Significance
Prolactin is a hormone secreted by the pituitary gland that controls changes in the breast to enable milk production after the baby is born. In some mothers with pregnancy-related high blood pressure (BP), the concentration of prolactin in the blood is higher than normal, but whether this causes the high BP or is a consequence of it is uncertain. To answer this question, we have generated experimental mice that produce prolactin in the liver when we feed them a substance, indole-3-carbinol (IC3), that is found in broccoli. When fed normal chow, the mice are well, but, when fed IC3, they develop high BP and heart problems. This suggests that pregnant women with abnormally high prolactin levels may need special attention.
Keywords: hypertension, lactation, protein kinase B/Akt
Abstract
Increased levels of a cleaved form of prolactin (molecular weight 16 kDa) have been associated with preeclampsia. To study the effects of prolactin on blood pressure (BP), we generated male mice with a single-copy transgene (Tg; inserted into the hypoxanthine-guanine phosphoribosyltransferase locus) that enables inducible hepatic production of prolactin and its cleavage product. The Tg is driven by the indole-3-carbinol (I3C)-inducible rat cytochrome P450 1A1 promoter. When the Tg mice were fed normal chow (NC), plasma prolactin concentrations were comparable to those in female WT mice in the last third of pregnancy, and BP was lower than in WT mice (∼95 mm Hg vs. ∼105 mm Hg). When the Tg mice were fed chow containing IC3, plasma prolactin concentrations increased threefold, BP increased to ∼130 mm Hg, and cardiac function became markedly impaired. IC3 chow did not affect the WT mice. Urinary excretion of nitrite/nitrate and the amount of Ser1177-phosphorylated endothelial nitric oxide (NO) synthase (eNOS) were significantly greater in the Tg mice fed NC than in WT mice, as they are during pregnancy. However, when I3C was fed, these indicators of NO production became significantly less in the Tg mice than in WT mice. The effects of increased plasma prolactin were abolished by a genetic absence of eNOS. Thus, a threefold increase in plasma prolactin is sufficient to increase BP significantly and to markedly impair cardiac function, with effects mediated by NO produced by eNOS. We suggest that pregnant women with abnormally high prolactin levels may need special attention.
Prolactin, a 23-kDa polypeptide hormone, is a potent multifunctional cytokine with a broad range of biological effects, including water and salt balance, lactogenesis, cell proliferation and differentiation, testicular Leydig cell function, T-cell immunity, pancreatic β-cell function, hematopoiesis, and adipogenesis (1). Prolactin is physiologically secreted mainly from the anterior lobe of pituitary gland. The secretion is negatively regulated by dopamine and positively regulated by prolactin-releasing peptide (2) synthesized in the hypothalamus. The levels of prolactin in the serum, urine, and amniotic fluids are significantly higher in patients with preeclampsia than in subjects with normal pregnancy (3–5), suggesting that prolactin is involved in the pathogenesis of pregnancy-associated hypertension. However, animal experiments have shown inconsistent effects of prolactin on blood pressure (BP). Thus, acute i.v. infusion of prolactin increased BP in rabbits (6), but, when ovine prolactin was chronically given i.p. via an osmotic minipump in rats, BP was decreased (7). It has also been reported that chronic prolactin infusion caused an increase in urinary sodium, potassium, and water excretion, but no significant changes in arterial pressure, in rats (8).
To study the chronic effects of different plasma concentrations of prolactin on BP and general well-being, we have generated male mice with a single-copy transgene [Tg; inserted into the hypoxanthine-guanine phosphoribosyltransferase (Hprt) locus] that enables hepatic production of prolactin and its cleavage product when the mice are fed indole-3-carbinol (I3C), a xenobiotic agent naturally present at high levels in broccoli and similar vegetables. The Tg is leaky, and, when the mice are fed normal chow (NC), it leads to basal prolactin levels comparable to those in the plasma of WT female mice in the last third of a normal pregnancy, accompanied by a decrease in BP and an increase in nitric oxide (NO) production, likewise similar to those occurring in females during pregnancy. When the Tg mice are fed a diet containing IC3, plasma levels become threefold basal, BP increases, and cardiac insufficiency develops. In the absence of endothelial NO synthase (eNOS), these changes did not occur.
Results
Generation of Tg Mice Producing Prolactin in the Liver.
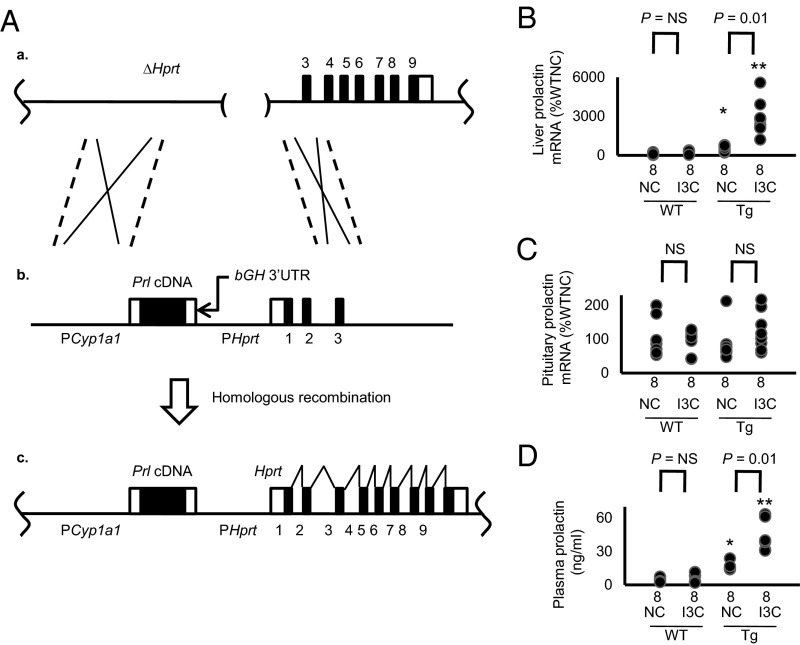
Gene targeting, as described previously (9), was used to insert a single-copy Tg into the Hprt locus of the X chromosome. The Tg is comprised of a copy of mouse cDNA for prolactin (Prl cDNA; Fig. 1A) driven by the rat cytochrome P450 1A1 promoter (PCyp1a1) (10), with the 3′-UTR of the bovine growth hormone gene (bGH) in place of its natural 3′-UTR. Male Tg mice fed NC have greater than WT expression of prolactin mRNA in the liver and a higher concentration of prolactin in the plasma (16.1 ± 1.1 ng/mL vs. 5.1 ± 0.6 ng/mL), indicating that the inducible Tg is leaky (Fig. 1 B and D). This basal plasma prolactin concentration is close to that we observe in female WT mice in the last third of pregnancy (15.3 ± 0.6 ng/mL). When the Tg mice were fed I3C, hepatic prolactin mRNA levels were markedly increased, and the plasma prolactin concentration became approximately three times basal (Fig. 1 B and D). The expression of prolactin in the pituitary gland was not significantly altered by the Tg or by the I3C diet (Fig. 1C).
Fig. 1.
Generation of drug-inducible hypermorphic mice for prolactin. (A) The gene-targeting strategy (*P < 0.05 and **P < 0.01 vs. WT; NS, not significantly different). (a) The target locus, into which the exogenous Tg is introduced by homologous recombination, is the mutated Hprt gene (ΔHprt) in the E14TG2a cells. (b) The targeting vector contains the rat Cyp1a1 promoter, mouse prolactin (Prl) cDNA, and the 3′-UTR of bGH. (c) The resulting locus after homologous recombination. The prolactin expression now is controlled by the Cyp1a1 promoter, which can be induced by I3C. Coding sequences of the prolactin gene are shown as black columns. (B) The mRNA levels of prolactin in the liver of WT and the Tg mice with and without I3C at the age of 12 wk. Coding sequences of the prolactin gene are shown as black columns. (C) The mRNA levels of prolactin in the pituitary gland are not changes by the Tg or by the administration of I3C. (D) Plasma levels of prolactin of WT and the Tg mice with and without I3C.
Opposite Changes in BP in the Tg Mice with Modestly and Substantially Increased Plasma Prolactin Levels.
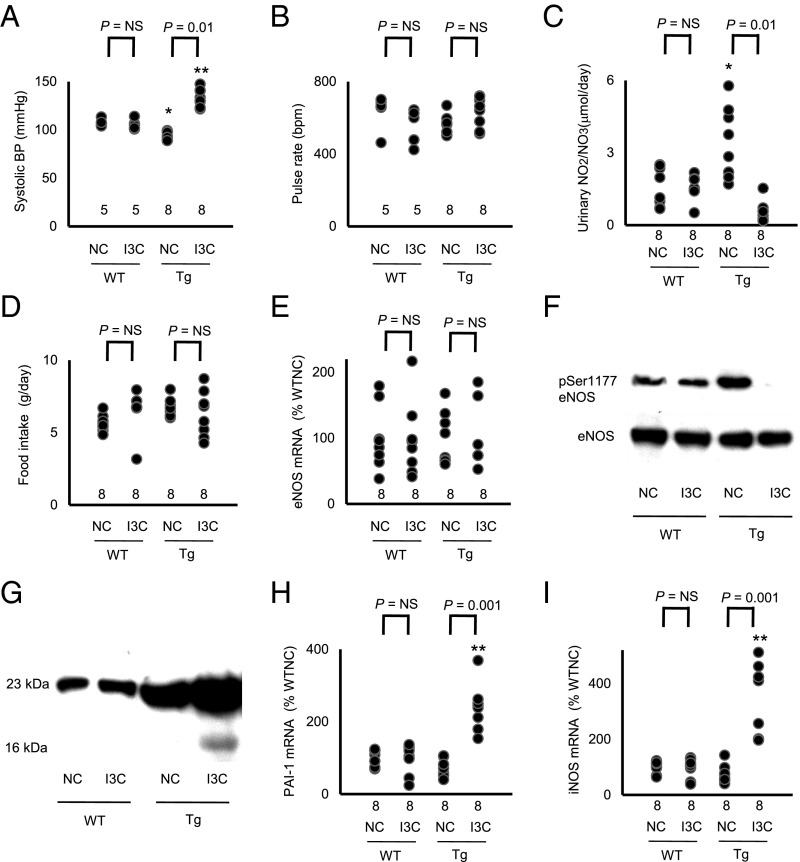
We found that the Tg mice fed NC had a significantly lower BP than WT mice fed NC [WT (n = 9), 108 ± 1 mm Hg vs. Tg (n = 11), 97 ± 3 mm Hg; P < 0.05; Fig. 2A and Fig. S1A], but the heart rate was not significantly different between Tg and WT [WT (n = 9), 635 ± 39 beats per minute (bpm) vs. Tg (n = 11), 561 ± 17 bpm; Fig. 2B and Fig. S1A].
Fig. 2.
Bidirectional change in arterial pressure by overexpression of prolactin is associated with changes in urinary excretion of nitrite/nitrate and in the expression of 16K prolactin and its downstream genes (*P < 0.05 and **P < 0.01 vs. WT; NS, not significantly different). (A) sBP in WT and prolactin Tg male mice on NC or on the I3C diet determined with a tail-cuff method. (B) Heart rate in WT and prolactin Tg mice fed NC or the I3C diet. (C) Urinary nitrite/nitrate excretion. (D) Food consumption. (E) Renal expression of eNOS in WT and the prolactin Tg mice without and with I3C administration. (F) Immunoblot for Ser1177-phosphorylated and total eNOS in the liver of WT and Tg mice without and with I3C administration. (G) Immunoblot for the full-length (23K) prolactin and 16K prolactin in the plasma of WT and the Tg mice without and with I3C administration. (H) Renal expression of PAI-1 in WT and prolactin Tg mice without and with I3C administration. (I) Renal expression of iNOS in WT and prolactin Tg mice without and with I3C administration.
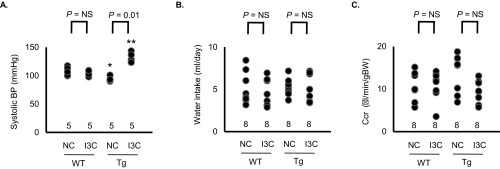
Fig. S1.
(A) sBP in WT and prolactin Tg female mice fed NC or I3C diet determined with a tail-cuff method (NS, not significantly different). (B) Water intake in WT and Tg male mice fed NC or I3C diet. (C) Creatinine clearance (Ccr) in WT and Tg mice fed NC or I3C diet.
When the Tg mice were fed chow containing 3.0% I3C (wt/wt), their BP was markedly higher than that in WT mice fed the I3C diet [WT (n = 9), 106 ± 2 mm Hg vs. Tg (n = 11), 132 ± 3 mm Hg; P < 0.01; Fig. 2A], but their heart rates were not significantly different [WT (n = 9), 555 ± 39 bpm vs. Tg (n = 11), 630 ± 25 bpm; Fig. 2B].
Urinary Excretion of Nitrite/Nitrate and Renal Expression of eNOS in the Tg Mice.
Renal excretion of nitrite/nitrate in the Tg mice fed NC was significantly higher than in WT mice fed NC [WT (n = 8), 1.60 ± 0.27 μmol/d vs. Tg (n = 8), 3.44 ± 0.49 μmol/d; P < 0.05; Fig. 2C]. However, when I3C was fed, the Tg mice had significantly less renal excretion of nitrite/nitrate than the WT mice [WT (n = 8), 1.55 ± 0.17 μmol/d vs. Tg (n = 8), 0.56 ± 0.15 μmol/d; P < 0.01; Fig. 2C].
Urinary nitrite/nitrate has both dietary and endogenous origins (10, 11). Because food consumption is not different in the WT and Tg mice with or without I3C (Fig. 2D), we conclude that the difference in urinary excretion of nitrite/nitrate is a result of differences in the endogenous production of NO. Water intake and creatinine clearance were not different among the 4 groups (Fig. S1 B and C).
Renal mRNA and protein levels of eNOS were not changed by the presence of the Tg or by the I3C diet (Fig. 2 E and F). However, the protein levels of Serine 1177-phosphorylated eNOS, which is the active form of eNOS, were increased in the Tg mice fed NC and decreased in the Tg mice fed the I3C diet (Fig. 2F).
Increased Expression of 16-kDa Prolactin in the Prolactin Tg Mice Fed I3C.
To determine total plasma prolactin (full-length plus 16 kDa), we performed an immunoblot with an antibody raised against the N-terminal peptide of prolactin. The result showed that total prolactin is greater in the Tg mice fed NC than in WT mice fed NC. The I3C diet increased total prolactin in the Tg mice but not in the WT mice (Fig. 2G). A faint band corresponding to the 16-kDa form of prolactin is visible in the plasma sample from the Tg mouse fed IC3, but whether this is because the cleaved form is now a greater proportion of the total or is just a reflection of the higher total amount is not clear.
This uncertainty was resolved by determining the renal expressions of genes for inducible NO synthase (iNOS) and plasminogen activator inhibitor-1 (PAI-1), both of which are induced by the 16-kDa prolactin but not by full-length prolactin (12–14). We found that the expressions of PAI-1 and iNOS were significantly increased only in the Tg mice fed IC3 (Fig. 2 H and I). We conclude that only the Tg mice fed IC3 express the 16-kDa fragment. Because it has been shown that the 16-kDa prolactin, but not the full-length protein, decreases the activity of eNOS (15, 16), this result suggests that 16-kDa prolactin plays a direct role in the increase in BP that occurs in the Tg mice fed IC3 by decreasing eNOS production of NO.
Deficiency of eNOS Abolishes the Effects of Prolactin on BP.
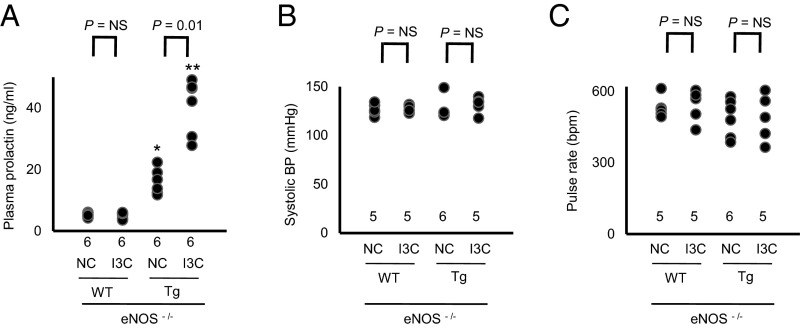
To study the role of eNOS in the regulation of BP by prolactin, we crossbred the prolactin Tg mice with mice deficient in eNOS (17). We found that the eNOS deficiency by itself did not significantly change plasma levels of prolactin (Fig. 3A), but the lack of eNOS abolished the differences in BP in the WT and Tg mice with and without I3C (Fig. 3 B and C).
Fig. 3.
Lack of eNOS abolishes the bidirectional change in arterial pressure by overexpression of prolactin (*P < 0.05 and **P < 0.01 vs. WT; NS, not significantly different). (A) Plasma levels of prolactin of eNOS-deficient prolactin WT mice and the eNOS-deficient prolactin Tg mice with and without I3C. (B) sBP in the eNOS-deficient prolactin WT mice and the eNOS-deficient prolactin Tg mice on NC or on the I3C diet. (C) Heart rate in the eNOS-deficient prolactin WT mice and the eNOS-deficient prolactin Tg mice on NC or on the I3C diet.
These findings show that eNOS plays a critical role in the decrease in BP observed in the Tg mice fed NC and the increase in BP seen in the Tg mice fed IC3.
Cardiac Dysfunction in the Mice with high Plasma Levels of Prolactin.
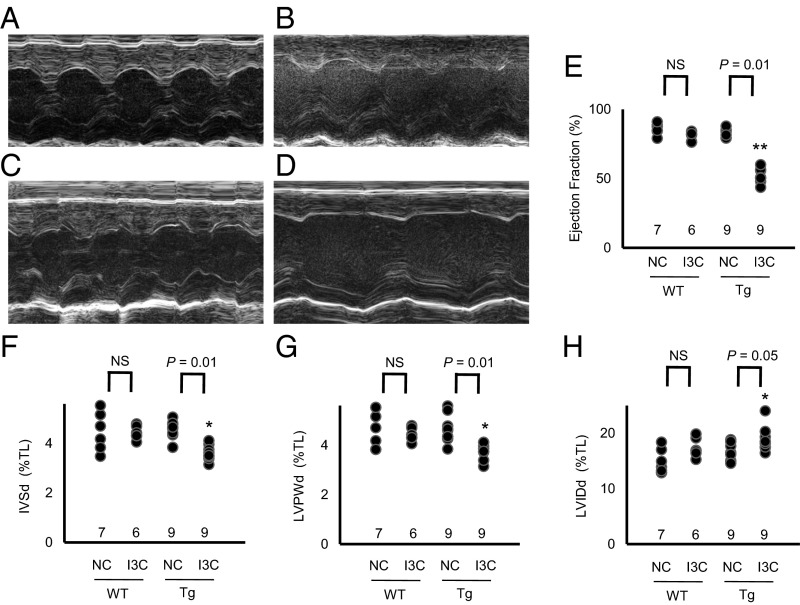
We used echocardiography.to determine the effects of the different levels of plasma prolactin on cardiac function and structure. We found that the ejection fraction of the left ventricle, an indicator of cardiac function, was decreased from ∼85% to ∼50% in the Tg mice fed IC3, but was not changed in the Tg mice fed NC (Fig. 4 A–E). The thicknesses of the intraventricular septum and the left ventricular posterior wall were also significantly reduced in the Tg mice fed I3C (Fig. 4 F and G). Thus, a threefold increase in plasma prolactin concentration is sufficient to severely impair cardiac function and structure.
Fig. 4.
Cardiac dysfunction by marked overexpression of prolactin (*P < 0.05 and **P < 0.01 vs. WT; NS, not significantly different; TL, tibia length). (A–D) M-mode echocardiography in WT and prolactin Tg mice with and without the I3C diet. (A) WT mice fed NC. (B) WT mice fed I3C diet. (C) Tg mice fed NC. (D) Tg mice fed I3C diet. (E) Ejection fraction of the left ventricle. (F) Thickness of the intraventricular septum in diastole (IVSd). (G) Thickness of the left ventricular posterior wall in diastole (LVPWd). (H) Left ventricular internal diameter in diastole (LVIDd).
Discussion
In the present study, we have generated male Tg mice that have a leaky prolactin Tg at the Hprt locus. The Tg is driven by a promoter that is inducible by feeding chow containing IC3. When the Tg mice are fed NC without IC3, their basal plasma prolactin concentration is approximately twice that of WT mice fed NC, their BP is approximately 10 mm Hg less than in WT mice, and their urinary excretion of nitrate/nitrite is approximately three times that of WT mice. When the Tg mice are fed chow containing IC3, their plasma prolactin concentration increases to approximately three times basal, their BP becomes approximately 25 mm Hg higher than in WT mice, and their cardiac function and structure are markedly compromised. In the absence of eNOS, feeding IC3 has no effects. We show that the effects of increased plasma concentrations of prolactin are mediated by its short form (16 kDa).
In humans, several studies have shown that increased levels of prolactin are associated with elevated arterial pressure (18, 19). In the opposite direction, a loss-of-function SNP in the GPR10 gene, which codes for the prolactin-releasing peptide receptor, is associated with reduced BP (20). Recently, a cohort study has demonstrated that a higher daytime plasma prolactin level is associated with an increased risk of incident hypertension among postmenopausal women (21). These results imply that circulating prolactin levels are positively correlated with BP in humans.
In animal experiments, whether changes in the expression of prolactin cause different BP levels has been debatable. Acute i.v. infusion of prolactin increases BP in rabbits (6). When ovine prolactin was chronically given i.p. via an osmotic minipump in rats, BP was decreased, which was associated with increased extracellular fluid volume (7). It has also been reported that chronic prolactin infusion caused an increase in urinary sodium, potassium, and water excretion, but no significant changes in arterial pressure in rats (8). A dopamine agonist lergotrile mesylate, which suppresses prolactin secretion (7), and a rabbit antiserum to rat prolactin significantly lowered BP in rats (22). In contrast, prolactin has been shown to reduce BP in rabbits with progesterone-induced hypertension (23) and in spontaneously hypertensive rats (24).
Prolactin increases NO production (25) by increasing intracellular calcium in mouse mammary epithelial cells (26) and by increasing the expression of carboxypeptidase-D, which releases the NOS substrate l-arginine from the C terminus of polypeptides (27, 28). Prolactin causes endothelium-dependent vasodilation via prolactin receptors in the rat aorta (29).
It has recently been demonstrated that the 16-kDa form of prolactin, which is cleaved out of prolactin by cathepsin-D and matrix metalloproteinases, has antiangiogenic effects, whereas the full-length prolactin has angiogenic effects (13, 30). Overexpression of the 16 kDa impairs cardiac function (31). The 16-kDa prolactin has also been demonstrated to inhibit eNOS activity by modulating intracellular calcium mobilization (15) and activating protein phosphatase 2A, leading to dephosphorylation and inactivation of endothelial NOS (16). The 16 kDa levels are elevated in plasma, urine, and amniotic fluid obtained from women with preeclampsia (4).
WT mice and healthy women have a decline in BP in pregnancy, but mice deficient in eNOS do not (32–34), suggesting that eNOS is involved in the pregnancy-induced decrease in BP. In the last third of pregnancy and during lactation, prolactin levels in female mice normally become three to four times higher than those in virgin females (32). Our Tg males fed NC have plasma prolactin concentrations comparable to this, and, like the pregnant mice, their BPs are less than in WT mice fed NC, and their cardiac parameters are normal. The situation is completely different when they are fed IC3; their plasma prolactin concentrations are threefold those of the Tg mice fed NC, their BPs are in the hypertensive range, and their hearts are damaged.
The changes in BP we observed in our Tg mice were accompanied by the changes in urinary excretion of NO metabolites nitrite/nitrate despite no changes in dietary intake of nitrite/nitrate, suggesting that endogenous production of NO is changed by the overproduction of prolactin. The total amount of eNOS was not changed by the higher plasma concentrations of prolactin. However, in agreement with published work showing that eNOS is activated when its Ser1177 is phosphorylated by Akt-kinase and that prolactin phosphorylates Akt-kinase (35–37), Ser1177-phosphorylated eNOS was increased in the Tg mice fed NC. In contrast, Ser1177 phosphorylation was decreased in the Tg mice fed I3C, in agreement with work showing that 16-kDa prolactin decreases phosphorylation of Ser1177 (16). These findings, together with our finding that a lack of eNOS almost totally abolished the alterations in BP by changes in plasma prolactin levels, lead us to conclude that the full-length and cleaved 16-kDa prolactin both change BP mainly via modulating activity of eNOS. We also observed an increase in iNOS expression, although lack of iNOS has no significant effects on plasma nitrite/nitrate levels (38), and the changes we observed in iNOS expression are minor compared with those that others have found to cause decreases in BP (39, 40).
The levels of 16-kDa prolactin in the plasma of the Tg mice fed I3C are more than those in WT, but are much less than those of the full-length prolactin. This situation is similar to the previous finding that the levels of 16-kDa prolactin are increased in the plasma, urine, and amniotic fluid of the patients with preeclampsia compared with those of women with normal pregnancy, although, as in our mice, the amount of 16-kDa prolactin is much less than that of the full-length prolactin (4). Why the hypertensive effect of the 16-kDa form can overcome the depressor effect of the full-length prolactin is poorly understood. The two forms clearly differ in many ways. Thus, PAI-1 and iNOS are downstream of 16-kDa prolactin but not of full-length prolactin (12–14). The 16-kDa form, but not the full-length form, requires the formation of a complex with PAI-1 to be active (41), and their intracellular signaling pathways differ (42).
Postpartum cardiomyopathy occurs within the ninth month of pregnancy to the fifth month postpartum, and is manifested by hypertrophy or dilatation with a low ejection fraction (43). Our findings that increased levels of full-length and 16-kDa prolactin cause hypertension and cardiac dysfunction are in agreement with the previously suggested link between the 16-kDa prolactin and preeclampsia/postpartum cardiomyopathy.
In summary, we find that different degrees of elevated plasma levels of prolactin have opposite effects: a decrease in BP caused by increased production of NO when plasma prolactin is modestly above normal, and an increase in BP together with cardiac malfunction caused by decreased production of NO when plasma prolactin is substantially increased. These findings, together with the known association of greater-than-normal prolactin levels with preeclampsia, suggest that pregnant women with high levels of prolactin may need special attention.
Materials and Methods
Animals.
We generated prolactin hypermorphic mice by inserting the single-copy Tg comprising the rat Cyp1a1 promoter, mouse prolactin cDNA (donated by Daniel I. H. Lintzer, Northwestern University, Evanston, IL), and the 3′-UTR of bGH into the Hprt locus in ES cells of the E14TG2a strain using homologous recombination (Fig. 1A) (9). After positive selection with hypoxanthine (16 μg/mL), aminopterin (0.175 mg/mL), and thymidine (4.8 mg/mL), the gene targeting was confirmed by Southern blot by using genomic DNA digested by BamHI (9). After generation of the animals, genotyping of prolactin hypermorphic mice was performed based on the presence or absence of the junction between prolactin cDNA and the 3′-UTR of bGH with conventional PCR using the toe DNA as a template. The primers used for the genotyping are as follows: forward, 5′-CAAGGTCATCAATGACTGCC-3′; reverse, 5′-ACAGTGGGAGTGGCATCTT-3′. The targeted mice were backcrossed into the C57BL/6 strain at least eight times before use.
IC3.
Normal mouse chow supplemented with 0.3% IC3 was obtained from Envigo.
Measurement of Biological Parameters.
We measured systolic BP (sBP) and pulse rate with the tail-cuff method (44). Plasma levels of prolactin were studied with ELISA (Mouse Prolactin DuoSet; R&D Systems). Urine nitrite/nitrate levels were studied with the Griess method (Nitrite/Nitrate Colorimetric Assay; Cayman). Metabolic balance studies were performed by using metabolic cages (Solo Mouse Metabolic Cage; Tecniplast). All animal protocols have been approved by the University of North Carolina Institutional Animal Care and Use Committee.
Immunoprecipitation/Western Blot.
The kidney was homogenated in immunoprecipitation buffer (50 mM Tris⋅HCl, pH 7.4, 150 mM NaCl, 50 mM β-glycerophosphate, 30 mM NaF, 2 mM EDTA, 2 mM EGTA, 30 mM Na4P2O7, 2 mM Na3VO4, 1% Triton X-100) and protease inhibitors (1 mM PMSF, 5 μg/mL leupeptin, 5 μg/mL antipain, 5 μg/mL pepstatin A, and 10 μg/mL aprotinin). The homogenate was centrifuged at 1,000 × g to pellet the nucleus and debris. Equal amounts of protein were incubated with an appropriate antiserum and protein G agarose for 16 h at 4 °C. The proteins were fractionated by SDS/PAGE (Criterion; Bio-Rad) and detected with Western blot by chemiluminescence (Supersignal West Pico; Thermo). The polyclonal antibody against the N terminus of prolactin (Cell Signaling) and the monoclonal antibody against GAPDH (Cell Signaling) were used for Western blot.
Quantitative RT-PCR.
Total RNA was extracted from different tissues, and the mRNAs were assayed by quantitative RT-PCR as previously described (45). The primers and probes used to measure mRNAs are shown in Table S1.
Table S1.
Primers and probes for quantification of mRNA with real-time quantitative RT-PCR
| Gene symbol | Primer | Sequence |
| Prl (Prolactin) | Forward | 5′-CTG GCT ACA CCT GAA GAC AA-3′ |
| Reverse | 5′-CAC CAA ACT GAG GAT CAG GT-3′ | |
| Probe | 5′-FAM-ACA AGC CCT GAA AGT CCC TCC GGA-Tamra-3′ | |
| Nos2 (iNOS) | Forward | 5′-TGAAGAGCGTGCCCTACTTC-3′ |
| Reverse | 5′-AGGGACAGATTGTGGCGAAT-3′ | |
| Probe | 5′-FAM-TCCAGCCCGCCGAGCTGTTGCT-Tamra-3′ | |
| Nos3 (eNOS) | Forward | 5′-ACG CAC AGC AGC TGG GAA GA-3′ |
| Reverse | 5′-ACA CCA GTG TCG TGC TCT AG-3′ | |
| Probe | 5′-FAM-TTC CGG AAG GCG TTT GAT CCC C-Tamra-3′ | |
| Serpine2 (PAI-1) | Forward | 5′-CGA CTT CAC AAG TCT TTC CG-3′ |
| Reverse | 5′-TCT CGT TTA CCT CGA TCC TG-3′ | |
| Probe | 5′-FAM-AGC AGC TCT CTG TAG CAC AGG CAC-Tamra-3′ | |
| Actb | Forward | 5′-AAG AGC TAT GAG CTG CCT GA-3′ |
| Reverse | 5′-ACG GAT GTC AAC GTC ACA CT-3′ | |
| Probe | 5′-FAM-CAC TAT TGG CAA CGA GCG GTT CCG-Tamra-3′ |
Echocardiography.
For analysis of heart function in mice, we used the Vevo 2100 v1.1.2 ultrasonograph system (VisualSonics) and a 30-MHz transducer. Diastolic and systolic wall thickness, end-diastolic dimensions, and end-systolic chamber dimensions of the left ventricle were measured by using a parasternal short-axis view. All measurements were done in the University of North Carolina Rodent Advanced Surgical Models Core according to the American Society of Echocardiography guidelines.
Statistical Analysis.
Data are expressed as means ± SEs. To compare groups, we used one-factor ANOVA. Post hoc pairwise comparisons were performed by Tukey–Kramer honestly significance differences test with JMP software (version 5.1.2; SAS). When the number of animals was fewer than five, a nonparametric Kruskal–Wallis test was used to compare the groups.
Acknowledgments
This work was supported by NIH Grants HL49277, HL70523, and HL71266 and by Career Development Award 2006-102 from the Juvenile Diabetes Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1615051113/-/DCSupplemental.
References
- 1.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: Actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19(3):225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- 2.Hinuma S, et al. A prolactin-releasing peptide in the brain. Nature. 1998;393(6682):272–276. doi: 10.1038/30515. [DOI] [PubMed] [Google Scholar]
- 3.Leaños-Miranda A, et al. Urinary prolactin as a reliable marker for preeclampsia, its severity, and the occurrence of adverse pregnancy outcomes. J Clin Endocrinol Metab. 2008;93(7):2492–2499. doi: 10.1210/jc.2008-0305. [DOI] [PubMed] [Google Scholar]
- 4.González C, et al. Elevated vasoinhibins may contribute to endothelial cell dysfunction and low birth weight in preeclampsia. Lab Invest. 2007;87(10):1009–1017. doi: 10.1038/labinvest.3700662. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima R, et al. Elevated vasoinhibin derived from prolactin and cathepsin D activities in sera of patients with preeclampsia. Hypertens Res. 2015;38(12):899–901. doi: 10.1038/hr.2015.99. [DOI] [PubMed] [Google Scholar]
- 6.Horrobin DF, Manku MS, Burstyn PG. Effect of intravenous prolactin infusion on arterial blood pressure in rabbits. Cardiovasc Res. 1973;7(5):585–587. doi: 10.1093/cvr/7.5.585. [DOI] [PubMed] [Google Scholar]
- 7.Mills DE, Buckman MT, Peake GT. Effect of prolactin administration and suppression on blood pressure and body fluid compartments in the rat. Endocrinology. 1981;109(5):1590–1596. doi: 10.1210/endo-109-5-1590. [DOI] [PubMed] [Google Scholar]
- 8.Ibarra F, et al. Prolactin, a natriuretic hormone, interacting with the renal dopamine system. Kidney Int. 2005;68(4):1700–1707. doi: 10.1111/j.1523-1755.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 9.Bronson SK, et al. Single-copy transgenic mice with chosen-site integration. Proc Natl Acad Sci USA. 1996;93(17):9067–9072. doi: 10.1073/pnas.93.17.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantachuvesiri S, et al. Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem. 2001;276(39):36727–36733. doi: 10.1074/jbc.M103296200. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Brown MA, Tam SH, Chan MC, Whitworth JA. Effects of diet on measurement of nitric oxide metabolites. Clin Exp Pharmacol Physiol. 1997;24(6):418–420. doi: 10.1111/j.1440-1681.1997.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee H, Struman I, Clapp C, Martial J, Weiner RI. Inhibition of urokinase activity by the antiangiogenic factor 16K prolactin: Activation of plasminogen activator inhibitor 1 expression. Endocrinology. 1998;139(9):3696–3703. doi: 10.1210/endo.139.9.6194. [DOI] [PubMed] [Google Scholar]
- 13.Struman I, et al. Opposing actions of intact and N-terminal fragments of the human prolactin/growth hormone family members on angiogenesis: An efficient mechanism for the regulation of angiogenesis. Proc Natl Acad Sci USA. 1999;96(4):1246–1251. doi: 10.1073/pnas.96.4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbacho AM, et al. Proteolytic cleavage confers nitric oxide synthase inducing activity upon prolactin. J Biol Chem. 2000;275(18):13183–13186. doi: 10.1074/jbc.275.18.13183. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez C, et al. 16K-prolactin inhibits activation of endothelial nitric oxide synthase, intracellular calcium mobilization, and endothelium-dependent vasorelaxation. Endocrinology. 2004;145(12):5714–5722. doi: 10.1210/en.2004-0647. [DOI] [PubMed] [Google Scholar]
- 16.García C, et al. Vasoinhibins prevent retinal vasopermeability associated with diabetic retinopathy in rats via protein phosphatase 2A-dependent eNOS inactivation. J Clin Invest. 2008;118(6):2291–2300. doi: 10.1172/JCI34508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shesely EG, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1996;93(23):13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka S, et al. Plasma prolactin levels in patients with essential hypertension, malignant hypertension and secondary hypertension. Jpn J Med. 1985;24(1):19–23. doi: 10.2169/internalmedicine1962.24.19. [DOI] [PubMed] [Google Scholar]
- 19.Oney T, Bellmann O, Kaulhausen H. Relationship between serum prolactin concentration, vascular angiotensin sensitivity and arterial blood pressure during third trimester pregnancy. Arch Gynecol Obstet. 1988;243(2):83–90. doi: 10.1007/BF00932973. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharyya S, et al. Association of polymorphisms in GPR10, the gene encoding the prolactin-releasing peptide receptor with blood pressure, but not obesity, in a U.K. Caucasian population. Diabetes. 2003;52(5):1296–1299. doi: 10.2337/diabetes.52.5.1296. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Curhan GC, Forman JP. Plasma prolactin level and risk of incident hypertension in postmenopausal women. J Hypertens. 2010;28(7):1400–1405. doi: 10.1097/HJH.0b013e328339f254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills DE, Buckman MT, Peake GT. Neonatal treatment with antiserum to prolactin lowers blood pressure in rats. Science. 1982;217(4555):162–164. doi: 10.1126/science.7089550. [DOI] [PubMed] [Google Scholar]
- 23.Mati JK, Mugambi M, Odipo WS, Nguli K. Prolactin and hypertension. Am J Obstet Gynecol. 1977;127(6):616–619. doi: 10.1016/0002-9378(77)90360-x. [DOI] [PubMed] [Google Scholar]
- 24.Ryszka F, et al. Hypotensive action of prolactin in rats with spontaneous hypertension. Acta Physiol Pol. 1984;35(3):215–217. [PubMed] [Google Scholar]
- 25.Meli R, et al. Recombinant human prolactin induces protection against Salmonella typhimurium infection in the mouse: Role of nitric oxide. Immunopharmacology. 1996;34(1):1–7. doi: 10.1016/0162-3109(95)00052-6. [DOI] [PubMed] [Google Scholar]
- 26.Bolander FF., Jr Prolactin activation of mammary nitric oxide synthase: Molecular mechanisms. J Mol Endocrinol. 2002;28(1):45–51. doi: 10.1677/jme.0.0280045. [DOI] [PubMed] [Google Scholar]
- 27.Abdelmagid SA, Too CK. Prolactin and estrogen up-regulate carboxypeptidase-d to promote nitric oxide production and survival of mcf-7 breast cancer cells. Endocrinology. 2008;149(10):4821–4828. doi: 10.1210/en.2008-0145. [DOI] [PubMed] [Google Scholar]
- 28.Thomas LN, Morehouse TJ, Too CK. Testosterone and prolactin increase carboxypeptidase-D and nitric oxide levels to promote survival of prostate cancer cells. Prostate. 2012;72(4):450–460. doi: 10.1002/pros.21446. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez C, et al. The prolactin family hormones regulate vascular tone through NO and prostacyclin production in isolated rat aortic rings. Acta Pharmacol Sin. 2015;36(5):572–586. doi: 10.1038/aps.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macotela Y, et al. Matrix metalloproteases from chondrocytes generate an antiangiogenic 16 kDa prolactin. J Cell Sci. 2006;119(pt 9):1790–1800. doi: 10.1242/jcs.02887. [DOI] [PubMed] [Google Scholar]
- 31.Hilfiker-Kleiner D, et al. Acathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128(3):589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 32.Guillou A, et al. Assessment of lactotroph axis functionality in mice: Longitudinal monitoring of PRL secretion by ultrasensitive-ELISA. Endocrinology. 2015;156(5):1924–1930. doi: 10.1210/en.2014-1571. [DOI] [PubMed] [Google Scholar]
- 33.Reckelhoff JF, Hennington BS, Moore AG, Blanchard EJ, Cameron J. Gender differences in the renal nitric oxide (NO) system: Dissociation between expression of endothelial NO synthase and renal hemodynamic response to NO synthase inhibition. Am J Hypertens. 1998;11(1 pt 1):97–104. doi: 10.1016/s0895-7061(97)00360-9. [DOI] [PubMed] [Google Scholar]
- 34.Hefler LA, Tempfer CB, Moreno RM, O’Brien WE, Gregg AR. Endothelial-derived nitric oxide and angiotensinogen: Blood pressure and metabolism during mouse pregnancy. Am J Physiol Regul Integr Comp Physiol. 2001;280(1):R174–R182. doi: 10.1152/ajpregu.2001.280.1.R174. [DOI] [PubMed] [Google Scholar]
- 35.Al-Sakkaf KA, Mooney LM, Dobson PR, Brown BL. Possible role for protein kinase B in the anti-apoptotic effect of prolactin in rat Nb2 lymphoma cells. J Endocrinol. 2000;167(1):85–92. doi: 10.1677/joe.0.1670085. [DOI] [PubMed] [Google Scholar]
- 36.Bailey JP, et al. Prolactin and transforming growth factor-beta signaling exert opposing effects on mammary gland morphogenesis, involution, and the Akt-forkhead pathway. Mol Endocrinol. 2004;18(5):1171–1184. doi: 10.1210/me.2003-0345. [DOI] [PubMed] [Google Scholar]
- 37.Fulton D, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399(6736):597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook S, et al. Increased eNO and pulmonary iNOS expression in eNOS null mice. Eur Respir J. 2003;21(5):770–773. doi: 10.1183/09031936.03.00121203. [DOI] [PubMed] [Google Scholar]
- 39.MacMicking JD, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81(4):641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi N, et al. Time-dependent expression of renal vaso-regulatory molecules in LPS-induced endotoxemia in rat. Peptides. 2006;27(9):2258–2270. doi: 10.1016/j.peptides.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 41.Bajou K, et al. PAI-1 mediates the antiangiogenic and profibrinolytic effects of 16K prolactin. Nat Med. 2014;20(7):741–747. doi: 10.1038/nm.3552. [DOI] [PubMed] [Google Scholar]
- 42.Halkein J, et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest. 2013;123(5):2143–2154. doi: 10.1172/JCI64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Futterman LG, Lemberg L. Peripartum cardiomyopathy: An ominous complication of pregnancy. Am J Crit Care. 2000;9(5):362–366. [PubMed] [Google Scholar]
- 44.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25(5):1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 45.Kim HS, Lee G, John SW, Maeda N, Smithies O. Molecular phenotyping for analyzing subtle genetic effects in mice: Application to an angiotensinogen gene titration. Proc Natl Acad Sci USA. 2002;99(7):4602–4607. doi: 10.1073/pnas.072083799. [DOI] [PMC free article] [PubMed] [Google Scholar]