Significance
Cold storage is widely used to extend shelf-life of agriculture products. For tomato, this handling results in reduced flavor quality. Our work provides major insights into the effects of chilling on consumer liking, the flavor metabolome and transcriptome, as well as DNA methylation status. Transcripts for some key volatile synthesis enzymes and the most important ripening-associated transcription factors are greatly reduced in response to chilling. These reductions are accompanied by major changes in the methylation status of promoter regions. Transient increases in DNA methylation occur during chilling. Our analysis provides insight into the molecular mechanisms of tomato fruit flavor loss caused by chilling.
Keywords: fruit quality, methylome, transcriptome
Abstract
Commercial tomatoes are widely perceived by consumers as lacking flavor. A major part of that problem is a postharvest handling system that chills fruit. Low-temperature storage is widely used to slow ripening and reduce decay. However, chilling results in loss of flavor. Flavor-associated volatiles are sensitive to temperatures below 12 °C, and their loss greatly reduces flavor quality. Here, we provide a comprehensive view of the effects of chilling on flavor and volatiles associated with consumer liking. Reduced levels of specific volatiles are associated with significant reductions in transcripts encoding key volatile synthesis enzymes. Although expression of some genes critical to volatile synthesis recovers after a return to 20 °C, some genes do not. RNAs encoding transcription factors essential for ripening, including RIPENING INHIBITOR (RIN), NONRIPENING, and COLORLESS NONRIPENING are reduced in response to chilling and may be responsible for reduced transcript levels in many downstream genes during chilling. Those reductions are accompanied by major changes in the methylation status of promoters, including RIN. Methylation changes are transient and may contribute to the fidelity of gene expression required to provide maximal beneficial environmental response with minimal tangential influence on broader fruit developmental biology.
The modern commercial tomato is widely perceived as lacking flavor and is a major source of consumer dissatisfaction. Postharvest handling and retail systems are major contributors to poor flavor, particularly the commonly used practice of chilling fruit. Many consumers store purchased fruits in the refrigerator, further contributing to flavor deterioration (1). Tomato flavor is produced by a combination of sugars, acids, and volatiles (2, 3). Production of flavor-associated volatiles is sensitive to temperatures below 12 °C and loss of volatiles has been observed during cold storage (4, 5). In contrast, taste-related chemicals, sugars, and acids, are not significantly affected by cold storage (6, 7).
Flavor-imparting volatiles are derived from amino acids, fatty acids, and carotenoids, and multiple genes essential for their synthesis have been functionally validated (8). For example, C6 volatiles are synthesized by a lipoxygenase, LoxC (9), hydroperoxide lyase (HPL) (10), and ALCOHOL DEHYDROGENASE2 (ADH2) (11). Volatile esters are synthesized by an ALCOHOL ACETYLTRANSFERASE1 (AAT1) (12). The CAROTENOID CLEAVAGE DIOXYGENASE1 (CCD1) contributes to synthesis of volatile apocarotenoids (13). Synthesis of many tomato flavor volatiles increases during fruit ripening. The ripening mutants, Colorless nonripening (Cnr) and nonripening (nor) produce substantially lower levels of lipid-derived volatiles than wild-type (14). One of the main transcription factors (TFs) mediating ripening is RIPENING INHIBITOR (RIN). RIN-binding sites are frequently demethylated upon ripening and binding occurs in concert with demethylation (15), indicating that promoter methylation state influences expression of RIN-dependent genes.
Transcriptomic analysis has identified genes associated with tomato fruit chilling. For example, 14-d chilling of Micro-Tom fruit resulted in differential expression of many genes related to photosynthesis, lipid metabolism, cell wall modification, and antioxidant production (16). Although this work has provided insights into chilling-induced gene expression, it did not directly address the molecular basis for loss of flavor volatiles. Several groups have analyzed changes in LOX activity and C6 volatiles in response to cold storage; reduced production of C6 volatiles cannot be directly explained by LOX activity alone (1, 5).
These observations provide a framework for analysis of the molecular mechanism underlying chilling-induced loss of tomato flavor. In addition to the economic impact of flavor quality loss, the tomato fruit provides an ideal system in which to examine the effects of environmental stress on a genome scale. Here, we provide a comprehensive analysis of the effect of chilling on the transcriptome and flavor metabolome. The large transcriptional reprogramming that occurred in response to chilling and following a recovery period was correlated with alterations in DNA methylation.
Results
Cold Storage Influences Flavor-Related Volatiles.
Chilling is known to significantly reduce flavor-associated volatile content (1, 4, 5). To understand the molecular mechanisms responsible for volatile loss during cold storage, we first determined the length of time needed to significantly alter volatile synthesis in two tomato cultivars, Ailsa Craig and FLA 8059, an heirloom variety and a modern inbred (17), respectively. Red ripe fruits were stored at 5 °C for 1, 3, or 7 d followed by a recovery period of either 1 or 3 d at 20 °C, and volatiles were measured. Compared with the day of harvest (day 0), no significant loss of flavor volatile content was observed after 1 or 3 d of cold storage, but after 7 d in the cold, volatile contents were significantly lower in both cultivars (SI Appendix, Fig. S1A). Even after a 3-d recovery period, volatiles remained significantly lower than the day 0 sample. To determine whether the reduction in volatile contents following cold storage affected flavor quality, a consumer panel was conducted. Ailsa Craig fruits chilled for 7 d followed by a 1-d recovery at 20 °C were compared with fruits harvested at the same stage of ripeness on the day before the consumer panel. Chilled fruits were rated significantly lower for overall liking (P < 0.03), indicating that the chilling treatment negatively affected flavor.
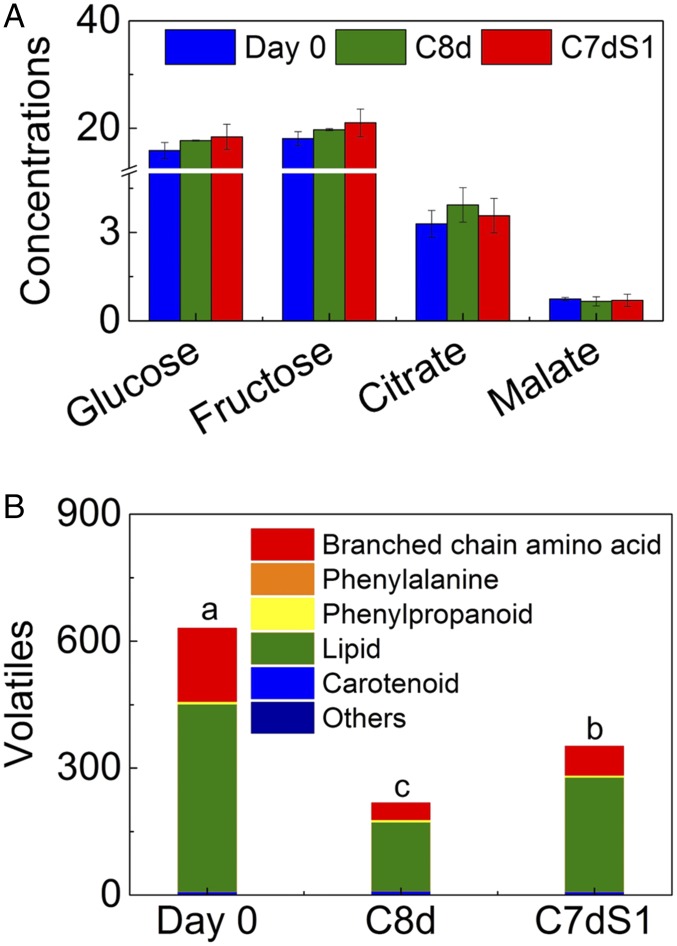
Because fruit flavor is determined by a combination of sugars, acids, and volatiles, contents of these compounds were analyzed in Ailsa Craig fruits. Consistent with previous studies (6, 8), there were no significant differences in glucose, fructose, malic acid, and citric acid (Fig. 1A), indicating that the lower flavor scores in chilled fruit are a result of reduced volatile content. Sixty-six volatiles were selected for further analysis (SI Appendix, Table S1). Based on volatile contents, fruits at different stages were clearly separated by principal component analysis (PCA) and biological replicates clustered together (SI Appendix, Fig. S2). Day 0 fruit had the highest volatile content. Volatiles in 8-d chilled fruits were decreased by 65% relative to day 0 (Fig. 1B). Although a 1-d recovery at 20 °C resulted in increased volatile content, the level was still significantly lower than on the day of harvest.
Fig. 1.
Changes in Ailsa Craig sugars, organic acids (mg⋅g−1 FW) and volatiles (ng⋅g−1 FW h−1) in response to cold storage. (A) Effect of cold storage on sugar and organic acid content. (B) Changes in volatiles derived from various pathways. Day 0, red ripe tomato fruit at harvest; C8d, fruit following 8 d of cold storage; C7dS1, fruit stored in the cold for 7 d followed by a 1-d recovery at 20 °C. Significant differences (P < 0.05) are denoted by letters. Error bars indicate means ± SE; three biological replicates.
Considering that fruit volatile content is sensitive to environmental factors and can vary between cultivars (3), we examined the effects of chilling over two seasons in two different cultivars, identifying 12 volatiles that were significantly influenced by cold storage in both seasons and cultivars (SI Appendix, Fig. S1B). These volatiles grouped into two clusters, one having the highest contents at day 0 and the other having the highest contents during cold storage. Volatiles with significantly lower content during and after cold storage included those derived from amino acids (isovaleraldehyde, 3-methyl-1-butanol, 2-methyl-1-butanol and butyl acetate) and lipids [hexanal, (Z)-3-hexenal and (E)-2-hexenal]. Contents of the carotenoid-derived volatiles 6-methyl-5-hepten-2-one (MHO) and geranial significantly increased during cold storage. Cold-induced increases were also observed for other carotenoid-derived volatiles, including neral, geranylacetone, and pseudoionone, as well as phenylalanine-associated volatiles (methylsalicylate, 1-nitro-2-phenylethane and phenylacetaldehyde), although significant differences were not always observed in both cultivars in both seasons (SI Appendix, Table S1).
Response of the Transcriptome to Chilling.
To investigate the molecular basis for flavor volatile changes following cold storage, transcriptome analysis was performed using RNA sequencing (RNA-Seq). Differentially expressed genes (DEGs) were grouped into MapMan BINs (18). The percentage of up- and down-regulated genes in each sample within each BIN is indicated in SI Appendix, Fig. S3. A Wilcoxon test was applied to comprehensively investigate changes in functional categories between samples using PageMan (19). Functional classes associated with major carbohydrate metabolism and photosynthesis were significantly down-regulated during cold storage and subsequent transfer to ambient temperature (SI Appendix, Fig. S4). Similar suppression was also found for lipid metabolism, amino acid metabolism, and secondary metabolism BINs. Meanwhile, functional categories with high representation in fruit during and after cold storage included RNA and protein metabolism (SI Appendix, Fig. S4).
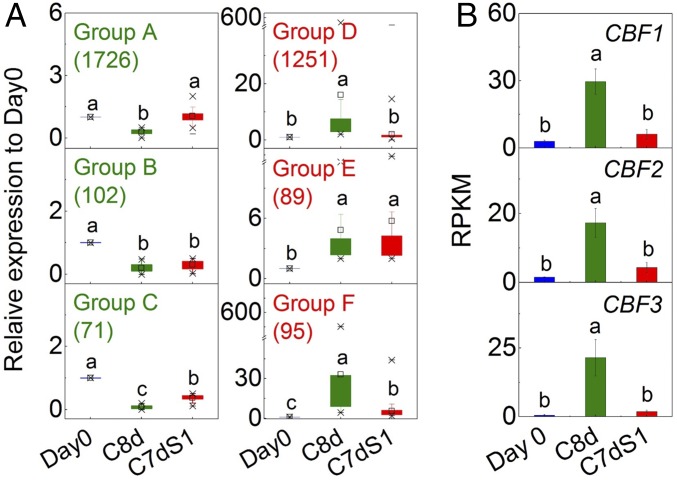
There were 25,879 genes whose expression was detected in fruit. The number of those genes whose expression was significantly altered in response to chilling is shown in SI Appendix, Fig. S5A. Fruit samples could be clearly separated using PCA (SI Appendix, Fig. S5B), similar to the pattern derived from volatile content (SI Appendix, Fig. S2). DEGs were classified into six groups based on their fruit expression patterns (Fig. 2A). There were 1,899 significantly less-abundant transcripts (groups A, B, and C) and 1,435 significantly more abundant transcripts (groups D, E, and F) following cold storage (C8d) compared with day 0 [>twofold change, false-discovery rate (FDR) < 0.05]. After 1 d of recovery (C7dS1), 184 transcripts were significantly more abundant and 173 transcripts were less abundant compared with the fruit at day 0 (Fig. 2A). Gene ontology (GO) term enrichment analysis indicated that genes involved in lipid metabolism and transcription regulation were highly enriched (SI Appendix, Table S2). These data indicate that expression of a large number of genes is significantly but transiently regulated by cold, with expression of many of them restored to levels close to those of day 0 upon returning to ambient temperature.
Fig. 2.
Transcriptional analysis of Ailsa Craig fruit in response to cold storage. (A) Box plot of relative expression of six classes of DEGs. RPKM at day 0 was set as 1. The number of genes in each group is shown in parentheses. (B) Expression of CBF genes in response to cold storage. Blue bar: day 0, red ripe tomato fruit at harvest; green bar: C8d, 8 d of cold storage; red bar: C7dS1, 7 d of cold storage followed by one day recovery at 20 °C. Significant differences (FDR < 0.05) are denoted by different letters. Error bars indicate means ± SE; three biological replicates.
The C-repeat binding factor(CBF)/dehydration responsive-element binding factor-mediated cold signaling regulatory cascade is conserved in plants (20). Three CBF genes—CBF1, CBF2, and CBF3—have been characterized in tomato (21). Their transcripts were all significantly higher during chilling, returning to unchilled levels 1 d after return of the fruit to 20 °C (Fig. 2B). Examination of the tomato homologs of a set of CBF-regulated Arabidopsis genes, the CBF regulon (22), indicated that a subset of those genes (27 of 172) were significantly altered in expression although the direction of the alteration was often opposite to what was observed in Arabidopsis (SI Appendix, Table S3). The return of CBF-associated genes to day 0 levels following 1 d of recovery indicates that the chilling response was activated and subsequently appropriately shut off.
Expression of Genes Related to Volatile Synthesis.
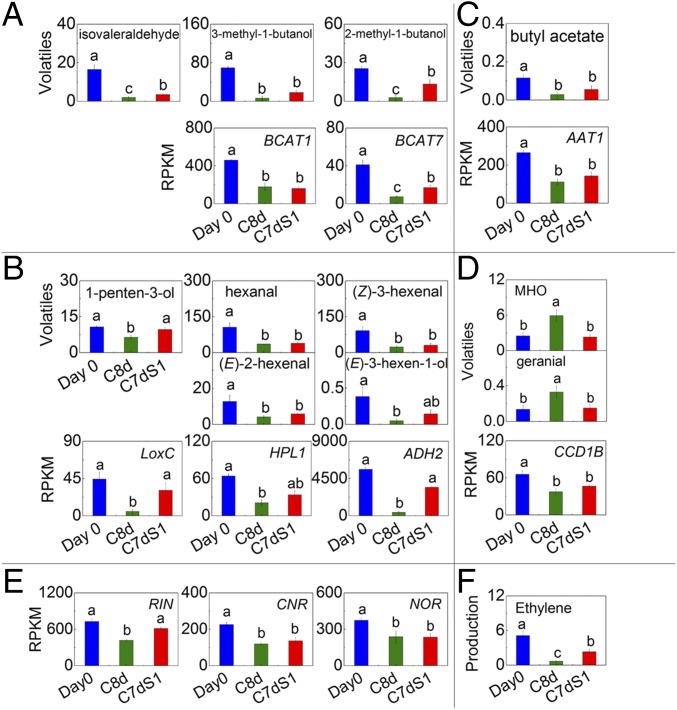
We next looked at the expression of genes encoding enzymes involved in synthesis of volatiles that were significantly altered in response to chilling. Isovaleraldehyde, 3-methyl-1-butanol, and 2-methyl-1-butanol are believed to be derived from the immediate precursors of branched-chain amino acids. Branched-chain aminotransferases (BCATs) catalyze the last step in synthesis and the first step in catabolism of these amino acids (23, 24). Transcripts of two members of the BCAT gene family were significantly lower after 8 d of chilling and remained low after 1 d of recovery, in accord with the volatile contents (Fig. 3A). The C5 volatile 1-penten-3-ol is dependent upon LoxC action (10), whereas the C6 volatiles require sequential activity of LoxC and HPL (9). The final step in the pathway, conversion of aldehydes to alcohols, requires ADH2 activity (11). Transcripts encoding all three of these enzymes were significantly lower in the C8d samples and partially recovered after 1 d at room temperature (Fig. 3B). Butyl acetate and AAT1 transcript similarly showed parallel reductions following chilling and did not return to day 0 levels after the 1-d recovery (Fig. 3C).
Fig. 3.
Relationships of volatiles and associated transcripts in response to chilling of red ripe Ailsa Craig fruit. Volatiles (ng⋅g−1 FW h−1) derived from (A) branched-chain amino acids, (B), fatty acids, (C) esters, (D) carotenoids. (E) Expression of ripening-related TFs. (F) Ethylene production. Blue bars, day 0; green bars, C8d; red bars, C7dS1. Significant differences in volatiles, ethylene (P < 0.05) and gene expression (FDR < 0.05) are denoted by different letters. Error bars indicate means ± SE; three biological replicates.
Compared with fruits at day 0, transcripts associated with carotenoid metabolism were less abundant during and after cold storage (Fig. 3D and SI Appendix, Fig. S6). We previously showed that CCD1B cleaves multiple carotenoids to release apocarotenoid volatiles (15). CCD1B was reduced following chilling. However, concentrations of MHO and geranial, two apocarotenoid volatiles derived from lycopene, were significantly higher following cold storage (Fig. 3D and SI Appendix, Fig. S1B). These results suggest that production of these volatiles may occur via an independent pathway, most likely nonenzymatic oxidation of carotenoids.
Transcriptional and Hormonal Mediators of Ripening.
Many of the most important regulators of ripening-associated transcription have been identified (25, 26). We examined the patterns of expression of a set of the most important TFs controlling ripening and identified several whose transcript abundance was significantly altered in response to chilling (Fig. 3E). RIN mRNA levels were lowest during cold storage and recovered after transfer to 20 °C, consistent with both extended shelf-life in cold storage and reduced volatile and quality characteristics. Similarly, reduced low-temperature transcript content was observed for direct targets of RIN, including FUL1, HB-1, NOR, and CNR (Fig. 3E and SI Appendix, Fig. S7). Changes in these ripening-related TFs are consistent with reduced expression of many of their downstream targets during cold storage.
Ethylene regulates many aspects of ripening (27). Contents of multiple volatiles were significantly reduced in the ethylene-insensitive mutant Nr compared with its isogenic parent, Pearson (SI Appendix, Table S4). Ethylene synthesis was significantly reduced during cold storage with partial recovery after transfer to 20 °C (Fig. 3F), showing the same pattern as was observed for total volatile content (Fig. 1C). The main contributors to ripening-associated ethylene synthesis are aminocyclopropane-1-carboxylate synthase (ACS2 and ACS4) and aminocyclopropane-1-carboxylate oxidase (ACO1) (28–30). There were no significant differences in ACS2 or ACO1 transcripts and ACS4 transcript was higher in chilled fruit (SI Appendix, Table S5), suggesting that ethylene synthesis during chilling is likely posttranscriptionally regulated.
DNA Methylation Dynamics in Response to Chilling.
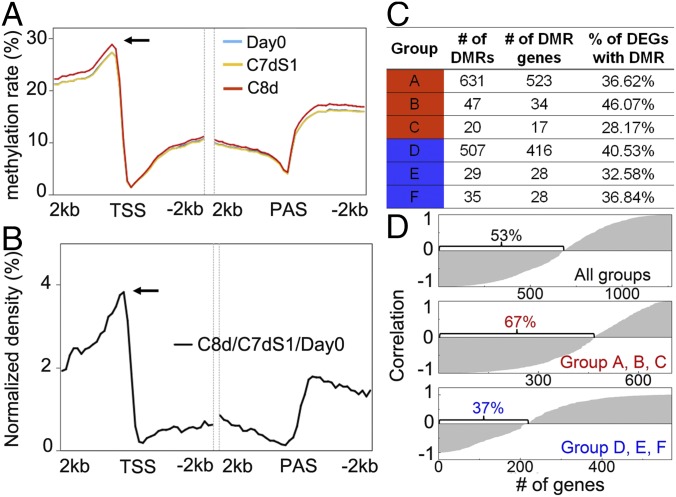
Epigenetic regulation is associated with stress responses, such as drought, salinity, heat, and cold in plants (31). Because DNA methylation also has an essential role in control of tomato fruit ripening (15), we examined potential alterations in DNA methylation associated with chilling and, specifically, with chilling-responsive genes. Whole-genome bisulfite sequencing was performed on the samples used for metabolite and RNA-Seq analysis to assess occurrence of cytosine methylation. Average cytosine methylation rates increased in promoter regions after 8 d of chilling and returned after 1 d of recovery to levels similar to day 0 (Fig. 4A). A total of 30,918 differentially methylated regions (DMRs) were identified in the three analyzed tissues, of which 9,951 DMRs were observed in promoter regions. As in fruit development (15), DMRs were highly enriched in the gene-rich euchromatin regions (SI Appendix, Fig. S8) and in gene regions highly enriched in promoters, peaking at 300–400 bp upstream of transcription start sites (TSS) (Fig. 4B). DMRs were classified into groups based on transcript abundance patterns in response to chilling shown in Fig. 2A (Fig. 4C and SI Appendix, Table S6). GO enrichment analysis showed that “metabolic process,” glycolysis, and lipid metabolism were highly enriched for groups A, B, and C, consistent with the categories for DEGs (Fig. 2A and SI Appendix, Table S2). For groups D, E, and F, GO terms associated with protein ubiquitination and cellular glucan metabolic process were enriched (SI Appendix, Table S6). Notably, there are multiple DMRs in certain genes upstream of the TSS, indicating that some genes are highly methylated in response to chilling or removal from chilling. For groups A, B, and C, approximately 67% of genes exhibited a negative correlation between the level of methylation and transcript abundance (Fig. 4D and SI Appendix, Table S7).
Fig. 4.
DNA methylation analysis of Ailsa Craig fruit in response to cold storage. (A) Change in cytosine methylation rate in genic regions (bin size, 100 bp). Arrow indicates methylation peak at 300–400 bp upstream of the TSS; PAS, polyadenylation site. (B) Distribution of DMRs in regions of gene promoters and bodies (bin size, 100 bp). (C) Number of DMRs and genes with DMRs in the promoter regions in the six groups defined in Fig. 2A. Percentage of DEGs with DMR was represented as a ratio between number of DMR genes and number of DEGs in each group shown in Fig. 2A. Groups A, B, and C are genes whose transcripts were significantly reduced by cold storage, whereas groups D, E, and F are genes induced by cold storage. (D) Correlation of methylation and transcripts. Percentage is the number of genes with negative correlation between methylation and transcripts divided by total number of genes in group.
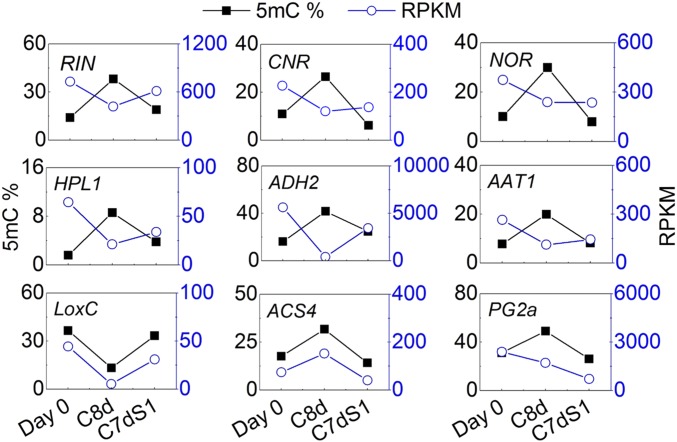
Inverse patterns between transcripts and methylation level were observed for RIN, and some of its direct targets, including CNR, NOR, HPL1, ADH2, and AAT1 (15, 32); cold storage increased methylation and decreased transcripts (Fig. 5). Other RIN targets, such as HB-1, FUL1, and PSY1 also showed a similar pattern (SI Appendix, Table S8). This pattern was not universal; LoxC, ACS4, and PG2a did not show this pattern (Fig. 5). Although we cannot attribute a causal relationship between cytosine methylation changes and transcript abundance with the data in hand, the results clearly indicate a major alteration in DNA methylation in response to chilling stress, often coinciding with expression changes of genes known to contribute to fruit maturation and volatile synthesis (SI Appendix, Fig. S9).
Fig. 5.
Methylation level and transcript abundance of RIN-interacting genes in Ailsa Craig fruit in response to cold storage. Black line represents methylation level. Blue line represents transcript abundance.
DML2, encoding the DEMETER-like DNA demethylase, is highly induced at the onset of fruit ripening and its repression resulted in DNA hypermethylation and substantially delaying ripening (33). Therefore, we analyzed DML2 expression in response to chilling and subsequent recovery at 20 °C. Reduced transcript level of DML2 was observed during cold storage with an increase in fruit transferred to 20° for 1 d (SI Appendix, Fig. S10), consistent with the changes in whole-genome cytosine methylation levels. As such, DML2 may contribute to both developmental and chilling-associated changes in fruit DNA methylation, with corresponding effects on both overall fruit maturation and specific quality characteristics, including flavor-associated volatiles.
Discussion
Although modern high-yielding varieties of tomatoes are not as flavorful as older varieties (8), a significant part of the perceived problem in modern commercial tomatoes can be attributed to postharvest chilling (4). Exposure to temperatures as low as 4 °C causes severe damage to flavor quality (1). To determine the underlying molecular basis for that flavor deterioration, we undertook a systematic analysis of fruits exposed to chilling temperature, examining changes in the flavor metabolome, the transcriptome, and alterations in patterns of DNA methylation.
Chilling did not alter fruit sugar and acid contents (Fig. 1). However, significant loss of flavor volatiles was observed for fruit stored at 5 °C for 8 d. Even after a 1-d recovery period at 20 °C, volatile composition was still significantly lower than in unchilled fruit, resulting in lower overall consumer liking. Twelve volatiles were significantly altered by cold storage in multiple seasons and cultivars. We observed significant reductions in the contents of volatiles associated with the C5/C6, branched-chain amino acid and ester pathways (Fig. 1C). These reductions were correlated with significantly lower transcript abundance of genes whose products are essential for their synthesis. Although transcript content of some of these genes increased after moving fruits to room temperature for 24 h, most remained significantly lower than in unchilled fruits (Fig. 3). Unlike sugars and acids, volatiles are freely diffusible through the stem scar and must be constantly replenished to maintain appropriate levels within harvested fruit. Because expression of genes encoding essential biosynthetic enzymes is significantly lower at 5 °C, chilling leads to depletion of important flavor volatiles and reduced flavor quality.
Although total fruit volatile content was significantly lower in chilled fruits, a subset of flavor volatiles increased during chilling. The contents of two volatiles derived from lycopene cleavage, MHO and geranial, were higher after chilling (Fig. 3D and SI Appendix, Fig. S1B). These volatiles are produced by oxidative cleavage of lycopene, which makes up ∼85% of the carotenoid pool in a ripe fruit (13, 34, 35). Cleavage can be either enzymatic, catalyzed by carotenoid cleavage dioxygenases, or nonenzymatic. The CCD1B transcript is significantly lower in chilled fruit, as are transcripts of multiple carotenoid biosynthetic pathway genes (Fig. 3D and SI Appendix, Fig. S6). The most likely explanation for increased MHO and geranial content is chilling-induced nonenzymatic carotenoid oxidation. Production of reactive oxygen species is one of the main responses of fruit subjected to abiotic stresses, such as high light and cold, and carotenoids were reported to be the main quenchers for singlet oxygen (36, 37). Nonenzymatic oxidation of carotenoids is the main mechanism for production of apocarotenoid volatiles in Arabidopsis exposed to high light stress (38).
Compared with ripe fruit on the day of harvest, RNA-Seq analysis detected 5,413 DEGs during cold storage, and 528 DEGs after recovery at ambient temperature (SI Appendix, Fig. S5A), indicating that expression of many genes is sensitive to temperature shift. A global view of transcriptional changes showed that carbohydrate metabolism was significantly down-regulated (Fig. 2A), indicating that energy metabolism is suppressed during cold storage. Our transcriptome data are consistent with previously described proteome changes in chilled tomato fruit, in which proteins belonging to energy metabolism BINs were significantly suppressed (7). Functional classes associated with amino acids, fatty acids, and secondary metabolism were reduced by cold storage followed by a recovery after transfer to 20 °C.
In line with the major reprogramming of gene expression during chilling, multiple TFs associated with fruit development exhibited significantly altered transcript abundance in response to chilling (Fig. 3E). In particular, TFs that are essential to ripening are down, including RIN (39), NOR (40), and CNR (41). Reduced expression of these TFs in response to chilling would be expected to globally reduce many ripening-associated processes, permitting the organ to redirect metabolic resources into more suitable stress responses. In addition to these TFs, transcripts of FUL1, a RIN-interacting MADS domain protein affecting aspects of ripening, including volatile synthesis (42), as well as HB-1, a positive regulator of ethylene synthesis (43), are down during chilling. Expression of other TFs that regulate specific aspects of fruit development, including TAGL1 (44, 45) and AP2a (46, 47), increased during chilling. Notably, AP2a is a negative regulator of ethylene synthesis and fruit ripening. Thus, an increase in its expression is consistent with the observed reduction in expression of ethylene synthesis genes.
The tomato chilling response also includes CBF transcriptional activators. Transcripts of all three CBF genes (CBF1–3) were significantly elevated in response to chilling and returned to basal levels upon return to ambient temperature (Fig. 2B). Previous comparison of CBF action in tomato indicated that the tomato CBF regulon is considerably smaller than its Arabidopsis counterpart (21). We examined chilling-induced expression of all of the closest homologs of the Arabidopsis regulon. The Arabidopsis regulon consists of 133 up-regulated and 39 down-regulated genes (22). Of the combined 172 genes, 27 exhibited significantly different expression during chilling and that differential expression was often in the opposite direction as in Arabidopsis (SI Appendix, Table S4). Thus, although there is a CBF-associated response to chilling, that response is substantially different from the chilling tolerant Arabidopsis.
Finally, tomato fruit ripening is associated with major alterations in DNA (cytosine) methylation, particularly in RIN-binding promoter regions, likely mediated by DML2. This epigenetic reprogramming is essential for ripening (15, 33). We identified 30,918 DMRs in chilled fruits, with 9,951 being in promoter regions. Interestingly, much of the methylation was transient, returning to a prechilled state after 24 h at room temperature. Notably, RIN and many—although not all—of its target genes, displayed an inverse correlation between promoter methylation and transcript abundance (Fig. 5 and SI Appendix, Fig. S6). Our data do not permit us to conclude whether DNA methylation is the cause or an effect of reduced expression. We can conclude that chilling stress causes major changes in the methylation status of the genome and many of these changes occur in promoters of genes known to contribute to ripening, quality, and flavor volatile synthesis, and are altered in response to chilling. It is possible that this methylation associated with reduced expression is an added level of insurance against expression of genes that are not essential to respond to this environmental stress. This point is especially relevant, given that many of the chilling-induced alterations in transcriptome activity appear to operate through broadly acting fruit developmental regulators, such as RIN. It will be interesting to determine whether stress-induced changes in methylation are fruit-specific or generalized to other biotic and abiotic stresses in the tomato plant.
In conclusion, we have demonstrated that chilling of ripe tomato fruits results in significantly reduced flavor quality. That reduction is associated with major alterations in contents of volatiles associated with consumer liking. Reduced levels of specific volatiles are associated with greatly reduced levels of transcripts for some key volatile synthesis enzymes. Expression of genes encoding TFs that are essential for ripening, including RIN, NOR, and CNR, are also reduced in response to chilling and may be responsible for reduced transcript levels in the many genes during chilling. Those reductions are accompanied by major changes in the methylation status of promoters, including those of the aforementioned TFs, and may contribute to the fidelity of gene expression required to provide maximal beneficial environmental response with minimal tangential influence on broader fruit developmental biology.
Materials and Methods
Tomato Fruit Treatment.
Tomatoes were grown in a greenhouse on the University of Florida campus. Fruit at the full red ripe stage, free of visual defects, and uniform in size were selected, washed with water, and air dried. Tomatoes were divided into three groups: (i) stored at 5 °C with 92% relative humidity for 7 d, and then transferred to 20 °C for 1-d recovery; (ii) held at 5 °C for 8 d without recovery at ambient temperature; and (iii) fruit on the day of harvest as controls. The first two groups were harvested 8 d before the third group, and fruit were subjected to consumer test on the day of the third harvest.
Consumer Tests Analysis.
All consumer tests were approved by the University of Florida Institutional Review Board. Taste panels consisted of 76 persons. Panelists rated overall liking of chilled and unchilled Ailsa Craig tomatoes using a hedonic general labeled magnitude scale, as described previously (3). Chilled tomatoes were less liked than unchilled tomatoes when panelists’ liking scores were compared as matched pairs using a one-tailed t test, sign test, or Wilcoxon signed rank test. After measurement of flavor quality, pericarp tissue was frozen in liquid nitrogen and stored at −80 °C until sugar and acid analysis.
Ethylene Production Analysis.
Fruit were sealed in 500-mL containers for 1 h, and 1 mL of headspace gas samples were analyzed using a HP5890 series II gas chromatograph (Hewlett Packard) equipped with a flame ionization detector. The temperature program was 110 °C for oven, 110 °C for injection port, and 130 °C for detector.
Volatile Analysis.
Volatile analysis was performed according to the method described previously (48), with three biological replicates of six pooled fruit each. Chopped ripe tomatoes were enclosed in glass tubes flowing with filtered air for 1 h, and volatiles were extracted using a Super Q column. Volatiles were eluted with methylene chloride using nonyl acetate as an internal control, and separated on an Agilent 6890N gas chromatograph equipped with a DB-5 column (Agilent). Retention times were compared with authentic standards, and volatile contents were calculated as ng⋅g−1 fresh weight (FW) h−1.
Sugars and Acids Analysis.
Contents of glucose, fructose, malic acid, and citric acid were determined as described previously (35). Analysis was performed on three biological replicates, each consisting of six fruit.
RNA Isolation and High-Throughput Sequencing.
RNA was extracted using an RNeasy Mini kit (Qiagen) following the manufacturer’s instructions, and quality was monitored by gel electrophoresis and A260/A280. Libraries for high-throughput Illumina strand-specific RNA-Seq were prepared as described previously (49). Three biological replicates for each treatment were prepared, each consisting of multiple pooled fruits. The statistics of sequencing quality and correlation data illustrated the global relative relationship between biological replicates and among fruit samples are presented in SI Appendix, Tables S10 and S11.
DNA Sequencing.
Illumina DNA sequencing was performed on a HiSeq2500 using reagents and protocols provided by Illumina, alignment to the reference tomato genome (v2.40) and determination of expression for each gene were performed as described previously (17).
DNA Methylation.
Genomic DNA was extracted using a Qiagen DNeasy Plant Mini Kit (https://www.qiagen.com/us), and quality was monitored by gel electrophoresis and a ratio of A260/A280. Bisulfite conversion of tomato genomic DNA and Illumina sequencing were performed as described previously (15). All of the chilling-related methylome data generated for this paper are archived at single-base resolution in the tomato epigenome database at ted.bti.cornell.edu/epigenome/.
Statistical Analysis.
Volatile emissions were subjected to one-way ANOVA analysis (OriginPro 9.0, Microcal Software). PCA was selected to provide an overview of changes in detected volatiles and global gene-expression patterns in response to cold storage (www.metaboanalyst.ca). DEGs were defined by reads per kilobase per million (RPKM) fold-change > 2 and FDR < 0.05. GO enrichment (geneontology.org/) was performed. DEGs were classified into functional categories defined by MapMan BINs (mapman.gabipd.org). Wilcoxon test and Benjamini–Hochberg correction were performed to provide a statistical-based graphic display of enriched BINs (19).
Supplementary Material
Acknowledgments
We thank Drs. Charles Sims and Asli Odabasi for their help with the consumer panel; Dawn Bies for help with volatile collection; Mark Taylor for help with plant care; and Michael Thomashow for invaluable advice. This work was supported by National Science Foundation Grants IOS-0923312 (to H.J.K., J.J.G., and Z.F.); National Key Research and Development Program 2016YFD0400101; Program of International Science and Technology Cooperation Grant 2011DFB31580; the New Star Program from Zhejiang University; and the China Scholarship Council.
Footnotes
The authors declare no conflict of interest.
Data deposition: All of the chilling-related methylome data generated for this paper are archived at single-base resolution in the tomato epigenome database at ted.bti.cornell.edu/epigenome/. Raw sequence reads of methylome and transcriptome have also been deposited in NCBI Sequence Read Archive (SRA) under accession numbers SRP082267 and SRP082266, respectively.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613910113/-/DCSupplemental.
References
- 1.Renard CM, Ginies C, Gouble B, Bureau S, Causse M. Home conservation strategies for tomato (Solanum lycopersicum): Storage temperature vs. duration—Is there a compromise for better aroma preservation? Food Chem. 2013;139(1-4):825–836. doi: 10.1016/j.foodchem.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin EA, Scott JW, Shewmaker CK, Schuch W. Flavor trivia and tomato aroma: Biochemistry and possible mechanisms for control of important aroma components. HortScience. 2000;35(6):1013–1022. [Google Scholar]
- 3.Tieman D, et al. The chemical interactions underlying tomato flavor preferences. Curr Biol. 2012;22(11):1035–1039. doi: 10.1016/j.cub.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Maul F, et al. Tomato flavor and aroma quality as affected by storage temperature. J Food Sci. 2000;65(7):1228–1237. [Google Scholar]
- 5.Bai J, et al. Chilling and heating may regulate C6 volatile aroma production by different mechanisms in tomato (Solanum lycopersicum) fruit. Postharvest Biol Technol. 2011;60(2):111–120. [Google Scholar]
- 6.Raffo A, et al. Impact of different distribution scenarios and recommended storage conditions on flavor related quality attributes in ripening fresh tomatoes. J Agric Food Chem. 2012;60(42):10445–10455. doi: 10.1021/jf3028528. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Bel P, et al. Proteome changes in tomato fruits prior to visible symptoms of chilling injury are linked to defensive mechanisms, uncoupling of photosynthetic processes and protein degradation machinery. Plant Cell Physiol. 2012;53(2):470–484. doi: 10.1093/pcp/pcr191. [DOI] [PubMed] [Google Scholar]
- 8.Klee HJ, Tieman DM. Genetic challenges of flavor improvement in tomato. Trends Genet. 2013;29(4):257–262. doi: 10.1016/j.tig.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Chen G, et al. Identification of a specific isoform of tomato lipoxygenase (TomloxC) involved in the generation of fatty acid-derived flavor compounds. Plant Physiol. 2004;136(1):2641–2651. doi: 10.1104/pp.104.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen J, et al. A 13-lipoxygenase, TomloxC, is essential for synthesis of C5 flavour volatiles in tomato. J Exp Bot. 2014;65(2):419–428. doi: 10.1093/jxb/ert382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speirs J, et al. Genetic manipulation of alcohol dehydrogenase levels in ripening tomato fruit affects the balance of some flavor aldehydes and alcohols. Plant Physiol. 1998;117(3):1047–1058. doi: 10.1104/pp.117.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goulet C, et al. Divergence in the enzymatic activities of a tomato and Solanum pennellii alcohol acyltransferase impacts fruit volatile ester composition. Mol Plant. 2015;8(1):153–162. doi: 10.1016/j.molp.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Simkin AJ, Schwartz SH, Auldridge M, Taylor MG, Klee HJ. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles beta-ionone, pseudoionone, and geranylacetone. Plant J. 2004;40(6):882–892. doi: 10.1111/j.1365-313X.2004.02263.x. [DOI] [PubMed] [Google Scholar]
- 14.Kovács K, et al. Effect of tomato pleiotropic ripening mutations on flavour volatile biosynthesis. Phytochemistry. 2009;70(8):1003–1008. doi: 10.1016/j.phytochem.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Zhong S, et al. Single-base resolution methylomes of tomato fruit development reveal epigenome modifications associated with ripening. Nat Biotechnol. 2013;31(2):154–159. doi: 10.1038/nbt.2462. [DOI] [PubMed] [Google Scholar]
- 16.Cruz-Mendívil A, et al. Transcriptional changes associated with chilling tolerance and susceptibility in ‘Micro-Tom’ tomato fruit using RNA-Seq. Postharvest Biol Technol. 2015;99:141–151. [Google Scholar]
- 17.Scott JW, et al. Fla. 8153 hybrid tomato; Fla. 8059 and Fla. 7907 breeding lines. HortScience. 2008;43(7):2228–2230. [Google Scholar]
- 18.Urbanczyk-Wochniak E, et al. Conversion of MapMan to allow the analysis of transcript data from Solanaceous species: Effects of genetic and environmental alterations in energy metabolism in the leaf. Plant Mol Biol. 2006;60(5):773–792. doi: 10.1007/s11103-005-5772-4. [DOI] [PubMed] [Google Scholar]
- 19.Usadel B, et al. A guide to using MapMan to visualize and compare Omics data in plants: A case study in the crop species, Maize. Plant Cell Environ. 2009;32(9):1211–1229. doi: 10.1111/j.1365-3040.2009.01978.x. [DOI] [PubMed] [Google Scholar]
- 20.Thomashow MF. Molecular basis of plant cold acclimation: Insights gained from studying the CBF cold response pathway. Plant Physiol. 2010;154(2):571–577. doi: 10.1104/pp.110.161794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, et al. Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis. Plant J. 2004;39(6):905–919. doi: 10.1111/j.1365-313X.2004.02176.x. [DOI] [PubMed] [Google Scholar]
- 22.Park S, et al. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 2015;82(2):193–207. doi: 10.1111/tpj.12796. [DOI] [PubMed] [Google Scholar]
- 23.Maloney GS, et al. Characterization of the branched-chain amino acid aminotransferase enzyme family in tomato. Plant Physiol. 2010;153(3):925–936. doi: 10.1104/pp.110.154922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochevenko A, Klee HJ, Fernie AR, Araújo WL. Molecular identification of a further branched-chain aminotransferase 7 (BCAT7) in tomato plants. J Plant Physiol. 2012;169(5):437–443. doi: 10.1016/j.jplph.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Gapper NE, McQuinn RP, Giovannoni JJ. Molecular and genetic regulation of fruit ripening. Plant Mol Biol. 2013;82(6):575–591. doi: 10.1007/s11103-013-0050-3. [DOI] [PubMed] [Google Scholar]
- 26.Liu M, Pirrello J, Chervin C, Roustan JP, Bouzayen M. Ethylene control of fruit ripening: Revisiting the complex network of transcriptional regulation. Plant Physiol. 2015;169(4):2380–2390. doi: 10.1104/pp.15.01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klee HJ, Giovannoni JJ. Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet. 2011;45(1):41–59. doi: 10.1146/annurev-genet-110410-132507. [DOI] [PubMed] [Google Scholar]
- 28.Lincoln JE, et al. LE-ACS4, a fruit ripening and wound-induced 1-aminocyclopropane-1-carboxylate synthase gene of tomato (Lycopersicon esculentum). Expression in Escherichia coli, structural characterization, expression characteristics, and phylogenetic analysis. J Biol Chem. 1993;268(26):19422–19430. [PubMed] [Google Scholar]
- 29.Barry CS, et al. Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J. 1996;9(4):525–535. doi: 10.1046/j.1365-313x.1996.09040525.x. [DOI] [PubMed] [Google Scholar]
- 30.Barry CS, Llop-Tous MI, Grierson D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol. 2000;123(3):979–986. doi: 10.1104/pp.123.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JM, Sasaki T, Ueda M, Sako K, Seki M. Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front Plant Sci. 2015;6:114. doi: 10.3389/fpls.2015.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujisawa M, Nakano T, Shima Y, Ito Y. A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell. 2013;25(2):371–386. doi: 10.1105/tpc.112.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu R, et al. A DEMETER-like DNA demethylase governs tomato fruit ripening. Proc Natl Acad Sci USA. 2015;112(34):10804–10809. doi: 10.1073/pnas.1503362112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel JT, Tan BC, McCarty DR, Klee HJ. The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions. J Biol Chem. 2008;283(17):11364–11373. doi: 10.1074/jbc.M710106200. [DOI] [PubMed] [Google Scholar]
- 35.Vogel JT, et al. Carotenoid content impacts flavor acceptability in tomato (Solanum lycopersicum) J Sci Food Agric. 2010;90(13):2233–2240. doi: 10.1002/jsfa.4076. [DOI] [PubMed] [Google Scholar]
- 36.Ramel F, et al. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc Natl Acad Sci USA. 2012;109(14):5535–5540. doi: 10.1073/pnas.1115982109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Havaux M. Carotenoid oxidation products as stress signals in plants. Plant J. 2014;79(4):597–606. doi: 10.1111/tpj.12386. [DOI] [PubMed] [Google Scholar]
- 38.Ramel F, Mialoundama AS, Havaux M. Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J Exp Bot. 2013;64(3):799–805. doi: 10.1093/jxb/ers223. [DOI] [PubMed] [Google Scholar]
- 39.Vrebalov J, et al. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science. 2002;296(5566):343–346. doi: 10.1126/science.1068181. [DOI] [PubMed] [Google Scholar]
- 40.Osorio S, et al. Systems biology of tomato fruit development: Combined transcript, protein, and metabolite analysis of tomato transcription factor (nor, rin) and ethylene receptor (Nr) mutants reveals novel regulatory interactions. Plant Physiol. 2011;157(1):405–425. doi: 10.1104/pp.111.175463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manning K, et al. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet. 2006;38(8):948–952. doi: 10.1038/ng1841. [DOI] [PubMed] [Google Scholar]
- 42.Bemer M, et al. The tomato FRUITFULL homologs TDR4/FUL1 and MBP7/FUL2 regulate ethylene-independent aspects of fruit ripening. Plant Cell. 2012;24(11):4437–4451. doi: 10.1105/tpc.112.103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin Z, et al. A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening. Plant J. 2008;55(2):301–310. doi: 10.1111/j.1365-313X.2008.03505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vrebalov J, et al. Fleshy fruit expansion and ripening are regulated by the Tomato SHATTERPROOF gene TAGL1. Plant Cell. 2009;21(10):3041–3062. doi: 10.1105/tpc.109.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Itkin M, et al. TOMATO AGAMOUS-LIKE 1 is a component of the fruit ripening regulatory network. Plant J. 2009;60(6):1081–1095. doi: 10.1111/j.1365-313X.2009.04064.x. [DOI] [PubMed] [Google Scholar]
- 46.Chung MY, et al. A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. Plant J. 2010;64(6):936–947. doi: 10.1111/j.1365-313X.2010.04384.x. [DOI] [PubMed] [Google Scholar]
- 47.Karlova R, et al. Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. Plant Cell. 2011;23(3):923–941. doi: 10.1105/tpc.110.081273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tieman DM, et al. Identification of loci affecting flavour volatile emissions in tomato fruits. J Exp Bot. 2006;57(4):887–896. doi: 10.1093/jxb/erj074. [DOI] [PubMed] [Google Scholar]
- 49.Zhong S, et al. High-throughput illumina strand-specific RNA sequencing library preparation. Cold Spring Harb Protoc. 2011;2011(8):940–949. doi: 10.1101/pdb.prot5652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.