Significance
One of the most important agronomic traits in crop breeding is yield, which includes increased seed size and weight in grain crops and leaf biomass in forage crops. In this work, we demonstrate that a transcription regulator encoded by the BIG SEEDS1 (BS1) gene from the model legume Medicago truncatula, negatively regulates primary cell proliferation in plants. The deletion of this gene in M. truncatula and down-regulation of its orthologs in soybean (Glycine max) lead to significant increases in the size of plant organs, including leaf and seed. Understanding the BS1 gene function and its regulatory mechanism offers an opportunity for increasing plant yield in legumes and other grain crops.
Keywords: plant organ size, seed size, forage quality, Medicago, soybean
Abstract
Plant organs, such as seeds, are primary sources of food for both humans and animals. Seed size is one of the major agronomic traits that have been selected in crop plants during their domestication. Legume seeds are a major source of dietary proteins and oils. Here, we report a conserved role for the BIG SEEDS1 (BS1) gene in the control of seed size and weight in the model legume Medicago truncatula and the grain legume soybean (Glycine max). BS1 encodes a plant-specific transcription regulator and plays a key role in the control of the size of plant organs, including seeds, seed pods, and leaves, through a regulatory module that targets primary cell proliferation. Importantly, down-regulation of BS1 orthologs in soybean by an artificial microRNA significantly increased soybean seed size, weight, and amino acid content. Our results provide a strategy for the increase in yield and seed quality in legumes.
As a key crop worldwide, soybean not only provides up to 69% of proteins and 30% of oils to the human diet (1) but also requires a low input of fertilizers due to its symbiotic nitrogen fixation ability (2), making it a highly valuable crop to secure global food supplies while contributing to sustainable agriculture. It is clear that the rapid increase of world population requires significant increases in crop production (3).
Seed size is a major agronomic trait that has been selected in crop plants during their domestication (4–6). Due to whole genome duplication events that occurred ∼59 and ∼14 Mya (million years ago) (2), soybean has a complex genome structure. Thus, the isolation of key genes or quantitative trait loci (QTL) that control seed size and weight in soybean using conventional approaches can be a challenge and has not been reported to date. To overcome this challenge, we took a molecular genetics approach, using the legume plant Medicago truncatula as a genetic model. We identified BIG SEEDS1 (BS1) as a key gene that controls size of lateral organs, including seeds, seed pods, and leaves, in M. truncatula. Based on these results, we further identified two BS1 orthologs in soybean (Glycine max). Our objectives were to understand the role and regulatory mechanism of the BS1 gene in lateral organ size control in legume plants. Down-regulation of soybean BS1 genes using an artificial microRNA resulted in increased size of seeds, seed pods, and leaves, thus revealing a key and conserved role of BS1 in the control of organ size in legumes.
Results and Discussion
Isolation and Characterization of M. truncatula big seeds1 Mutants.
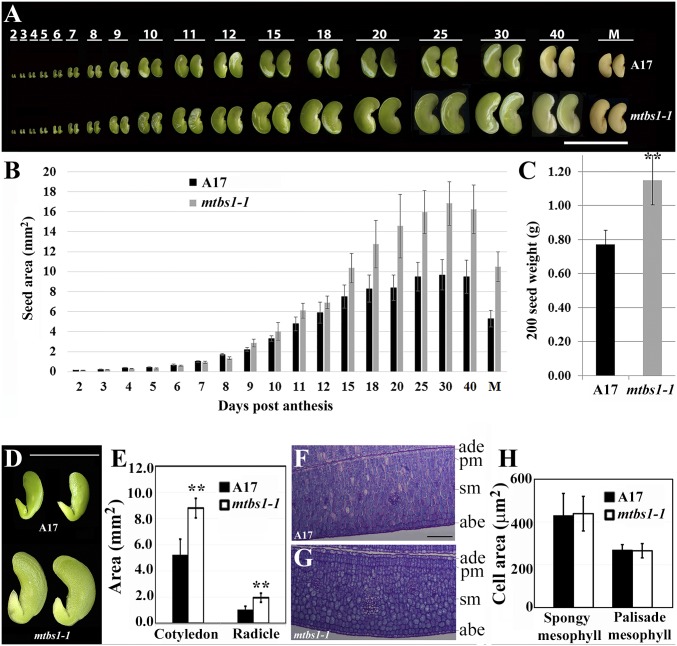
In a screen of the fast neutron bombardment (FNB)-induced deletion mutant collection of M. truncatula [cultivar (cv.) Jemalong A17] (7), a unique mutant with large seeds was isolated and named M. truncatula big seeds1-1 (mtbs1-1) (Fig. 1A). The mtbs1-1 mutant also exhibited larger seed pods and leaves than WT plants, suggesting a key role of BS1 in determining lateral organ size (SI Appendix, Figs. S1 and S2). Time-course experiments showed that developing seeds at stages before 9 d postanthesis (DPA) were indistinguishable between WT and the mtbs1-1 mutant (Fig. 1A). After 9 DPA, mtbs1-1 seeds were significantly larger than WT seeds (Fig. 1 A and B). Seed pods were also larger in the mutant, even at stages before 9 DPA (SI Appendix, Fig. S1). In agreement with the observed increase in seed size, mature seeds of the mtbs1-1 mutant were 49% heavier than WT seeds (Fig. 1C).
Fig. 1.
M. truncatula bs1-1 mutant exhibited increased seed size and weight. (A) Time-course analysis of seed development in WT (A17; Top) and mtbs1-1 mutant (Bottom). Numbers denote days post anthesis (DPA). M, mature stage. Two representative seeds are shown for each time point. The panel is composed of multiple images that were taken at different time points and aligned in Photoshop. (B) Measurements of seed area during seed development. Shown are means ± SD for n = 12. (C) Measurements of 200 seed weight. Shown are means ± SD for n = 3. **P < 0.001, Student’s t test. (D) Morphology of cotyledon and radicle of seeds 25 DPA, after removing seed coat. The panel is composed of images of two genotypes. (E) Measurements of cotyledon and radicle area. Shown are means ± SD for n = 7. **P < 0.001, Student’s t test. (F and G) Cross-sections of cotyledon of seeds 25 DPA in A17 (F) and mtbs1-1 (G). abe, abaxial epidermis; ade, adaxial epidermis; pm, palisade mesophyll; sm, spongy mesophyll. (H) Measurements of mesophyll cell area of cotyledon. Shown are means ± SD for n = 60. **P < 0.001, Student’s t test. (Scale bars: C, 1 cm; D, 0.5 cm; F and G, 100 μm.
To understand how BS1 functions to control seed size, we first examined embryogenic processes in A17 and mtbs1-1. Microscopic imaging analyses showed that, from early stages to the torpedo stage when cotyledons start to expand, embryo and endosperm in mtbs1-1 were indistinguishable from those in A17 (SI Appendix, Fig. S3), indicating that BS1 does not affect early embryogenesis. This finding is consistent with the morphological observation that seeds were larger in the mtbs1-1 mutants than in WT only at a late developmental stage (Fig. 1 A and B). To identify which tissue was mostly affected in mtbs1-1 seeds, we removed the seed coat from seeds 25 DPA. We found that both cotyledon and radicle were significantly larger in mtbs1-1 seeds than in WT seeds (Fig. 1 D and E). These results are consistent with the notion that seed size in legume species is strongly and positively correlated with cotyledon size (8, 9). Analyses of cross-sections showed that the size of spongy and palisade mesophyll cells of cotyledons was indistinguishable between the mtbs1-1 mutant and WT (Fig. 1 F–H). Further measurements showed that cotyledon size was significantly larger in a 3-wk-old mtbs1-1 mutant than in WT plants (SI Appendix, Fig. S4 A and B). Epidermal cell number, but not cell size, was significantly increased in cotyledons of 3-wk-old mtbs1-1 mutant than WT (SI Appendix, Fig. S4 C–F). In addition, epidermal cell pattern and stomatal index (10) were indistinguishable in expanded cotyledons between the mtbs1-1 mutant and WT (SI Appendix, Fig. S4 C, D, and G).
In the mtbs1-1 mutant, both terminal and lateral leaflets of trifoliate leaves were dramatically increased in length, width, perimeter, and area compared with WT counterparts (SI Appendix, Fig. S2). In addition, the shape of leaflets was also altered. In contrast to the flat leaflets of WT plants, mtbs1-1 leaflets were dome-shaped, with a positive Gaussian curvature (11), and could be flattened only by cutting the edges, suggesting uneven lamina growth (SI Appendix, Fig. S2). Strikingly, stipules in the mtbs1-1 mutant were more than 10 times larger than WT stipules (SI Appendix, Fig. S5), showing the potential of morphogenetic changes imposed by the mtbs1-1 mutation. Based on the dramatic increases in organ size in the mtbs1-1 mutant, we concluded that BS1 is a key regulator of organ size.
Because M. truncatula is a forage model (12), we tested whether forage quality and biomass yield were altered in the mtbs1-1 mutant. Wet chemistry analyses showed that acid detergent fiber (ADF) and neutral detergent fiber (NDF) were significantly decreased in three month-old mtbs1-1 plants compared with WT (SI Appendix, Fig. S6A). Consistently, total digestible nutrients (TDN), net energy for body maintenance (NEm), body weight gain (NEg), and lactation (NEl) and metabolizable energy for lactation (MEl) were significantly increased whereas crude protein (CP) was not significantly changed (SI Appendix, Fig. S6 A and B). Because leaf tissues are of higher forage quality than stem tissues, we tested whether the leaf/stem ratio was altered in the mtbs1-1 mutant. As expected, mtbs1-1 mutant plants had a significantly increased leaf/stem ratio compared with WT (SI Appendix, Fig. S6E). In addition, total biomass based on fresh and dry weight measurements was also significantly increased in 3-mo-old mtbs1-1 plants compared with WT (SI Appendix, Fig. S6 C and D). These results indicate that the mtbs1-1 mutant improved forage quality and increased biomass yield.
An increase in organ size may result from an increase in cell proliferation and/or cell expansion, two successive processes contributing to the final organ size (13, 14). Measurements showed that epidermal cells of the seed coats of mature seeds and stipules of fully expanded leaves were indistinguishable in size between mtbs1-1 and WT (A17) plants (SI Appendix, Fig. S7), suggesting that cell proliferation rather than cell expansion was altered in the mtbs1-1 mutant. Leaf primordia are initiated from the periphery of the shoot apical meristem (SAM) and initially consist of cells that undergo coordinated division without changes in cell size. As the leaf grows, cells at the distal tip cease dividing and differentiate, resulting in a cell cycle arrest front moving from the tip to base. Eventually, all cells cease dividing and the leaf reaches its final size (11, 15, 16). To further examine the developmental processes affected in the mtbs1-1 mutant, we followed leaf development over time. After emerging from the shoot apex, young leaves [plastochron (P) 5] of both WT and the mtbs1-1 mutant similarly expanded along the proximodistal (length) and mediolateral (width) axes (SI Appendix, Fig. S8). The expansion of WT leaves along the length and width axes reached a plateau ∼2 d later (SI Appendix, Fig. S8). By contrast, leaves of the mtbs1-1 mutant continuously expanded and did not reach a plateau until ∼6 d later (SI Appendix, Fig. S8). Scanning electron microscopic (SEM) analyses showed that SAM and leaf primordia were not different in size between mtbs1-1 and WT (SI Appendix, Fig. S9), suggesting that the mtbs1-1 mutation does not affect the size of SAM and leaf primordia.
Cell division rate measurements showed that the first, newly emerged leaf (L1) in both WT (A17) and mtbs1-1 was similarly undergoing rapid cell proliferation with the highest cell division rate (SI Appendix, Fig. S10). In the second and third leaves (L2 and L3), cell division rate was rapidly decreased in A17 (12% and 6% of that of L1, respectively). By contrast, cell division rate was gradually decreased in L2 and L3 in the mtbs1-1 mutant (62% and 22% of that of L1, respectively), supporting an extended cell proliferation activity in developing leaves in the mtbs1-1 mutant compared with WT (SI Appendix, Fig. S10). In agreement with the observed cell division rates, the size of epidermal cells in L1 was not different between A17 and mtbs1-1 (SI Appendix, Fig. S10H). By contrast, epidermal cells in L2 were larger in WT than the mtbs1-1 mutant, consistent with the notion that a majority of epidermal cells undergo cell proliferation in L2 in the mtbs1-1 mutant and that a majority of epidermal cells already undergo cell expansion in L2 in WT (SI Appendix, Fig. S10). Collectively, these results show that the enlarged organ phenotype was caused by a prolonged cell proliferation activity in the mtbs1-1 mutant, which is also consistent with the observation that seed and seed pod maturation was similarly delayed in the mtbs1-1 mutant compared with WT (Fig. 1A and SI Appendix, Fig. S1).
Cloning and Characterization of the BS1 Gene.
To genetically characterize the mtbs1-1 mutant phenotype, we performed reciprocal crosses between the mtbs1-1 mutant and WT (A17). The results showed that F1 seeds from A17 (♀) × mtbs1-1 (♂) crosses were indistinguishable from A17 seeds whereas seeds from mtbs1-1 (♀) × A17 (♂) crosses were indistinguishable from mtbs1-1 seeds (SI Appendix, Fig. S11), suggesting a maternal effect on seed size (SI Appendix, Fig. S11). On the other hand, regardless of seed phenotypes, F1 plants from the reciprocal crosses displayed WT phenotype. Segregation analyses of an F2 population from self-pollination of F1 backcrossed plants showed that WT and mtbs1-1 mutant segregated in a 3:1 ratio (158 WT and 44 mutants; χ2 test, P = 0.29), indicating that the enlarged organ size phenotype of mtbs1-1 is caused by a single recessive mutation.
Using a map-based approach with a mapping population of 518 plants, we located the BS1 locus to the south arm of chromosome 1 between two simple sequence repeat (SSR) markers, h2-49g13b and 005E04 (SI Appendix, Fig. S12 and Table S1) (17). The MTIC61 marker was tightly linked to the mutation (SI Appendix, Fig. S12 and Table S1). Based on the latest Medicago genome sequence (Version 4), we estimated that there are ∼160 genes located in the mapped region. To identify the gene, we compared transcript profiles in vegetative shoot buds and young leaves between WT and the mutant. Among all of the microarray probesets mapped to the region, only one probeset, Mtr.44029.1.S1_at, was significantly down-regulated in both shoot buds and young leaves in the mtbs1-1 mutant (SI Appendix, Table S2), suggesting that it may correspond to the BS1 gene. Accordingly, reverse transcription (RT)-PCR amplification detected the corresponding transcript in WT plants but not in the mtbs1-1 mutant (Fig. 2B), indicating that Mtr.44029.1.S1_at was deleted in the mutant. We recovered deletion borders using PCR-based chromosomal walking and thermal asymmetric interlaced (TAIL)-PCR (SI Appendix, Fig. S13). Sequencing results showed that the right deletion border occurred in the second exon at 398 bp downstream from the translation initiation codon ATG of the candidate gene. However, the left border did not match to any available sequences and was likely located in the gap region upstream of the gene (Fig. 2A).
Fig. 2.
Cloning and characterization of Medicago BS1 gene. (A) Map-based cloning leads to identification of a deletion at the BS1 locus. Shown are introns (horizontal lines), exons (solid boxes), and 5′ and 3′ untranslated regions (open boxes) of the BS1 locus, and the deletion region delimited by vertical lines. (B) RT-PCR analysis shows lack of BS1 transcripts in the mtbs1-1 mutant, in contrast to WT (A17). The expression of an ACTIN gene is present in both WT and mtbs1-1, serving as an internal control. (C and D) BS1-GFP fusion protein is localized to the nucleus in tobacco leaf epidermal cells. Shown are a confocal image of a tobacco leaf transiently expressing 35S::BS1-GFP and an overlay with a Normaski image. (E and F) As a control, free GFP driven by the 35S promoter was localized to the cytoplasm. (G–J) RNA in situ hybridization shows that BS1 transcripts were detected in SAM and leaf primordia as early as P0 in vegetative shoot bud (G) and in developing petal (p), carpel (c) and ovule (o) during reproductive development (I). No signals were detected in neighboring tissue sections when a sense probe was used (H and J). (K). Amino acid sequence alignments of BS1 homologs from alfalfa (M. sativa), soybean (G. max), and L. japonicus with M. truncatula BS1. TIFY and CCT2 domains are underlined in red and blue, respectively.
To confirm the candidate gene, we isolated two additional mutant lines, from the FNB mutant collection, that exhibited phenotypes similar to the mtbs1-1 mutant (SI Appendix, Fig. S14). Genomic PCR and RT-PCR analyses showed that the candidate BS1 locus was completely deleted in both mutants (SI Appendix, Fig. S14); therefore, these two lines were named mtbs1-2 (FN 1860) and mtbs1-3 (FN 2876). Additionally, the mtbs1-1 phenotype was completely rescued by introducing the BS1 coding sequence under the control of its native promoter (SI Appendix, Fig. S15). These results confirm that Mtr.44029.1.S1_at corresponds to BS1.
BS1 encodes a group II member of the TIFY family of transcription regulators (18, 19). It is related to two tandemly repeated genes in Arabidopsis, At4g14713 (PEAPOD1 or PPD1) and At4g14720 (PPD2), that have been shown to regulate the size and shape of leaves and siliques but not seeds (20, 21). Protein subcellular localization showed that BS1-GFP fusion proteins were localized to the nucleus (Fig. 2 C–F and SI Appendix, Fig. S16), consistent with a role of a putative transcription regulator. Database searches and PCR amplification identified BS1 homologous sequences from diverse eudicot species, including alfalfa (Medicago sativa), Lotus japonicus, and soybean (G. max) (Fig. 2K).
Search results of the M. truncatula Gene Expression Atlas showed that BS1 was expressed in all major organs, including seeds, seed pods, flowers, shoot buds, and leaves (SI Appendix, Fig. S17). RNA in situ hybridization showed that BS1 transcripts were present in the shoot apical meristem (SAM), lamina tissues, and leaf primordia as early as P0 (Fig. 2 G and H). In reproductive tissues, BS1 transcripts were detected in developing petal, carpel, and ovule (Fig. 2 I and J).
BS1 Represses Primary Cell Proliferation.
Arabidopsis PPD1 and PPD2, the BS1 orthologs, repress meristemoid cell division in leaves (20, 21)). In loss-of-function and knock-down ppd mutants, numerous small cells that surround stomata were generated, giving rise to enlarged and dome-shaped leaves (20–22) (SI Appendix, Fig. S18 A and B). In leaves of ppd deletion mutants, epidermal cell pattern was substantially changed, and the stomatal index was significantly reduced due to the presence of numerous small, nonstomatal cells (20, 21) (SI Appendix, Fig. S18). By contrast, in the mtbs1-1 mutant, epidermal cells of seed coats, leaf blades, stipules, and cotyledons were not changed in size and pattern from those in WT plants (SI Appendix, Figs. S4, S7, and S10). In addition, the stomatal index was not changed in all tissues tested (SI Appendix, Figs. S4, S10, and S18). Taken together, these results indicate that Medicago BS1 represses primary cell proliferation in the control of lateral organ size, a mechanism different from that of the redundant PPD1 and PPD2, which repress the asymmetric division of meristemoids in Arabidopsis.
Transcription Profiling Analysis of the mtbs1-1 Mutant.
To further elucidate the mechanisms by which BS1 regulates primary cell proliferation, we analyzed transcript profiles in both vegetative shoot buds and young leaves in WT and mtbs1-1 (Fig. 3). Interestingly, a large number of core cell cycle (CCC) and histone genes were significantly up-regulated in young leaves, but not in vegetative shoot buds, in mtbs1-1 compared with WT (Fig. 3A and SI Appendix, Tables S3 and S4). Comparison of transcript levels in vegetative shoot buds and young leaves showed that the expression of CCC genes was dramatically down-regulated in the leaf tissues in both WT and mtbs1-1 plants (SI Appendix, Fig. S19). However, the down-regulation of CCC genes was significantly attenuated in the mtbs1-1 mutant relative to WT plants (SI Appendix, Fig. S19), suggesting insufficient suppression of CCC gene expression in expanding leaves in the mtbs1-1 mutant. This finding is consistent with the increase in cell proliferation observed in the mtbs1-1 mutant.
Fig. 3.
Gene expression analysis of the mtbs1-1 mutant. (A) Transcript profiling analysis of core cell cycle genes in expanding leaves in mtbs1-1. Shown is a heat map of log2 fold changes in gene expression in vegetative shoot bud (VB) and young leaf (YL) samples between mtbs1-1 and A17. (B and C) Quantitative RT-PCR analysis of expression of Medicago HISTONE 4 (B) and CYCD3;3 (C) in young leaf, stipule, and seed samples in A17 and mtbs1-1 plants. Shows are means ± SD for n = 3. **P < 0.001, Student’s t test. (D) Transcript profiling analysis of genes whose homologs are known to regulate organ growth in A. thaliana. Shown is a heat map of log2 fold changes in gene expression between mtbs1-1 and A17. (E and F) qRT-PCR analysis of expression of Medicago GRF5 (E) and GIF1 (F) in young seed, leaf, and stipule samples in A17 and mtbs1-1 plants. Shows are means ± SD for n = 3. **P < 0.001, Student’s t test. RNA samples were prepared from developing seeds at 6 DPA and leaflets and stipules of the second visible leaf from the shoot apex (B, C, E, and F). (G–J) RNA in situ hybridization shows that transcripts of MtGIF1 were detected in the shoot apical meristem (SAM), leaf primordia as early as P0 and lamina tissues in A17 (G) and mtbs1-1 (H). A sense probe for MtGIF1 did not detect any signals in neighboring tissue sections in A17 (I) and mtbs1-1 (J). (Scale bars: 100 µm.)
Next, we examined expression levels of genes known to regulate organ growth in Arabidopsis thaliana, using the transcript profiling data (Fig. 3D). Interestingly, the top 12 most up-regulated genes included 5 genes related to Arabidopsis GROWTH REGULATING FACTOR5 (GRF5) and 2 genes related to Arabidopsis GRF-INTERACTING FACTOR1 (GIF1) [also known as ANGUSTIFOLIA3 (AN3)] (Fig. 3D and SI Appendix, Table S5). The Medicago genome has nine GRF members and three GIF members. We designated the top two up-regulated genes that are closely related to the Arabidopsis GRF5 as MtGRF5 and MtGRF1, and the two up-regulated genes related to the Arabidopsis GIF1 as MtGIF1 and MtGIF2, respectively. By quantitative RT-PCR, we confirmed up-regulation of MtH4 (Fig. 3B), MtCYCD3;3 (Fig. 3C), MtGRF5 (Fig. 3E), MtGIF1 (Fig. 3F), and MtGRF1 and MtGIF2 (SI Appendix, Fig. S20) in developing seed, leaf, and stipule samples. RNA in situ hybridization showed that MtGIF1 signals were detected in leaf primordia as early as P0, expanding leaves, shoot apical meristem (SAM), and vascular tissues in the mtbs1-1 mutant (Fig. 3 G and H). In developing leaves, MtGIF1 signals were relatively stronger in the mtbs1-1 mutant than WT (Fig. 3 G and H). As negative controls, sense probes of MtGIF1 did not detect any signals in neighboring tissue sections (Fig. 3 I and J). In Arabidopsis, GRF5 and GIF1 are known to be interacting partners (23). Overexpression of either gene leads to significantly increased leaf size and altered leaf shape (23), a phenotype resembling that of the mtbs1-1 mutant leaves. The significant up-regulation of GIF and GRF, and CCC genes in expanding leaves, but not in vegetative shoot buds in the mtbs1-1 mutant, is consistent with the mtbs1-1 mutant phenotype and suggests that up-regulation of the GIF and GRF genes may contribute to the prolonged cell proliferation activity and lateral organ growth in the mtbs1-1 mutant.
BS1 Interacts with Medicago NINJA.
As a group II member of the TIFY family of proteins, BS1 does not contain any known DNA-binding domains. Some TIFY proteins interact with Novel Interactor of JAZ (NINJA), an adaptor protein that interacts with the transcription corepressors TOPLESS (TPL) and TOPLESS-RELATED PROTEINs (TPRs) to suppress downstream gene expression (24, 25). Using yeast two-hybrid assays, we showed that BS1 strongly interacts with Medicago NINJA, but not with Medicago MYC2, a transcription activator that physically interacts with Jasmonate JIM-domain (JAZ) repressor proteins to regulate jasmonic acid signaling (25) (SI Appendix, Fig. S21A). As a control, Medicago JAZ3, another TIFY protein, strongly interacted with both Medicago MYC2 and NINJA via its JAZ domain (SI Appendix, Fig. S21 B and C). These results suggest that BS1 likely forms a repressor complex with NINJA and its corepressors, but not with MYC2, to negatively control the expression of downstream target genes.
Down-Regulation of BS1 Orthologs Resulted in Increased Seed Size and Quality in Soybean.
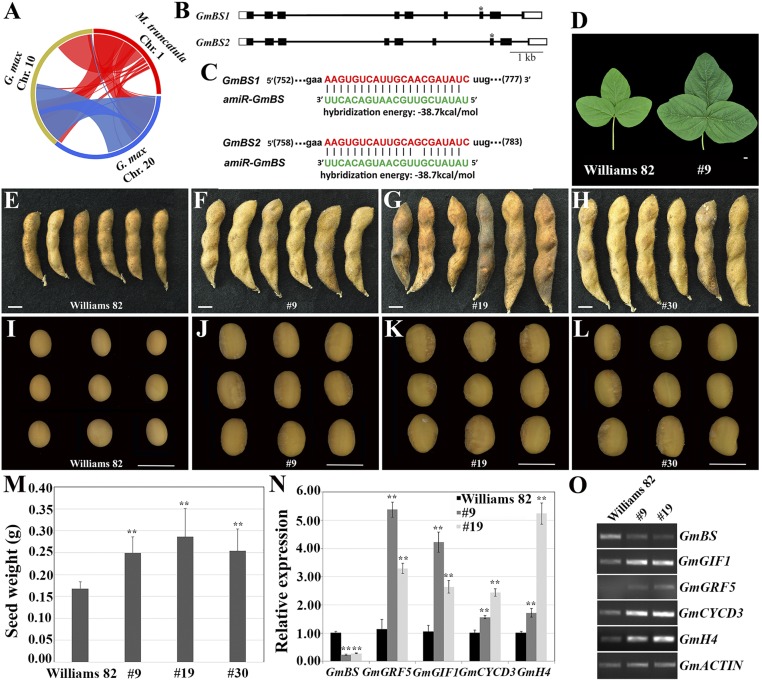
Given a close phylogenetic relationship between Medicago and soybean, we hypothesized that BS1 orthologs may play a similar role in soybean. Synteny analysis showed that Medicago chromosome 1, particularly a region in the south arm where BS1 is located, is highly syntenic to regions in chromosomes 10 and 20 in soybean (Fig. 4A and SI Appendix, Fig. S22). We identified two soybean BS1 orthologs named GmBS1 (Glyma10g38970) and GmBS2 (Glyma20g28840) from these microsyntenic regions (Fig. 2K and SI Appendix, Fig. S22). Next, we designed an artificial microRNA that targets a conserved coding sequence of GmBS1 and GmBS2 (Fig. 4 B and C). The artificial microRNA driven by a constitutive, double strength CaMV35S promoter (2 × 35S::preamiR) was introduced and stably integrated into the soybean genome. We performed phenotypic analysis of three independent transgenic lines (lines 9, 19, and 30) over five generations. As shown in Fig. 4 D–M, these independent lines displayed dramatically increased size and weight of plant organs, including seeds, seed pods, and leaves, compared with those of WT plants.
Fig. 4.
Down-regulation of BS1 orthologs resulted in increased organ size and seed weight in soybean. (A) Soybean chromosomes 10 and 20 are syntenic to Medicago chromosome 1, where BS1 is located. (B) GmBS1 and GmBS2 gene structures and artificial microRNA target sites. Shown are introns (horizontal lines), exons (solid boxes), and 5′ and 3′ untranslated regions (open boxes). *, artificial microRNA target sites. (C) Design of an artificial microRNA targeting a conserved coding sequence of GmBS1 and GmBS2. (D) Representative images of trifoliate leaves (D) of WT (Williams 82; Left) and the transgenic line 9 (Right). The panel is composed of two images. (E–L) Representative images of mature seed pods (E–H) and seeds (I–L) of WT (Williams 82) and independent transgenic lines that overexpress the artificial microRNA, showing enlarged seed pods and seeds in transgenic lines. Individual seed images in (I–L) were aligned in Photoshop. (M) Measurements show that seed weight was significantly increased in the transgenic lines. Shown are means ± SD for n = 5–11 plants. **P < 0.001, Student’s t test. (N and O) Quantitative RT-PCR (N) and RT-PCR (O) analyses show that soybean BS1 genes were significantly down-regulated, whereas GRF5, GIF1, CYCD3;3, and HISTONE4 genes were significantly up-regulated in young leaves in transgenic lines 9 and 19. Shown in N are means ± SD for n = 3. **P < 0.001, Student’s t test. (Scales bars: D–L, 1 cm.)
Gene expression analysis showed that the expression of the GmBS genes was significantly down-regulated in these independent transgenic plants. On the other hand, the expression of soybean GRF5, GIF1, CYCD3;3, and HISTONE4 genes was significantly up-regulated in the independent transgenic plants, similar to that in the mtbs1-1 mutant (Fig. 4 N and O). Amino acid analysis showed that the content of all 16 amino acids analyzed was significantly increased in seeds in transgenic lines 19 and 30 whereas six amino acids were significantly increased in transgenic line 9 (SI Appendix, Table S6). However, contents of the five common fatty acids were not significantly changed in seeds in these transgenic lines compared with WT (SI Appendix, Table S7).
PPD1 and PPD2, two tandemly duplicated BS1 orthologs in Arabidopsis, regulate the size and shape of leaves and siliques, but not seeds, by repressing the secondary cell proliferation, asymmetric division of meristemoids (20, 21). In ppd1 ppd2 deletion and knock-down mutants, asymmetric cell division and the number of small cells are increased in leaves (20, 21), leading to a substantially altered epidermal cell pattern and a significantly reduced stomatal index. This result is in contrast to the phenotype seen in the mtbs1-1 mutants in which only cell number, but not cell size and pattern and stomatal index, is changed. In addition, transcriptomic analysis indicates that, although a large number of core cell cycle genes are up-regulated in the ppd silenced lines, the expression of GIF1 and GRF5 genes is not altered (21). In Arabidopsis, GIF1 and GRF5 are positive regulators of primary cell proliferation (23, 26, 27). Our results show that, in the mtbs1-1 mutant, the increased size of lateral organs, including leaves, cotyledon, seeds, and seed pods, was due to prolonged primary cell proliferation, which is consistent with the significant up-regulation of Medicago GIF and GRF genes in expanding leaves. Our results suggest that Medicago BIG SEEDS1 negatively regulates organ size by repressing GIF and GRF gene expression in developing organs, a mechanism different from that of the PPD proteins in Arabidopsis.
Taken together, our work demonstrates that loss-of-function mtbs1 mutant plants exhibited several favorable agronomic traits, such as increased biomass, forage quality, and seed size. Down-regulation of soybean BS1 orthologs also resulted in similar phenotypes as the Medicago bs1 mutants, supporting a conserved role of BS1 in the control of organ size in legumes. In addition, down-regulation of soybean BS1 orthologs resulted in higher seed amino acid contents, another favorable agronomic trait. Thus, our findings that BS1 encodes a key regulator of plant organ size provide opportunities to develop effective strategies to significantly increase seed yield and quality in soybean and possibly other grain crops.
Materials and Methods
Plant Materials.
Homozygous big seeds1-1 (mtbs1-1; M477), mtbs1-2 (FN 1860), and mtbs1-3 (FN 2876) mutants were isolated from a fast neutron bombardment (FNB)-induced deletion mutant collection of M. truncatula cv. Jemalong A17. The mtbs1-1 allele was backcrossed to WT (A17). The BC1 mutant and its descendants were used for phenotypic characterization. F2 mapping populations were generated from crosses between mtbs1-1 and polymorphic ecotype M. truncatula cv. Jemalong A20. A total of 518 individuals (432 WT-like and 86 mutants) were used in a bulk segregant and fine mapping analyses to construct a linkage map of the bs1 locus. Soybean cultivar G. max cv. Williams 82 was used for stable transformation. Arabidopsis ppd deletion mutant (CS16548) and WT (Ler) were grown in a growth chamber (16 h/8 h day/night light cycle; 22 °C/20 °C day/night temperature).
Phenotypic Analysis.
To measure leaf and seed parameters, digital images of leaves and seeds were obtained using a high-resolution scanner (Epson Perfection V700 Photo) and analyzed using ImageJ (https://imagej.nih.gov/ij/) and Tomato Analyzer (28). To accurately measure leaf area of mtbs1-1, we flattened leaflets by cutting the leaf margin before scanning. For time lapse analysis, the youngest emerging leaf of 6-wk-old plants was labeled and imaged daily during a 12-d time course. For analyzing seeds, flowers were labeled, and seed pods were collected at different time points after anthesis. Seeds were removed from seed pods and imaged. A digital balance was used to measure weights of leaves, stems, and seeds.
Forage Quality Analysis.
See SI Appendix for details.
DNA Isolation and PCR Amplification.
Total genomic DNA was isolated from fresh leaf tissues of individual plants grown in the greenhouse using the CTAB (cetyltrimethylammonium bromide) method (29). PCR amplification and PCR products separation using an LI-COR 4300 DNA sequencer were carried out as previously described (30).
Genetic Mapping.
Genetic mapping was carried out as previously described (7). See SI Appendix for details.
Scanning Electron Microscopy.
Scanning electron microscopic imaging analysis of the shoot apical meristem was performed as previously described (31). Hitachi Tabletop Scanning Electron Microscope TM3000 was used for imaging epidermal cells.
Light Microscopy.
Developing seeds dissected from seed pods were cleared in Visikol optical clearing agent for 3 h. Embryos were imaged using a light microscope (Olympus BX41). For cross-sectioning, seeds 25 DPA were fixed in 4% (vol/vol) paraformaldehyde in PBS buffer. After a gradient of alcohol dehydration, tissues were embedded in wax. After sectioning, samples were stained with toluidine blue for 30 s and imaged using a light microscope (Olympus BX41).
Cell Division Rate Measurements.
Leaves from 3-wk-old plants were labeled. One of the lateral leaflets (0 h) was first scanned using a digital scanner to measure the leaf area. Epidermal cells were imaged using Hitachi Tabletop Scanning Electron Microscope (SEM) TM3000. Twenty-four hours later, the other lateral leaflet (24 h) was scanned, and then epidermal cells were imaged. Cell area was measured using Photoshop and ImageJ software. Cell number was calculated by dividing the leaf area by average cell area. Cell division rate was calculated by dividing the increased cell number within 24 h by the cell number at time 0 h. Stomatal index was calculated by dividing the stomata number by the total cell number as previously described (10).
RNA Isolation, RT-PCR, and Quantitative PCR.
Total RNA was isolated using an RNeasy Plant Mini Kit (Qiagen). Reverse transcription was performed using a Qiagen SuperScript II Kit (Qiagen). Quantitative PCR was conducted on a 7900HT Fast Real-Time PCR system (Applied Biosystems) as previously described (31).
Microarray Analysis.
M. truncatula transcriptomic analysis was performed as previously described (32, 33). See SI Appendix for details.
RNA in Situ Hybridization.
RNA in situ hybridization was performed as previously described (31), with minor modifications (see SI Appendix for details).
Chromosome Synteny Analysis.
Chromosome synteny analysis was performed using SyMAP (34).
Plasmids and Plant Transformation.
See SI Appendix for details on plasmids and plant transformation. Primers used in this study are listed in SI Appendix, Table S8.
Amino Acid Content Analysis.
Amino acid content analysis was conducted at the Amino Acid Analysis Service Laboratory, Inc. Seed protein was hydrolyzed, and the hydrolyzed product was analyzed by a Hitachi L8900 Amino Acid Analyzer.
Fatty Acid Composition Analysis.
See SI Appendix for details of fatty acid composition analysis.
Yeast Two-Hybrid Assays.
Bait and prey constructs were cotransformed into the yeast two-hybrid strain GOLD (Clontech), using a Frozen-EZ Yeast Transformation II Kit (Zymo Research). Protein–protein interactions were tested on the SD medium containing X-α-Gal (40 ng/mL) and Aureobasidin A (125 ng/mL), without leucine, tryptophan, adenine, and histidine.
Protein Subcellular Localization.
The BS1-GFP fusion protein was either transiently expressed in tobacco leaves or stably expressed in transgenic Arabidopsis plants. The GFP signal was imaged, using the Leica SP2 laser confocal microscope as previously described (7, 31).
GenBank Accessions.
GenBank accession numbers are as follows: KM668032 (MtBS1), KM668033 (MsBS1), KM668027 (GmBS1), and KM668028 (GmBS2).
Supplementary Material
Acknowledgments
We thank Randy Allen (Oklahoma State University) for critical reading of the manuscript, Guifen Li and Yuhong Tang (Noble Foundation) for assistance with RNA in situ and microarray experiments, Jing Li and David Human (Noble Foundation) for fatty acid measurements, and Jay Gambee (Amino Acid Analysis Service Laboratory, Inc.) for amino acid analysis. Funding of this work was provided by the Samuel Roberts Noble Foundation. Development of the Medicago FNB mutant resources used in this study was funded by National Science Foundation Grant IOS-1127155 (to R.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.J.H. is a Guest Editor invited by the Editorial Board.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. KM668032 (MtBS1), KM668033 (MsBS1), KM668027 (GmBS1), and KM668028 (GmBS2)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611763113/-/DCSupplemental.
References
- 1.Lam HM, et al. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat Genet. 2010;42(12):1053–1059. doi: 10.1038/ng.715. [DOI] [PubMed] [Google Scholar]
- 2.Schmutz J, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463(7278):178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- 3.Masuda T, Goldsmith PD. World soybean production: Area harvested, yield, and long-term projections. Int Food Agribusiness Manage Rev. 2009;12(4):143–162. [Google Scholar]
- 4.Linkies A, Graeber K, Knight C, Leubner-Metzger G. The evolution of seeds. New Phytol. 2010;186(4):817–831. doi: 10.1111/j.1469-8137.2010.03249.x. [DOI] [PubMed] [Google Scholar]
- 5.Li N, Li Y. Ubiquitin-mediated control of seed size in plants. Front Plant Sci. 2014;5:332. doi: 10.3389/fpls.2014.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Li Y. Signaling pathways of seed size control in plants. Curr Opin Plant Biol. 2016;33:23–32. doi: 10.1016/j.pbi.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, et al. Control of dissected leaf morphology by a Cys(2)His(2) zinc finger transcription factor in the model legume Medicago truncatula. Proc Natl Acad Sci USA. 2010;107(23):10754–10759. doi: 10.1073/pnas.1003954107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marinos NG. Embryogenesis of the pea (Pisum sativum). I. The cytological environment of the developing embryo. Protoplasma. 1970;70(3):261–279. [Google Scholar]
- 9.Lemontey C, Mousset-Déclas C, Munier-Jolain N, Boutin JP. Maternal genotype influences pea seed size by controlling both mitotic activity during early embryogenesis and final endoreduplication level/cotyledon cell size in mature seed. J Exp Bot. 2000;51(343):167–175. doi: 10.1093/jexbot/51.343.167. [DOI] [PubMed] [Google Scholar]
- 10.Skirycz A, et al. Pause-and-stop: The effects of osmotic stress on cell proliferation during early leaf development in Arabidopsis and a role for ethylene signaling in cell cycle arrest. Plant Cell. 2011;23(5):1876–1888. doi: 10.1105/tpc.111.084160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nath U, Crawford BCW, Carpenter R, Coen E. Genetic control of surface curvature. Science. 2003;299(5611):1404–1407. doi: 10.1126/science.1079354. [DOI] [PubMed] [Google Scholar]
- 12.Nair RM, Howie JH, Delalande M. 2006 Medicago truncatula cultivars. The Medicago truncatula Handbook, eds Mathesius U, Journet EP, Sumner LW (Samuel Roberts Noble Foundation, Ardmore, OK). Available at www.noble.org/MedicagoHandbook.
- 13.Hepworth J, Lenhard M. Regulation of plant lateral-organ growth by modulating cell number and size. Curr Opin Plant Biol. 2014;17:36–42. doi: 10.1016/j.pbi.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez N, et al. Increased leaf size: Different means to an end. Plant Physiol. 2010;153(3):1261–1279. doi: 10.1104/pp.110.156018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG. Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol. 1999;215(2):407–419. doi: 10.1006/dbio.1999.9443. [DOI] [PubMed] [Google Scholar]
- 16.Poethig RS, Sussex IM. The cellular parameters of leaf development in tobacco: A clonal analysis. Planta. 1985;165(2):170–184. doi: 10.1007/BF00395039. [DOI] [PubMed] [Google Scholar]
- 17.Choi HK, et al. A sequence-based genetic map of Medicago truncatula and comparison of marker colinearity with M. sativa. Genetics. 2004;166(3):1463–1502. doi: 10.1534/genetics.166.3.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai Y, Meng Y, Huang D, Qi Y, Chen M. Origin and evolutionary analysis of the plant-specific TIFY transcription factor family. Genomics. 2011;98(2):128–136. doi: 10.1016/j.ygeno.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Vanholme B, Grunewald W, Bateman A, Kohchi T, Gheysen G. The tify family previously known as ZIM. Trends Plant Sci. 2007;12(6):239–244. doi: 10.1016/j.tplants.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 20.White DWR. PEAPOD regulates lamina size and curvature in Arabidopsis. Proc Natl Acad Sci USA. 2006;103(35):13238–13243. doi: 10.1073/pnas.0604349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez N, et al. A repressor protein complex regulates leaf growth in Arabidopsis. Plant Cell. 2015;27(8):2273–2287. doi: 10.1105/tpc.15.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU. Termination of asymmetric cell division and differentiation of stomata. Nature. 2007;445(7127):501–505. doi: 10.1038/nature05467. [DOI] [PubMed] [Google Scholar]
- 23.Horiguchi G, Kim GT, Tsukaya H. The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J. 2005;43(1):68–78. doi: 10.1111/j.1365-313X.2005.02429.x. [DOI] [PubMed] [Google Scholar]
- 24.Cuéllar Pérez A, et al. The non-JAZ TIFY protein TIFY8 from Arabidopsis thaliana is a transcriptional repressor. PLoS One. 2014;9(1):e84891. doi: 10.1371/journal.pone.0084891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pauwels L, et al. NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature. 2010;464(7289):788–791. doi: 10.1038/nature08854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ha Lee B, Hoe Kim J. Spatio-temporal distribution patterns of GRF-INTERACTING FACTOR expression and leaf size control. Plant Signal Behav. 2014;9:9. doi: 10.4161/psb.29697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JH, Choi D, Kende H. The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth in Arabidopsis. Plant J. 2003;36(1):94–104. doi: 10.1046/j.1365-313x.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 28.Brewer MT, et al. Development of a controlled vocabulary and software application to analyze fruit shape variation in tomato and other plant species. Plant Physiol. 2006;141(1):15–25. doi: 10.1104/pp.106.077867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA. 1984;81(24):8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu JB, Bai GH, Cai SB, Ban T. Marker-assisted characterization of Asian wheat lines for resistance to Fusarium head blight. Theor Appl Genet. 2006;113(2):308–320. doi: 10.1007/s00122-006-0297-z. [DOI] [PubMed] [Google Scholar]
- 31.Ge L, Peng J, Berbel A, Madueño F, Chen R. Regulation of compound leaf development by PHANTASTICA in Medicago truncatula. Plant Physiol. 2014;164(1):216–228. doi: 10.1104/pp.113.229914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tadege M, et al. STENOFOLIA regulates blade outgrowth and leaf vascular patterning in Medicago truncatula and Nicotiana sylvestris. Plant Cell. 2011;23(6):2125–2142. doi: 10.1105/tpc.111.085340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uppalapati SR, et al. Loss of abaxial leaf epicuticular wax in Medicago truncatula irg1/palm1 mutants results in reduced spore differentiation of anthracnose and nonhost rust pathogens. Plant Cell. 2012;24(1):353–370. doi: 10.1105/tpc.111.093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soderlund C, Bomhoff M, Nelson WM. SyMAP v3.4: A turnkey synteny system with application to plant genomes. Nucleic Acids Res. 2011;39(10):e68. doi: 10.1093/nar/gkr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.