Significance
The present study demonstrates the utility of global phosphoproteomic profiling of diseased cardiac tissue to identify signaling pathways and other biological processes disrupted in cardiomyopathy. Perturbed Notch-1 signaling was identified by bioinformatics analyses of phosphoprotein patterns present in affected cardiac tissue in a transgenic mouse model system of dilated cardiomyopathy and by complementary molecular biology and microscopy techniques. In addition, dozens of other disturbed signaling pathways offer an opportunity for novel therapeutic and/or diagnostic clinically applicable targets. Although this study was performed in mice, only minor adjustments to the experimental approach would be required for comparative analysis of analogous samples from human cardiac patients, potentially leading to even more clinically relevant data.
Keywords: phospholamban, proteomic, bioinformatics, heart disease, signaling
Abstract
Phospholamban (PLN) plays a central role in Ca2+ homeostasis in cardiac myocytes through regulation of the sarco(endo)plasmic reticulum Ca2+-ATPase 2A (SERCA2A) Ca2+ pump. An inherited mutation converting arginine residue 9 in PLN to cysteine (R9C) results in dilated cardiomyopathy (DCM) in humans and transgenic mice, but the downstream signaling defects leading to decompensation and heart failure are poorly understood. Here we used precision mass spectrometry to study the global phosphorylation dynamics of 1,887 cardiac phosphoproteins in early affected heart tissue in a transgenic R9C mouse model of DCM compared with wild-type littermates. Dysregulated phosphorylation sites were quantified after affinity capture and identification of 3,908 phosphopeptides from fractionated whole-heart homogenates. Global statistical enrichment analysis of the differential phosphoprotein patterns revealed selective perturbation of signaling pathways regulating cardiovascular activity in early stages of DCM. Strikingly, dysregulated signaling through the Notch-1 receptor, recently linked to cardiomyogenesis and embryonic cardiac stem cell development and differentiation but never directly implicated in DCM before, was a prominently perturbed pathway. We verified alterations in Notch-1 downstream components in early symptomatic R9C transgenic mouse cardiomyocytes compared with wild type by immunoblot analysis and confocal immunofluorescence microscopy. These data reveal unexpected connections between stress-regulated cell signaling networks, specific protein kinases, and downstream effectors essential for proper cardiac function.
Cardiovascular diseases (CVDs) leading to systolic/diastolic heart failure (HF), such as hypertensive/diabetic heart disease, stroke, and vascular atherosclerosis, are leading causes of death in the developed world (1). Many CVDs are associated with genetic predispositions. For example, in humans, the arginine to cysteine (R9C) substitution in phospholamban (PLN) has been shown to result in dilated cardiomyopathy (DCM) presenting in adolescence, leading to rapid deterioration of heart function and premature death (2). However, the etiology and molecular mechanisms of progression of DCM and other CVDs leading to HF are complex and still poorly understood, further complicating clinical assessment and management. From a biological and clinical perspective, the identification and characterization of clinically relevant, potentially druggable, pathways driving the maladaptive response in affected heart tissue are key challenges to improved diagnostic and therapeutic tools for earlier detection and preventative treatment of both inherited and chronic CVDs.
Cardiac muscle contraction is controlled by Ca2+ flux and signaling relays, which are perturbed in HF. Internal stores of Ca2+ required for the proper functioning of cardiomyocytes (CMs) are normally maintained through the function of the sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2) (3), which is responsible for the sequestration of Ca2+ resulting in muscle relaxation. SERCA2 activity is regulated through a reversible inhibitory interaction with PLN, which can be relieved by phosphorylation of PLN by protein kinase A (PKA) or Ca2+/calmodulin-dependent protein kinase II (CaMKII) (3).
Proteomic analyses have revealed changes in the abundance of other effector proteins in diverse biochemical pathways in DCM. Notably, shotgun proteomic analysis of membrane protein expression dynamics in heart microsomes isolated from mice overexpressing a superinhibitory (I40A) mutant of PLN revealed changes in G protein-coupled receptor-mediated pathways leading to activation of protein kinase C (PKC) (4). We previously reported quantitative changes in protein and cognate mRNA expression levels in cardiac ventricular tissue at different time points in the development of DCM in R9C-PLN mice representing clear clinical stages in the progression to HF (5). We showed that the latter maladaptive response was driven by elevated activity of MAPK signaling by the protein kinases p38 and JNK, in part through down-regulation of prosurvival microRNAs (6). However, the underlying upstream and downstream signaling events preceding HF were not fully explored.
In the present study, we report a systematic, large-scale quantitative phosphoproteomic analysis of dysregulated protein phosphorylation-dependent signaling occurring at the early symptomatic stages of DCM progression in whole hearts from R9C mutant mice compared with wild-type littermates.
Results
Comparative Phosphoproteome Analysis.
To achieve a comprehensive survey of cardiac signaling cascades impacted by DCM, we performed global quantitative phosphopeptide profiling on three independent biological replicates of whole-heart tissue isolated from 8-wk-old (early symptomatic) R9C mice and wild-type littermates (Fig. 1). A major experimental consideration was preservation of phosphorylation-site integrity, through use of phosphatase inhibitors, low temperature, and harsh denaturing sample-processing conditions. To achieve the deepest possible coverage, we performed chromatographic sample prefractionation steps, using hydrophilic interaction liquid chromatography to separate peptides bearing negatively charged phosphate moieties (7). Given the low relative abundance of phosphopeptides and their tendency to be undetectable due to ion competition/suppression during MS analysis, we performed differential affinity capture with immobilized metal oxide affinity chromatography (TiO2) to enrich for phosphopeptides. To measure differences in relative abundance, we applied label-free quantification, comparing the extracted parent-ion current intensities recorded in high-energy collision dissociation (HCD) spectra using a high-precision orbitrap instrument. In parallel, we measured total protein levels by shotgun sequencing.
Fig. 1.
General phosphoproteomics workflow. Three pooled hearts from R9C-PLN transgenic and wild-type littermates were analyzed by quantitative precision LC-MS/MS. PTM, posttranslational modification.
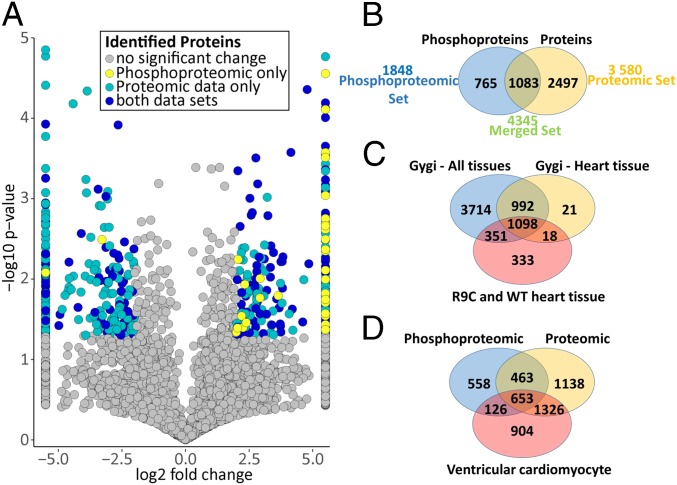
In total, our stringent data analysis workflow (Fig. S1) mapped 94,956 and 217,092 spectra to mouse reference protein sequences for the phosphoproteomic and background proteomic datasets, respectively. From this, we derived a set of high-confidence sequence matches [false discovery rate (FDR) ∼1% at both protein and peptide levels] corresponding to over 1,800 cardiac phosphoproteins and 3,900 phosphopeptides in normal and DCM hearts, and 15,436 unique peptides mapping to 3,580 unique proteins in the background proteomic dataset (Fig. 2 A and B, Fig. S1, and Datasets S1–S3). For the former, we confidently (site-localization probability >0.7, as described in SI Materials and Methods and Datasets S4 and S5) identified 7,589 unique putative phosphorylation sites (i.e., sites on identified phosphopeptides with supporting MS/MS data and localization probability >0.7), of which 6,855 mapped to serine, 674 mapped to threonine, and 60 mapped to tyrosine residues (consistent with the expected 90:9:1 cellular distribution ratios) on 1,848 distinct cardiac proteins (Fig. 2 A and B and Datasets S1 and S2). Our coverage is comparable to a pioneering study of the mouse cardiac phosphoproteome by Lundby et al. (8) and a recent phosphoproteomic analysis of in vivo effects of CaMKII inhibition in mouse hearts (9). Phosphoproteins identified in our study also showed considerable overlap with heart and other tissue profiles in the mouse phosphoproteomic atlas reported by the Gygi group (10), but 333 were uniquely identified in our study, 96 of which contained phosphopeptides significantly altered and/or solely identified in R9C hearts (Fig. 2C). A high degree of overlap with the mouse ventricular myocyte proteome by Sharma et al. (11) was also observed (Fig. 2D).
Fig. S1.
Overview of the data analysis pipeline including significance (P value) assignment-based ranking of the proteomic and phosphoproteomic datasets and subsequent gene set enrichment for the purpose of identification of signaling pathways and biological processes disrupted in R9C-PLN mutant relative to wild-type mouse hearts.
Fig. 2.
(A) Volcano plot indicating significantly altered proteins and phosphoproteins identified in the combined datasets. Log-transformed P values (t test) associated with individual peptides and phosphopeptides plotted against log-transformed fold change in abundance between the R9C-PLN and wild-type hearts. (B) Venn diagram depicting the total number of proteins identified in the corresponding proteomic and phosphoproteomic datasets. (C) Venn diagram depicting phosphoprotein overlap with mouse tissue phosphoproteomes from Huttlin et al. (10). (D) Venn diagram depicting the overlap of the R9C and WT combined phosphoproteome and proteome with the ventricular cardiomyocyte proteome from Sharma et al. (11).
Quantification and Ranking.
Based on reproducible measurements of precursor-ion intensity, we scored both the individual phosphopeptides and their consolidated cognate phosphorylation sites for differential relative abundance between the healthy and diseased samples. Based on a two-tailed Student’s t test (P < 0.05), the abundance of 211 phosphopeptides was differentially altered (elevated or reduced) between the R9C and WT hearts (Fig. 2A and Dataset S1), with 86% of these predominantly higher in the disease state. In comparison, 499 proteins showed differential expression, consistent with our previous report (5).
Systematic evaluation of the corresponding biological annotations revealed that the aberrant phosphorylation patterns occurred on proteins linked to disparate subcellular compartments, ranging from membrane-associated receptors to nuclear-localized proteins with established links to heart development, contractile function, and/or cardiomyopathy (Dataset S1). Consistent with expectation, the predominant phosphoform of PLN detected preferentially in R9C hearts was phosphorylated on serine 16, a site critical for inhibition of SERCA2 activity (3). We also detected alterations in the phosphorylation pattern of central kinases involved in cardiac signaling such as PKA. Additionally, the S112 site on the PKA type II regulatory beta subunit (PRKAR2B), which results in the lowering of the activation threshold of PKA (12), increased in R9C. Additionally, we detected abundance changes in a cardiac stress marker (FHL1), an indicator of the fetal gene program (MYH7), markers of ECM remodeling (POSTN, SPARC), and proteins involved in Ca2+ modulation (SLMAP, CASQ2) (Datasets S1 and S2).
Further examples of interest include the highest-ranked phosphorylated peptide (S64 + T69) from eIF4E-binding protein 1 (EIF4EBP), which negatively regulates translation initiation. Hypophosphorylated EIF4EBP acts as a repressor of eIF4E whereas its phosphorylation releases eIF4E, thereby up-regulating protein translation in response to signaling by diverse cell-surface receptors, including insulin, EGF, PDGF, and other ligands (13). In heart, EIF4EBP has been implicated in ischemia–reperfusion stress, cardiomyocyte survival, cardiac hypertrophy, and oxidative and nutritional stress responses (13).
Also significantly more highly phosphorylated in R9C hearts was a previously uncharacterized T187 site in EMMPRIN (BSG/CD147), an extracellular metalloprotease-inducing member of the Ig superfamily, consistent with its role in inflammatory processes in cardiac remodeling (14). In addition, the S2 phosphosite on transcription factor MAX (Myc-associated factor X) (15), a positive regulator of cardiac alpha-myosin heavy-chain gene expression in cardiomyocytes, was significantly altered in R9C mice. Phosphorylation of S2 inhibits DNA binding by MAX homodimers and association with MYC but is not known to affect its interaction with another cofactor, transcription enhancer factor 1 (TEF-1, TEAD1), or regulation of cardiac alpha-myosin heavy chain (16). In contrast, when phosphorylated, the S73 site of transcription factor JUN, likewise more highly phosphorylated in the R9C mutant mice, is linked to increased transcription of JUN target genes encoding components of a myriad of signaling pathways (17).
Phosphosites on two intercalated disk-associated Xin repeat proteins (XIRP1, XIRP2) with roles in cardiac development were also increased significantly in R9C hearts. XIRPs interact with catenins and ion channels in mature cardiac muscle and, although the significance of phosphorylation is unclear, gene knockout mice display early-onset cardiomyopathy (18).
Kinase Target Motif Analysis.
We used the MotifX algorithm (19) to identify consensus motifs overrepresented in the set of phosphopeptides identified exclusively in R9C heart tissue and/or those showing significant (P < 0.05) differential expression between R9C and wild-type mice (Table 1 and Dataset S1). Candidate consensus sequences were processed using PhosphoMotif Finder (20) to identify cognate kinases known or predicted to phosphorylate these sequences.
Table 1.
Top 10 ranked identified consensus motifs on phosphopeptides significantly altered and/or exclusively detected in R9C heart tissue with kinases predicted to target these sequences
| Motifs | Kinase | Motif score | Matches | Fold increase |
| ...RS.S...... | CamKII, PKA, PKC, CK2 | 27.34 | 59 | 4.76 |
| ......SD.E... | CK2 | 24.66 | 34 | 6.77 |
| ....SPT...... | GSK3, ERK1/2, CDK5, CK2, DNA-PKCs | 23.7 | 27 | 7.75 |
| .R..S.S...... | CamK2, PKA, PKC, CK2 | 23.62 | 34 | 5.64 |
| ....SDS...... | CK2, BARK | 17.65 | 21 | 6.41 |
| ......SP.... | GSK3, ERK1/2, CDK5 | 16 | 275 | 2.34 |
| ...S..S...... | CK1/2 | 16 | 258 | 1.74 |
| ...R..S...... | CamK2, PKA, PKC | 12.45 | 123 | 2.01 |
| ......TP..... | GSK3, ERK1/2, CDK5 | 9.46 | 63 | 2.35 |
| ..S...S...... | GPCRK1, MAPKAPK2K, GSK3 | 8.1 | 120 | 1.7 |
The number of matches in the query set and the fold increase over the normal occurrence of consensus sequence are also provided.
We found central kinases involved in cardiac contractility through transient Ca2+ regulation among the most overrepresented compared with the whole mouse proteome. Two consensus motifs identified were targeted by PKA and CaMKII (Table 1), one enriched 4.76-fold (59 matches) over background and the other 5.64-fold (34 matches). Phosphorylation of PLN by PKA on S16, or by CaMKII on T17, reverses PLN-mediated inhibition of SERCA2a activity up to 3-fold during β-adrenergic stimulation (3). Up-regulation of these kinases is expected as a compensatory mechanism considering that R9C-PLN mutant molecules effectively trap PKA, inhibiting phosphorylation of wild-type protein and relieving SERCA2a inhibition (2).
Two consensus sequences targeted by extracellular signal-regulated kinases (ERK1/2) were identified on 275 significantly altered phosphopeptides (Table 1). ERK1/2 become activated in cardiac myocytes in response to a variety of stimuli, including progression of DCM (21). Likewise, we found consensus motifs associated with phosphorylation by casein kinases and GSK3 kinases, previously linked to cardiac pathology (22).
Pathway Network Enrichment Analysis.
For each phosphoprotein (and corresponding gene), the most differential peptide or modification site was selected for a systematic pathway enrichment analysis (SI Materials and Methods). Phosphopeptides were initially ranked based on the level of statistical significance (differential P value) rather than direction (up/down) of observed fold change. The focus of this analysis was to identify pathway-level alterations present in R9C mice compared with controls.
Three sets of enrichment analyses were performed to gain the most complete coverage, based on ranking (i) the phosphoproteomic dataset alone, (ii) the background proteomic dataset alone, and (iii) a merged combination of both (Fig. 2 A and B and Fig. S1), which included the significant differentially abundant proteins derived from both the proteomic background and differential phosphorylation events. This merged set of 4,345 unique proteins/genes (Fig. 2B) was included because signaling perturbations do not necessarily consist only of changes in phosphorylation state or protein expression alone but often rather both in concert. The most differentially expressed peptides or phosphorylation events (based on P value) from each protein were used to represent individual protein/gene products. The majority of enriched pathways found in the merged set (Dataset S3) reflected the presence of many proteins exclusively in the background proteome. Of the 2,039 gene sets enriched in the merged set, many (1,427) were likewise enriched in the global proteomic data alone (Fig. S1).
We chose to focus on a subset of 612 pathways and processes that reflected contributions from both datasets, rather than merely differential protein levels. To simplify analysis by minimizing redundancy in the annotation term labels (Dataset S1), we combined the 182 enrichments from the phosphoproteomic dataset (not in the background proteome) with the 526 gene sets unique to the merged set (Fig. S1 and Dataset S3). By visualizing the resulting 708 enriched gene sets as a single graphical enrichment map (23) (Dataset S6), we identified several dysregulated pathways of notable relevance to cardiac pathology (Table 2 and Fig. S2). For instance, reflecting a central role in cardiac physiology and pathology, dysregulated pathways associated with activation PKA- and cAMP-dependent kinases were prominently overrepresented in all gene set enrichment analyses (Datasets S1 and S3). Considering the ubiquity of many signaling pathways across different tissues and cell types, when examined as a whole (rather than individual components) it is not surprising that most pathways have associations with other disorders or processes. This is also partly a bias of existing annotations in curation databases.
Table 2.
List of select cardiac-relevant signaling pathways, from the gene set enrichment analysis, along with their relevant protein components, altered in R9C-PLN hearts
| Pathway | Identified components | Cardiac context |
| Notch-1 signaling | hdac7(s178), tle3(s216), snw1(s224,s232), tmed2, ncor2,* tbl1xr1, tbl1x, ncor1(s2351), ep300, adam10, crebbp, rbx1, numb, hdac5(s652), arrb1, rps27a, mib1 | Dilated cardiomyopathy, left ventricular noncompaction, cardiac development (36, 40) |
| VIP signaling | map3k1(s915), ppp3cb(s469), plcg1(s1263), nfatc2(s136), prkar2b(s112), prkacb, prkar2a, prkar1a, nfkb1, ppp3ca | Myocardial fibrosis, diabetic cardiomyopathy, ischemia–reperfusion (41) |
| H3-K4 histone methylation | ogt, kmt2a, snw1(s224,s232), nipbl(s2652), bcor(s423), men1, kdm1a, mt2b, dnmt1,* pml(s449), pygo2(t264), ctbp1, arrb1, ube2n, wbp2, lpin1 | Hypertrophic cardiomyopathy, fetal gene program (42) |
| BCR signaling, beta-cell activation and regulation | mif, irs2(s1165), thoc1(s560), sos1,* orai1, fas,* sash3,* ptprc(s801), lyn(s19), syk, itpr1(s1573), cd81, stim1,* ptpn6, nfatc2(s136), inpp5d(t701, s709), sh3kbp1(s193), aplf(s41), cbl, plcg1(s1263), tcf3(s517), bad(s112), itpr2, calm1, pawr, itpr3, bmi1, nck1 | Heart failure, hypertrophy, myocardial fibrosis, myocardial infarction, ischemia–reperfusion (43, 44) |
| TGF-β/smad signaling | glg1, pdpk1(s117), jun(s63), lemd3, fbn2, men1, zeb1, aspn, eng, snx6, zfyve9, cav3, sptbn1, bcl9l, strap, cav2,* ppm1a, htra1 | Dilated cardiomyopathy, heart failure, fibrosis, myocardial infarction, cardiac remodeling (24, 45) |
| PPAR-α signaling | med19(s226), txnrd1, ankrd1, wwtr1,* abca1(s2234), yap1, med24,* tbl1xr1, alas1, cdk19, tbl1x, apoa1, acox1, apoa2, smarcd3, agt, ncoa3,* ncoa2, nfya(s187), fhl2, sp1, ncoa6, acadm, acsl1, cd36, crebbp, slc27a1, cpt2, ncor1(s2351), plin2, me1, ep300 | Dilated cardiomyopathy, heart failure, diabetic cardiomyopathy (24, 46) |
| TLR signaling | hsp90b1(s306), lgmn, ikbkb, map2k1, app, nfkb2(t425), dhx9(s137), dnm2, ppp2r5d,* unc93b1, dnm1(s774), itgb2, rps6ka3, eea1, dusp3, tlr3, ctsb, nfkb1, ube2n, ppp2ca, cd36, mef2a, ppp2r1a | Dilated cardiomyopathy, heart failure, ischemia–reperfusion, hypertrophy, viral myocarditis (24, 47) |
| Wnt signaling | scyl2(s253), dab2ip(s854), ranbp3, cdh2, wwtr1,* rapgef1,* gsk3a, ppp2r3a, mcc,* dab2, limd1, ctnnb1, dact3(s165), bicc1, gsk3b, stk3(s246), ctnnd1(s813), g3bp1, cav1, ppp2r5a, pi4k2a(s462), cul3, mapk14,* dvl3,* hdac2(s394) | Dilated cardiomyopathy, fibrosis, heart failure, myocardial infarction, arrhythmia (24, 48) |
Phosphopeptides/proteins increased in R9C hearts are shown in bold, whereas components identified but showing a decrease in R9C are not bolded. The identified phosphorylation site of a particular protein is indicated in parentheses. For a full list of protein/gene names associated with each pathway, refer to Datasets S1–S3.
Proteins annotated as “ambiguous,” as described in SI Materials and Methods.
Fig. S2.
Simplified representations of selected pathways, and relevant protein components, perturbed in R9C-PLN transgenic hearts. Coloring of the protein name fonts indicates the trend in the R9C-PLN transgenic hearts, being either up- (red) or down-regulated (blue). The identified phosphorylation site of a particular protein is indicated in parentheses. Phosphoproteins with an ambiguous site are marked with an asterisk. For a full list of protein/gene names associated with each pathway, refer to Datasets S1–S3.
To identify disease signatures specifically relevant to DCM, we integrated and compared these pathways against a recent in-depth RNA sequencing-based gene expression study of the R9C-PLN model (Datasets S2 and S7) (24). Specifically, differential mRNA expression modules in the Seidman study (24) corresponding to cardiomyocyte and nonmyocyte cardiac cell populations at different stages of disease (represented by six individual nodes) were tested for overlap with our enrichment map using a stringent Mann–Whitney test cutoff (P < 0.01). Strikingly, we observed highly significant overlap between most of the disrupted biological processes and signaling pathways noted by the two studies, including (but not limited to) innate immunity, glucose metabolism, TGF-β signaling, PPAR signaling, Wnt/β-catenin signaling, and TLR signaling (Dataset S8). Notably, the general pathways involved in metabolic and profibrotic processes determined by the Seidman study to differentiate between DCM and hypertrophic cardiomyopathy (HCM) were likewise detected as differentially perturbed in R9C hearts in our merged data.
Notch-1 Signaling.
Distinct from the Seidman study, however, we identified evidence for significant disruption in homeostatic Notch-1 signaling in R9C hearts, with most phosphosites never reported before in the context of DCM. For instance, uncharacterized sites on SNW1 and CREBBP, members of the Notch-1 transcription coactivator complex (25), were hyperphosphorylated in the R9C mice. Similarly, we detected previously unreported phosphorylation sites on both MAX and NCOR1/2, components of a downstream transcriptional corepressor complex known to inhibit transcription of Notch target genes (26).
To directly determine the differential state of Notch receptor signaling, we performed immunoblots (Fig. 3A) using a panel of antibodies targeting different Notch receptor isoforms (Notch1/3), activating and inhibiting ligands (DLL1/4, JAGG1/2, NUMB), processing enzymes (ADAM9/17), and key downstream effector transcription factors (HEY2, HES3, SOX9). Strikingly, a very substantive and specific decrease in total Notch-1 receptor levels was seen in the R9C hearts, whereas levels of Notch-3 receptor were unchanged (Notch-2 was undetectable, as was the cleaved Notch-1 intracellular domain).
Fig. 3.
(A) Immunoblots of components of the Notch-1 signaling pathway. (B) Immunofluorescence images of Notch-1, ADAM9, and Notch-3 in ventricular sections of WT and R9C-PLN hearts. (C) Representative reconstructed images showing nuclear localization of the Notch-1 intracellular domain and a bar graph showing quantification of relative distribution (spots identified/nuclear volume as determined by Hoechst staining) in WT and R9C-PLN hearts (mean ± SEM, *P = 0.00282).
Of the activating Notch ligands, DLL1 was down-regulated in R9C whereas JAGG1 showed no change (DLL4 and JAGG2 were not detected). Conversely, DLL3 and NUMB, which can suppress Notch signaling, were up-regulated in R9C hearts. Two metalloproteinases (ADAM9 and TACE/ADAM17) required for extracellular processing and/or degradation of the Notch-1 receptor were similarly up-regulated in R9C. Conversely, the downstream Notch-1–regulated transcription factors HES1 and HEY2 were down-regulated in R9C.
Immunofluorescence visualization of components of the Notch signaling pathway in the ventricular wall of R9C and wild-type hearts (Fig. 3B) established down-regulation of Notch-1 receptor expression in R9C cardiomyocytes along with up-regulation of the ADAM9 metalloproteinase (Notch-3 was unchanged). Imaris-based quantification (Fig. 3C) of signal intensity and localization of the Notch-1 intracellular domain (N1CID) in cardiomyocyte nuclei, indicative of Notch-1 activation, revealed a significant decrease (P < 0.003) in overall nuclear localization of N1CID in cells in the ventricular sections of R9C hearts compared with controls (due to gross changes in tissue morphology in R9C hearts, a large proportion of nuclei showed no N1CID colocalization). These results verify Notch-1 receptor signaling inhibition in affected heart tissue during early-stage disease (Fig. 4).
Fig. 4.
Proposed schematic model of Notch-1 signaling in normal and DCM cardiomyocytes populated with differential proteins identified with different lines of evidence in whole-heart lysates or ventricular sections, including proteomic data, phosphoproteomic data, immunoblotting, and immunofluorescence (IF).
SI Materials and Methods
Mouse Heart Tissue Sample Preparation.
Mice (three R9C, three control wild-type littermates) were killed by CO2 asphyxiation at 8 wk of age (2). Whole hearts were collected rapidly, washed in ice-cold PBS, snap-frozen in liquid nitrogen, and stored at −80 °C until use. Frozen hearts were placed in 1 mL 8 M urea containing protease (Sigma) and phosphatase inhibitors (PhosSTOP; Roche) and subjected to mechanical homogenization using a homogenizer (PRO 200; PRO Scientific) at the highest setting. Cell debris and protein aggregates were removed by centrifugation at 13,000 × g for 10 min (repeated three times). Total protein content in each sample was determined by the Bradford total protein assay (Bio-Rad). Two milligrams of protein from each sample was reduced by addition of DTT to a final concentration of 2.5 mM for 60 min at room temperature and alkylated by the addition of iodoacetamide (5 mM) and incubation at room temperature for 30 min in the dark. The samples were then diluted eightfold in 50 mM ammonium bicarbonate, and 100 µg of sequencing-grade trypsin (Promega) was added. Digestion was allowed to proceed overnight at 37 °C and stopped by the addition of formic acid (to 1% in solution). The resulting tryptic peptides were isolated and desalted using a C18 TopTip (Glygen) as per the manufacturer’s instructions.
Fractionation and Phosphopeptide Enrichment.
Hydrophilic interaction liquid chromatography (HILIC) was performed on a 2.0 × 150-mm 5-μm particle TSKgel Amide-80 column (Tosoh Bioscience) connected to an Agilent 1100 HPLC system. Buffer A was 98% (vol/vol) acetonitrile (ACN) with 0.1% TFA, whereas buffer B was composed of 2% (vol/vol) ACN with 0.1% TFA in HPLC water. One milligram of peptide digests was suspended in 80% (vol/vol) buffer A with 20% (vol/vol) buffer B and loaded onto the column at a flow rate of 250 μL/min. The liquid chromatography method was as follows: 3-min loading period in 20% (vol/vol) buffer B, from 3 to 30 min a gradient of 20–40% (vol/vol) buffer B, 3-min gradient of 40–100% (vol/vol) buffer B, 100% (vol/vol) buffer B for 5 min, a gradient of 100–20% (vol/vol) buffer B for 2 min, and finally 20% (vol/vol) buffer B for 10 min. Eluted fractions were collected in 1.5-mL tubes at 2-min intervals and lyophilized to dryness. TiO2-coated magnetic beads (Pierce) were used for phosphopeptide enrichment as per the manufacturer’s protocol. The lyophilized peptide mixture from each HILIC fraction was resuspended in 200 μL 80% (vol/vol) ACN with 2% (vol/vol) formic acid before incubation with 10 μL TiO2 magnetic beads for 1 min. Subsequently, the tubes were placed on a magnetic plate for 1 min, the supernatant was removed, and the beads were washed three times with 200 μL binding buffer provided in the kit. Phosphopeptides were eluted for 10 min with 30 μL elution buffer provided in the kit and lyophilized to dryness before LC-MS analysis.
Liquid Chromatography-Mass Spectrometry Identification.
Bead-enriched phosphopeptide mixtures from each HILIC fraction were individually loaded onto a 2.5-cm-long silica microcapillary (75-μm diameter) containing a reverse-phase resin (5-μm Luna C18 beads; Phenomenex) precolumn and separated on a 10-cm analytical column (75-μm diameter) containing 3-μm Luna C18 stationary phase (Phenomenex) using an EASY-nano LC system (Proxeon). The trap was constructed in a 25-mm × 75-μm-i.d. silica capillary packed with 5-μm Luna C18 stationary phase (Phenomenex). A fine tip on the analytical column was pulled with a column puller (Sutter Instrument). The nanoflow gradient consisted of buffer A, composed of 5% (vol/vol) ACN with 0.1% formic acid, and buffer B, which contained 95% (vol/vol) ACN with 0.1% formic acid. Nanoflow liquid chromatography was performed for 120 min at a flow rate of 300 nL/min, with a gradient of 2–6% (vol/vol) buffer B for 1 min, followed by a 6–24% (vol/vol) gradient of buffer B for 89 min, 24–100% (vol/vol) gradient of buffer B for 16 min, 100% (vol/vol) buffer B for 5 min, 100–0% (vol/vol) gradient of buffer B for 1 min, and finishing with 0% buffer B for 8 min.
Peptides were directly ionized using a nanospray ion source (Proxeon) into an LTQ-Orbitrap Velos hybrid mass spectrometer (Thermo Fisher Scientific). Ten MS2 data-dependent scans in centroid mode were acquired per single profile mode full-scan mass spectrum using HCD fragmentation. Full scans were performed at 30,000 resolution, with a maximum injection time of 250 ms and an ion-packet setting of 1 × 106 for automatic gain control. MS2 scans were performed at 7,500 resolution, with an ion-packet setting of 3 × 104 for automatic gain control, maximum injection time of 150 ms, and 0.1 ms activation time, and using 40% total normalized collision energy. The dynamic exclusion list was set to exclude a maximum of 500 ions over 30 s.
Resulting RAW files were searched using MaxQuant (version 1.5.0; www.coxdocs.org/doku.php?id=maxquant:start) under standard settings using the UniProt mouse database (www.uniprot.org/taxonomy/10090) allowing for two missed trypsin cleavage sites, variable modifications for protein phosphorylation at S, T, and Y residues, N-terminal acetylation, and methionine oxidation. Carbamidomethylation of cysteine residues was set as a fixed modification. Ion tolerances of 20 and 6 ppm were set for first and second searches, respectively. Candidate (phospho)peptides, proteins, and phosphorylation-site identifications were filtered based on a 1% false discovery rate threshold based on searching of the reverse sequence database. Data are deposited and publicly available at the PRIDE Archive, proteomics data repository (European Bioinformatics Institute, European Molecular Biology Laboratory).
Statistical Analysis.
For the phosphoproteomic data, raw peptide-ion intensities were extracted from the MaxQuant evidence file. Phosphopeptide IDs (as entered in Dataset S4) with the same sequence and mass with site-localization probabilities of 0.7 (as assigned by the PTM Score algorithm in Andromeda/MaxQuant) or over were treated as confident “nonambiguous” identifications. We labeled the identified phosphopeptides as ambiguous or nonambiguous based on the following rules. For a phosphorylation site on any particular phosphopeptide to be designated as nonambiguous (Dataset S1), all phosphopeptide identifications in the MaxQuant evidence file (Dataset S4) had to meet the 0.7 probability cutoff for the same site. Any phosphopeptide of the same sequence and mass with even a single identification entry where the probability was less than 0.7 on all possible phosphorylation sites was labeled as ambiguous, and the intensities were combined as a single entry (as in Dataset S1). Additional phosphorylation site-localization scores based on the A-score algorithm (49) are provided in Dataset S5.
Using an in-house script, single posttranslational modifications mapping to a common site were collapsed and the corresponding ion intensities were added together to generate a single representative sequence (Dataset S1), whereas multiply phosphorylated peptides were entered as individual entities and scored independently. Candidates identified in only one biological replicate of either condition were discarded. Both the background proteomic and phosphoproteomic datasets were normalized using LOESS normalization in the Normalyzer package (50).
Proteins measured in each individual experimental dataset were tested for biological pathway enrichment using ROAST against a custom gene set annotation database (composed of Gene Ontology biological process terms and curated pathways; download.baderlab.org/EM_Genesets/) (23). Enriched gene sets were required to have between 5 and 500 associated gene products. ROAST scores each pathway according to a computed ranked list of proteins and assigns significance of this score relative to a randomized version of the dataset, using a rotation instead of permutation to calculate significance. P values were further corrected for multiple hypothesis testing using the Benjamini–Hochberg correction (6). For the background and merged gene set analyses, we used a more stringent threshold enrichment P ≤ 0.01 and FDR ≤ 0.05, whereas for the phosphoproteomic dataset, we applied an enrichment P < 0.01 and FDR ≤ 0.1 cutoff for further analysis. We calculated overlap between gene set (pathway) annotations using a combination of Jaccard and overlap coefficients, using a cutoff of 0.375 to reduce redundancy in pathway annotations. Signature gene sets were constructed from Burke et al. (24), supplemental table 3, representing cardiomyoctye and noncardiomyocytes at pre-DCM, DCM, and HF stages (Datasets S2 and S7). Signatures were added to pathway enrichment results using a postanalysis in Enrichment Map. Any pathway that had significant overlap with one of the signature sets (as determined using a two-sided Mann–Whitney test, P < 0.01) was connected to the signature set with a new pink edge.
Kinase Target Motif Analysis.
Phosphopeptide sequences with associated extracted intensities exclusive to R9C-PLN mouse tissue and those that were significantly altered between the experimental conditions (P < 0.05) were searched for consensus sequences using the MotifX algorithm (19, 51). Central residues were set as S or T with the “MS/MS” foreground format searched against the whole mouse proteome. Window width was set to 13 and the cutoff for minimal occurrences in the searched phosphopeptide sequences was set to 20. Significance was set to 0.000001. Identified consensus sequences were then searched for cognate kinases known to target these sequences using PhosphoMotif Finder (20).
Western blotting.
Whole-heart homogenates from three wild-type and three R9C-PLN mutant mice (from above) were diluted in water and 3× loading dye down to a final total protein concentration of 1.5 mg/mL (5). The samples (20 µL) were loaded and run on precast NuPAGE 12% Bis-Tris gels (10-well) in Mes/SDS running buffer as per the manufacturer’s protocol (Invitrogen) for 1 h at 200 V. The resolved proteins were transferred onto a nitrocellulose membrane using the iBlot Gel Transfer Device (Thermo Fisher). The membrane was blocked by incubation with TBS-T (0.1 M Tris⋅HCl buffer, pH 7.5, containing 0.15 M NaCl and 0.1% Tween 20) supplemented with 5% nonfat dry milk for 1 h at room temperature and probed with relevant primary antibodies for 1 h at room temperature. The membrane was washed three times for 15 min with TBS-T and incubated with the appropriate secondary antibody in 5% nonfat dry milk for 1 h at room temperature. Finally, the membrane was washed three times in TBS-T and the signal was detected by ECL (Pierce) using a MicroChemi imager (FroggaBio).
Immunofluorescence Microscopy.
WT and R9C hearts were snap-frozen and cryosectioned at 6 μm. Tissues were fixed with 4% paraformaldehyde at room temperature for 15 min (5 min on ice, 10 min off ice) and permeabilized with 90% methanol for 1 min on ice. Tissues were then blocked at room temperature with 5% horse serum in PBS for 1 h and probed with primary antibody overnight at 4 °C. Primary mouse monoclonal antibodies (Cell Signaling) were used at the following concentrations: Notch-1 (D1E11; 1:1,000), cleaved Notch-1 (D3B8; 1:1,000), Notch-2 (D76A6; 1:1,600), Notch-3 (D11B8; 1:1,000), ADAM9 (D64B5; 1:1,000), TACE (D22H4; 1:1,000), DLL1 (2588; 1:1,000), DLL3 (G93; 1:1,000), JAGGED1 (28H8; 1:1,000), and NUMB (C29G11; 1:400). Secondary anti-mouse antibody was conjugated to Alexa Fluor 488 (1:400) and used for visualization, followed by F-actin visualized with phalloidin (Thermo Fisher; R415; 1:1,000 and 1:500) and nuclear staining with Hoescht (1 mg/mL). Ventricular sections were visualized with a Zeiss spinning-disk confocal microscope at 50% laser power, with exposure time kept constant between conditions. Z-stack images were taken at 100% laser power for 3D reconstruction using Imaris (version 7.5.0; Bitplane). Background signals outside of nuclei were masked, and only signals within the nucleus were counted using the Imaris “Spots” function. A coefficient was calculated as the number of dots or signal per nuclear volume.
Discussion
During pathological cardiac remodeling, including DCM, disruptions in various signaling cascades have been reported, including AKT, ERKs, GSK3, JAKs, MAPKs, PI3K, PKA, PKC, and TGF-β. Activation or inhibition of signaling pathways can ultimately result in changes to cardiomyocyte cell growth, differentiation, proliferation, and/or survival (27). However, despite advances in understanding the molecular basis of cardiac disease, a clear global picture of system-wide signaling network perturbations does not exist for most conditions, including DCM. To address this gap, we applied quantitative precision mass spectrometry to glean the complex interplay of signaling events associated early during disease progression with a genetic cause of cardiac HF.
One-third of all cardiac proteins are known or predicted to be phosphorylated. Here, we mapped 7,589 phosphopeptides on 1,848 cardiac proteins, of which 211 were differentially abundant in the R9C model, providing unprecedented insights into the global dysregulation of signaling cascades in early-stage DCM. Many sites we identified are novel and of unknown biological significance. Although annotated repositories of experimental phosphoproteomic datasets have been established (e.g., PhosphoSitePlus) (28), establishing relevance to heart biology and CVD is not straightforward. Our present study aimed to first identify significantly altered individual phosphosites and then use the corresponding associated protein/gene annotations in conjunction with information gained from global proteomic analysis. This design was motivated by computational tools for signaling network analysis and visualization, which historically focused on gene expression datasets rather than individual phosphosites.
One especially intriguing finding was identification of Notch-1 signaling as prominently altered in DCM. The Notch pathway is a widely studied system crucial to “cell-fate” determination (29). Mammals express four Notch receptors (Notch-1 to Notch-4) and five ligands (DLL1/3/4, JAGG1/2) (30). Notch-1 is expressed across a broad variety of cell types, including cardiomyocytes. All members of the Notch family are translated as single chains but are subsequently processed in the Golgi system to produce extracellular and transmembrane subunits that remain bound together at the plasma membrane by noncovalent bonds. Generally, ligand binding triggers the selective cleavage and removal of the extracellular subunit by members of the ADAM protease family, followed by cleavage of the membrane-bound intracellular subunit by γ-secretase to release N1ICD, which translocates to the nucleus to modulate transcription (Fig. 4). However, this oversimplification does not reflect cell context, as different ligands can act as Notch inhibitors whereas processing enzymes can oppose Notch activation (29).
Changes in phosphorylation of Notch signaling components included an uncharacterized phosphorylation site on the polyubiquitin protein UBB required for homeostatic recycling of Notch-1, which is up-regulated in R9C mice (31), as well as members of the histone deacetylase family (HDAC4/7/9) and others (32). However, due to incomplete functional annotation, our cautious conclusion was that the Notch-1 pathway was disrupted but not the precise nature of the perturbation (i.e., activation/inhibition).
We verified changes in Notch-1 signaling by immunoblotting and immunofluorescence. We demonstrated down-regulation of Notch-1 and DLL1 in R9C. DLL3, a Notch-1 ligand previously shown to be a cis-acting inhibitor (33), was up-regulated along with NUMB, whose association with Notch-1 leads to proteasomal degradation (34). ADAM9 and TACE/ADAM17, normally necessary for activation of Notch-1, were also up-regulated in R9C. However, excessive expression of these proteases has also been shown to inhibit Notch signaling, whereas deficiency of a major associated inhibitor, TIMP3, leads to dilated cardiomyopathy in mice (35). Furthermore, expression and nuclear localization of the N1ICD domain of Notch-1 were significantly greater in wild-type cardiomyocytes compared with R9C. Together, these results show that Notch-1 signaling is down-regulated in myocardium in early-stage DCM.
Our findings are consistent with previous reports of development of DCM in vitro and in newborn mice treated with an inhibitor of Notch-1–dependent signaling (36), or more pronounced cardiac dysfunction, fibrosis, and apoptosis in adult hearts from an agonist-induced hypertrophy model or Notch-1 cardiac-specific null mice (37). Similarly, transgenic mice overexpressing the Notch ligand JAGGED1 were protected from pressure overload-induced cardiac hypertrophy (38).
Considering these unprecedented phosphoproteomics data, it is clear that a more complete understanding of Notch-1 signaling and other potentially relevant pathways in adult heart following injury, including in human patients, is warranted (39). Indeed, uncoupling of Notch-1 signaling in R9C (Fig. 4) suggests that Notch-1–activating compounds (i.e., activating antibodies, polypeptides, small molecules) may be clinically beneficial under DCM conditions, similar to that observed in hypertrophy (38) and potentially other pathological contexts.
Materials and Methods
Transgenic mice carrying the R9C mutation in the PLN gene were previously described (2) and experiments were approved by the Animal Use Committee at the University of Toronto. Workflows for heart sample preparation, processing, and (phospho)proteomic profiling, including chromatographic fractionation, MS data generation, phosphosite localization and scoring, statistical enrichment analyses, motif prediction, Western blotting, and immunofluorescence, are detailed in SI Materials and Methods and Table S1.
Supplementary Material
Acknowledgments
We thank Paul Taylor, Peter Backx, Gary Bader, Michael Moran, and David H. MacLennan for valuable advice. This project was funded by the Heart and Stroke Foundation of Ontario (T-6281; to A.O.G.), Canadian Institutes of Health Research (MOP-106538; to A.O.G.), Ontario Research Fund (Global Leadership in Genomics and Life Sciences Grant GL2-01012), and Heart and Stroke Richard Lewar Centre (A.O.G. and A.E.). A.O.G. is a Canada Research Chair in Cardiovascular Proteomics and Molecular Therapeutics. A.E. is the Ontario Research Chair in Biomarkers of Disease Management. U.K. received postdoctoral fellowship support from the Ted Rogers Centre for Heart Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: All mass spectrometry results reported in this paper have been uploaded to PRIDE, https://www.ebi.ac.uk/pride/archive/ (accession no. PXD004810). PRIDE is a public data repository for proteomics data, including protein and peptide identifications, posttranslational modifications, and supporting spectral evidence. PRIDE is a core member in the ProteomeXchange (PX) consortium.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606444113/-/DCSupplemental.
References
- 1.Mozaffarian D, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Schmitt JP, et al. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003;299(5611):1410–1413. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- 3.MacLennan DH, Kranias EG. Phospholamban: A crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4(7):566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 4.Pan Y, et al. Identification of biochemical adaptations in hyper- or hypocontractile hearts from phospholamban mutant mice by expression proteomics. Proc Natl Acad Sci USA. 2004;101(8):2241–2246. doi: 10.1073/pnas.0308174101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gramolini AO, et al. Comparative proteomics profiling of a phospholamban mutant mouse model of dilated cardiomyopathy reveals progressive intracellular stress responses. Mol Cell Proteomics. 2008;7(3):519–533. doi: 10.1074/mcp.M700245-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Isserlin R, et al. Systems analysis reveals down-regulation of a network of pro-survival miRNAs drives the apoptotic response in dilated cardiomyopathy. Mol Biosyst. 2015;11(1):239–251. doi: 10.1039/c4mb00265b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engholm-Keller K, Larsen MR. Technologies and challenges in large-scale phosphoproteomics. Proteomics. 2013;13(6):910–931. doi: 10.1002/pmic.201200484. [DOI] [PubMed] [Google Scholar]
- 8.Lundby A, et al. In vivo phosphoproteomics analysis reveals the cardiac targets of β-adrenergic receptor signaling. Sci Signal. 2013;6(278):rs11. doi: 10.1126/scisignal.2003506. [DOI] [PubMed] [Google Scholar]
- 9.Scholten A, et al. Phosphoproteomics study based on in vivo inhibition reveals sites of calmodulin-dependent protein kinase II regulation in the heart. J Am Heart Assoc. 2013;2(4):e000318. doi: 10.1161/JAHA.113.000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huttlin EL, et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143(7):1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma P, et al. Evolutionarily conserved intercalated disc protein Tmem65 regulates cardiac conduction and connexin 43 function. Nat Commun. 2015;6:8391. doi: 10.1038/ncomms9391. [DOI] [PubMed] [Google Scholar]
- 12.Terrin A, et al. PKA and PDE4D3 anchoring to AKAP9 provides distinct regulation of cAMP signals at the centrosome. J Cell Biol. 2012;198(4):607–621. doi: 10.1083/jcb.201201059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vary TC, Lang CH. Differential phosphorylation of translation initiation regulators 4EBP1, S6k1, and Erk 1/2 following inhibition of alcohol metabolism in mouse heart. Cardiovasc Toxicol. 2008;8(1):23–32. doi: 10.1007/s12012-008-9012-4. [DOI] [PubMed] [Google Scholar]
- 14.Venkatesan B, et al. EMMPRIN activates multiple transcription factors in cardiomyocytes, and induces interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB andMKK7/JNK/AP-1 signaling. J Mol Cell Cardiol. 2010;49(4):655–663. doi: 10.1016/j.yjmcc.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bousset K, Henriksson M, Lüscher-Firzlaff JM, Litchfield DW, Lüscher B. Identification of casein kinase II phosphorylation sites in Max: Effects on DNA-binding kinetics of Max homo- and Myc/Max heterodimers. Oncogene. 1993;8(12):3211–3220. [PubMed] [Google Scholar]
- 16.Gupta MP, Amin CS, Gupta M, Hay N, Zak R. Transcription enhancer factor 1 interacts with a basic helix-loop-helix zipper protein, Max, for positive regulation of cardiac alpha-myosin heavy-chain gene expression. Mol Cell Biol. 1997;17(7):3924–3936. doi: 10.1128/mcb.17.7.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karin M, Gallagher E. From JNK to pay dirt: Jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57(4–5):283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Lin JL, Chan SY, Lin JJ. The Xin repeat-containing protein, mXinβ, initiates the maturation of the intercalated discs during postnatal heart development. Dev Biol. 2013;374(2):264–280. doi: 10.1016/j.ydbio.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou MF, Schwartz D. 2011. Using the scan-x web site to predict protein post-translational modifications. Curr Protoc Bioinformatics Chapter 13:Unit 13.16.
- 20.Keshava Prasad TS, et al. Human Protein Reference Database—2009 update. Nucleic Acids Res. 2009;37(Database issue):D767–D772. doi: 10.1093/nar/gkn892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y. Mitogen-activated protein kinases in heart development and diseases. Circulation. 2007;116(12):1413–1423. doi: 10.1161/CIRCULATIONAHA.106.679589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juhaszova M, et al. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res. 2009;104(11):1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isserlin R, Merico D, Voisin V, Bader GD. Enrichment Map—A Cytoscape app to visualize and explore OMICs pathway enrichment results. F1000Res. 2014;3:141. doi: 10.12688/f1000research.4536.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke MA, et al. Molecular profiling of dilated cardiomyopathy that progresses to heart failure. JCI Insight. 2016;1(6):e86898. doi: 10.1172/jci.insight.86898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou S, et al. SKIP, a CBF1-associated protein, interacts with the ankyrin repeat domain of NotchIC to facilitate NotchIC function. Mol Cell Biol. 2000;20(7):2400–2410. doi: 10.1128/mcb.20.7.2400-2410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Espinosa L, Santos S, Inglés-Esteve J, Muñoz-Canoves P, Bigas A. p65-NFkappaB synergizes with Notch to activate transcription by triggering cytoplasmic translocation of the nuclear receptor corepressor N-CoR. J Cell Sci. 2002;115(Pt 6):1295–1303. doi: 10.1242/jcs.115.6.1295. [DOI] [PubMed] [Google Scholar]
- 27.Sun Z, Hamilton KL, Reardon KF. Phosphoproteomics and molecular cardiology: Techniques, applications and challenges. J Mol Cell Cardiol. 2012;53(3):354–368. doi: 10.1016/j.yjmcc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Ren J, et al. Computational analysis of phosphoproteomics: Progresses and perspectives. Curr Protein Pept Sci. 2011;12(7):591–601. doi: 10.2174/1389203711109070591. [DOI] [PubMed] [Google Scholar]
- 29.Morrisey EE. Weary of the stress: Time to put another notch in cardiomyopathy. Circ Res. 2010;106(7):1187–1189. doi: 10.1161/CIRCRESAHA.110.218974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizzo P, et al. The role of Notch in the cardiovascular system: Potential adverse effects of investigational Notch inhibitors. Front Oncol. 2015;4:384. doi: 10.3389/fonc.2014.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryu HW, Park CW, Ryu KY. Disruption of polyubiquitin gene Ubb causes dysregulation of neural stem cell differentiation with premature gliogenesis. Sci Rep. 2014;4:7026. doi: 10.1038/srep07026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Y, Boucher JM, Liaw L. Histone deacetylase activity selectively regulates Notch-mediated smooth muscle differentiation in human vascular cells. J Am Heart Assoc. 2012;1(3):e000901. doi: 10.1161/JAHA.112.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman G, Sparrow DB, Kremmer E, Dunwoodie SL. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Hum Mol Genet. 2011;20(5):905–916. doi: 10.1093/hmg/ddq529. [DOI] [PubMed] [Google Scholar]
- 34.Wu M, Li J. Numb family proteins: Novel players in cardiac morphogenesis and cardiac progenitor cell differentiation. Biomol Concepts. 2015;6(2):137–148. doi: 10.1515/bmc-2015-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fedak PW, et al. Altered expression of disintegrin metalloproteinases and their inhibitor in human dilated cardiomyopathy. Circulation. 2006;113(2):238–245. doi: 10.1161/CIRCULATIONAHA.105.571414. [DOI] [PubMed] [Google Scholar]
- 36.Urbanek K, et al. Inhibition of Notch1-dependent cardiomyogenesis leads to a dilated myopathy in the neonatal heart. Circ Res. 2010;107(3):429–441. doi: 10.1161/CIRCRESAHA.110.218487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Croquelois A, et al. Control of the adaptive response of the heart to stress via the Notch1 receptor pathway. J Exp Med. 2008;205(13):3173–3185. doi: 10.1084/jem.20081427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metrich M, et al. Jagged1 intracellular domain-mediated inhibition of Notch1 signalling regulates cardiac homeostasis in the postnatal heart. Cardiovasc Res. 2015;108(1):74–86. doi: 10.1093/cvr/cvv209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gude N, Sussman M. Notch signaling and cardiac repair. J Mol Cell Cardiol. 2012;52(6):1226–1232. doi: 10.1016/j.yjmcc.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Amato G, Luxán G, de la Pompa JL. Notch signalling in ventricular chamber development and cardiomyopathy. FEBS J. 2016 doi: 10.1111/febs.13773. [DOI] [PubMed] [Google Scholar]
- 41.Dvorakova MC, Kruzliak P, Rabkin SW. Role of neuropeptides in cardiomyopathies. Peptides. 2014;61:1–6. doi: 10.1016/j.peptides.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Zaidi S, et al. De novo mutations in histone-modifying genes in congenital heart disease. Nature. 2013;498(7453):220–223. doi: 10.1038/nature12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cordero-Reyes AM, et al. Full expression of cardiomyopathy is partly dependent on B-cells: A pathway that involves cytokine activation, immunoglobulin deposition, and activation of apoptosis. J Am Heart Assoc. 2016;5(1):e002484. doi: 10.1161/JAHA.115.002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofmann U, Frantz S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res. 2015;116(2):354–367. doi: 10.1161/CIRCRESAHA.116.304072. [DOI] [PubMed] [Google Scholar]
- 45.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51(4):600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finck BN. The PPAR regulatory system in cardiac physiology and disease. Cardiovasc Res. 2007;73(2):269–277. doi: 10.1016/j.cardiores.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 47.Vallejo JG. Role of Toll-like receptors in cardiovascular diseases. Clin Sci (Lond) 2011;121(1):1–10. doi: 10.1042/CS20100539. [DOI] [PubMed] [Google Scholar]
- 48.Dawson K, Aflaki M, Nattel S. Role of the Wnt-Frizzled system in cardiac pathophysiology: A rapidly developing, poorly understood area with enormous potential. J Physiol. 2013;591(6):1409–1432. doi: 10.1113/jphysiol.2012.235382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beausoleil SA, Villén J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol. 2006;24(10):1285–1292. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]
- 50.Chawade A, Alexandersson E, Levander F. Normalyzer: A tool for rapid evaluation of normalization methods for omics data sets. J Proteome Res. 2014;13(6):3114–3120. doi: 10.1021/pr401264n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz D, Chou MF, Church GM. Predicting protein post-translational modifications using meta-analysis of proteome scale data sets. Mol Cell Proteomics. 2009;8(2):365–379. doi: 10.1074/mcp.M800332-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.