Significance
Transport of macromolecules through nanometer-sized membrane pores is a ubiquitous theme in cell biology. Examples include the linear import of precurser proteins into mitochondria and DNA transport during bacterial gene transfer. However, little is known about the biophysical mechanisms that bias the direction of macromolecular movement within membrane pores. Here, we used a single-molecule approach for studying a key step of bacterial gene transfer, the import of macromolecular DNA from the environment into the periplasm of the bacterial pathogen Neisseria gonorrhoeae. We show that the force-dependent kinetics of DNA uptake are in remarkable agreement with a translocation ratchet model, whereby the periplasmic ComE protein acts as a chaperone that rectifies DNA diffusion through the outer membrane by reversible binding.
Keywords: molecular motor, translocation ratchet, bacterial transformation, gene transfer
Abstract
Horizontal gene transfer can speed up adaptive evolution and support chromosomal DNA repair. A particularly widespread mechanism of gene transfer is transformation. The initial step to transformation, namely the uptake of DNA from the environment, is supported by the type IV pilus system in most species. However, the molecular mechanism of DNA uptake remains elusive. Here, we used single-molecule techniques for characterizing the force-dependent velocity of DNA uptake by Neisseria gonorrhoeae. We found that the DNA uptake velocity depends on the concentration of the periplasmic DNA-binding protein ComE, indicating that ComE is directly involved in the uptake process. The velocity–force relation of DNA uptake is in very good agreement with a translocation ratchet model where binding of chaperones in the periplasm biases DNA diffusion through a membrane pore in the direction of uptake. The model yields a speed of DNA uptake of 900 bp⋅s−1 and a reversal force of 17 pN. Moreover, by comparing the velocity–force relation of DNA uptake and type IV pilus retraction, we can exclude pilus retraction as a mechanism for DNA uptake. In conclusion, our data strongly support the model of a translocation ratchet with ComE acting as a ratcheting chaperone.
The question of how polymers translocate across membranes is ubiquitous in cell biology. For example, precursor proteins are transported from the cytoplasm into mitochondria or the endoplasmic reticulum (1, 2). Furthermore, packaging and ejection of DNA into and out of viral capsids involve the translocation of DNA through narrow pores (3, 4). During horizontal gene transfer, DNA travels through narrow constrictions within the bacterial cell envelope (5, 6).
Various molecular models have been proposed to understand how directional movement of polymers through nanoscopic pores is generated (2). They include cyclic molecular motors that bind to and transport the translocating polymer via repeated conformational changes driven by ATP hydrolysis. The translocation ratchet model has been proposed by Peskin et al. (7) and Simon et al. (8). In this concept, the polymer diffuses within a membrane pore. Chemical asymmetries can bias the Brownian walk of the chain. Experimental examples for such asymmetries include compaction of DNA during DNA injection into host cells by the Agrobacterium tumefaciens type 4 secretion system (9) and calcium-induced folding of proteins exported by the type 1 secretion system of Bordetella pertussis (10). A conceptually simple mechanism for ratcheting would be the existence of molecules (chaperones) binding to the polymer only on one side of the membrane; they hinder backward diffusion and thus bias the polymer translocation. The model proposed by Peskin et al. (7) predicts a velocity vs. force relationship for this ratcheting mechanism that has not been tested experimentally so far.
DNA translocation across the cell envelope is crucial for bacterial transformation (11). Transformation is the import and inheritable integration of DNA from the environment. A large number of bacterial species are naturally competent for transformation (12). Although the genes essential for transformation are well described for various species, little is known about the molecular mechanism driving DNA import (6, 13). The transformation system shares several structural and functional features with the type 4 pilus system (T4PS) and the type 2 secretion system (T2SS) (14) (Fig. S1). The only known exception is Helicobacter pylori; it has adapted a type 4 secretion system (T4SS) for DNA uptake (15). DNA uptake by Gram-negative Neisseria gonorrhoeae requires the proteins known to be necessary for biogenesis of T4P (Fig. S1). They include the major pilin subunit (PilE) that polymerizes to form pili (16). In the outer membrane PilQ proteins form the secretin pore for the pilus (17). Both proteins are essential for DNA uptake (17). The secretin assembly has DNA-binding properties and is part of a complex that spans the outer and inner membranes (18, 19). In the cytoplasm, the ATPase PilF is required for pilus biogenesis (20). In addition to the proteins required for T4P assembly, various additional proteins are essential for DNA import. In the cytoplasm the pilus retraction ATPase PilT is necessary for transformation (21). Within the periplasm, the DNA-binding protein ComE is essential for DNA uptake (22). In the absence of transforming DNA, ComE is distributed homogeneously within the periplasm and rapidly colocalizes with imported DNA (23, 24). ComE governs the carrying capacity of the periplasm for DNA in a gene-dosage–dependent way (23). In N. gonorrhoeae and other competent species, transport through the outer membrane is uncoupled from transport through the inner membrane (25–27). Single-stranded DNA is transported through a pore formed by ComA through the inner membrane (28). The probability of DNA uptake is strongly enhanced by the presence of the DNA uptake sequence (DUS) (29). This 12-bp-long sequence occurs at high frequency on the gonococcal genome, conveying species-specific recognition of DNA. Double-stranded but not single-stranded DUS enhances the probability of DNA uptake (30). The minor pilin ComP is responsible for binding the DUS and DNA import is strongly inhibited when comP is deleted (31, 32). PilV has an antagonistic character, and its deletion increases the probability for DNA binding and uptake (33).
Fig. S1.
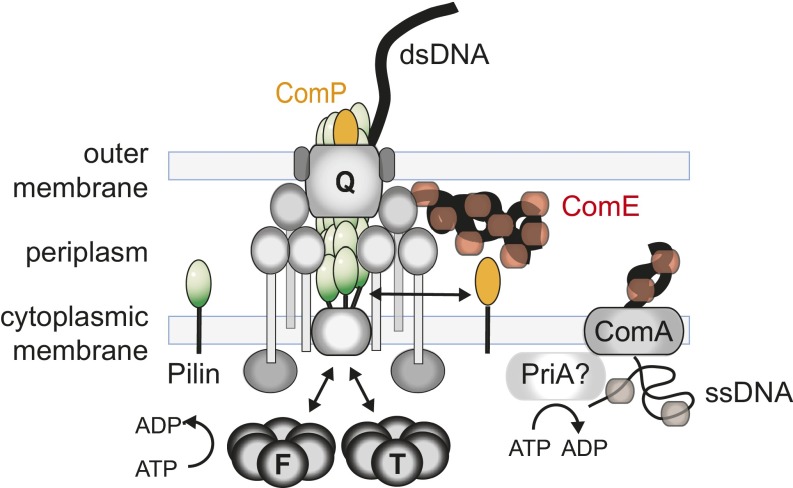
Putative molecular picture of DNA uptake machine. The proteins forming the T4P system are required for DNA uptake. They include the major pilin subunits PilE (green), the cytoplasmic ATPases PilF (F) and PilT (T), the PilQ proteins forming the outer membrane export/putative DNA import channel (Q), and various structural proteins depicted in gray. The minor pilin ComP and the periplasmic protein ComE are required for DNA uptake into the periplasm. The inner membrane pore or channel formed by ComA is essential for transport from the periplasm to the cytoplasm by an unknown mechanism. Only a single DNA strand enters the cytoplasm.
Previously, we have shown that a strong molecular machine drives uptake of DNA in Gram-positive Bacillus subtilis. B. subtilis shares most of the genes essential for T4P assembly with N. gonorrhoeae, but it lacks an outer membrane. DNA uptake proceeded at a velocity of ∼80 bp⋅s−1 up to external forces of 50 pN (34). In contrast, DNA uptake was reversible at forces of ∼20 pN in the Gram-negative H. pylori (26). The velocity of DNA uptake, however, was considerably faster with 1.3 kbp⋅s−1 at 10 pN. H. pylori has adapted a type IV secretion system for DNA uptake (15). Therefore, characterizing the biophysical properties of the T4P/T2SS-based DNA uptake system in a Gram-negative species will close an important gap of knowledge.
We hypothesized that DNA is imported into the periplasm through a translocation ratchet mechanism based on the following data: (i) Uptake of DNA into the periplasm decouples from uptake into the cytoplasm (23). Because ATP is not available in the periplasm, it is unlikely that an ATP-dependent motor drives DNA uptake. Similarly, no ion gradient is maintained over the outer membrane. (ii) The periplasmic DNA-binding protein ComE is essential for DNA import into the periplasm (22, 23) and depicts an ideal candidate for biasing the direction of DNA movement through the membrane. (iii) Single-cell studies of DNA uptake show that the secondary structure of DNA has only a minor effect on uptake kinetics (30), arguing against a machine that requires a tight fit on its substrate. Here, we characterized the velocity–force relation of single-DNA import and found that it is consistent with the model of a translocation ratchet. Moreover, we show that reducing the concentration of ComE reduces the speed of DNA uptake, in agreement with its role as a ratcheting chaperone.
Results
DNA Binding and Uptake.
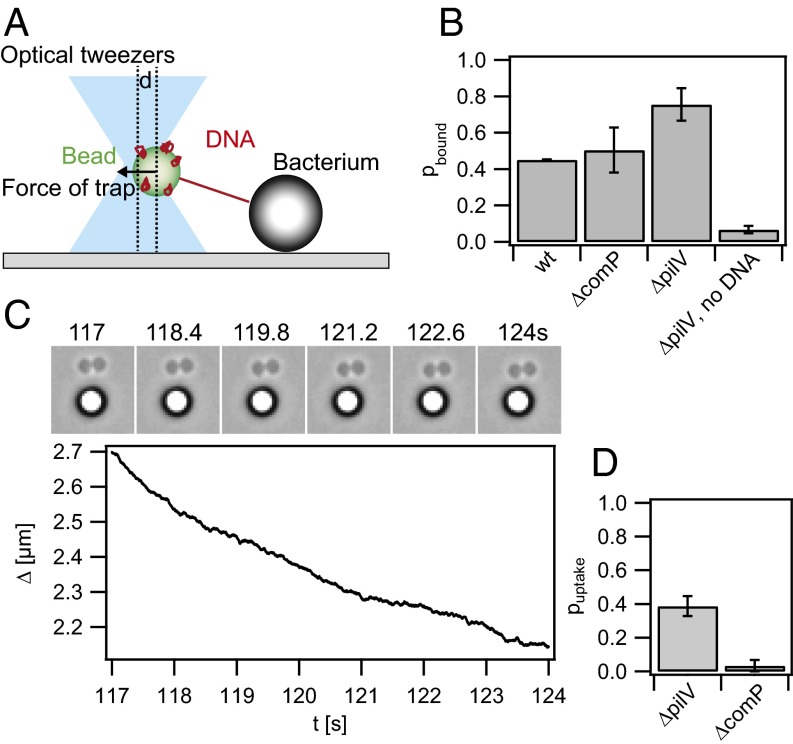
We aimed at quantifying the velocity of DNA uptake at the single-molecule level. To this end, we generated 10-kbp DNA fragments that contained the 12-bp DUS (29) at one extremity and multiple biotin tags at the other end (Fig. S2). Streptavidin-coated beads were incubated with the modified DNA as described in Materials and Methods. In the first step, we determined the binding probability of DNA to gonococci. To this end, a DNA-coated bead was trapped in the optical trap and placed in close proximity to a diplococcus. After about 30 s, the trapped bead was moved away from the cell in 100-nm steps to test for deflection of the bead and thus binding (Fig. 1A). A binding event was defined as a deflection of the bead from the center of the trap exceeding 20 pN. Roughly half of the attempts with WT cells resulted in DNA binding (Fig. 1B). In all of our strains (Table S1) recA expression is repressed to inhibit gene conversion in the pilin locus (35). Using uncoated beads that were treated like DNA-coated beads but without adding DNA, only 7% of the attempts resulted in binding, indicating that binding was mostly specific to DNA. Next, we tested whether the presence of the minor pilin ComP affected the probability of binding. ComP was shown to bind specifically to the DUS (31) but its abundance within the pilus is low compared with the major pilin PilE. With our assay we found no significant difference in binding probability between a comP deletion strain and the WT (Fig. 1B), in agreement with mostly unspecific binding of DNA to either T4P or the cell surface in general (32). Finally, we investigated the binding probability to gonococci that had the gene for the minor pilin pilV deleted. pilV deletion is known to increase the binding and uptake probability (33). With this strain, the binding probability was significantly higher than for WT (Fig. 1B), suggesting that specific binding occurs more frequently.
Fig. S2.
DNA construct. The DNA fragment attached to the beads is a 10-kbp PCR derivative of λ DNA. On one end three biotin residues are introduced as part of the PCR primer, one covalently attached to the 5′ end and two more covalently attached to thymines within the primer sequence. On the opposite end a DUS is introduced to face away from the bead and to be recognized for DNA uptake.
Fig. 1.
Probability of DNA binding and uptake. (A) Scheme of the experimental setup. A bead coated with DNA is trapped in an optical trap and placed close to a bacterium. (B) A binding event is defined as a deflection of the bead from the center of the trap exceeding 20 pN while the bead was moved away from the bacterium. Shown are binding probabilities of WT (Ng003), ΔcomP (Ng031), ΔpilV (Ng005), and ΔpilV (Ng005) with plain beads. (C) Typical DNA uptake event at F = 4 pN. (C, Upper) Time lapse; (C, Lower) distance Δ between bacterium and bead as a function of time. (D) Probability of DNA uptake subsequent to binding with ΔpilV and ΔcomP.
Table S1.
Strains
| Strain | Relevant genotype | Source | |
| N400 (Ng003) | recA6ind(tetM) | (20, 54) | |
| GV1 (Ng005) | recA6ind(tetM) pilVfs (G-1) | (55) | |
| MW104 (Ng031) | comP::m-tn3 erm recA6ind(tetM) | (56) | |
| ΔcomE234ΔpilV (Ng052) | comE4::Kan | This work | |
| comE3::Clm | This work | ||
| comE2::Erm | This work | ||
| recA6ind(tetM) pilVfs | This work | ||
| GV1 ΔcomA (Ng054) | recA6ind(tetM) pilVfs (G-1) comA | (23) |
For the following reasons, the ΔpilV strain was used to study DNA uptake. First, the binding probability is higher than for the WT, which did not show comP-dependent binding in our tests. Second, it is known that the DNA uptake efficiency is drastically enhanced (33) and therefore the success rate of the single-molecule experiment is much higher. Third, pilV deletion strongly inhibits surface motility compared with WT, which substantially benefits the assay. Finally, the binding and retraction probabilities of T4P to the beads are strongly reduced. WT gonococci would bind to and retract beads at a frequency of ∼1 s−1 (36), interfering with the quantification of DNA uptake.
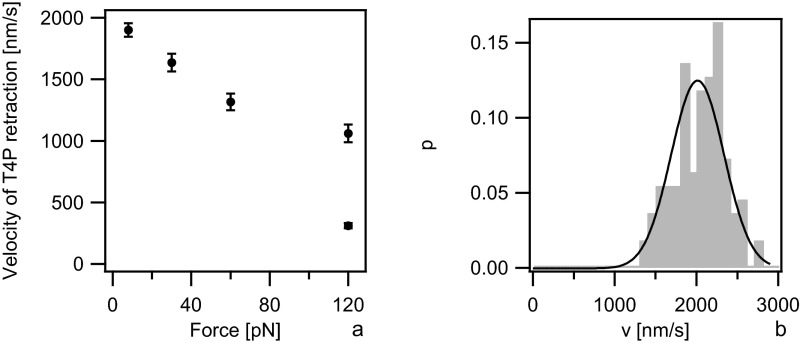
We detected DNA uptake as follows. A DNA-coated bead was trapped in the laser trap. Subsequently, binding was tested as described above. If the deflected bead was at a distance sufficient for observing DNA uptake, the measurement was started at a force of 10 pN as determined by four-quadrant photodiode (QPD) detection. If necessary, the force was reduced to trigger DNA uptake. If DNA uptake could not be directly started, attempts to extract DNA possibly already imported into the cell were made by applying higher forces (DNA extraction is described in detail in Fig. 3). If extraction was successful, the force was decreased once more to allow for reuptake of the extracted DNA. A typical DNA uptake event at an external force of F = 4 pN is shown in Fig. 1C. While DNA is imported, the length of the tether between the bead and the bacterium, Δ, shortens continuously. DNA uptake can be clearly distinguished from T4P retraction events because the speed is significantly slower. The speed of WT T4P retraction was v(8 pN) = (2,050 ± 30) nm/s (Fig. S3A) (36, 37). To ensure that deletion of pilV did not affect the speed of T4P retraction, we characterized T4P retraction in the ΔpilV strain and found vΔpilV(8 pN) = (2,010 ± 30) nm/s (Fig. S3B) in agreement with the WT data.
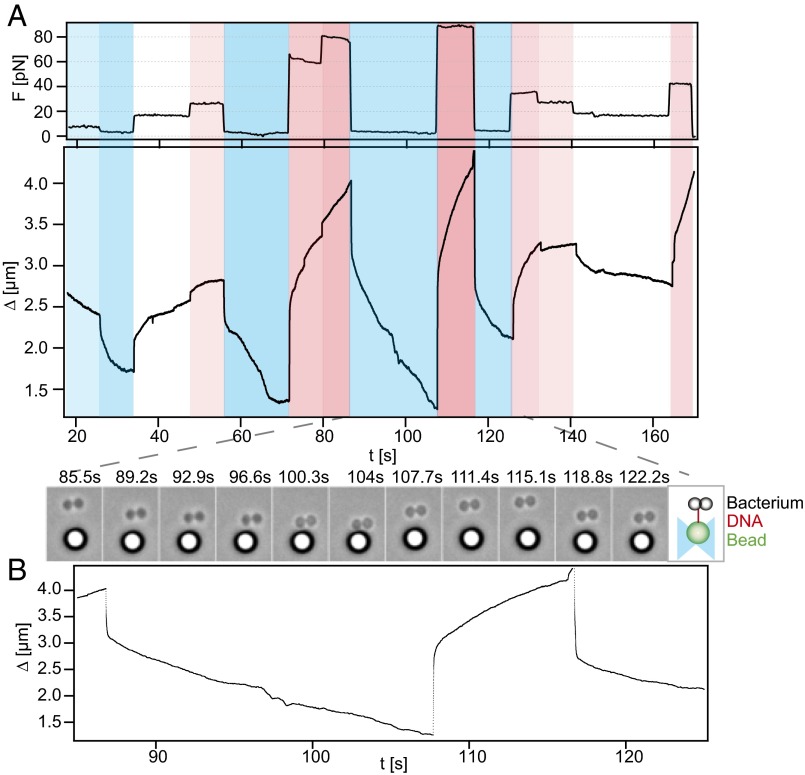
Fig. 3.
DNA uptake is reversible upon application of force. Typical time series of DNA uptake and extractions (Ng005) are shown. (A, Upper) Force; (A, Lower) distance between bead and bacterium Δ as a function of time. For presentation, the data were down-sampled to 1 Hz. Colors guide the eye. Events marked in blue are retraction, and those in red are extraction. More opaque colors signify stronger forces. At Δ < 1.3 µm, tracking was not reliable because bacterium and bead were in contact. (B, Lower) Zoom-in to the distance between bead and bacterium. (B, Upper) Corresponding time-lapse microscopic images.
Fig. S3.
Velocity vs. force relationship of T4P retraction. The speed of T4P retraction was determined by placing uncoated beads close to a bacterium at a force clamped to different values. Pili bound to the bead and the speed was measured during retraction as described in ref. 57. (A) Average speed of T4P retraction at different forces of WT gonococci. Data are taken from ref. 36. (B) Distribution of T4P retraction speeds at F = 8 pN for ΔpilV (Ng005).
As a control showing that the DNA uptake events observed were specific to the gonococcal DNA uptake system, we attempted to observe DNA-tether shortening in a ΔcomP strain. Deletion of comP has been shown to inhibit DNA uptake (32). To this end, a DNA-coated bead was trapped in the optical trap and placed adjacent to a diplococcus, followed by the procedure to establish DNA uptake described above. For each change of force, the bead was monitored for a sufficient amount of time to decide whether DNA uptake might have started. Whereas the probability that DNA binding resulted in uptake was pΔpilV = (0.39 ± 0.06) for the ΔpilV strain (Fig. 1D), the probability for the ΔcomP strain was pΔpilV = (0.03 ± 0.03), i.e., in agreement with full inhibition of DNA uptake.
The beads were coated with multiple DNA molecules to increase the probability of detecting a DNA-uptake event. Even so, only a fraction of pDNAup = 0.39 of the binding events resulted in DNA uptake. The probability that two DNA molecules were taken up simultaneously would be (pDNAup · pDNAbind)2 = 0.09. Here, the binding probability is most likely overestimated, because transient binding events were included in Fig. 1B, but were not quantified for determining the DNA uptake probability (Fig. 1D). Even if two uptake events had started within a short period, it would have been very unlikely that both uptake events were detected because the DNA tethers would have different lengths and only the shortest tether would register. We conclude that less than 10% of the analyzed DNA uptake events were caused by the import of more than one DNA molecule and that these events were very unlikely to affect our data analysis in a significant way.
In summary, we were able to characterize the kinetics of gonococcal DNA uptake during transformation as a function of force at the level of single DNA molecules.
The Velocity of DNA Uptake Depends on the Periplasmic DNA-Binding Protein ComE.
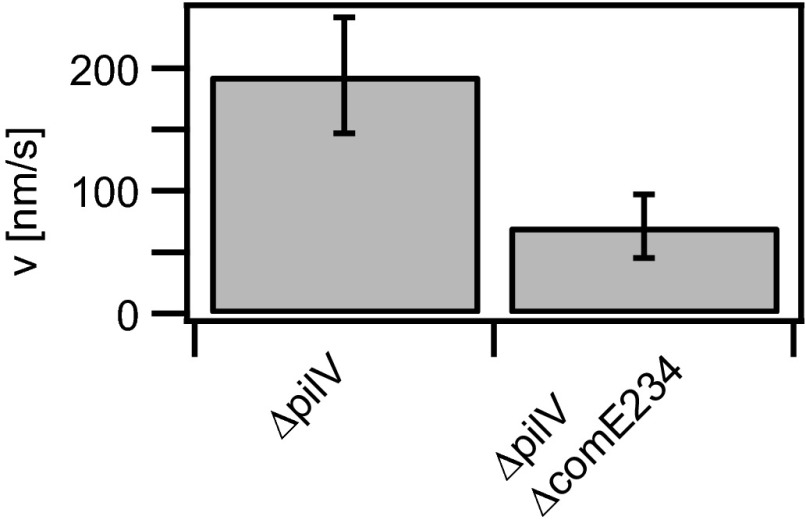
The periplasmic DNA-binding protein ComE is necessary for DNA import into the periplasm and for transformation (22). If ComE acted as a chaperone, biasing diffusion of DNA by binding, then its concentration would be expected to affect the translocation time and speed (38). We tested whether the concentration of ComE affected the speed of DNA import by deleting three of the four identical copies of comE generating ΔpilV ΔcomE234. In bulk experiments, the ΔcomE234 strain showed severe reduction of DNA uptake and transformation efficiencies and of ComE concentration, but their levels were above background levels (22). We found that the speed at F = 4 pN was reduced from v = (194 ± 47) nm/s in the ΔpilV strain to v = (71 ± 26) nm/s in the ΔpilV ΔcomE234 strain (Fig. 2). We conclude that the concentration of ComE affects the speed of DNA import.
Fig. 2.
The uptake velocity depends on the concentration of ComE. Shown is average speed of DNA uptake for ΔpilV (Ng005, n = 22) and ΔpilV ΔcomE234 (Ng052, n = 10) at F = 4 pN.
Gonococcal DNA Uptake Is Reversible at High Force.
DNA uptake by the Gram-positive B. subtilis using the T4P system is irreversible up to high forces exceeding F = 50 pN (34). On the other hand, DNA uptake by Gram-negative H. pylori using the T4S system is reversible at F = 23 pN (26). Here, we tested whether DNA uptake in Gram-negative N. gonorrhoeae using the T4P system is reversible. To this end, we used the force-clamp mode for changing the external force during a DNA uptake event (Fig. 3). We found that DNA previously imported at low force could be extracted by increasing the force. Fig. 3A shows a typical trace of the length change Δ of the DNA tether between the bacterium and the bead. While the force was kept constant, there was little variation in the speed of DNA uptake or extraction. Upon changing the force, jumps in the tether length were observed; these rapid length changes can be assigned to the elastic properties of DNA (Fig. 3B). Multiple rounds of import and extractions were observed.
We note that we observed rare events of continuous reduction of DNA uptake speed (Fig. 3A, t ∼ 30 s) or abrupt stalling of DNA uptake (Fig. 3A, t ∼ 70 s). There are various explanations for this behavior. When the distance fell below Δ < 1.3 µm, then bead and bacterium were in close contact and the data were dismissed from further analysis. We have shown previously that the periplasm is saturable with 40 kbp DNA and that ComK governs the carrying capacity in a gene-dosage–dependent fashion (23). With our 10-kbp DNA substrate we do not expect to saturate the periplasm. However, we cannot rule out that free DNA (whose concentration we reduced to a minimal level by extensive washing) is taken up and causes saturation occasionally.
In conclusion, DNA uptake in N. gonorrhoeae is reversible under application of external force.
The Velocity vs. Force Relationship Is in Agreement with a Translocation Ratchet Model.
Conceptually, the simplest mechanism for translocation of a DNA molecule through a pore is the translocation ratchet (see Fig. 5A) (8). The idea behind this model is that DNA diffuses through the pore in the outer membrane. The diffusion process is rectified by binding of proteins (chaperones) that are present in the periplasm but not within the extracellular space. In this scenario, the movement is generated by Brownian motion and the energy required for biasing the direction is provided by the binding energy of the chaperones. In the imperfect translocation ratchet model proposed by Peskin et al. (7), a rod diffusing in the pore is considered with a diffusion coefficient D. The rod carries ratchet sites with a spacing a between two sites for chaperones. A ratchet site can freely cross the origin from the extracellular side to the periplasm, but it is reflected when it attempts to cross the origin from the periplasm to the extracellular space provided that a chaperone is bound, with a probability p. This probability is related to the dissociation constant For K > 0, the direction of DNA translocation is reversible when the extracellular force is sufficiently high. With increasing K, the directional bias (and thus the DNA uptake speed) is expected to decrease. The model describes the velocity–force relation of polymer translocation (7) (Supporting Information, SI Description of Translocation Ratchet Model)
| [1] |
where
Fig. 5.
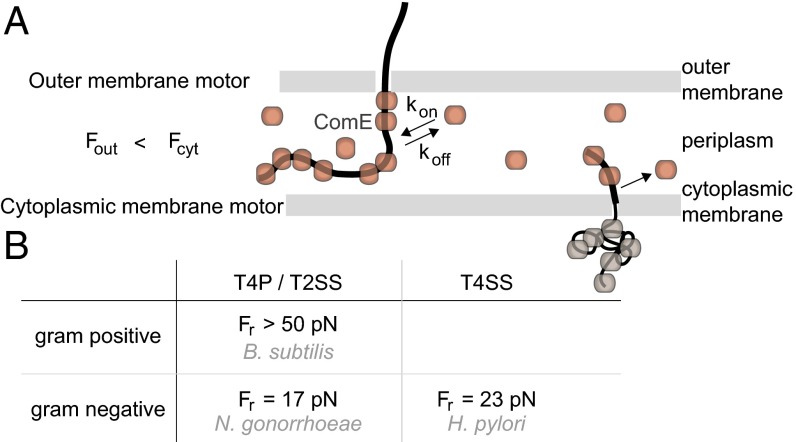
Force generation by cytoplasmic and outer membrane motors. (A) Hypothetical model for DNA transport through the Gram-negative cell envelope. A translocation ratchet drives uptake of DNA from the environment into the periplasm by reversible ComE binding. For transport across the inner membrane to occur, ComE must unbind. B. subtilis data suggest that in agreement with this prerequisite, the force generated by the cytoplasmic motor is considerably larger. (B) Reversal forces for B. subtilis (34), N. gonorrhoeae, and H. pylori (26). T4P/T2SS, type 4 pilus/type 2 secretion system; T4SS, type 4 secretion system.
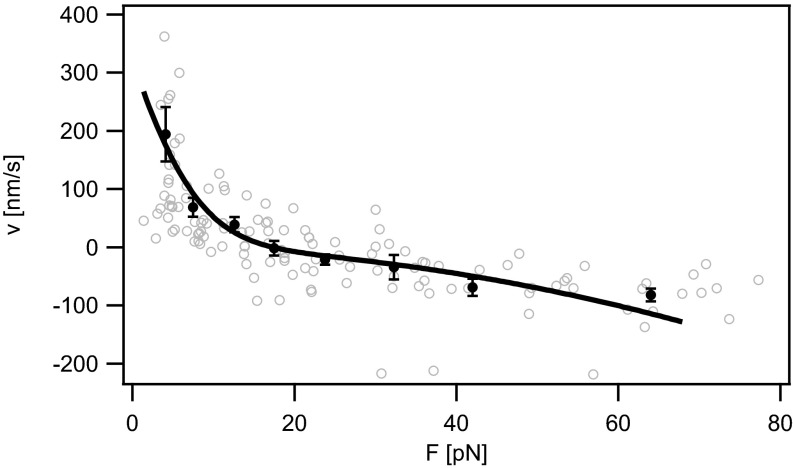
We tested this velocity vs. force relation by plotting velocity data (obtained from data shown in Fig. 3 and other DNA uptake events) as a function of the external force (Fig. 4). The fit to Eq. 1 was restricted to the region F < 65 pN, because we expect the transition of the secondary structure of DNA around F ∼ 65 pN (39). Eq. 1 describes the velocity–force relation of DNA uptake in N. gonorrhoeae well with a = (1.6 ± 0.3) nm, D = (250 ± 100) nm2⋅s−1, K = 0.0012 ± 0.0008.
Fig. 4.
Velocity vs. force relationship of DNA uptake (Ng005). Shaded open circles, raw data; solid circles, data binned over 6–25 data points. Error bars: SEM. Solid line: fit to Eq. 1 with a = (1.6 ± 0.3) nm, D = (250 ± 100) nm2⋅s−1, K = 0.0012 ± 0.0008.
Using these fit parameters, we can estimate the speed v0 at which the DNA would be taken up in the absence of external force applied by the laser tweezers, i.e., F = 0 (7) (Supporting Information, SI Description of Translocation Ratchet Model). Without external force, Moreover, we obtain the reversal force Fr, where, on average, the system switches from uptake to extraction
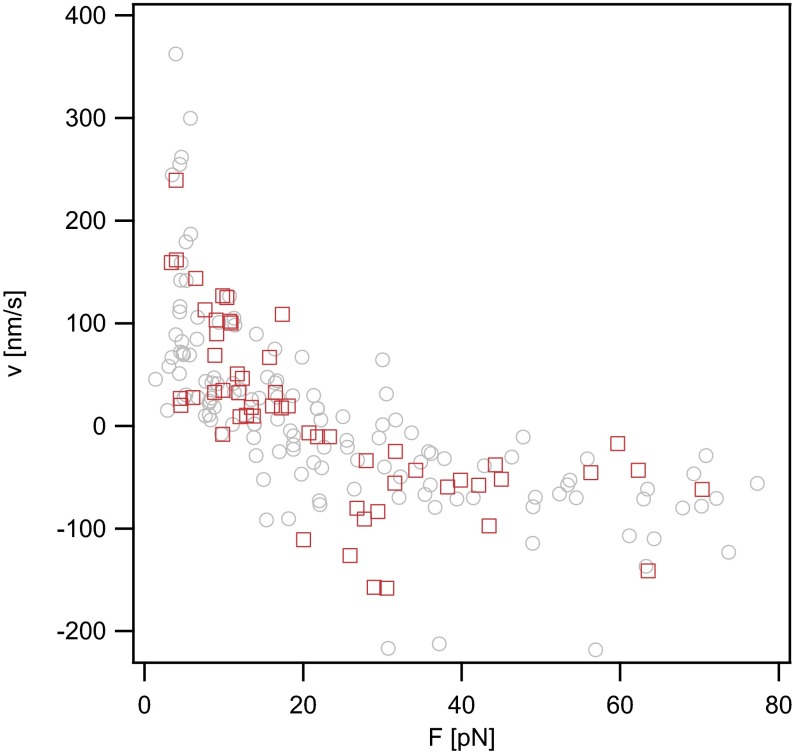
To confirm that the kinetics of DNA uptake observed in this study were independent of transport through the cytoplasmic membrane, we repeated the experiment using a comA deletion strain. ComA is essential for transport of DNA through the cytoplasmic membrane but does not affect DNA uptake into the periplasm (28). We found that the force-dependent velocity was independent of comA (Fig. S4), indicating that we observed transport of DNA through the outer membrane with our assay.
Fig. S4.
Velocity vs. force relationship of DNA uptake for ΔcomA. Gray open circles, ΔpilV recAind (Ng005); red open squares, ΔcomA ΔpilV recAind (Ng054).
To summarize, the velocity vs. force relation of DNA is in very good agreement with the model of the translocation ratchet.
Discussion
The Force-Dependent Velocity Is Consistent with a Translocation Ratchet Driven by ComE.
By measuring the velocity of DNA length change at varying force we found that DNA import into the periplasm of N. gonorrhoeae is reversible. The v(F) relation is in very good agreement with the relation predicted for an imperfect translocation ratchet mechanism (7). As there is no ATP in the bacterial periplasm and no ion motive force is maintained across the outer membrane, various other mechanisms could contribute to rectifying thermal motion of a polymer through a pore, including binding and dissociation of chaperones, chain coiling, or cross-linking (8). Our data are consistent with ComE acting as a chaperone for the following reasons. We have shown that the ComE concentration affects the translocation speed. Reduction of the translocation speed with decreased concentration of chaperones is in agreement with Langevin dynamics simulations of chaperone-assisted polymer translocation (38). Moreover, previous reports have shown that ComE binds DNA and is essential for DNA uptake (22). In the absence of periplasmic DNA it is homogeneously distributed within the periplasm and relocates to the incoming DNA within minutes (23). The binding energy of ComE to DNA most likely provides the fuel for biasing diffusion of DNA through the secretin in the outer membrane. Considering the low dissociation constant of K = 0.0012 provokes the question of how ComE is recycled from the periplasmic DNA. ComE bound to periplasmic DNA is likely to dissociate with time, because DNA is either transported into the cytoplasm or degraded by the thermonuclease Nuc (30). These processes can release ComE, making it available for driving import of new DNA. Thus, previous reports together with our present findings indicate that ComE is an important chaperone, ratcheting DNA from the environment to the periplasm during gonococcal transformation.
The model assumes a constant length a between two binding sites (7). ComE binds to double-stranded DNA without apparent sequence specificity (22). Thus, we expect that the size of ComE exceeds the distance between two possible binding sites, namely the distance between two base pairs (0.34 nm). Therefore, the lower limit would be given by the size of the protein. The structure of the ComE homolog of Thermus thermophilus HB8, ComEA, shows a protein diameter of ∼2 nm (https://www.ncbi.nlm.nih.gov/Structure/mmdb/mmdbsrv.cgi?uid=52976). Thus, the value of a = 1.6 nm obtained from the fit to our data is a reasonable value. The low dissociation constant K = 0.0012 indicates that ComK has a high affinity to DNA in the periplasm. This observation is in good agreement with recent cell biological experiments showing that ComE forms foci at the entry site of transforming DNA (23). Various other parameters are expected to affect the free energy barrier for DNA translocation. For example, the change of chain entropy can play a role during the translocation process (40). However, because multiple (∼70) binding sites for the ratcheting protein are present on a single Kuhn segment, we expect that this effect is negligible. The fact that the simple translocation ratchet model proposed by Peskin et al. (7) fits our data very well further indicates that molecular crowding within the periplasm (41) is most likely negligible. Different theoretical approaches to molecular motor modeling have been developed (42). Inserting mutations in ComE would allow for testing whether the model of the imperfect translocation ratchet used to describe the data in this study applies over a range of binding energies or whether other (more sophisticated) molecular motor models must be employed.
Type IV Pilus Retraction Does Not Directly Drive DNA Import.
Genes that are essential for T4P biogenesis and retraction are also essential for DNA uptake (11). The mechanistic role of the T4P proteins in DNA uptake remains unclear. Two major scenarios have been proposed. First, the T4P “fishes” for DNA at the extracellular side and by retraction through depolymerization, it takes the DNA along into the periplasm (5, 6, 43). Extended competence-associated T4P polymers have been found in Streptococcus pneumoniae and Vibrio cholerae but not in B. subtilis (27, 44, 45). A second scenario assumes the formation of an alternative DNA-uptake complex by T4P proteins.
By comparing the force-dependent kinetics of DNA uptake characterized in this study with the kinetics of T4P retraction, we can exclude T4P retraction as a mechanism for DNA uptake. If DNA was brought into the periplasm along with the retracting pilus, then we would expect that the velocity–force relations of T4P retraction and DNA uptake were comparable. Single T4P retract at a speed of v ∼ 2 µm⋅s−1 at F = 8 pN and the maximum force of T4P retraction exceeds 100 pN (46) (Fig. S3). The characteristic length of a T4P is ∼1 µm (47). Therefore, if DNA was transported by binding to retracting T4P, we would expect to find periods of time during which DNA uptake proceeds at v ∼ 2 µm⋅s−1 followed by pauses. This result is in disagreement with our experimental observations.
Our data are consistent with a role of T4P proteins in forming a specific DNA uptake complex that allows DNA to diffuse through the outer membrane into the periplasm where the diffusion is biased by ComE. The role of the T4P retraction ATPase may be in remodeling T4P to DNA uptake complexes. Alternatively, the T4P may be essential for opening the outer membrane secretin pore formed by PilQ (19, 48), to bind DNA at the extracellular side and enable threading into the pore.
DNA Uptake into the Periplasm Is Reversible for Gram-Negative Bacteria.
Transformation occurs in dissociable steps, including DNA binding, transport through the outer membrane, transport through the inner membrane, and homologous recombination (11). In the periplasm, DNA can be massed for extended periods of time (23, 26, 49). All of the data we have acquired in this study indicate that DNA uptake from the environment to the periplasm is powered by reversible binding of ComE (Fig. 5A). The next step of the transformation process is the transport of transforming DNA from the periplasm to the cytoplasm. Therefore, the associated cytoplasmic machine must work against the force generated by ComE binding in the periplasm. Importantly, we showed in a recent study that the DNA uptake machine of B. subtilis for cytoplasmic transport generates force exceeding F > 50 pN (34). Because the genes essential for DNA uptake in B. subtilis are homologous to N. gonorrhoeae, the cytoplasmic motor of N. gonorrhoeae most likely generates higher forces than the outer membrane motor.
Mechanistically, the proteins required for the transport through the cytoplasmic membrane have not been identified. It is conceivable that a translocation ratchet driven by DNA-binding proteins in the cytoplasm generates a force exceeding the force generated by ComE binding in the periplasm. Transforming DNA entering the cytoplasm is coated with various single-strand binding proteins that protect DNA from degradation and mediate homologous recombination with the chromosome (50). These proteins may fulfill the second purpose of biasing DNA translocation from the periplasm to the cytoplasm. In B. subtilis the DEAD-box helicase ComFA is important for DNA uptake (51). It is tempting to speculate that it performs a dual role in converting dsDNA into ssDNA and transporting ssDNA into the cytoplasm. A homolog of ComFA in N. gonorrhoeae is the primosome assembly protein (PriA) (52). PriA is central to the restart of chromosomal replication when replication fork progression is disrupted, is involved in homologous recombination and DNA repair, and is essential for transformation (53).
Interestingly, the proteins forming the pore for DNA translocation through the cytoplasmic membrane are conserved for N. gonorrhoeae (T4P/T2SS, Gram-negative), B. subtilis (T4P/T2SS, Gram-positive), and H. pylori (T4SS, Gram-negative) (12, 52). It is tempting to speculate that the kinetics and forces generated by the machine that drives import through the inner membrane are also conserved. For both Gram-negative systems studied so far, DNA uptake was reversible at forces in the range of F ∼ 20 pN (Fig. 5B). The speed of DNA uptake was ∼10-fold lower for N. gonorrhoeae at F ∼ 10 pN, suggesting that reversibility is a characteristic feature of uptake across the outer membrane and that the speed is determined by the specific DNA uptake system.
Conclusion
In this study we used single-molecule techniques for characterizing the speed of gonococcal DNA uptake during transformation as a function of the external force. Our data agree remarkably well with a basic translocation ratchet model with the periplasmic protein ComE as a chaperone. Thus, we provide a mechanistic model for a key step of bacterial gene transfer. Comparison with data from different species strongly suggests that DNA uptake through the outer membrane is driven by weak and reversible molecular motors whereas transport through the cytoplasmic membrane employs a strong and irreversible machine.
Materials and Methods
SI Materials and Methods contains details including bacterial strains and growth conditions, generation of bacterial strains, preparation of biotinylated DNA fragments, preparation of DNA-coated beads for optical tweezer assays, laser tweezers setup and data analysis, and data acquisition.
SI Materials and Methods
Bacterial Strains and Growth Conditions.
N. gonorrhoeae was grown at 37 °C and 5% CO2 on agar plates containing gonococcal base agar [10 g/L Bacto agar (BD Biosciences), 5 g/L NaCl (Roth), 4 g/L K2HPO4 (Roth), 1 g/L KH2PO4 (Roth), 15 g/L Bacto Proteose Peptone No. 3 (BD), 0.5 g/L soluble starch (Sigma-Aldrich)] and the following supplements: 1 g/L d-Glucose (Roth), 0.1 g/L l-glutamine (Roth), 0.289 g/L l-cysteine-HCL×H20 (Roth), 1 mg/L thiamine pyrophosphate (Sigma-Aldrich), 0.2 mg/L Fe(NO3)3 (Sigma-Aldrich), 0.03 mg/L thiamine HCl (Roth), 0.13 mg/L 4-aminobenzoic acid (Sigma-Aldrich), 2.5 mg/L β-nicotinamide adenine dinucleotide (Roth), and 0.1 mg/L vitamin B12 (Sigma-Aldrich).
Our bacterial strains were derived from MS11 (Table S1). Strain GV1 (Ng005) carried an IPTG (isopropyl β-d-1-thiogalactopyranoside)-inducible recAind copy (20, 54) and a frameshift mutation in the minor pilin pilV (55). RecA is essential for homologous recombination. It does not influence the rate of DNA uptake, but inhibits pilin antigenic variation. Deletion of pilV increases the amount of imported DNA. This strain was used for the experiments unless otherwise noted. Strain MW104 (Ng031) was generated from the recAind strain by transposon insertion into comP (56). Strain GV1 ΔcomA (Ng054) was generated by transformation of GV1 with chromosomal DNA from ΔcomA [derived by transformation of N400 with the ΔcomA allele originally detailed in Facius and Meyer (28)].
Generation of the ΔcomE234 Strain.
Genomic DNA of a complete knockout of all four comE copies in MS11 was kindly provided by Ines Chen, Public Health Research Institute, Newark, NJ. The strain is described in ref. 22 with different antibiotic markers. The genomic DNA was transformed into N400 and the cells were plated separately on the four different antibiotic markers: erythromycin for ΔcomE2, chloramphenicol for ΔcomE3, and kanamycin for ΔcomE4. Genomic DNA was isolated from ΔcomE2, ΔcomE3, and ΔcomE4 in the N400 background strains for further constructions of knockouts in GV1. For the construction of multiple comE knockouts, the genomic DNA of these single knockouts in GV1 was isolated. Then, a successive transformation of genomic DNA produced the following gradual knockouts: ΔcomE34 by transforming genomic DNA from ΔcomE3 into ΔcomE4 and ΔcomE234 by transforming ΔcomE2 into ΔcomE34. All single and gradual comE knockout strains were controlled via PCR for the correct genotype in all three comE loci after transformation and selection on the respective antibiotic markers.
Preparation of Biotinylated DNA Fragments.
A 10-kbp fragment from λ DNA (Invitrogen) was amplified by LongAmp polymerase (NEB), using primer 5′-ATGCCGTCTGAACAGGTGGTAAGCACTTCCTGCTC (Sigma-Aldrich) containing the DUS and primer 5′-[Btn]AAAA[BtndT]TT[BtndT]CCGGTTTAAGGCG TTTCCGTTCTTCTTCGTCATAAC (Sigma-Aldrich) containing three biotin modifications. The PCR sample was precipitated at room temperature (RT), using 0.1 vol 3 M sodium acetate, pH 5, and 0.7 vol isopropanol. After precipitation, the DNA was washed with 70% ethanol at RT and resuspended in buffer EB (Qiagen) to yield a concentration of 0.5–1 µg/µL. The DNA was stored in solution at 4 °C.
Preparation of DNA-Coated Beads for Optical Tweezer Assays.
A total of 40 µL of 2.0 µm streptavidin-coated polystyrene beads (Kisker) was washed with 400 µL DNA coupling buffer (0.1 M Na-PO4, pH 7.4, 150 mM NaCl, 1% BSA) three times. Subsequently, the washed beads were resuspended in 40 µL DNA coupling buffer and incubated with 2 µg of biotinylated DNA overnight at 4 °C head-over-tail. To remove unbound DNA, the DNA-coated beads were washed four times with PBS, pH 7.45 (tablets; Life Technologies) and stored head-over-tail at 4 °C.
Laser Tweezers Setup and Data Analysis.
The sample was mounted on a temperature-controlled microscope (37 °C) (Zeiss Axiovert 200). The optical tweezers setup was described before (57). In brief, positional data of the bead trapped in the laser beam were acquired at 20-kHz time resolution with a QPD. Positional data were verified by recording videos at 10 Hz and subsequent tracking of both bead and bacterium using a MatLab routine, using a Hough grid (58). Force feedback was activated after force reached a preprogrammed level, and the position of the piezo table was adjusted so that the force remained constant but controllable by the user. The trap stiffness was calibrated by the power spectrum analysis of the bead’s Brownian motion and verified by the viscous drag method. Video tracking was used to calculate the actual forces for the evaluation. Data from the stage movement were down-sampled to 20 data points per second by MatLab routines, leading to velocity values for 50-ms intervals after differentiation. Each data point in Fig. 4 depicts the average velocity between two consecutive changes of the external force illustrated in Fig. 3A. The error bars of the velocities in Figs. 2 and 4 are the SEs over N uptake events. Due to the bead radius of 1 µm, the bead has a velocity component in the vertical direction. As a consequence, the actual velocities depend on the length of the DNA tether between the bead and the bacterium and are slightly underestimated.
The probability of DNA binding was determined as follows. A DNA-coated bead was trapped in the optical trap and placed in close proximity to a diplococcus. After about 30 s, the trapped bead was moved away from the cell in 100-nm steps to test for deflection of the bead. A binding event was defined as a deflection of the bead from the center of the trap exceeding 20 pN. The fraction of events that resulted in bead deflection beyond 20 pN was determined on three different days and the mean and SD are shown in Fig. 1B.
The probability that DNA binding resulted in DNA uptake was determined as follows. A DNA-coated bead was trapped in the optical trap and placed adjacent to a diplococcus, followed by the procedure to establish DNA uptake described above. For each change of force, the bead was monitored for a sufficient amount of time to decide whether DNA uptake might have started. In total, k = 26 of n = 67 binding events resulted in DNA uptake for ΔpilV (Ng005) and kp = 1 of np = 30 binding events resulted in DNA uptake for ΔcomP (Ng031). The probability of DNA uptake was then calculated as p = k/n. Considering p as a point estimate, the error bars are the SEs assuming a binomial distribution of k.
Data Acquisition.
After 24 h of growth, 10 piliated colonies were picked and transferred to a new plate. After 16–20 h of growth, about five clones were picked and resuspended in 100 µL DNA uptake retraction assay medium (7 mM MgCl2, 0.4 mg/mL BSA). The DNA-coated beads were diluted 1:5 in PBS and 1 µL of beads was mixed with 20 µL of bacterial suspension and mounted on the optical tweezers setup. A bead was trapped in the optical tweezers and placed next to a diplococcus. After 10–30 s it was slowly removed from the cell in 100-nm steps. If a persistent deflection of the bead due to a binding event could be detected, the acquisition was started. Force-clamp mode at a starting force of 10 pN was triggered automatically, either instantly due to the initial deflection of the bead or eventually due to the increased deflection by moving the bead farther away from the cell. If DNA uptake could not be readily detected, the force was decreased stepwise down to 2 pN. If DNA uptake did not start upon decreasing the force, it was increased to extract DNA potentially already taken up into the periplasm. Once DNA could be successfully extracted, the force was decreased again to observe uptake. An attempt was ceased if extraction of DNA failed or the extracted DNA could not be reimported. During DNA uptake, the force was varied to observe DNA uptake or extraction velocity at different forces. DNA uptake at a constant force was monitored for up to 30 s, depending on a reasonable time frame for the observed velocity. Ideally, varying forces were applied such that they permitted multiple rounds of extraction and elongation of a single DNA fragment.
SI Description of Translocation Ratchet Model
The model yielding Eq. 1 is described in detail in Peskin et al. (7). In short, a rod diffusing along the x axis is considered with a diffusion coefficient D. The rod carries ratchet sites with a spacing a between two sites for chaperones. A ratchet site can freely cross the origin from the extracellular side to the periplasm, but it is reflected when it attempts to cross the origin from the periplasm to the extracellular space provided that a chaperone is bound, with a probability p. The variable x(t) = position of the first site within the periplasm, so that x(t) is always within (0, a) is introduced. c(x,t) is the density of the variable x(t). The flux of rods at point x is
| [S1] |
where F is the external force. In our experiments, F is applied by the optical tweezers at the extracellular side and therefore it always counteracts DNA uptake. The density and flux satisfy the conservation equation:
| [S2] |
The boundary conditions are as follows:
Please refer to ref. 7 for derivation of the boundary conditions. Considering only steady states, the flux J is constant. By solving Eq. S1 with the boundary conditions, the solution is (7)
| [S3] |
where a is the distance between two binding sites, D is the diffusion constant of DNA within the pore, and is the dissociation constant.
From the fit parameters obtained in Fig. 4, we can derive the speed at which translocation would proceed in the absence of external force. Setting the drift term in Eq. S1 and solving Eq. S1 with the boundary conditions described above, we obtain
Acknowledgments
We thank Michael Koomey and Ines Chen for the donation of bacterial strains, Lena Dewenter for experimental support, and Kerstin Stingl, Heike Gangel, Stephanie Müller, David Dubnau, Stefan Klumpp, and the B.M. laboratory for highly valuable discussions. This work was supported by the Deutsche Forschungsgemeinschaft through Grant MA3898.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608110113/-/DCSupplemental.
References
- 1.Neupert W, Brunner M. The protein import motor of mitochondria. Nat Rev Mol Cell Biol. 2002;3(8):555–565. doi: 10.1038/nrm878. [DOI] [PubMed] [Google Scholar]
- 2.Neupert W. A perspective on transport of proteins into mitochondria: A myriad of open questions. J Mol Biol. 2015;427(6 Pt A):1135–1158. doi: 10.1016/j.jmb.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, et al. A viral packaging motor varies its DNA rotation and step size to preserve subunit coordination as the capsid fills. Cell. 2014;157(3):702–713. doi: 10.1016/j.cell.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inamdar MM, Gelbart WM, Phillips R. Dynamics of DNA ejection from bacteriophage. Biophys J. 2006;91(2):411–420. doi: 10.1529/biophysj.105.070532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allemand JF, Maier B. Bacterial translocation motors investigated by single molecule techniques. FEMS Microbiol Rev. 2009;33(3):593–610. doi: 10.1111/j.1574-6976.2009.00166.x. [DOI] [PubMed] [Google Scholar]
- 6.Burton B, Dubnau D. Membrane-associated DNA transport machines. Cold Spring Harb Perspect Biol. 2010;2(7):a000406. doi: 10.1101/cshperspect.a000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peskin CS, Odell GM, Oster GF. Cellular motions and thermal fluctuations: The Brownian ratchet. Biophys J. 1993;65(1):316–324. doi: 10.1016/S0006-3495(93)81035-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon SM, Peskin CS, Oster GF. What drives the translocation of proteins? Proc Natl Acad Sci USA. 1992;89(9):3770–3774. doi: 10.1073/pnas.89.9.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grange W, et al. VirE2: A unique ssDNA-compacting molecular machine. PLoS Biol. 2008;6(2):e44. doi: 10.1371/journal.pbio.0060044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bumba L, et al. Calcium-driven folding of RTX domain β-rolls ratchets translocation of RTX proteins through type I secretion ducts. Mol Cell. 2016;62(1):47–62. doi: 10.1016/j.molcel.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Chen I, Dubnau D. DNA uptake during bacterial transformation. Nat Rev Microbiol. 2004;2(3):241–249. doi: 10.1038/nrmicro844. [DOI] [PubMed] [Google Scholar]
- 12.Johnston C, Martin B, Fichant G, Polard P, Claverys JP. Bacterial transformation: Distribution, shared mechanisms and divergent control. Nat Rev Microbiol. 2014;12(3):181–196. doi: 10.1038/nrmicro3199. [DOI] [PubMed] [Google Scholar]
- 13.Berry JL, Pelicic V. Exceptionally widespread nanomachines composed of type IV pilins: The prokaryotic Swiss Army knives. FEMS Microbiol Rev. 2015;39(1):134–154. doi: 10.1093/femsre/fuu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korotkov KV, Sandkvist M, Hol WG. The type II secretion system: Biogenesis, molecular architecture and mechanism. Nat Rev Microbiol. 2012;10(5):336–351. doi: 10.1038/nrmicro2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofreuter D, Odenbreit S, Haas R. Natural transformation competence in Helicobacter pylori is mediated by the basic components of a type IV secretion system. Mol Microbiol. 2001;41(2):379–391. doi: 10.1046/j.1365-2958.2001.02502.x. [DOI] [PubMed] [Google Scholar]
- 16.Long CD, Tobiason DM, Lazio MP, Kline KA, Seifert HS. Low-level pilin expression allows for substantial DNA transformation competence in Neisseria gonorrhoeae. Infect Immun. 2003;71(11):6279–6291. doi: 10.1128/IAI.71.11.6279-6291.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drake SL, Koomey M. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol Microbiol. 1995;18(5):975–986. doi: 10.1111/j.1365-2958.1995.18050975.x. [DOI] [PubMed] [Google Scholar]
- 18.Assalkhou R, et al. The outer membrane secretin PilQ from Neisseria meningitidis binds DNA. Microbiology. 2007;153(Pt 5):1593–1603. doi: 10.1099/mic.0.2006/004200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang YW, et al. Architecture of the type IVa pilus machine. Science. 2016;351(6278):aad2001. doi: 10.1126/science.aad2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freitag NE, Seifert HS, Koomey M. Characterization of the pilF-pilD pilus-assembly locus of Neisseria gonorrhoeae. Mol Microbiol. 1995;16(3):575–586. doi: 10.1111/j.1365-2958.1995.tb02420.x. [DOI] [PubMed] [Google Scholar]
- 21.Wolfgang M, et al. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol Microbiol. 1998;29(1):321–330. doi: 10.1046/j.1365-2958.1998.00935.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen I, Gotschlich EC. ComE, a competence protein from Neisseria gonorrhoeae with DNA-binding activity. J Bacteriol. 2001;183(10):3160–3168. doi: 10.1128/JB.183.10.3160-3168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gangel H, et al. Concerted spatio-temporal dynamics of imported DNA and ComE DNA uptake protein during gonococcal transformation. PLoS Pathog. 2014;10(4):e1004043. doi: 10.1371/journal.ppat.1004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seitz P, et al. ComEA is essential for the transfer of external DNA into the periplasm in naturally transformable Vibrio cholerae cells. PLoS Genet. 2014;10(1):e1004066. doi: 10.1371/journal.pgen.1004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Facius D, Fussenegger M, Meyer TF. Sequential action of factors involved in natural competence for transformation of Neisseria gonorrhoeae. FEMS Microbiol Lett. 1996;137(2–3):159–164. doi: 10.1111/j.1574-6968.1996.tb08099.x. [DOI] [PubMed] [Google Scholar]
- 26.Stingl K, Müller S, Scheidgen-Kleyboldt G, Clausen M, Maier B. Composite system mediates two-step DNA uptake into Helicobacter pylori. Proc Natl Acad Sci USA. 2010;107(3):1184–1189. doi: 10.1073/pnas.0909955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seitz P, Blokesch M. DNA-uptake machinery of naturally competent Vibrio cholerae. Proc Natl Acad Sci USA. 2013;110(44):17987–17992. doi: 10.1073/pnas.1315647110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Facius D, Meyer TF. A novel determinant (comA) essential for natural transformation competence in Neisseria gonorrhoeae and the effect of a comA defect on pilin variation. Mol Microbiol. 1993;10(4):699–712. doi: 10.1111/j.1365-2958.1993.tb00942.x. [DOI] [PubMed] [Google Scholar]
- 29.Frye SA, Nilsen M, Tønjum T, Ambur OH. Dialects of the DNA uptake sequence in Neisseriaceae. PLoS Genet. 2013;9(4):e1003458. doi: 10.1371/journal.pgen.1003458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hepp C, Gangel H, Henseler K, Günther N, Maier B. Single-stranded DNA uptake during gonococcal transformation. J Bacteriol. 2016;198(18):2515–2523. doi: 10.1128/JB.00464-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cehovin A, et al. Specific DNA recognition mediated by a type IV pilin. Proc Natl Acad Sci USA. 2013;110(8):3065–3070. doi: 10.1073/pnas.1218832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aas FE, et al. Competence for natural transformation in Neisseria gonorrhoeae: Components of DNA binding and uptake linked to type IV pilus expression. Mol Microbiol. 2002;46(3):749–760. doi: 10.1046/j.1365-2958.2002.03193.x. [DOI] [PubMed] [Google Scholar]
- 33.Aas FE, Løvold C, Koomey M. An inhibitor of DNA binding and uptake events dictates the proficiency of genetic transformation in Neisseria gonorrhoeae: Mechanism of action and links to type IV pilus expression. Mol Microbiol. 2002;46(5):1441–1450. doi: 10.1046/j.1365-2958.2002.03265.x. [DOI] [PubMed] [Google Scholar]
- 34.Maier B, Chen I, Dubnau D, Sheetz MP. DNA transport into Bacillus subtilis requires proton motive force to generate large molecular forces. Nat Struct Mol Biol. 2004;11(7):643–649. doi: 10.1038/nsmb783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rotman E, Seifert HS. The genetics of Neisseria species. Annu Rev Genet. 2014;48:405–431. doi: 10.1146/annurev-genet-120213-092007. [DOI] [PubMed] [Google Scholar]
- 36.Marathe R, et al. Bacterial twitching motility is coordinated by a two-dimensional tug-of-war with directional memory. Nat Commun. 2014;5:3759. doi: 10.1038/ncomms4759. [DOI] [PubMed] [Google Scholar]
- 37.Kurre R, Maier B. Oxygen depletion triggers switching between discrete speed modes of gonococcal type IV pili. Biophys J. 2012;102(11):2556–2563. doi: 10.1016/j.bpj.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu W, Luo K. Chaperone-assisted translocation of a polymer through a nanopore. J Am Chem Soc. 2011;133(34):13565–13570. doi: 10.1021/ja204892z. [DOI] [PubMed] [Google Scholar]
- 39.van Mameren J, et al. Unraveling the structure of DNA during overstretching by using multicolor, single-molecule fluorescence imaging. Proc Natl Acad Sci USA. 2009;106(43):18231–18236. doi: 10.1073/pnas.0904322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sung W, Park PJ. Polymer translocation through a pore in a membrane. Phys Rev Lett. 1996;77(4):783–786. doi: 10.1103/PhysRevLett.77.783. [DOI] [PubMed] [Google Scholar]
- 41.Gopinathan A, Kim YW. Polymer translocation in crowded environments. Phys Rev Lett. 2007;99(22):228106. doi: 10.1103/PhysRevLett.99.228106. [DOI] [PubMed] [Google Scholar]
- 42.Bressloff PC, Newby JM. Stochastic models of intracellular transport. Rev Mod Phys. 2013;85(1):135–196. [Google Scholar]
- 43.Matthey N, Blokesch M. The DNA-uptake process of naturally competent Vibrio cholerae. Trends Microbiol. 2016;24(2):98–110. doi: 10.1016/j.tim.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Laurenceau R, et al. A type IV pilus mediates DNA binding during natural transformation in Streptococcus pneumoniae. PLoS Pathog. 2013;9(6):e1003473. doi: 10.1371/journal.ppat.1003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen I, Provvedi R, Dubnau D. A macromolecular complex formed by a pilin-like protein in competent Bacillus subtilis. J Biol Chem. 2006;281(31):21720–21727. doi: 10.1074/jbc.M604071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maier B, Wong GC. How bacteria use type IV pili machinery on surfaces. Trends Microbiol. 2015;23(12):775–788. doi: 10.1016/j.tim.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Holz C, et al. Multiple pilus motors cooperate for persistent bacterial movement in two dimensions. Phys Rev Lett. 2010;104(17):178104. doi: 10.1103/PhysRevLett.104.178104. [DOI] [PubMed] [Google Scholar]
- 48.Gold VA, Salzer R, Averhoff B, Kühlbrandt W. Structure of a type IV pilus machinery in the open and closed state. eLife. 2015;4:07380. doi: 10.7554/eLife.07380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seitz P, Blokesch M. DNA transport across the outer and inner membranes of naturally transformable Vibrio cholerae is spatially but not temporally coupled. MBio. 2014;5(4):e01409–e01414. doi: 10.1128/mBio.01409-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Claverys JP, Martin B, Polard P. The genetic transformation machinery: Composition, localization, and mechanism. FEMS Microbiol Rev. 2009;33(3):643–656. doi: 10.1111/j.1574-6976.2009.00164.x. [DOI] [PubMed] [Google Scholar]
- 51.Londoño-Vallejo JA, Dubnau D. Mutation of the putative nucleotide binding site of the Bacillus subtilis membrane protein ComFA abolishes the uptake of DNA during transformation. J Bacteriol. 1994;176(15):4642–4645. doi: 10.1128/jb.176.15.4642-4645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krüger NJ, Stingl K. Two steps away from novelty--principles of bacterial DNA uptake. Mol Microbiol. 2011;80(4):860–867. doi: 10.1111/j.1365-2958.2011.07647.x. [DOI] [PubMed] [Google Scholar]
- 53.Kline KA, Seifert HS. Mutation of the priA gene of Neisseria gonorrhoeae affects DNA transformation and DNA repair. J Bacteriol. 2005;187(15):5347–5355. doi: 10.1128/JB.187.15.5347-5355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tønjum T, Freitag NE, Namork E, Koomey M. Identification and characterization of pilG, a highly conserved pilus-assembly gene in pathogenic Neisseria. Mol Microbiol. 1995;16(3):451–464. doi: 10.1111/j.1365-2958.1995.tb02410.x. [DOI] [PubMed] [Google Scholar]
- 55.Winther-Larsen HC, et al. Neisseria gonorrhoeae PilV, a type IV pilus-associated protein essential to human epithelial cell adherence. Proc Natl Acad Sci USA. 2001;98(26):15276–15281. doi: 10.1073/pnas.261574998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolfgang M, van Putten JP, Hayes SF, Koomey M. The comP locus of Neisseria gonorrhoeae encodes a type IV prepilin that is dispensable for pilus biogenesis but essential for natural transformation. Mol Microbiol. 1999;31(5):1345–1357. doi: 10.1046/j.1365-2958.1999.01269.x. [DOI] [PubMed] [Google Scholar]
- 57.Clausen M, Koomey M, Maier B. Dynamics of type IV pili is controlled by switching between multiple states. Biophys J. 2009;96(3):1169–1177. doi: 10.1016/j.bpj.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rizon M, et al. Object detection using circular Hough transform. Am J Appl Sci. 2005;2:1606–1609. [Google Scholar]