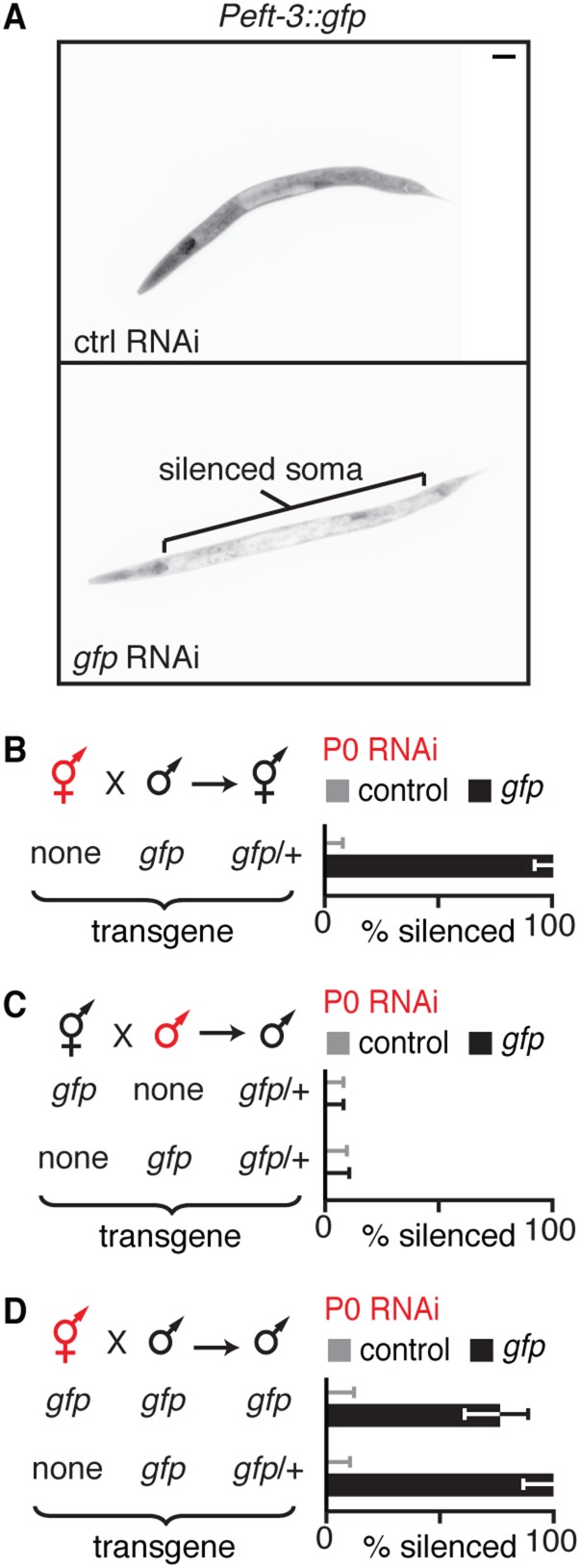
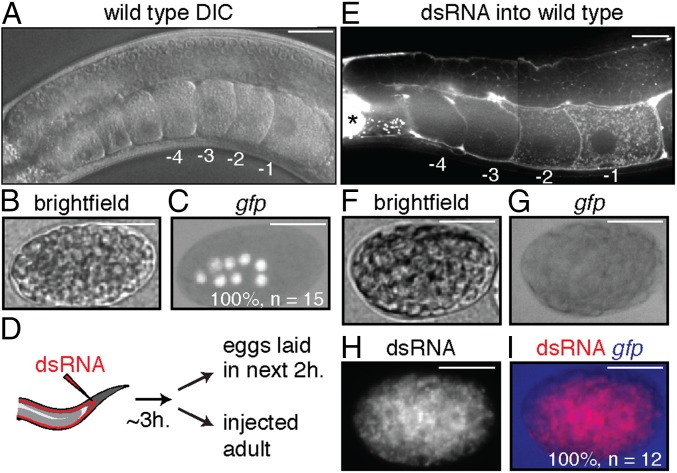
To evaluate gene silencing in progeny upon ingestion of dsRNA, we fed worms bacteria that express dsRNA, removed the bacteria, and examined silencing in progeny (Fig. 1A). Using this assay, all silencing of endogenous genes, as well as transgenes detected in progeny, was due to the inheritance of a silencing signal from parents to progeny (Fig. S2). We found that ingestion of dsRNA by animals from hatching until their fourth larval (L4) stage resulted in silencing in only ∼10% of progeny, but ingestion beyond the L4 stage for a 24-h period resulted in silencing in ∼100% of progeny (Fig. 1B). Silencing occurred in all animals among early progeny but was observed in progressively fewer animals among later progeny (Fig. 1C), as is the case when limiting amounts of dsRNA are introduced by injection into the germline (25). This reduction of inherited silencing is consistent with the dilution of silencing signals by two known processes: cytoplasmic streaming within the germline (26) and the flow of material from the intestine into oocytes (e.g., yolk) (27). Such dilution is expected to be progressive in oocytes, which are made continuously during adulthood, but not in sperm, which are made in a single batch during the fourth larval stage (Fig. S3) (28). Furthermore, unlike the ∼100% silencing that could be observed in progeny of hermaphrodites that ingested dsRNA, silencing was not detectable in any progeny of males that ingested dsRNA (Fig. 1D), despite the detection of SID-1–dependent silencing within the germline of male parents (Fig. 1D and Fig. S4). Together, these results suggest that ingested dsRNA or dsRNA-derived silencing signals that can be progressively diluted are transported to progeny through oocytes.
Fig. 1.
Ingested dsRNA or dsRNA-derived silencing signals can be transported to progeny through oocytes by parents that lack DNA of matching sequence. (A) Schematic of assay to assess silencing in progeny (F1) by parental ingestion of dsRNA (P0 RNAi, red). Also see Fig. S2. (B and C) Silencing of multicopy gfp transgenes in progeny by ingested dsRNA. (B) Robust silencing of Psur-5::sur-5::gfp in intestinal cells required parental ingestion of gfp-dsRNA during adulthood. L1 to L4, larval stages; yA, young adult. (C) Silencing of Pmyo-3::gfp in muscle cells after parental ingestion of gfp-dsRNA was detectable in all early progeny (0–12 h post-RNAi) but only in diminishing fractions of later progeny (12–34 h post-RNAi). (D) Males showing silencing of gfp (Pgtbp-1::gtbp-1::gfp) within the germline (sil.) did not transmit silencing to any cross-progeny (Right). Males fed control RNAi did not show any silencing. n/a, not applicable. (E) Silencing of a single-copy gfp transgene (Peft-3::gfp) in cross-progeny was detected in somatic cells even when only hermaphrodites (red) that lack gfp (none) ingested gfp-dsRNA (black bars). Error bars indicate 95% confidence interval (CI); L4-staged animals were assayed [n > 80 (B), n > 84 (C), n > 40 (D), n > 56 (E)]; and gray bars indicate silencing in progeny of animals that ingested control dsRNA (D and E).
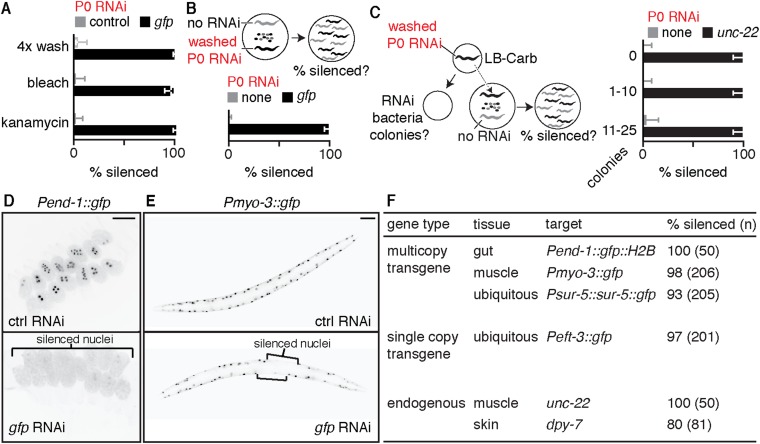
Fig. S2.
Washing worms that ingested bacteria expressing dsRNA is sufficient to ensure that silencing in progeny is caused by inheritance of a silencing signal from parents and not by the ingestion of dsRNA by progeny. (A) Silencing (% silenced) of a multicopy gfp transgene (Psur-5::sur-5::gfp) in intestinal cells of progeny was detectable when the bacteria expressing gfp-dsRNA (P0 RNAi) were removed from parents by washing four times with buffer (4x wash) or by killing (bleaching P0 animals or placing animals on kanamycin plates). (B) A worm that was fed bacteria that express gfp-dsRNA and subsequently washed with buffer (washed P0 RNAi, black) was placed along with a worm that was not fed dsRNA but was marked with pharyngeal gfp expression (no RNAi, gray), and progeny from both worms were assessed for silencing of a multicopy gfp transgene (Pmyo-3::gfp) in muscle cells (Top). Silencing (% silenced) was detectable only in muscle cells of progeny from parents fed gfp-dsRNA (P0 gfp RNAi, black bar) (Bottom). (C) WT animals that were washed as in A, after being fed carbenicillin-resistant RNAi bacteria (washed P0 RNAi, black), were allowed to crawl on carbenicillin plates for 1 h before being cultured along with worms marked with a fluorescent marker, and the carbenicillin plates were incubated overnight to identify colonies generated by any residual bacteria that were not removed by the washes (RNAi bacteria colonies) (Left). Although washing parent worms fed P0 RNAi did not eliminate a few RNAi bacteria in some cases (5 of 15 plates had 0 colonies; 5 of 15 plates had 1 to 10 colonies; and 5 of 15 plates had 11 to 25 colonies), silencing (% silenced) of an endogenous gene (unc-22) was detectable only in progeny from parents fed unc-22-dsRNA (P0 unc-22 RNAi, black bars) and not in the cocultured fluorescently marked worms (no RNAi, gray) in all cases (Right). (D) Ingestion of bacteria that express gfp-dsRNA by animals that express a gfp transgene in intestinal cells (Pend-1::gfp, black circles) caused silencing in embryos held in utero. (E) Representative developed progeny from animals that ingested control dsRNA (Top) or from those that ingested gfp-dsRNA (Bottom) showing silencing in muscle cells (regions within square brackets) of a multicopy gfp transgene (Pmyo-3::gfp). (F) Table summarizing cases where silencing was observed in self progeny when hermaphrodites ingest dsRNA against multicopy transgenes, single-copy transgenes, or endogenous genes using the inheritance assay described above and in Materials and Methods. Error bars indicate 95% CI (A–C), L4-staged animals were assayed [n > 46 (A), n > 104 (B), n > 50 (C)]. (Scale bars: D and E, 50 µm.) Gray bars are progeny from parent worms fed control RNAi (A) or no RNAi (B and C). Bleaching gravid adult worms only allows analysis of the few progeny embryos that are protected by their egg shell and held in utero. These results establish serial washing as a viable alternative.
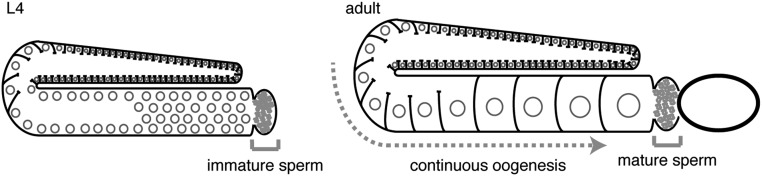
Fig. S3.
Schematic of gamete production in Caenorhabditis elegans. Sperm is made in one batch within the gonad during the L4-stage (Left) whereas oocyte production begins after the L4-stage and continues throughout adulthood (Right).
Fig. S4.
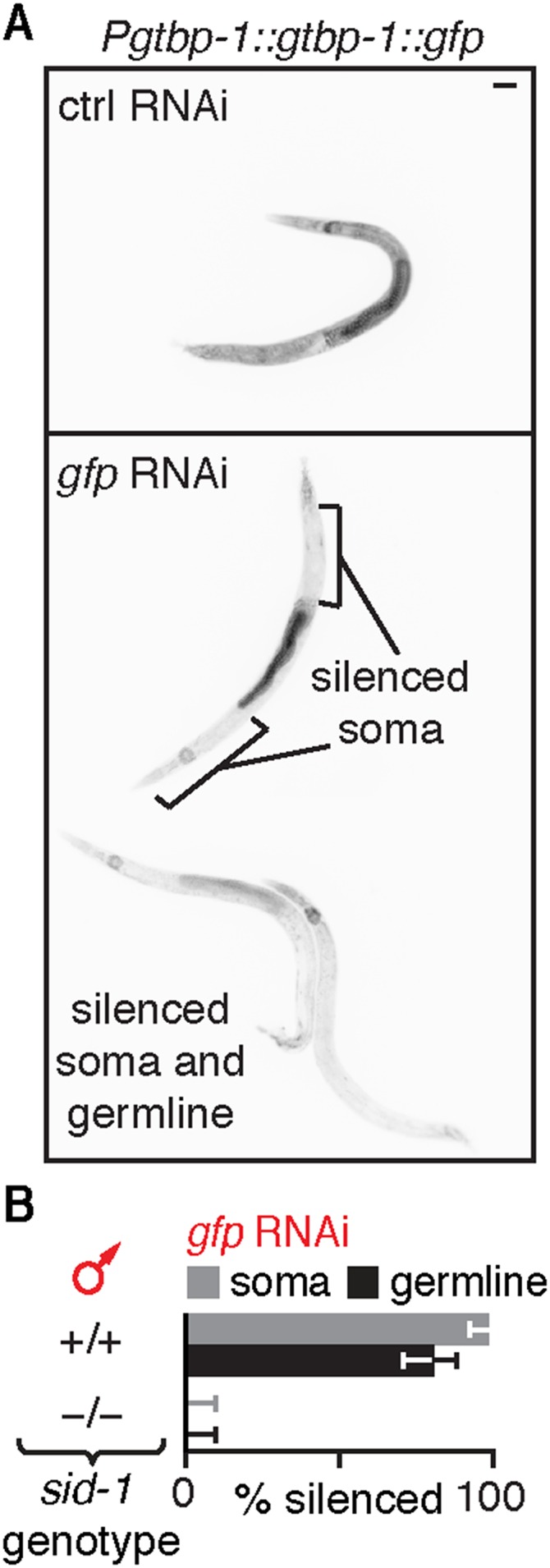
Silencing of the male germline is dependent on SID-1 but is not detectable in all males that ingest gfp-dsRNA. (A) Ingestion of bacteria that express gfp-dsRNA (gfp RNAi) by animals with Pgtbp-1::gtbp-1::gfp caused silencing within the germline in some male animals (bottom two animals in Lower) but not in others (top animal in Lower). (B) Silencing (% silenced) of gfp fused to a genomic locus (Pgtbp-1::gtbp-1::gfp) in the somatic cells (gray bars) and in the germline (black bars) of males that ingested gfp-dsRNA was dependent on the presence of sid-1. Error bars indicate 95% CI; L4-staged animals were assayed [n > 43 (A)]. (Scale bar: A, 50 µm.)
Silencing in Progeny Does Not Require Parents That Ingest dsRNA to Have DNA of Matching Sequence.
The progressive dilution of silencing in progeny is consistent either with the inheritance of small RNAs synthesized using mRNA templates, as was previously proposed in response to injected dsRNA (29) and ingested dsRNA (23), or with the inheritance of ingested dsRNA or its derivatives independent of any homologous sequence. To test whether homologous sequences are required in animals that ingest dsRNA for silencing in progeny, we exposed hermaphrodites lacking target sequences that match ingested dsRNA and examined silencing in cross-progeny after introducing the target sequence through males. Silencing was detected in ∼100% of progeny when gfp-dsRNA was ingested by hermaphrodite animals lacking a gfp transgene (Fig. 1E and Fig. S5). Thus, for ingested dsRNA to cause silencing of a matching gene in progeny, that gene need not be present in the parent that ingests the dsRNA.
Fig. S5.
Ingested dsRNA does not require matching DNA in parents to silence genes in progeny. (A and B) Silencing of a single-copy gfp transgene (Peft-3::gfp) in cross-progeny was detected in somatic cells even when gfp-dsRNA was ingested by hermaphrodites that lack gfp. (A) Representative images of cross-progeny for data shown in Fig. 1E. (B) Data for hermaphrodite cross-progeny. (C) Silencing (% silenced) of a single-copy gfp transgene (Peft-3::gfp) in all somatic cells in cross-progeny was not detected when males (red) that either lacked the gfp transgene (none) or that had the gfp transgene ingested gfp-dsRNA (black bars). (D) Silencing (% silenced) of a multicopy gfp transgene (Pmyo-3::gfp) in cross-progeny was detected in muscle cells even when hermaphrodites (red) that lacked gfp (none) ingested gfp-dsRNA (black bars). (Scale bar: A, 50 µm.) Error bars indicate 95% CI (B–D); L4-staged animals were assayed [n > 56 (B), n > 47 (C), n > 48 (D)]; and gray bars are as in Fig. 1.
Forms of dsRNA Reach Progeny and Spread Between Cells in the Embryo.
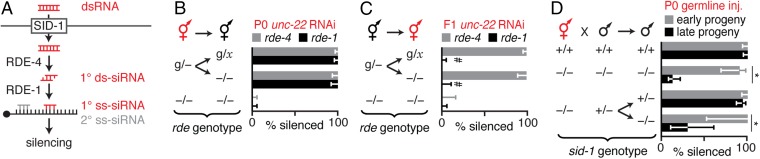
The simplest hypothesis explaining the ability of ingested dsRNA to cause silencing in progeny even when parents lack matching sequences is that either the ingested dsRNA itself or a processed derivative is delivered into progeny. Processing of dsRNA can begin upon entry of dsRNA into the cytosol through SID-1 (Fig. 2A and reviewed in ref. 22). The dsRNA is bound by the dsRNA-binding protein RDE-4 and recruited to the endonuclease Dicer, which processes it into primary double-stranded short-interfering RNAs (1° ds siRNAs). One of the strands of 1° ds siRNAs is eliminated by the Argonaute RDE-1 to generate primary single-stranded short-interfering RNAs (1° ss siRNAs), which are used as guides to identify mRNAs of matching sequence. Subsequent recruitment of RNA-dependent RNA polymerases generates numerous secondary small RNAs (2° ss siRNAs), which are used for silencing. Thus, although secondary RNAs require mRNAs of matching sequence for synthesis, all primary RNAs can be made independent of any homologous sequence.
Fig. 2.
Inherited dsRNA spreads between cells in the embryo to cause silencing. (A) Model of RNA silencing in C. elegans. Extracellular long dsRNA (red) enters the cytosol of cells through SID-1 and is processed by proteins (RDE-4 and RDE-1) into primary RNA species (1° ds siRNA and 1° ss siRNA, red) that are used to find target mRNA and trigger the synthesis of secondary RNA species (2° ss siRNA, gray), which results in gene silencing. (B and C) Silencing of unc-22 in progeny of animals with rde-4 (gray bars) or rde-1 (black bars) expressed within the germline and the intestine under the mex-5 promoter [g, Pmex-5::rde(+)] progeny genotypes (−/− or g/x, where x = + or g) and type of feeding RNAi (P0 unc-22 RNAi or F1 unc-22 RNAi) are indicated. #, much weaker silencing in all animals (Movies S1–S3). (D) Presence (+) of sid-1 was necessary in late progeny (laid 72 h postinjection, black) for ∼100% silencing of unc-22 when unc-22-dsRNA was injected into the germline of hermaphrodite parents (red, P0 germline inj.). Asterisks indicate P < 0.05 (Student’s t test); error bars indicate 95% CI (B–D); and L4-staged animals were assayed [n > 56 (B), n > 42 (C), and n > 22 (D)].
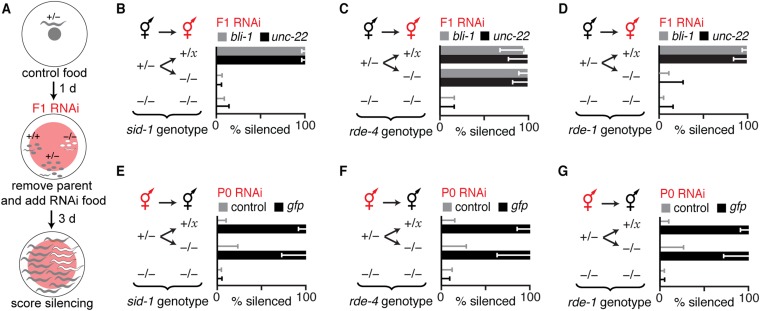
To determine the requirements for dsRNA processing in parent and in progeny to silence genes in response to ingested dsRNA, we examined silencing when SID-1, RDE-4, or RDE-1 was each present in either the parent or the progeny (Figs. S6 and S7 and Materials and Methods). Analysis of sid-1 mutants revealed that the entry of dsRNA into cells in parents or during early development in progeny is sufficient for silencing in progeny (Fig. S6 A, B, and E). Analysis of rde-4 mutants revealed that recruitment of ingested dsRNA into the RNAi pathway can occur in animals that ingest dsRNA or in their progeny at any stage during development (Figs. S6 C and F and S7) (29, 30). However, analysis of rde-1 mutants revealed that the production of 1° ss siRNAs must occur in animals that ingest dsRNA or in their progeny before larval development for silencing in progeny (Fig. S6 D and G) (30). Therefore, ingested dsRNA and all primary RNAs derived from it can be processed in the animal that ingests dsRNA or in its progeny during early development for silencing in progeny.
Fig. S6.
Requirements for SID-1, RDE-4, and RDE-1 for gene silencing in progeny upon ingestion of dsRNA by parent or by progeny. (A) Schematic of F1 RNAi. Heterozygous parents (+/−, gray) were allowed to lay progeny on a small amount of control food. One day (1 d) later, the parents were removed, and RNAi food (pink) was added to progeny (+/− or +/+, gray and −/−, white). Three days later, the animals on RNAi food were scored for silencing. (B) Presence (+) of sid-1 in parents was not sufficient for silencing (% silenced) of endogenous genes (hypodermal gene bli-1, gray bars; muscle gene unc-22, black bars) in sid-1(−) progeny when only progeny ingested matching dsRNA (F1 RNAi, red). (C and D) Presence (+) of rde-4 (C) but not of rde-1 (D) in parents was sufficient for silencing (% silenced) of endogenous genes (hypodermal gene bli-1, gray bars; muscle gene unc-22, black bars) in mutant progeny that lack the corresponding rde gene when only progeny ingest dsRNA (F1 RNAi, red). (E) Presence (+) of sid-1 in parents was sufficient for silencing (% silenced) of a single-copy gfp transgene (Peft-3::gfp) in progeny when parents ingested gfp-dsRNA. (F and G) Presence (+) of rde-4 (F) and rde-1 (G) in parents was sufficient for silencing (% silenced) of a single-copy gfp transgene (Peft-3::gfp) in the soma of progeny when only parents ingested gfp-dsRNA. Error bars indicate 95% CI (B–G); x = + or − (B–G); L4-staged animals were assayed [n > 13 (B), n > 31 (E), n > 25 (C), n > 14 (D), n > 9 (F), n > 13 (G)]; and gray bars in E–G are as in Fig. 1. Entry of dsRNA into cytosol through SID-1, processing by RDE-4, and processing by RDE-1 can all occur in parents or during early development in progeny and be sufficient for silencing in progeny when parents ingest dsRNA. Parental RDE-4 can enable silencing in rde-4(−) progeny when progeny ingest dsRNA as larvae.
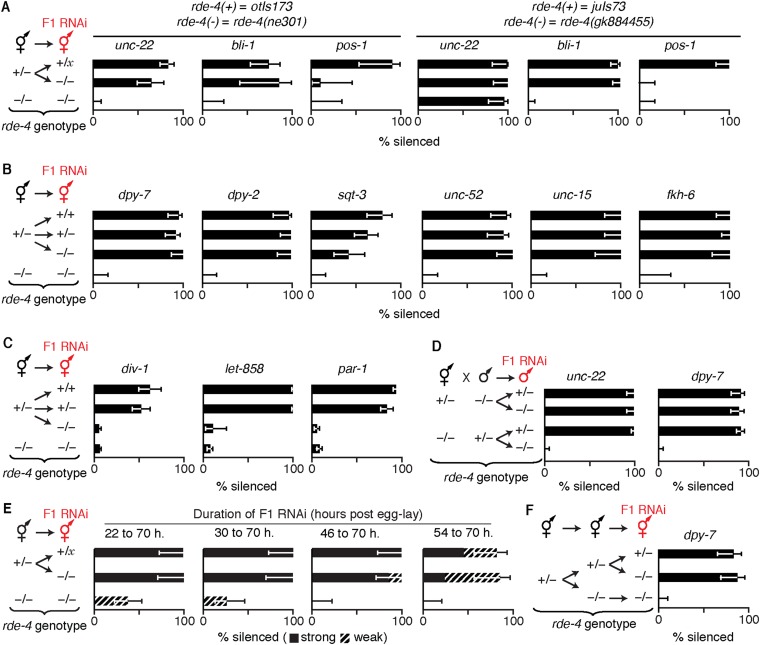
Fig. S7.
Presence of rde-4 in parents is sufficient for silencing somatic but not germline genes in rde-4(−) progeny when only progeny ingest dsRNA. (A) Presence (+) of rde-4 in parents was sufficient for silencing (% silenced) of the somatic genes unc-22 and bli-1 but not the germline gene pos-1 when only progeny (−/− or +/x, where x = + or −) ingested the corresponding dsRNA (F1 RNAi, red). Similar results were observed for both the strong ne301 mutant allele (Left, balanced with the fluorescent transgene otIs173) and the weak gk884455 mutant allele (Right, balanced with the fluorescent transgene juIs73) of rde-4. (B) Presence (+) of rde-4 in parents was sufficient for silencing (% silenced) of the somatic genes dpy-7, dpy-2, sqt-3, unc-52, unc-15, and fkh-6 in response to F1 RNAi. (C) Presence (+) of rde-4 in parents was not sufficient for silencing (% silenced) of the germline genes div-1, let-858, and par-1 in response to F1 RNAi. (D) Presence (+) of rde-4 in hermaphrodite but not male parents was sufficient for silencing (% silenced) of the endogenous genes unc-22 (Left) and dpy-7 (Right) when only cross-progeny ingested the corresponding dsRNA (F1 RNAi, red). (E) Presence (+) of rde-4 in parents was sufficient for silencing (% silenced) of unc-22 when progeny ingested unc-22-dsRNA even as late as 54 h post-egg lay. (F) Presence (+) of rde-4 in hermaphrodite grandparents was not sufficient for silencing (% silenced) of dpy-7 when only mutant progeny of mutant progeny (i.e., mutant grandprogeny) ingested dpy-7 dsRNA (F1 RNAi, red). Error bars indicate 95% CI, and L4-staged animals were assayed [n > 11 (A), n > 13 (B), n > 10 (C), n > 26 (D), n > 13 (E), n > 28 (F)]. Sufficient parental RDE-4 is present in progeny to enable silencing of genes expressed in somatic tissues—even when feeding RNAi is initiated beyond the fourth larval stage of progeny (unc-22).
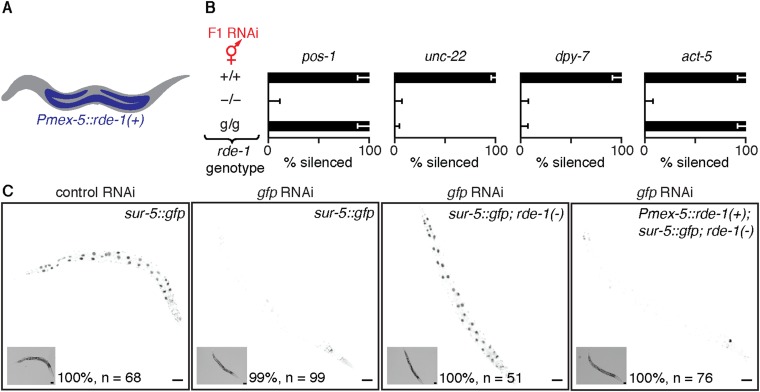
If dsRNA is processed in a parental cell containing homologous mRNA—e.g., muscle cells for unc-22-dsRNA—processed derivatives of dsRNA could interact with mRNA to generate secondary small RNAs (Fig. 2A). But, if unc-22-dsRNA is processed in cells without homologous mRNA, such as the germline, the dsRNA can be processed only to primary single-stranded siRNA (1° ss siRNA) (Fig. 2A). To allow processing only in cells that lack matching mRNA and to allow subsequent inheritance of silencing signals to progeny, we expressed RDE-4 or RDE-1 under the control of a germline promoter (Pmex-5) and examined silencing in response to ingestion of unc-22-dsRNA. This promoter enabled expression within the germline and additionally within the intestine, but not within the muscle or hypodermis (Fig. S8). Expression of RDE-4 under the control of Pmex-5 enabled silencing of unc-22 in rde-4(−) progeny when either the parent with RDE-4 expression or progeny lacking RDE-4 expression ingested dsRNA, reflecting the persistence of parental RDE-4 in progeny (Fig. 2 B and C). However, such expression of RDE-1 under the control of Pmex-5 enabled silencing in rde-1(−) progeny only when the parent with RDE-1 ingested dsRNA (Fig. 2 B and C). These results suggest that processing of ingested dsRNA by RDE-4 and subsequent processing by RDE-1 within cells that lack target mRNA in parents or within progeny during early development is sufficient for silencing in progeny. Thus, some primary RNAs, which could include long dsRNA, 1° ds siRNA, and 1° ss siRNA, are inherited from parents to progeny.
Fig. S8.
Expression of RDE-1 under the control of the mex-5 promoter enables silencing within the germline and intestinal cells but not within hypodermal or muscle cells. (A) Schematic of worm showing the expression of rde-1 restricted to the germline (blue) with a promoter reported to be specific to the germline [Pmex-5::rde-1(+)]. (B) Expression of rde-1 under the control of Pmex-5 (g) was sufficient for silencing (% silenced) the germline gene pos-1 and the intestinal gene act-5, but not the muscle gene unc-22 or the hypodermal gene dpy-7 when animals ingested the corresponding dsRNA (F1 RNAi, red). Error bars indicate 95% CI, and L4-staged animals were assayed (n > 42). (C) Expression of rde-1 under the control of Pmex-5 (g) also supports silencing of gfp expression within the intestine. Silencing of gfp by F1 RNAi in animals that express sur-5::gfp in a WT, rde-1(−), or rde-1(−); Pmex-5::rde-1(+) background was scored, and representative worms were imaged (the percentage indicates animals with similar phenotype and n indicates number of L4 animals scored). Insets are bright-field images. (Scale bars: 50 µm.) Silencing of gfp was not observed upon F1 RNAi in ∼25% of progeny from sur-5::gfp; rde-1(−); Pmex-5::rde-1(+)/+ parents (n > 25 F1s each from five P0 animals; total n = 206 L4 animals), consistent with lack of silencing in sur-5::gfp; rde-1(−) progeny and with lack of rescue from rde-1(+) expression within the parental germline. These results suggest that our strain, with expression of rde-1(+) under the control of a mex-5 promoter, supports gene silencing by feeding RNAi in germline and intestinal cells but not in in muscle or hypodermal cells.
When radioactively labeled dsRNA is injected into the germline, a large fraction of the dsRNA remains as high molecular weight material in progeny (31, 32), consistent with substantial delivery of long dsRNA into progeny. When unc-22-dsRNA was similarly delivered directly into the parental germline, and thus into the cytosol of cells in the embryo, potent silencing occurred in early-born progeny in WT and in sid-1(−) animals (Fig. 2D), suggesting that sufficient dsRNA was delivered into all cells in early progeny. In contrast, later-born progeny, which are expected to receive smaller doses of dsRNA because of dilution within the germline (26), required SID-1 for efficient silencing (Fig. 2D). This need for SID-1–dependent spread of dsRNA between cells for efficient silencing in later progeny is consistent with forms of dsRNA (long dsRNA and/or 1° ds siRNA) being inherited from the injected parents to progeny.
Taken together, these results suggest that intracellular primary small RNAs—including forms of dsRNA—can be inherited from parent to progeny and explain the surprising observation that, when animals that lack DNA of matching sequence ingest dsRNA, they can still transmit a silencing signal to progeny.
Extracellular dsRNA Can Cause Silencing in Progeny Without SID-1–Dependent Entry in Parents.
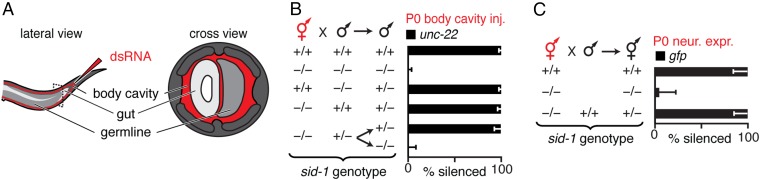
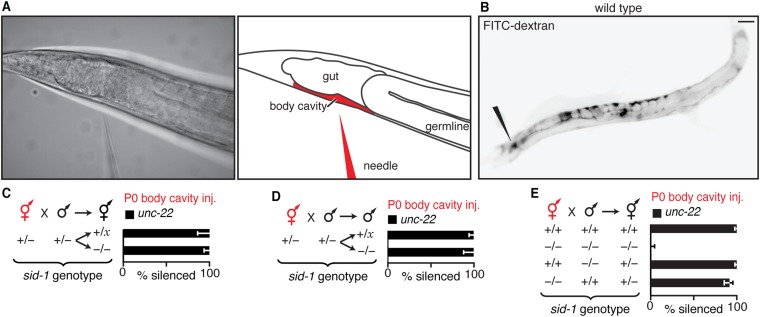
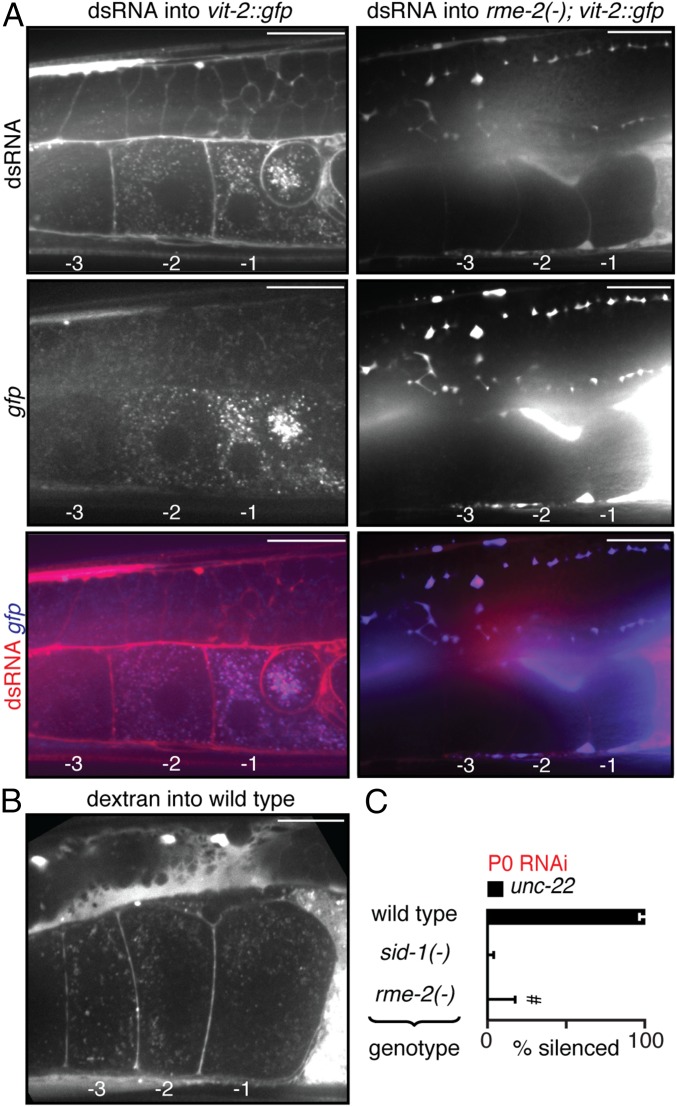
Ingested dsRNA can cross the intestine into the fluid-filled body cavity that surrounds all tissues, including the germline, without SID-1–dependent entry into the cytosol of intestinal cells (33–35). To determine where subsequent entry into the cytosol in parents is required for silencing in progeny, we delivered dsRNA into the extracellular space by injecting into the body cavity beyond the bend of the posterior gonad arm (Fig. 3A and Fig. S9A). Such injection resulted in the immediate spread of the injected material by diffusion throughout the entire body cavity, as evidenced by injection of fluorescently labeled dextran (Fig. S9B). Entry of dsRNA from the body cavity into the cytosol of a cell is expected to occur only in cells that have the dsRNA importer SID-1 (19). Consistent with results from feeding RNAi, presence of SID-1 in parents was sufficient for silencing in progeny in response to dsRNA injected into the body cavity (Fig. S9 C and D). However, when SID-1 was restricted to either sperm [sid-1(+) male] or to all cells except sperm [sid-1(+) hermaphrodite], injection of dsRNA targeting the muscle gene unc-22 (unc-22-dsRNA) into the body cavity of hermaphrodites enabled unc-22 silencing in cross-progeny (Fig. 3B and Fig. S9E), as was reported earlier (19). Taken together with the dynamics of silencing by ingested dsRNA (Fig. 1 B–D), these results suggest that injected extracellular dsRNA can be transported to the next generation through oocytes, presumably within intracellular vesicles, from which the entry of dsRNA into the cytosol through SID-1 occurs in progeny. Consistently, any paternal SID-1 protein or mRNA present in sperm from heterozygous males was not sufficient for silencing, and zygotic expression of SID-1 in progeny was required for silencing (Fig. 3B, compare fifth bar with sixth bar). Similarly, in the case of dsRNA transported from neurons (15), the neuronal dsRNA from animals that lack SID-1 could cause silencing in cross-progeny when mated with WT animals (Fig. 3C). Thus, these results suggest that extracellular dsRNA does not need to enter the cytosol in parental tissues to cause silencing in progeny—cytosolic entry within the progeny is sufficient.
Fig. 3.
Extracellular dsRNA does not need to enter the cytosol of any cell in parents to cause silencing in progeny. (A) Schematic showing injection of dsRNA into the body cavity. (B) Presence (+) of sid-1 in progeny was sufficient for silencing of an endogenous gene (unc-22) in the muscles of progeny when dsRNA matching the gene (unc-22-dsRNA, red) was injected into the body cavity of hermaphrodite parents. Also see Fig. S9. (C) Presence (+) of sid-1 in hermaphrodite parents was not required for silencing of a single-copy gfp transgene (Pmex-5::gfp) in the germline of progeny when gfp-dsRNA was expressed within parental neurons (P0 neur. expr., red) of hermaphrodite parents. Error bars indicate 95% CI (B and C), and L4-staged animals were assayed [n > 49 (B), n > 38 (C)].
Fig. S9.
Presence of SID-1 in parents or progeny is sufficient for silencing in progeny when dsRNA is injected into the body cavity of parents. (A) Injection of dsRNA into the body cavity (Figs. 3–6) was performed by inserting a microinjection needle containing dsRNA (needle) past the bend of the posterior gonad arm. Representative image of set up (Left) and schematic (Right) for injection are shown. (B) Representative image of fluorescence (black) in a WT adult animal from fluorescein-labeled 10-kDa dextran injected into the body cavity (black needle indicates site of injection). (C and D) Presence (+) of sid-1 in parents was sufficient for silencing (% silenced) of an endogenous gene (unc-22) in the muscles of hermaphrodite (C) and male (D) progeny when dsRNA matching the gene (unc-22-dsRNA, red) was injected into the body cavity of hermaphrodite parents. (E) Injections and crosses were performed as described in Fig. 3 A and B, and hermaphrodite cross-progeny were assayed for silencing. (Scale bar: B, 50 µm.) Error bars indicate 95% CI (C–E); x = + or − (D–E); and L4-staged animals were assayed [n > 31 (C), n > 35 (D), n > 139 (E)].
Extracellular dsRNA Accumulates in Proximal Oocytes and Persists in Embryos to Silence a Gene of Matching Sequence.
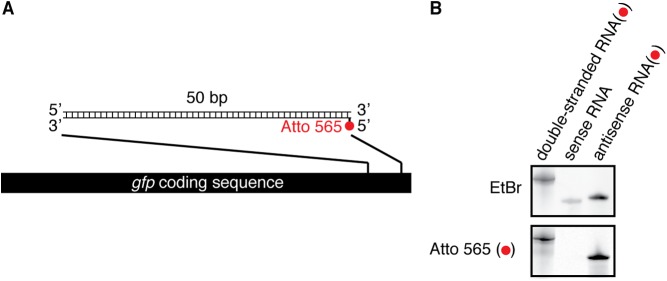
Genetic analyses collectively suggest that extracellular dsRNA can reach progeny to silence a matching gene (Figs. 1–3). To track extracellular dsRNA between generations, we labeled 50-bp gfp-dsRNA with a fluorophore at a 5′ end (Fig. S10), injected the fluorescently labeled gfp-dsRNA into the body cavity of transgenic worms with embryonic expression of GFP, and imaged the germline (Fig. 4A) of injected animals as well as embryos (Fig. 4 B and C) laid by injected adults (Fig. 4D). Fluorescently labeled dsRNA injected into the body cavity accumulated as puncta within oocytes in adult animals (Fig. 4E), and intracellular accumulation could be detected ∼7 min after injection (Movie S4). Levels of fluorescent dsRNA decreased from proximal to distal oocytes such that the oocyte located proximal to the spermatheca (−1 oocyte) had the highest fluorescence and most of the fluorescence within oocytes was limited to the two most proximal oocytes (Fig. 4E). Fluorescent RNA could also be detected in embryos (Fig. 4 F–I), suggesting that extracellular dsRNA imported into oocytes persists in embryos. Furthermore, all embryos with fluorescent dsRNA also showed silencing of gfp expression (compare Fig. 4C with Fig. 4G). Together, these results demonstrate the entry of extracellular dsRNA into oocytes and their persistence in embryos to silence genes of matching sequence.
Fig. S10.
Annealing sense RNA and Atto 565-labeled antisense RNA generates fluorescent dsRNA. (A) Schematic of fluorescent gfp-dsRNA. The Atto 565 label is attached to the 5′ end of the antisense strand of dsRNA. (B) RNA was run in a 12% polyacrylamide gel and imaged for fluorescence (Bottom, Atto 565) and stained with ethidium bromide (Top, EtBr).
Fig. 4.
Extracellular dsRNA accumulates in proximal oocytes and subsequently within embryos where it can silence genes of matching sequence. (A–C) Images of adult germline showing proximal oocytes (−1 through −4 with respect to the spermatheca) (A) and embryos (B) expressing GFP in intestinal cells (Pend-1::gfp) (C). (D) Strategy to visualize silencing by fluorescently labeled gfp-dsRNA. (E) In injected WT animals, dsRNA concentrated in proximal oocytes (−1 and −2). Slices from Z-series (Movies S5 and S6) were spliced. Asterisk indicates brightly fluorescent intestinal cell. (F–I) WT embryos inherited the gfp-dsRNA, and silencing of Pend-1::gfp occurred. (Scale bars: 20 µm.) Multiple adults (n = 4 in E) and embryos (n = 15 in B and C, and n = 12 in F–I) were imaged for each experiment.
Extracellular dsRNA Enters Oocytes Along with Yolk.
The accumulation of dsRNA in two proximal oocytes (Fig. 4E) is reminiscent of the accumulation of yolk proteins (Vitellogenins), which also similarly accumulate in proximal oocytes (27). To directly compare dsRNA import with vitellogenin import, we injected fluorescently labeled dsRNA into the body cavity of animals that express a GFP-tagged vitellogenin, VIT-2::GFP (Fig. 5 A, Left). Both dsRNA and VIT-2::GFP accumulated as puncta within the proximal oocytes (Fig. 5 A, Left), consistent with their import being mediated by the same process. The import of yolk into C. elegans oocytes occurs through endocytosis and requires the receptor RME-2, a member of the low-density lipoprotein receptor superfamily (27). In animals that lack RME-2, both GFP-tagged vitellogenin and fluorescently labeled dsRNA accumulated extracellularly in the body cavity without import into oocytes (Fig. 5 A, Right). The coaccumulation of dsRNA with yolk is likely to be through nonspecific import of material from the body cavity during the uptake of the ∼0.5-µm-sized yolk granules (36) because fluorescently labeled dextran (10 kDa) also accumulated in punctate structures within oocytes (Fig. 5B). Consistent with the results from injected dsRNA, RME-2 was required for silencing in progeny when parents ingested dsRNA (Fig. 5C). Together, these results suggest that the import of dsRNA into oocytes occurs through the same pathway that imports yolk in C. elegans—RME-2–mediated endocytosis.
Fig. 5.
Import of dsRNA into oocytes relies on RME-2–mediated endocytosis. (A) Import of dsRNA and of the Vitellogenin VIT-2 into oocytes both require RME-2. Fluorescently labeled dsRNA (Top) and VIT-2::GFP (Middle, gfp) accumulate similarly in proximal oocytes (merge, Bottom) in WT (Left) but not in rme-2(−) (Right) animals. Also, see Movie S7. (B) Fluorescent dextran from the body cavity also accumulates in punctate structures within oocytes. (Scale bars: 20 µm.) Proximal oocytes are numbered as in Fig. 4. Multiple adults (n = 4 in A, Left; n = 5 in A, Right; n = 3 in B) were imaged for each experiment. (C) Ingestion of dsRNA by animals that lack rme-2 [rme-2(−)] does not result in detectable silencing in progeny. #, much weaker silencing in 2 of 23 animals. Error bars and gray bars are as in Fig. 1, and n > 22 L4-staged animals.
Extracellular dsRNA Can Reach Embryos Without SID-1–Dependent Entry.
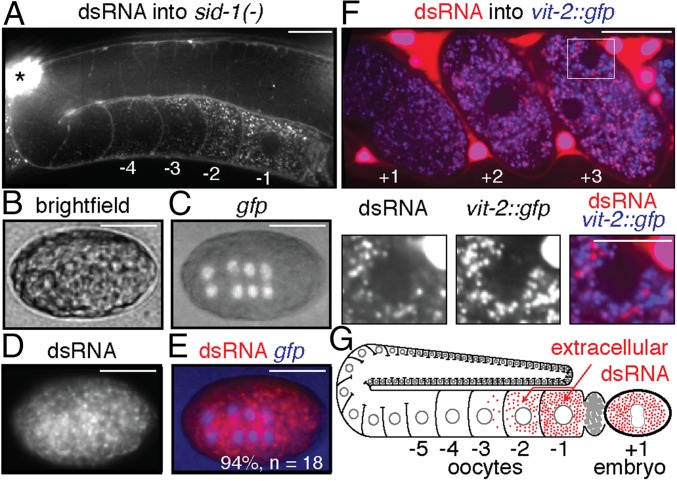
Despite import into oocytes through RME-2–mediated endocytosis, dsRNA is expected to require SID-1 for entry into the cytosol and subsequent silencing. Injection of fluorescent dsRNA into WT animals resulted in silencing in embryos (Fig. 4), consistent with cytosolic entry either in oocytes or in the embryo. To determine whether extracellular dsRNA can reach embryos without cytosolic entry, as predicted by genetic analyses (Fig. 3B), we injected fluorescently labeled gfp-dsRNA into the body cavity of sid-1(−) animals that express GFP in embryos and imaged the germline of injected animals, as well as embryos laid by the injected animals (as schematized in Fig. 4D). The dsRNA accumulated in proximal oocytes of sid-1(−) adults (Fig. 6A), as was observed in WT animals. The dsRNA also accumulated in embryos, but, unlike in WT embryos, such accumulation was not accompanied by silencing of gfp expression in sid-1(−) embryos (Fig. 6 B–E). Thus, extracellular dsRNA can be imported into oocytes and can reside in presumed intracellular vesicles that reach embryos without SID-1–dependent entry into the cytosol.
Fig. 6.
Extracellular dsRNA can accumulate without cytosolic entry in proximal oocytes and subsequently within embryos. (A) In injected sid-1(−) animals, dsRNA concentrated in proximal oocytes (−1 and −2). Slices from Z-series (Movies S8 and S9) were spliced. Asterisk indicates brightly fluorescent coelomocyte. (B–E) The sid-1(−) embryos inherited the gfp-dsRNA, but no silencing of Pend-1::gfp occurred. (Scale bars: 20 µm.) Proximal oocytes are numbered as in Fig. 4. Multiple adults (n = 4 in A) and embryos (n = 18 in B–E) were imaged for each experiment. (F) Colocalization of fluorescently labeled dsRNA and VIT-2::GFP is reduced as the embryo develops. Single confocal slice of an adult animal showing accumulation of fluorescently labeled dsRNA that was injected into the body cavity of a strain with vit-2::gfp in embryos held in utero (n = 6 <4-cell embryos and n = 10 ≥4-cell embryos). (Top) Merged image showing +1, +2, and +3 embryos after fertilization. (Scale bar: 20 µm.) (Bottom) Zoomed image of highlighted region (white box) in Top image for individual channels of dsRNA and VIT-2::GFP fluorescence and merge. (Scale bar: 10 µm.) (G) Model illustrating that extracellular dsRNA can be transported through oocytes to progeny with or without entry into the cytosol.
In C. elegans embryos, yolk is secreted by blastomeres and reimported by intestinal cells, resulting in enrichment within the intestinal primordium (37, 38). We did not observe a similar intestinal enrichment of labeled dsRNA in embryos (e.g., Fig. 4H and Fig. 6D), suggesting that yolk and dsRNA are trafficked differently in progeny. Consistent with this possibility, the colocalization between fluorescently labeled dsRNA and VIT-2::GFP is greatly reduced in embryos that are beyond the four-cell stage (Fig. 6F). These results raise an intriguing hypothesis that is worthy of further investigation: C. elegans embryos have mechanisms to distinguish inherited nutrition (yolk) from inherited information (dsRNA) when both reside within the same putative intracellular vesicle.
Discussion
Using genetic analyses and fluorescently labeled RNA, we have established that extracellular dsRNA is imported into oocytes along with yolk and can reach embryos with or without entry into the cytosol (Fig. 6G). Cytosolic entry in embryos of dsRNA from parental circulation and spread between cells of dsRNA processed within the parental germline or during early development in progeny results in robust gene silencing.
Implications for the Inheritance of RNA Silencing.
The direct transfer of extracellular and intracellular dsRNA from parents to progeny when parents ingest dsRNA, when dsRNA is injected into parents, or when dsRNA is expressed within neurons in parents demonstrates that the trigger for RNAi is transported between generations in C. elegans. Therefore, when multigenerational silencing is observed for genes expressed within the germline (Fig. S1), the mechanisms that are required for transgenerational stability of silencing could be initiated in progeny—potentially during germline development. Thus, the production of secondary small RNAs and deposition of chromatin modifications proposed to be required for transgenerational gene silencing (15, 24, 29, 39–41) could be initiated in progeny when parents encounter dsRNA. These considerations also impact the interpretation of experiments evaluating the duration of transgenerational inheritance in response to RNAi (25, 42).
Inherited silencing of genes expressed in somatic cells in C. elegans, which typically lasts for precisely one generation (23) (Fig. S1), could simply reflect silencing triggered by dsRNA in progeny without engaging any transgenerational gene silencing machinery within the parent germline. Similar direct delivery to progeny could underlie parental RNAi in insects (reviewed in ref. 14), when dsRNA is delivered into the hemocoel (e.g., ref. 43) or through ingestion (e.g., ref. 44) to initiate RNAi. Additional studies are required to discover the evolutionarily selected function, if any, for the delivery of ingested material—including regulatory RNA—directly into progeny.
RNAs in Circulation as Carriers of Gene-specific Information Between Generations.
Molecules that can cross generational boundaries can cause apparent intergenerational effects. Exposure to some chemicals can cause multigenerational effects in mammals [e.g., endocrine disruptors (45) or odors (46)]. Examination of how many generations the molecules used to trigger a response persist within an animal could inform mechanisms underlying multigenerational effects. Alternatively, intergenerational effects could result if RNAs carry sequence-specific information to gametes through circulation from distant tissues that experience chemicals, changes in diet, or stress. In support of this possibility, studies focused on intergenerational and transgenerational effects in mammals implicate RNA in the inheritance of gene expression states across generations (47), report changes in small RNAs in gametes (48, 49), and report changes in RNAs acquired during gamete maturation from surrounding epithelia (50, 51). However, in all these cases, direct effects of a treatment—e.g., diet—on gametes and surrounding support tissues that alter RNA composition in gametes have not been ruled out. Furthermore, although extracellular RNAs have been detected in mammals, their biology is not well-understood and is under intense investigation (see ref. 52 for a recent review). Using genetic mutants and fluorescently labeled RNAs to control and follow the traffic of extracellular RNAs, our results demonstrate their direct transfer between generations in an animal—an inheritance that can potentially vary based on parental experience.
Materials and Methods
All C. elegans strains (Table S1) were maintained at 20 °C and were fed Escherichia coli OP50 (53). Oligonucleotides were used for injections and genotyping as necessary (Table S2). Transgenic strains with RDE-4 and RDE-1 restricted to the germline were generated using Mos1-mediated single copy insertion (MosSCI) (54). Transgenes were moved into different genetic backgrounds using genetic crosses and were either balanced with visible markers or sequenced for verification. Parent (P0) and progeny (F1) RNAi experiments (Table S3) were performed using RNAi E. coli clones for endogenous genes from the Ahringer library (55). Feeding RNAi and soaking animals in gfp-dsRNA are expected to cause similar silencing (56). RNAi clones and bacteria expressing gfp-dsRNA provided by the Hamza laboratory, University of Maryland, College Park, MD. Fluorescent transgenes were imaged using a Nikon AZ100 microscope using exposure times just under saturation upon control RNAi of each genetic background. dsRNA that was injected into animals was transcribed in vitro or purchased as single-stranded oligos that were then annealed. Worms injected with fluorescently labeled dsRNA were imaged using a Nikon Eclipse Ti spinning disk confocal microscope and processed using ImageJ (NIH). Detailed procedures are provided in SI Materials and Methods.
Table S1.
Strains used
| Strain | Genotype |
| AMJ8 | juIs73 [Punc-25::gfp] III |
| AMJ12 | ccIs4251 [pSAK2 (Pmyo-3::nlsGFP-LacZ) & pSAK4 (Pmyo-3::mitoGFP), & dpy-20] I [4x] |
| AMJ180 | mIs10 [Pmyo-2::GFP, Ppes-10::gfp, PF22B7.9::gfp] V |
| AMJ190 | oxSi221 [Peft-3::GFP + cb-unc-119(+)] II; rde-4(ne301)III |
| AMJ256 | rde-4(gk884455) III |
| AMJ286 | jamSi1 [Pmex-5::rde-4(+)] II; rde-4(ne301) III |
| AMJ291 | oxSi221 II; unc-119(ed9) III; sid-1(qt9) V |
| AMJ325 | oxSi221 II; unc-119(ed9) III; rde-1(ne219) V |
| AMJ345 | jamSi2 [Pmex-5::rde-1(+)] II; rde-1(ne219) V |
| AMJ542 | sid-1(qt9) V; jamEx140 [Prgef-1::gfp-hpRNA, Pmyo-2::DsRed] |
| AMJ593 | oxSi487 dpy-2(e8) II; unc-119(ed3)? III; sid-1(qt9) V |
| AMJ598 | oxSi487 dpy-2(e8) II; unc-119(ed3)? III; sid-1(qt9) V; jamEx140 |
| AMJ794 | unc-119(ed3) III (?); sid-1(qt9) V; teIs46 [Pend-1::gfp::H2B] |
| AMJ795 | rme-2(b1008) IV; pwIs23 [Pvit-2::vit-2::gfp] |
| DH1390 | rme-2(b1008) IV |
| EG4322 | ttTi5605 II; unc-119(ed9) III |
| EG6070 | oxSi221 II; unc-119(ed9) III |
| EG6787 | oxSi487 II; unc-119(ed3) III |
| HC195 | nrIs20 [Psur-5::sur-5::gfp::unc-54 3′ UTR] IV |
| HC196 | sid-1(qt9) |
| HC738 | nrIs20 IV; rde-1(ne219) V |
| JH3197 | K08F4.2(ax2053[K08F4.2::gfp]) |
| N2 | WT |
| RT130 | pwIs23 |
| TX691 | unc-119(ed3) III; teIs46 |
| WM27 | rde-1(ne219) V |
| WM49 | rde-4(ne301) III |
| AMJ843 | jamSi2 II; nrIs20 IV; rde-1(ne219) V |
Table S2.
Oligonucleotides used (5′ to 3′) (IDT)
| Oligonucleotide | Sequence |
| P1 (Pmex-5::rde-4 fwd) | gatccaactcctcgacgcac |
| P2 (Pmex-5::rde-4 rev) | cgttagtttggttaaatccattctctgtctgaaacattc |
| P3 (Pmex-5::rde-4 fwd_nested) | tctctcactagtacttccgcagagacaaccatc |
| P4 (rde-4 fwd) | gaatgtttcagacagagaatggatttaaccaaactaacg |
| P5 (rde-4 rev) | cactgcagagaatgagtgtg |
| P6 (rde-4 rev_nested) | gagagaactagtgtagaggtcagaggcatag |
| P7 (Pmex-5::rde-1 fwd) | agtcagtgagcgaggaagc |
| P8 (Pmex-5::rde-1 rev) | tcgggaaaattcgaggacattctctgtctgaaacattcaatt |
| P9 (Pmex-5::rde-1 fwd_nested) | tgtaaaacgacggccagt |
| P10 (rde-1 fwd) | aattgaatgtttcagacagagaatgtcctcgaattttcccga |
| P11 (rde-1 rev) | tcacacttctccagttgagc |
| P12 (rde-1 rev_nested) | gagagacctgcagggaagtcgtgaaatcacctgc |
| P13 (Pmex-5 genotype fwd) | ccgtactccgtttgtttgatc |
| P14 (rde-4 genotype rev) | tcgggaaggcttcataggaac |
| P15 (rde-1 genotype rev) | tgccgtcgcatttaccagtg |
| P16 (T7 fwd) | taatacgactcactataggg |
| P17 (gfp sense ssRNA) | ugguccuucuugaguuuguaacagcugcugggauuacacauggcauggau |
| P18 (5′ Atto 565 labeled gfp antisense ssRNA) | 5′Atto 565-auccaugccauguguaaucccagcagcuguuacaaacucaagaaggacca |
| P19 [rme-2(b1008) genotype fwd] | acaaccactacgccagaagg |
| P20 [rme-2(b1008) genotype rev] | actcggcgacagaacattcc |
Table S3.
Scoring of gene-specific silencing
| Gene | Site of expression | Defect scored upon RNAi |
| bli-1 | Hypodermis | Presence of fluid-filled blisters on adults |
| div-1 | Germline | Dead (unhatched) eggs |
| dpy-2 | Hypodermis | Short, fat L4 animals |
| dpy-7 | Hypodermis | Short, fat L4 animals |
| fkh-6 | Somatic gonad | Dead (unhatched) eggs |
| gfp | Muscle | L4-staged worms had dimmed or absent GFP expression when viewed using Olympus fluorescent scope compared with worms fed control RNAi food. |
| Intestine | ||
| Ubiquitous | ||
| Germline | ||
| sqt-3 | Hypodermis | Adults that roll |
| let-858 | Germline | Dead (unhatched) eggs |
| par-1 | Germline | Dead (unhatched) eggs |
| pos-1 | Germline | Dead (unhatched) eggs |
| unc-15 | Body-wall muscle | Slow/lethargic movement of L4 animals |
| unc-22 | Body-wall muscle | Weak: L4 or young adults occasionally twitch within 1 min in response to 3 mM levamisole (Sigma Aldrich) |
| Strong: L4 or young adults continuously twitch within 1 min in response to 3 mM levamisole (Sigma Aldrich) | ||
| unc-52 | Hypodermis | Slow/lethargic movement, including paralysis, of L4 animals |
SI Materials and Methods
Strains, Transgenesis, and Oligonucleotides.
All strains used are listed in Table S1, and all oligonucleotides used are listed in Table S2.
Expression of RDE-4 in the germline [Pmex-5::rde-4(+)].
The promoter for mex-5 (Pmex-5) was amplified (Phusion polymerase; NEB) from N2 genomic DNA (gDNA) using the primers P1 and P2. The rde-4 gene was amplified (Phusion polymerase; NEB) from N2 gDNA using the primers P4 and P5. Using these two amplicons as template, Pmex-5::rde-4(+) was generated (Phusion polymerase; NEB) with primers P3 and P6. This final product [Pmex-5::rde-4(+)] was purified (QIAquick PCR Purification Kit; Qiagen) and cloned into pCFJ151 using the SpeI (NEB) restriction site to generate pJM1. pJM1 (22.5 ng/µL) and the coinjection markers pJL43.1 (50 ng/µL), pMA122 (10 ng/µL), pGH8 (10 ng/µL), pCFJ90 (2.5 ng/µL), and pCFJ104 (5 ng/µL) (plasmids described in ref. 54) were injected into the germline of adult EG4322 animals. One transgenic line was isolated as described earlier (54) and crossed into rde-4(ne301) animals to generate AMJ286. The integration of Pmex-5::rde-4(+) in AMJ286 was verified by genotyping AMJ286 using primers P13 and P14.
Expression of RDE-1 in the germline [Pmex-5::rde-1(+)].
The promoter for mex-5 (Pmex-5) was amplified (Phusion polymerase; NEB) from pJA252 (54) using the primers P7 and P8. The gene rde-1 was amplified (Phusion polymerase; NEB) from N2 gDNA using the primers P10 and P11. Using these two amplicons as template, Pmex-5::rde-1(+) was generated (Phusion polymerase; NEB) with primers P9 and P12. This final product [Pmex-5::rde-1(+)] was purified (QIAquick PCR Purification Kit; Qiagen) and cloned into pCFJ151 using the AflII and SpeI (NEB) restriction sites to make pJM2. The pJM2 plasmid (22.5 ng/µL) and the coinjection markers pJL43.1 (50 ng/µL), pMA122 (10 ng/µL), pGH8 (10 ng/µL), pCFJ90 (2.5 ng/µL), and pCFJ104 (5 ng/µL) (plasmids described in ref. 54) were injected into the germline of adult EG4322 animals. One transgenic line was isolated and crossed into an rde-1(ne219) background to make AMJ345. The integration of Pmex-5::rde-1(+) in AMJ345 was verified by genotyping AMJ345 using primers P13 and P15.
Feeding RNAi.
Control RNAi by feeding Escherichia coli containing the empty dsRNA-expression vector (pL4440), which does not produce dsRNA against any gene, was done in parallel with all RNAi assays, and all silencing defects were scored (Table S3) in comparison with that observed (if any) upon pL4440 feeding.
Inheritance assay in response to P0 RNAi.
RNAi bacteria were grown in LB-carbenicillin overnight, and 100 µL was seeded on RNAi plates [NG agar plate supplemented with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) (Omega) and 25 µg/mL carbenicillin (MP Biochemicals)]. Seeded RNAi plates were incubated at room temperature for 1–2 d before L4-staged worms were added. The plates were then incubated at 20 °C for 1 d. RNAi bacteria were then removed in one of the following ways.
Four times wash.
The RNAi-fed worms were suspended in 1 mL of M9 buffer in a 1.5-mL microcentrifuge (VWR) tube and spun at 5,510 × g for 30 s. After removing 800 µL of the old buffer, an equal volume of fresh M9 buffer was added. This washing was repeated four times, and the final 200 µL of M9 buffer with worms was placed on plates seeded with OP50 and incubated for 1 h at room temperature before each worm was moved to a fresh plate seeded with OP50.
Bleach.
The RNAi-fed worms were placed into a small drop of 0.6% NaOCl [10% (vol/vol) of Chlorox] in 1.5 M NaOH (Sigma-Aldrich) on individual OP50-seeded agar plates.
Kanamycin.
The RNAi-fed worms were washed with buffer as described above in Four times wash and then placed onto individual NG-kanamycin plates (50 µg/mL kanamycin; EMD Millipore) seeded with 100 µL of OP50. Plates were checked each day for remaining OP50, and more OP50 was added if needed.
For all of the above RNAi bacteria removal methods, the earliest L4-staged progeny were scored (∼20 per worm) for inherited gene silencing by assaying gene-specific effects (typically 2 to 3 d later, except in the case of the slow-growing rme-2(−) strain, which was scored 5 d later).
Silencing assay in response to F1 RNAi.
A single L4-staged animal (P0) was placed on an RNAi plate [NG agar plate supplemented with 1 mM IPTG (Omega) and 25 µg/mL carbenicillin (MP Biochemicals)] seeded with 5 µL of OP50 E. coli and allowed to lay eggs. After 1 d, the P0 animal was removed, leaving the F1 progeny embryos. Then, 100 µL of an overnight culture of RNAi food (E. coli that express dsRNA against a gene of choice) was added to the plate. The earliest L4-staged progeny were scored for gene silencing by assaying gene-specific effects (Table S3). For F1 RNAi of males, the starting P0 was a single gravid adult-staged animal from a mating plate that was started with three L4-staged hermaphrodites and nine males.
Balancing Loci.
Integrated transgenes expressing gfp were used to balance mutations in heterozygous animals. Progeny of heterozygous animals were scored as homozygous mutants if they lacked both copies of the transgene. The rde-4(ne301) allele on chromosome III (Chr III) was balanced by juIs73 or otIs173 (Fig. S7). About 99% (153/155) of the progeny of rde-4(ne301)/juIs73 that lacked fluorescence were found to be homozygous rde-4(ne301) animals either by Sanger sequencing (96 animals) or by resistance to pos-1 RNAi (59 animals). The rde-1(ne219) and sid-1(qt9) alleles on Chr V were balanced by mIs10. About 94% (63/67) progeny of rde-1(ne219)/mIs10 that lacked fluorescence were found to be homozygous rde-1(ne219) by Sanger sequencing. The jamSi1 and jamSi2 alleles integrated into the ttTi5605 Mos site on Chr II were balanced by oxSi221, which is a transgene that is also integrated at the ttTi5605 Mos site on Chr II. Worms homozygous for juIs73 or oxSi221 were brighter than worms hemizygous for juIs73 or oxSI221 and could be reliably distinguished. For otIs173 and mIs10, homozygous transgenic animals could not be distinguished from hemizygous animals and were thus grouped together (i.e., +/+ and +/− genotypes for rde).
Inferences from Genetic Analyses.
We found that the presence of SID-1 in parents was not sufficient for silencing in progeny when only progeny ingested dsRNA (Fig. S6 A and B), suggesting that parental SID-1 does not persist in larval progeny to enable the import of ingested dsRNA. On the other hand, the presence of SID-1 in parents was sufficient for silencing in progeny when only parents ingested dsRNA (Fig. S6E), suggesting that entry of dsRNA into cells in parents or during early development in progeny is sufficient for silencing in progeny.
We found that the presence of RDE-4 in parents was sufficient for silencing genes expressed in somatic tissues of progeny (Fig. S6F), as noted for injected dsRNA in early experiments (30). Unlike in the case of SID-1, however, the presence of RDE-4 in parents enabled silencing in progeny when progeny ingested dsRNA as larvae (Fig. S6C). Silencing was robust for somatic genes but undetectable for germline genes (Fig. S7 A–D), consistent with the failure to detect any maternal rescue of RDE-4 when dsRNA against germline genes was injected into progeny (29). Silencing of somatic genes however, could be detected even when progeny only began ingesting dsRNA ∼54 h after egg laying (Fig. S7E) but could not be enabled by grandparental RDE-4 (Fig. S7F), consistent with the persistence of parental RDE-4 in progeny. Thus, detectable silencing in progeny when an animal ingests dsRNA matching a somatic gene requires entry into the cytosol in the animal that ingests the dsRNA or during early development of its progeny, but subsequent processing by RDE-4 can occur even in late-staged progeny. Similar experiments using RDE-1 revealed that, when an animal ingests dsRNA matching a somatic gene, processing by RDE-1 must occur in that animal or during early development of its progeny for silencing in progeny (Fig. S6 D and G), consistent with observations using injected dsRNA (30).
Injection of dsRNA.
unc-22-dsRNA.
The unc-22 sequence with flanking T7 promoters was amplified (Phusion polymerase; NEB) from the unc-22 RNAi vector using the P16 primer. The product was purified (QIAquick PCR Purification Kit; Qiagen), and dsRNA was transcribed in vitro (T7 High Yield RNA Synthesis Kit; NEB). Transcribed dsRNA product was purified (QIAquick PCR Purification Kit; Qiagen), treated with RNase A (Omega Bio-Tek), and purified (QIAquick PCR Purification Kit; Qiagen).
gfp-dsRNA.
ssRNA oligos P17 and P18 were resuspended in nuclease free 1× Tris ethylenediaminetetraacetic acid (TE) (IDT) to ∼1,635 ng/µL. Equal volumes of each ssRNA were mixed in nuclease-free duplex buffer (IDT) to a final concentration of ∼490 ng/µL of each ssRNA. This mixture was heated to 95 °C and cooled at 1 min per degree to 10 °C, resulting in ∼980 ng/µL dsRNA. P17, P18, and the annealed dsRNA were run in a 12% denaturing polyacrylamide gel. The gel was first imaged with the Typhoon Trio Variable Mode Imager (GE Healthcare) using a 532-nm laser and then stained with ethidium bromide (Amresco), followed by imaging with the Molecular Imager Gel Doc XR (Bio-Rad) using UV light. Fluorescein-labeled 10-kDa dextran (D-1821; Life Technologies) was used as a marker for bulk-phase endocytosis.
Injection.
Adult animals (24 h post-L4 stage) were injected with 159 ng/µL unc-22-dsRNA or ∼325 to 980 ng/µL gfp-dsRNA into the body cavity past the bend of the posterior arm of the gonad (Figs. 3B, 4E, 5, and 6A and Fig. S9 C–E) or with 159 ng/µL unc-22-dsRNA into both arms of the germline (Fig. 2D). Hermaphrodites injected with unc-22-dsRNA were crossed with males that express gfp to distinguish self and cross-progeny. The earliest progeny (Fig. 3B and Fig. S9 C–E) or both early progeny that were L4-staged 3 d after injection and late progeny that were L4-staged 4 d after injection were scored (Fig. 2D) for silencing of unc-22 (Table S3). Hermaphrodites injected with gfp-dsRNA were either imaged ∼3 h postinjection or allowed to lay progeny, and the resultant progeny were imaged (Figs. 4–6).
Fluorescence Imaging.
RNAi-fed worms and embryos from adults injected with fluorescent gfp-dsRNA.
Fourth-larval stage (L4) animals in 3 mM tetramisole hydrochloride (Sigma) or embryos laid by adults 3–5 h postinjection on agar plates were individually imaged at fixed magnification on an AZ100 microscope (Nikon) with a Cool SNAP HQ2 camera (Photometrics). A C-HGFI Intensilight Hg Illuminator was used to excite GFP (filter cube: 450 to 490 nm excitation, 495 dichroic, and 500 to 550 nm emission) or DsRed/Atto 565 (filter cube: 530 to 560 nm excitation, 570 dichroic, and 590 to 650 nm emission). Exposure times were scaled for control RNAi-fed worms or control embryos laid by uninjected adults to just under saturation for each genetic background and then gfp RNAi-fed worms or embryos laid by injected adults were imaged using the same exposure time. Corresponding bright-field images were taken using auto-exposure. Images were adjusted for display using ImageJ (NIH).
Adults injected with fluorescent gfp-dsRNA.
At 2.5 to 3 h postinjection, adults were placed in 3 mM tetramisole hydrochloride (Sigma) and imaged using the Eclipse Ti spinning disk confocal microscope (Nikon) with a 60× objective lens. Atto 565 was excited using a 561-nm laser, and fluorescence was collected through a 415- to 475-nm and 580- to 650-nm emission filter. GFP was excited using a 488-nm laser, and fluorescence was collected through a 500- to 550-nm emission filter. Images were adjusted for display using ImageJ (NIH). A large circular enclosure of cytoplasmic material was observed, typically during ovulation, in 8 of 29 of the −1 oocytes imaged across multiple genotypes (e.g., Fig. 5 A, Left). This structure is either a normal feature of ovulation that is seen when the extracellular space is fluorescently labeled or is the result of constrained ovulation under a coverslip.
Supplementary Material
Acknowledgments
We thank Yinglun Wu for work on the inheritance assay; Sindhuja Devanapally for Fig. 3C; Pravrutha Raman for part of Fig. S8; Leslie Pick, Steve Mount, and members of the A.M.J. laboratory for critical reading of the manuscript; the Andrews laboratory (University of Maryland) for use of a confocal microscope; the Caenorhabditis elegans Genetic Stock Center, the Hunter laboratory (Harvard University), and the Seydoux laboratory (Johns Hopkins University) for some worm strains; and the Hamza laboratory (University of Maryland) for bacteria that express dsRNA. This work was supported by National Institutes of Health Grant R01GM111457 (to A.M.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608959113/-/DCSupplemental.
References
- 1.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell. 2014;157(1):95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bale TL. Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci. 2015;16(6):332–344. doi: 10.1038/nrn3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nestler EJ. Transgenerational epigenetic contributions to stress responses: Fact or fiction. PLoS Biol. 2016;14(3):e1002426. doi: 10.1371/journal.pbio.1002426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rando OJ. Intergenerational transfer of epigenetic information in sperm. Cold Spring Harb Perspect Med. 2016;6(5):a022988. doi: 10.1101/cshperspect.a022988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pembrey ME, et al. ALSPAC Study Team Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14(2):159–166. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- 6.Carone BR, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143(7):1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rechavi O, et al. Starvation-induced transgenerational inheritance of small RNAs in C. elegans. Cell. 2014;158(2):277–287. doi: 10.1016/j.cell.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franklin TB, et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68(5):408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 9.Dietz DM, et al. Paternal transmission of stress-induced pathologies. Biol Psychiatry. 2011;70(5):408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33(21):9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arroyo JD, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell PS, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 14.Jose AM. Movement of regulatory RNA between animal cells. Genesis. 2015;53(7):395–416. doi: 10.1002/dvg.22871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devanapally S, Ravikumar S, Jose AM. Double-stranded RNA made in C. elegans neurons can enter the germline and cause transgenerational gene silencing. Proc Natl Acad Sci USA. 2015;112(7):2133–2138. doi: 10.1073/pnas.1423333112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 17.Tabara H, Grishok A, Mello CC. RNAi in C. elegans: Soaking in the genome sequence. Science. 1998;282(5388):430–431. doi: 10.1126/science.282.5388.430. [DOI] [PubMed] [Google Scholar]
- 18.Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395(6705):854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- 19.Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science. 2002;295(5564):2456–2459. doi: 10.1126/science.1068836. [DOI] [PubMed] [Google Scholar]
- 20.Feinberg EH, Hunter CP. Transport of dsRNA into cells by the transmembrane protein SID-1. Science. 2003;301(5639):1545–1547. doi: 10.1126/science.1087117. [DOI] [PubMed] [Google Scholar]
- 21.Shih JD, Hunter CP. SID-1 is a dsRNA-selective dsRNA-gated channel. RNA. 2011;17(6):1057–1065. doi: 10.1261/rna.2596511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billi AC, Fischer SEJ, Kim JK. Endogenous RNAi pathways in C. elegans. WormBook. 2014:1–49. doi: 10.1895/wormbook.1.170.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton NO, Burkhart KB, Kennedy S. Nuclear RNAi maintains heritable gene silencing in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2011;108(49):19683–19688. doi: 10.1073/pnas.1113310108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu SG, et al. Amplification of siRNA in Caenorhabditis elegans generates a transgenerational sequence-targeted histone H3 lysine 9 methylation footprint. Nat Genet. 2012;44(2):157–164. doi: 10.1038/ng.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alcazar RM, Lin R, Fire AZ. Transmission dynamics of heritable silencing induced by double-stranded RNA in Caenorhabditis elegans. Genetics. 2008;180(3):1275–1288. doi: 10.1534/genetics.108.089433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolke U, Jezuit EA, Priess JR. Actin-dependent cytoplasmic streaming in C. elegans oogenesis. Development. 2007;134(12):2227–2236. doi: 10.1242/dev.004952. [DOI] [PubMed] [Google Scholar]
- 27.Grant B, Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell. 1999;10(12):4311–4326. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubbard EJ, Greenstein D. Introduction to the germ line. WormBook. 2005:1–4. doi: 10.1895/wormbook.1.18.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grishok A, Tabara H, Mello CC. Genetic requirements for inheritance of RNAi in C. elegans. Science. 2000;287(5462):2494–2497. doi: 10.1126/science.287.5462.2494. [DOI] [PubMed] [Google Scholar]
- 30.Tabara H, et al. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999;99(2):123–132. doi: 10.1016/s0092-8674(00)81644-x. [DOI] [PubMed] [Google Scholar]
- 31.Parrish S, Fire A. Distinct roles for RDE-1 and RDE-4 during RNA interference in Caenorhabditis elegans. RNA. 2001;7(10):1397–1402. [PMC free article] [PubMed] [Google Scholar]
- 32.Parrish S, Fleenor J, Xu S, Mello C, Fire A. Functional anatomy of a dsRNA trigger: Differential requirement for the two trigger strands in RNA interference. Mol Cell. 2000;6(5):1077–1087. doi: 10.1016/s1097-2765(00)00106-4. [DOI] [PubMed] [Google Scholar]
- 33.Jose AM, Smith JJ, Hunter CP. Export of RNA silencing from C. elegans tissues does not require the RNA channel SID-1. Proc Natl Acad Sci USA. 2009;106(7):2283–2288. doi: 10.1073/pnas.0809760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calixto A, Chelur D, Topalidou I, Chen X, Chalfie M. Enhanced neuronal RNAi in C. elegans using SID-1. Nat Methods. 2010;7(7):554–559. doi: 10.1038/nmeth.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinas A, Wright AJ, Hunter CP. SID-5 is an endosome-associated protein required for efficient systemic RNAi in C. elegans. Curr Biol. 2012;22(20):1938–1943. doi: 10.1016/j.cub.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paupard MC, Miller A, Grant B, Hirsh D, Hall DH. Immuno-EM localization of GFP-tagged yolk proteins in C. elegans using microwave fixation. J Histochem Cytochem. 2001;49(8):949–956. doi: 10.1177/002215540104900803. [DOI] [PubMed] [Google Scholar]
- 37.Bossinger O, Schierenberg E. The use of fluorescent marker dyes for studying intercellular communication in nematode embryos. Int J Dev Biol. 1996;40(1):431–439. [PubMed] [Google Scholar]
- 38.Yu X, Odera S, Chuang CH, Lu N, Zhou Z. C. elegans Dynamin mediates the signaling of phagocytic receptor CED-1 for the engulfment and degradation of apoptotic cells. Dev Cell. 2006;10(6):743–757. doi: 10.1016/j.devcel.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Vastenhouw NL, et al. Gene expression: Long-term gene silencing by RNAi. Nature. 2006;442(7105):882. doi: 10.1038/442882a. [DOI] [PubMed] [Google Scholar]
- 40.Buckley BA, et al. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature. 2012;489(7416):447–451. doi: 10.1038/nature11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashe A, et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell. 2012;150(1):88–99. doi: 10.1016/j.cell.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houri-Ze’evi L, et al. A tunable mechanism determines the duration of the transgenerational small RNA inheritance in C. elegans. Cell. 2016;165(1):88–99. doi: 10.1016/j.cell.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 43.Bucher G, Scholten J, Klingler M. Parental RNAi in Tribolium (Coleoptera) Curr Biol. 2002;12(3):R85–R86. doi: 10.1016/s0960-9822(02)00666-8. [DOI] [PubMed] [Google Scholar]
- 44.Khajuria C, et al. Parental RNA interference of genes involved in embryonic development of the western corn rootworm, Diabrotica virgifera virgifera LeConte. Insect Biochem Mol Biol. 2015;63:54–62. doi: 10.1016/j.ibmb.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 45.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci. 2014;17(1):89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rassoulzadegan M, et al. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature. 2006;441(7092):469–474. doi: 10.1038/nature04674. [DOI] [PubMed] [Google Scholar]
- 48.Gapp K, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci. 2014;17(5):667–669. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci USA. 2015;112(44):13699–13704. doi: 10.1073/pnas.1508347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma U, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351(6271):391–396. doi: 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Q, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351(6271):397–400. doi: 10.1126/science.aad7977. [DOI] [PubMed] [Google Scholar]
- 52.Abels ER, Breakefield XO. Introduction to extracellular vesicles: Biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeiser E, Frøkjær-Jensen C, Jorgensen E, Ahringer J. MosSCI and gateway compatible plasmid toolkit for constitutive and inducible expression of transgenes in the C. elegans germline. PLoS One. 2011;6(5):e20082. doi: 10.1371/journal.pone.0020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamath RS, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421(6920):231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 56.Timmons L, Tabara H, Mello CC, Fire AZ. Inducible systemic RNA silencing in Caenorhabditis elegans. Mol Biol Cell. 2003;14(7):2972–2983. doi: 10.1091/mbc.E03-01-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.