Significance
Antimicrobial thiopeptides constitute a broad family of natural compounds that are ribosomally synthesized and posttranslationally modified. This paper reports the characterization of a biosynthetic pathway for the production of the canonical thiopeptide micrococcin. These biochemical details were acquired by reconstituting the pathway in a genetically manipulable laboratory organism and then isolating and characterizing partially synthesized thiopeptide intermediates. Our findings highlight strict sequential order of biochemical conversions leading to the micrococcin product and pave the way toward using this understanding to engineer antimicrobial compounds with clinically useful properties.
Keywords: thiopeptide, affinity-tagged intermediates, biosynthesis, antibiotic, RiPP
Abstract
Thiopeptides, including micrococcins, are a growing family of bioactive natural products that are ribosomally synthesized and heavily modified. Here we use a refactored, modular in vivo system containing the micrococcin P1 (MP1) biosynthetic genes (TclIJKLMNPS) from Macrococcus caseolyticus str 115 in a genetically tractable Bacillus subtilis strain to parse the processing steps of this pathway. By fusing the micrococcin precursor peptide to an affinity tag and coupling it with catalytically defective enzymes, biosynthetic intermediates were easily captured for analysis. We found that two major phases of molecular maturation are separated by a key C-terminal processing step. Phase-I conversion of six Cys residues to thiazoles (TclIJN) is followed by C-terminal oxidative decarboxylation (TclP). This TclP-mediated oxidative decarboxylation is a required step for the peptide to progress to phase II. In phase II, Ser/Thr dehydration (TclKL) and peptide macrocycle formation (TclM) occurs. A C-terminal reductase, TclS, can optionally act on the substrate peptide, yielding MP1, and is shown to act late in the pathway. This comprehensive characterization of the MP1 pathway prepares the way for future engineering efforts.
Thiazolyl peptides (thiopeptides) constitute a family of ribosomally synthesized and posttranslationally modified peptides (RiPPs), with precursor peptides comprised of an N-terminal leader peptide and a C-terminal Cys/Ser/Thr-rich core (1, 2). The core peptides are extensively modified with formation of azol(in)es, dehydroalanine(Dha)/dehydrobutyrine(Dhb) and a pyridine/piperidine, en route to macrocyclization and cleavage of the leader to yield a bioactive product. Although the mature products possess potent activity toward several targets, most notably Gram-positive bacteria, most lack sufficient solubility to be useful pharmaceuticals (3). Their ribosomal synthesis, however, makes them attractive targets for genetic engineering of both the core peptide and the biosynthetic enzymes to create diverse thiopeptide chemical libraries. Previous efforts to explore pathway promiscuity and define enzyme function in vivo have coupled expression of core-peptide variants and/or gene deletions with organic extraction of pathway products (4–10). These studies succeed in identifying extractable products and intermediates that have been prematurely cleaved from the leader peptide, but overlook peptide intermediates that are chemically very different from product molecules. In vitro studies of thiopeptide biosynthetic components have provided information about functional sets of enzymatic processing (11, 12), but complete reconstitution remains a challenging endeavor. As an alternative approach, we recently reported an in vivo heterologous expression system that allows both efficient genetic changes in the core and biosynthetic enzymes and isolation and characterization of biochemical intermediates in micrococcin biosynthesis (13).
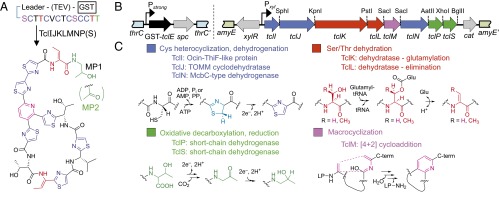
Micrococcin (Fig. 1A), an archetypal thiopeptide, contains six thiazoles, two Thr-derived Dhb residues, and a central pyridine ring formed from two Ser-derived Dha residues. It is additionally oxidatively decarboxylated at the C-terminal Thr residue, initially to a ketone to form micrococcin P2 (MP2), which may be rereduced to an alcohol, yielding micrococcin P1 (MP1) (13). Micrococcin/thiocillin (tcl) biosynthetic gene clusters have been identified in Macrococcus caseolyticus strain 115 (Mc) and Bacillus cereus ATCC 14579 (Bc) (14, 15). The Bc cluster is comparatively large (24 chromosomal genes) and controls the synthesis of a mixture of eight micrococcin/thiocillin variants, whereas the simpler Mc gene cluster (12 plasmid-encoded genes) dictates the synthesis of a single micrococcin (MP1) product (14, 15). Mc requires only 9 of its 12 tcl genes to synthesize MP1 (SI Appendix, Fig. S1) (13), and these genes can be easily manipulated as synthetic gene clusters (Fig. 1B) in our heterologous Bacillus subtilis producer strain expressing the precursor peptide TclE and the eight biosynthetic proteins TclIJKLMNPS (Fig. 1A). The functions of these proteins are straightforward to predict by their homology with proteins from other RiPP pathways (Fig. 1C). TclI is an Ocin–ThiF-like protein with a proposed RiPP precursor peptide recognition element (16) and, together with TclJ, a Thiazole/Oxazole-Modified Microcin (TOMM) family cyclodehydratase, converts core-peptide cysteines to thiazolines. TclN, an FMN-binding McbC-type dehydrogenase, should subsequently oxidize thiazolines to thiazoles. TclK and TclL show homology to the N- and C-terminal domains, respectively, of NisB, a lantibiotic Ser/Thr dehydratase (17), implicated in dehydrating core-peptide Ser/Thr residues by glutamylation (TclK) followed by elimination (TclL). The TclM homolog from the Bc pathway has been shown to catalyze a [4+2] cycloaddition reaction that forms the central pyridine ring, simultaneously closing the peptide macrocycle and releasing the leader peptide (5, 12). TclP and TclS, both short-chain dehydrogenases, are proposed to catalyze the C-terminal oxidative decarboxylation (TclP) and subsequent rereduction (TclS) (13).
Fig. 1.
Micrococcin structure and posttranslational processing. (A) Diagram depicting conversion of the TclE precursor peptide to mature MP1 when all eight biosynthetic proteins are expressed or to MP2 in the absence of TclS. (B) Engineered gene clusters for micrococcin production in B. subtilis. Left, the constitutive Pstrong promoter drives expression of GST–TclE from a cassette integrated into the thrC locus; Right, the xylose-inducible Pxyl promoter drives expression of tcl biosynthesis genes from a cassette integrated into the amyE locus. A TEV-protease cleavage site is incorporated between the GST-affinity tag and the TclE precursor peptide. (C) Summary of the seven chemical transformations required in micrococcin maturation and the enzymes predicted to carry them out.
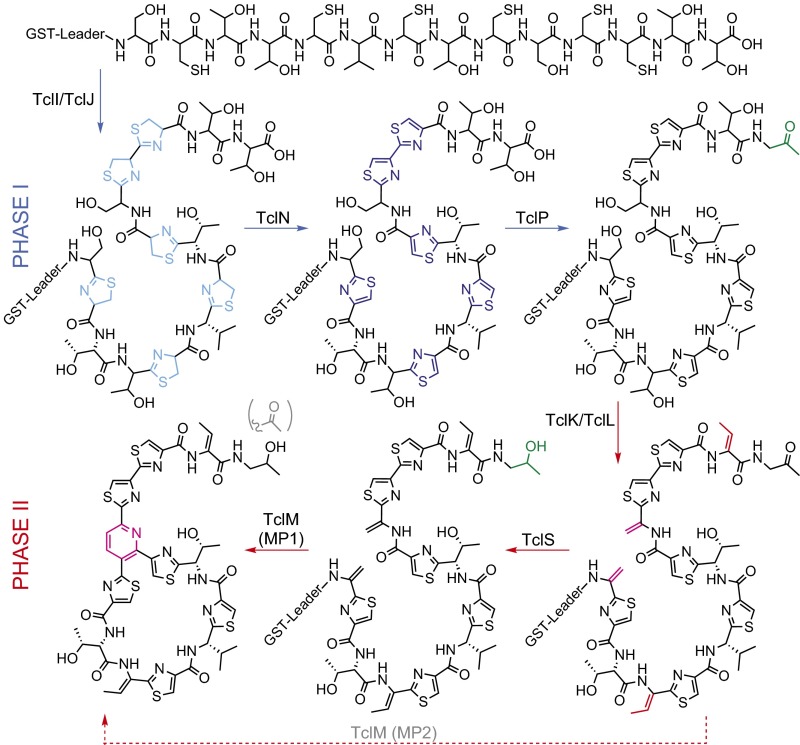
Although a foundational understanding of micrococcin biosynthesis exists, we still have an incomplete understanding of the requirements and ordering of the Tcl protein activities. In implementing this heterologous expression system, we made two key observations (13): (i) TclP, predicted to be involved only in C-terminal tailoring, is absolutely required for production of an extractable bioactive product, and (ii) an N-terminal GST fusion to the TclE precursor peptide does not interfere with micrococcin biosynthesis, allowing nonextractable intermediates to be isolated and characterized. To investigate the role of TclP-mediated oxidative decarboxylation in the overall processing of micrococcin, we used this system to capture pathway intermediates from strains genetically blocked for specific Tcl enzyme activities. This unique capability to capture GST-tagged intermediates has allowed us to discern in great detail the ordering of micrococcin-processing steps (see proposed model in Fig. 2), with the striking observation that the two major phases of core-peptide processing (phase I: TclIJN-mediated Cys cyclization and phase II: TclKL-mediated Ser/Thr dehydration and TclM-mediated macrocyclization) are punctuated by a TclP-dependent decarboxylation event. This oxidative decarboxylation constitutes a biochemical checkpoint that can occur only after Cys cyclization and is required for substrate peptide progression to phase II reactions.
Fig. 2.
A proposed model for micrococcin biosynthesis, indicating the two major phases delineated by TclP-mediated oxidative decarboxylation, and illustrating TclS bypass (dotted arrow) to yield MP2.
Results
Prediction and Creation of Catalytically Inactivated Tcl Enzyme Variants.
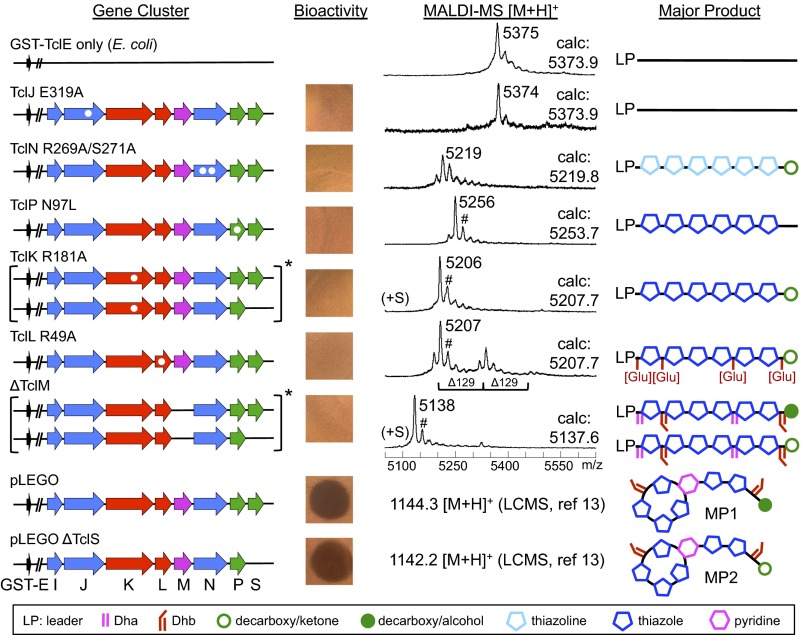
The ability of the Mc biosynthetic enzymes to process the affinity-tagged GST–TclE precursor peptide provides an avenue for isolation and analysis of all biosynthetic intermediates along the pathway before the macrocyclization step. To capitalize on this opportunity, we generated separate B. subtilis strains, each of which couples expression of GST–TclE with either a point mutation(s) or gene deletion designed to knock out the activity of one of the seven enzymes in the pathway (Fig. 1 and SI Appendix, Fig. S2). Mutational predictions were achieved by aligning Tcl sequences with proteins of known structure using HHpred (18) and using the top-scoring alignments to generate structural models using Modeller (19) (SI Appendix, Figs. S3–S8). Loss of micrococcin formation in GST–TclE strains harboring individual mutations was then verified by evaluating the bioactivity of methanol extracts against a Staphylococcus aureus indicator strain (13).
TclJ is homologous with the Mg2+–ATP-dependent TOMM cyclodehydratases, which have been shown to lose activity upon substitution of Mg2+-binding glutamic acid residues with alanine (20, 21). Our structural model, based on LynD from the cyanobactin RiPP pathway (21) (SI Appendix, Fig. S3), predicted alteration of TclJ E319 would have the same effect, and a TclJ E319A mutation abolished formation of extractable bioactive compounds (Fig. 3). TclK and TclL correspond to the well-characterized N- and C-terminal domains of the Ser/Thr dehydratase NisB from the nisin RiPP pathway (17, 22), which allowed us to design mutations in TclK (R181A) and TclL (R49A) that disrupt enzymatic activity and abolish bioactivity in extracts (Fig. 3 and SI Appendix, Figs. S4–S5). Structural alignment of TclN, a putative FMN-dependent nitroreductase, with another nitroreductase (Ava_2154 from Anabaena variabilis) revealed two conserved residues that likely coordinate the phosphate of FMN (SI Appendix, Fig. S6). Expression of a double-substitution variant of TclN (R269A/S271A) abolished formation of bioactive product (Fig. 3). The structure of TclP was modeled with (R)-hydroxypropyl–coenzyme M dehydrogenase (23) (SI Appendix, Fig. S7), and of the conserved active site residues (24), N97, S126, and Y139, only the N97L mutation gave complete loss of activity in extracts and was used in this study (Fig. 3). Because the cycloaddition enzyme TclM does not have homologs of known structure, and its catalytic mechanism is not well understood, we evaluated sequence alignments with related enzymes, including TbtD involved in thiomuracin biosynthesis. Based on these alignments (SI Appendix, Fig. S8) and in vitro mutational studies with TbtD (11), we predicted that TclM Y222A and R235A substitutions might perturb activity. Extracts from both of these mutants retained bioactivity (SI Appendix, Fig. S8); thus we used a deletion mutant (ΔTclM) for these studies (Fig. 3). The above mutations were all introduced in the presence of TclS. Because we previously showed that macrocycle formation can occur in the absence of TclS (13), where appropriate, the above mutations were also incorporated into the pathway lacking the tclS gene.
Fig. 3.
Dissection of micrococcin biosynthesis by genetic disruption, capture of biosynthetic intermediates, and mass analysis. Amino acid substitutions are indicated with white dots within tcl genes (arrows). Bioassays of methanol extracts from each strain are shown along with MALDI-MS data for modified peptides obtained after TEV-cleavage of GST-tagged intermediates. Observed m/z values are given next to major peaks, and calculated m/z values (calc) are indicated. Products consistent with mass values of the major peaks are diagrammed on the right, with chemical group symbols shown in the accompanying key. Asterisk (*) denotes mass comparisons that required high-resolution ESI-LCMS (see Fig. 4). Nonproton adduct ions were observed (#) and found to be +17 amu via ESI-LCMS. Previous studies showed only MP1 is produced from the full set of genes, whereas only MP2 is formed in the ∆TclS strain (13).
TclP Mediates the Transition Between Two Phases of Core-Peptide Processing.
GST–TclE peptides isolated from the different genetically trapped B. subtilis producer strains were cleaved with TEV (tobacco etch virus) protease and analyzed for changes in mass that, coupled with knowledge of activities of orthologs from other RiPP systems, allow us to deduce the type and extent of posttranslational modification that occur upstream of the genetically defective step. Matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) was initially used for analysis of pathway intermediates, and high-resolution electrospray ionization liquid chromatography–mass spectrometry (ESI-LCMS) was used to further resolve the identity of processed peptides. This approach gave rise to a well-resolved model for the sequential processing events leading to micrococcin product formation (Fig. 2).
From our modeling, TclJ is expected to convert the six core-peptide Cys residues to thiazolines, and by analogy to results from the recent in vitro thiomuracin study (11), may initiate processing. Indeed, the TclE peptide remains unmodified in the TclJ-defective strain (Fig. 3), confirming its role in catalyzing the first step in the pathway. TclN is expected to carry out the FMN-dependent oxidation of thiazolines to thiazoles. Isolated peptide from the TclN-defective strain gave an envelope of ion peaks discussed in more detail below; however, the major ion peak has a mass consistent with a peptide containing six thiazolines and, surprisingly, a decarboxylated C terminus (Fig. 3). This result confirms both the roles of TclJ and TclN and the timing of thiazole installation as the first step in the pathway. However, the observation of decarboxylated peptide suggests TclP also acts very early in the pathway. Strikingly, the TclP-inactivated strain accumulates core peptide modified only with six thiazoles (Fig. 3), indicating that not only is TclP responsible for the decarboxylation step, it is required for robust downstream Ser/Thr dehydration and subsequent macrocyclization. TclP-mediated decarboxylation therefore appears to act as a functional link between two distinct phases of the overall pathway: TclIJN-dependent thiazole installation with TclP-dependent decarboxylation (phase I) and TclKLM-dependent dehydration/macrocyclization (phase II).
Characterization of Phase II Processing by TclK, TclL, and TclM.
The TclK enzyme is expected to initiate Ser/Thr dehydration steps via side-chain glutamylation, so peptide from the TclK-defective strain identifies all processing steps that precede Ser/Thr dehydration. This strain accumulated peptide with a mass consistent with six thiazoles and complete removal of the C-terminal carboxyl group (Fig. 3), confirming the placement of the TclP-mediated decarboxylation before Ser/Thr dehydration. TclL is expected to complete the Ser/Thr dehydration by elimination of glutamyl ester (Glu) adducts introduced by TclK. Thus, the TclL-defective strain should accumulate decarboxylated and thiazole-modified peptide intermediates containing one or more glutamylations. MALDI-MS data from this strain show a series of peaks [Δ129 atomic mass units (amu)] consistent with decarboxylated peptides harboring six thiazoles and up to three glutamylations (Fig. 3 and SI Appendix, Fig. S5). A similar pattern of multiple glutamylations has previously been observed for nonthiopeptide RiPP pathways (22, 25); however, in the in vitro reconstituted thiomuracin system, only a monoglutamylated intermediate accumulates when the TclL ortholog (TbtC) is omitted (11). This discrepancy may be due to the different reaction conditions (in vitro versus in vivo) or to an intrinsic difference in how this two-step dehydration process is coordinated in the two biosynthetic systems. To shed light on this, we repeated our analysis using a strain with TclL deleted rather than catalytically inactivated. Isolated peptide from the TclL-deletion strain also exhibited multiple glutamylations (SI Appendix, Fig. S5), suggesting there may be some inherent difference in the way glutamylation/elimination steps are coordinated between the two systems.
TclM from the Bc pathway has been shown to complete the thiocillin/micrococcin maturation process by catalyzing formation of the central pyridine ring from the Dha residues generated by the TclKL dehydratase module (12). Thus, a TclM-defective strain should accumulate a linear peptide with complete side-chain processing. In initial experiments with this strain grown for 16 h, we detected only TclE leader peptide lacking the C-terminal core (SI Appendix, Fig. S8). This result is consistent with the complementary findings from the Bc tclM deletion strain that after their typical 3-d growth, the major extractable compound was the fully modified but linear core resulting from proteolytic cleavage between the leader and modified core (5). Taken together, we postulated this results from a slow, unidentified proteolytic process and that a shortened culture time would allow capture of the nonproteolyzed intermediate. Indeed, by decreasing culture time from 16 to 8 h, we successfully isolated the expected fully processed linear core still attached to the leader (Fig. 3).
Timing of TclS-Mediated C-Terminal Reduction.
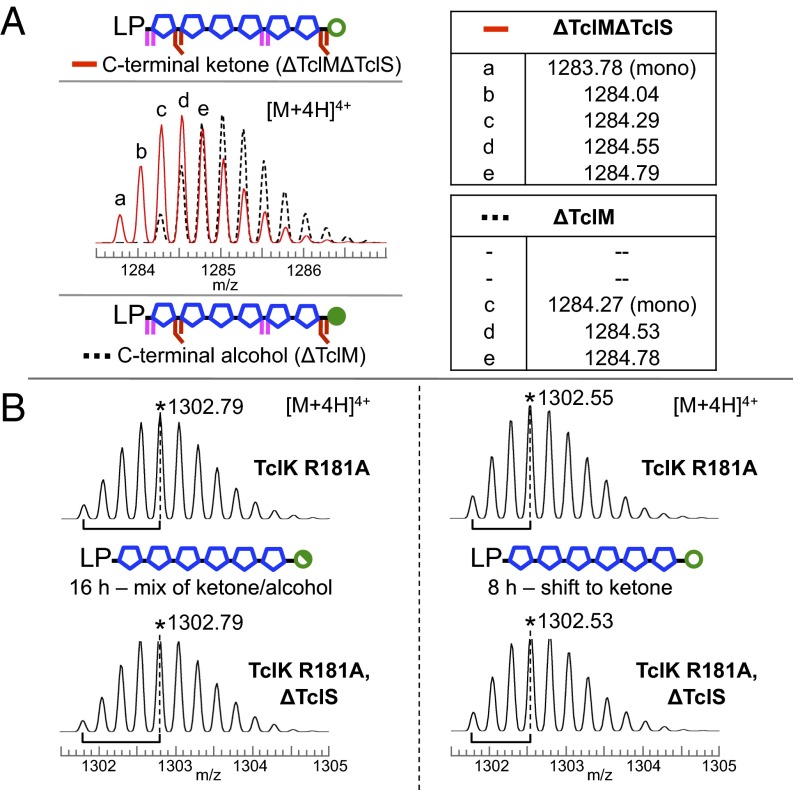
Thus far, we have mapped the timing of six of the seven enzymatic reactions in the pathway. The remaining step involves the TclS-catalyzed rereduction of the TclP-generated C-terminal ketone to an alcohol, a change in mass of only 2 amu that requires high-resolution ESI-LCMS to distinguish in peptides of this size. Although the TclS-catalyzed reaction is not required for macrocycle formation, when TclS is present, only the alcohol product is observed, suggesting the enzyme activity is robust. Here we address the placement of TclS activity with respect to the other Tcl enzymatic steps. We first observed that peptide from the ∆TclM strain had a C-terminal alcohol and confirmed the reduction was TclS-mediated using a ∆TclM∆TclS strain, which yielded a peptide with a C-terminal ketone, as expected (Fig. 4A). Next we tested whether TclS acted before phase II by analyzing peptides from TclK-defective strains with and without TclS. In this case, the results were less straightforward. Both TclK-defective strains (±TclS) grown for 16 h had peptide peaks with identical isotope patterns that were consistent with a mixture of C-terminal ketone and alcohol (Fig. 4B). To test for possible slow-acting, TclS-independent reduction, we shortened the culture time to 8 h and indeed found a shift toward the oxidized form for both strains (Fig. 4B). This TclS-independent, slow-acting redox phenomenon was also observed in TclL- and TclN-defective strains (SI Appendix, Fig. S5 and Fig. 5) and may be attributable to weak reductase activity by TclP or to some unknown cellular component. Taken together, these observations show that TclS activity is biochemically distinct and temporally uncoupled from that of TclP, that it requires a Ser/Thr-dehydrated substrate, and that it does not require prior macrocyclization (Fig. 2).
Fig. 4.
ESI-LCMS analysis of C-terminal redox state in peptides from TclM- and TclK-defective strains. (A) Data for peptides captured from ΔTclM (black dashed) and ΔTclMΔTclS (red) strains. Relative intensities of isotopic peaks indicate the presence of a single product in each case. (B) In the TclK-defective strain, the maximum abundance isotope shifts to higher mass with longer culture time (16 vs. 8 h) in both the presence and absence of TclS. Asterisk (*) denotes m/z for the most abundant isotope for [M+4H]4+ ions.
Fig. 5.
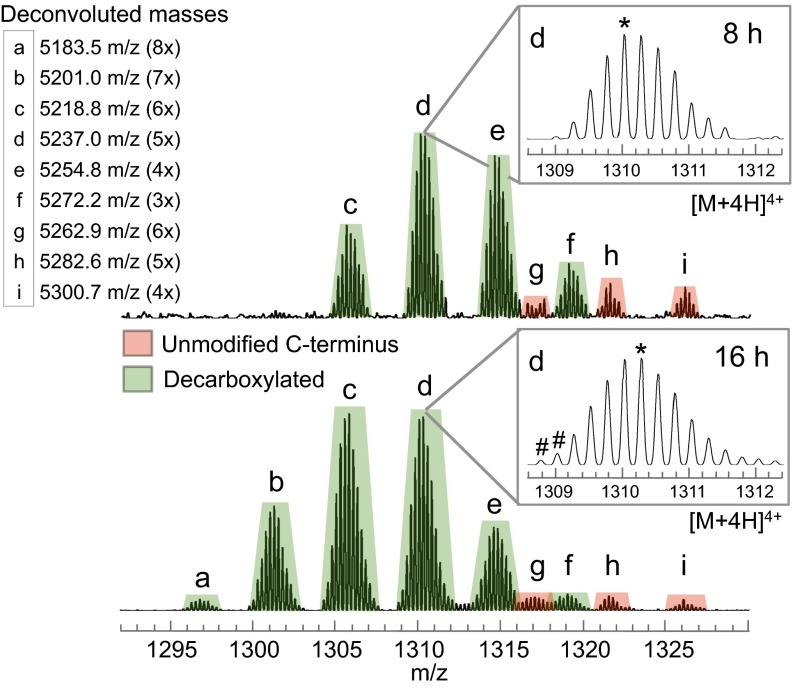
ESI-LCMS data for modified peptides isolated from the TclN-defective strain after shorter (8 h, Top) or longer (16 h, Bottom) culture times. Decarboxylated peptide series (green peaks) and nondecarboxylated series (red peaks) display within-series mass differences (deconvoluted) of 18 amu. Inset table reports deconvoluted masses of peaks a–i and the degree of dehydration (3x–8x) for each peak. Subtle isotopic details (d, Insets) provide additional information about peptide processing (see text), including a minor amount of thiazole formation (#) and a shift in the most abundant isotope (*).
TclN-Dependent Thiazoline Oxidation Facilitates Efficient Progression to Downstream Modifications.
In addition to the timing of TclS, analysis of the ESI-LCMS data for peptide from TclN-defective strains at 8 and 16 h allowed us to probe more deeply the transition from phase I to phase II. Disruption at this step gives rise to a complex mix of products that vary in the number of modifications and in C-terminal decarboxylation status, both of which were found to be dependent on culture time (Fig. 5). After 8 h, ESI-LCMS data show two series of peaks corresponding to decarboxylated intermediates (more intense, green peaks) and nondecarboxylated intermediates (less intense, red peaks). Within each series, peaks are separated by 18 amu (deconvoluted), indicative of products with different numbers of dehydrations up to six—suggesting all are due to cyclodehydration of cysteines to thiazolines. Several differences emerge with 16 h of culture time: (i) the proportion of decarboxylated peptides (i.e., ratio of green:red) increases, (ii) decarboxylated products shift to higher degrees of dehydration, up to eight (Fig. 5, peaks a and b), and (iii) a trace of thiazole formation is detected (Fig. 5, 16 h, Inset). The two additional degrees of dehydration may be explained by Ser/Thr conversion to either Dha/Dhb or to oxazolines. Of these two types of modifications, only Dha/Dhb are resistant to mild acid hydrolysis. Incomplete hydrolysis of 16-h peptides treated with 10% formic acid supports the accumulation of ∼2 Dha/Dhb residues (SI Appendix, Fig. S6). Additionally, we observe ∼∆307 satellite peaks that likely arise from glutathione addition to Dha/Dhb residues during the GST purification process. Taken together, these results indicate that blocking the pathway at the thiazoline stage still allows TclP-mediated decarboxylation, albeit less efficiently, but without thiazole formation, progress to Ser/Thr dehydration is very limited. Therefore, robust phase II Ser/Thr processing requires both decarboxylation and complete thiazole installation.
Discussion
This genetically refactored system is the first thiopeptide analytical system that allows for in vivo processing of a tagged precursor peptide and isolation of intermediates at each step in the pathway. Using this approach, we have determined the sequential functions of all Mc Tcl enzymes in the micrococcin biosynthetic pathway and resolved the specific placement of TclP and TclS C-terminal oxidoreductase activities. Our GST–TclE pull-down data support the following model (Fig. 2): First, TclI/TclJ cyclizes Cys residues to thiazolines. Although the data suggest all six thiazolines can be installed without oxidation, the installation appears to be slow and most likely occurs in a distributive fashion in conjunction with TclN-dependent oxidation. Once TclN converts thiazolines to thiazoles (or potentially a subset of the six), TclP readily oxidizes the C terminus, and decarboxylation occurs, licensing the core peptide for phase II modifications. Next, two Ser and two Thr residues are dehydrated to Dha and Dhb, respectively, by the coordinated activities of TclK and TclL. TclS reduces the C-terminal ketone to an alcohol, an optional step that occurs only after Dha/Dhb formation. We show TclS can act on the fully processed linear peptide, but the data are inconclusive as to whether it can also act after macrocyclization. Finally, TclM performs the macrocyclization step, cleaving the leader from the final MP1 (alcohol) or MP2 (ketone) product.
Perhaps the most striking principle emerging from these experiments is that the TclP-catalyzed oxidative decarboxylation step is a prerequisite for phase II core-peptide processing. At present it is unknown whether TclP catalyzes just the oxidation of the C-terminal Thr, or if it additionally catalyzes the decarboxylation step. Whether TclP is completely absent (∆TclP) or catalytically inactivated (TclP N97L), the accumulating core-peptide intermediate is the same (SI Appendix, Fig. S7), strongly suggesting that TclP catalytic activity (and not just its presence to stabilize a multiprotein complex) is crucial for progression to phase II processing. The presence of only trace amounts of Ser/Thr dehydration from TclP-defective strains indicates that TclK (the initiator of phase II reactions) requires substrate with a decarboxylated C terminus.
Our finding that C-terminal decarboxylation is an obligatory step is consistent with results obtained with the thiopeptide TP-1161, where disruption of the single dehydrogenase, TpaJ, also abolished formation of detectable products in extracts (6), although in this case an accumulated intermediate was not characterized. Previous data from the Bc Tcl system showed core-peptide variants T14A and ∆T14 greatly impacted the ability to form extractable product (4), further corroborating our results. The other major C-terminal modifications found in thiopeptides are amides and carboxylic acids, mostly arising from cleavage of C-terminal residues (26). These modifications, or lack thereof, appear to be more permissive to processing (9, 26), suggesting differences in the substrate recognition requirements for Ser/Thr dehydratases from different thiopeptide pathways.
Analysis of the peptides from the TclN-defective strain gives us further understanding of the substrate requirements for TclP and TclK. The phase I TclN-catalyzed oxidation of thiazolines to thiazoles results in substantial rigidification of core-peptide structure (4), which influences the suitability of the core peptide as a substrate for both TclP and TclK. Comparison of the accumulated peptides from TclN-defective versus TclK-defective strains showed incomplete decarboxylation in the less rigid thiazoline-rich peptide intermediates but complete decarboxylation in the more rigid thiazole-containing intermediate, suggesting that core-peptide rigidification by TclN results in a better substrate for TclP-dependent decarboxylation. A rigidified substrate is also preferred by TclK, as evidenced by very slow and incomplete accumulation of Dha/Dhb groups in the decarboxylated peptides from the TclN-defective strain.
From the ESI-LCMS data, we also found accumulation of peptide species in the TclN-defective strain with fewer than six thiazolines, whereas blocking the pathway after TclN results in robust installation of all six thiazoles. It is possible thiazoline hydrolysis during workup is partially responsible for the envelope of peaks (<6 thiazolines); regardless, there is still a difference between 8 and 16 h, suggesting full Cys cyclization is impaired without TclN. These observations support a model in which TclIJ and TclN act in a coordinated fashion, distributively processing each Cys residue before C-terminal decarboxylation. This reflects similar conclusions from studies with TOMM (27) and microcin B17 (28) heterocyclization enzymes. However, further study is needed to unambiguously identify the mixture of peptides arising from the TclN-defective strain and contextualize them within the proposed cooperativity of TclIJN.
The comprehensive study reported here demonstrates the capability of this analytical system for investigation of the principles of thiopeptide biosynthesis. This work paves the way for further inquiry into the mechanisms of Tcl enzymes with respect to substrate specificity and provides an opportunity to further engineer the Tcl core peptide and biosynthetic genes to generate diverse thiopeptide derivatives.
Materials and Methods
Generation of tcl Expression Plasmids and Bioassays.
All tcl mutant strains were constructed by making alterations in pLEGO, which harbors all of the biosynthetic genes (SI Appendix, Fig. S2), and transforming the resulting plasmids into a B. subtilis Pstrong-GST-tclE recipient. Appropriate mutations were made in tcl genes using overlap-extension PCR. Amplified mutant alleles were reintroduced into pLEGO using restriction digestion to remove the native gene and ligating the altered gene in its place. Detailed descriptions of pLEGO-derived plasmid primers used in their construction are summarized in SI Appendix, Tables S1 and S2. For pLEGO derivatives containing tcl gene deletions, DNA segments were removed by restriction digestion followed by treatment with Klenow polymerase and religation. All pLEGO alterations were confirmed by PCR and Sanger sequencing. Successful integration into B. subtilis was confirmed by PCR with primers spanning chromosomal integration junctions. Bioassays of methanolic extracts from B. subtilis micrococcin expression strains were conducted as described previously (13, 14).
Purification of Processed Peptide Intermediates.
B. subtilis tcl mutant or deletion strains were grown as previously described for either 8 or 16 h (13). More details are included in the SI Appendix, Text, but briefly, cell pellets were resuspended, sonicated, and centrifuged, and the clarified lysate was incubated with glutathione resin. After washing, the GST-peptide was eluted from the resin beads with 10 mM glutathione, buffer-exchanged, concentrated, and stored at –80 °C. For ∆TclM strains only, the TEV protease cleavage was carried out on the resin.
Sample Preparation for Mass Spectrometry.
The concentration of peptide was estimated using the value supplied by GE Healthcare for GST (1 Abs280 ≈ 0.5 mg/mL GST) before cleavage. Each 10–30 µL aliquot of GST-peptide was cleaved with 2 µg TEV protease [pRK793 (29)] and 0.5 mM DTT at room temperature for 1 h. In samples from 16-h cultures, TEV and GST were removed by precipitation with acetonitrile (ACN) (50% final concentration) and centrifugation (2 min, 9503 × g). Supernatant with soluble peptide was diluted with water to final 20% ACN concentration. To minimize loss of lower yields of isolated peptides from 8-h cultures, TEV and GST were not precipitated before MS analysis. These samples were acidified with 0.1% TFA, desalted, and concentrated with a C18 Zip-Tip into 75% ACN: 25% H2O (0.1% TFA).
MALDI-MS.
A 0.5 µL saturated α-cyano–4-hydroxycinnamic acid (CHCA) matrix in 50:50 ACN:H2O (0.1% TFA) was mixed with 0.5 µL of Zip-Tipped peptide sample on the MALDI plate. Calibration of the MALDI-MS instrument (AXIMA Performance, Shimadzu Biotech) was performed with a mixture of insulin (Sigma Aldrich), calibration mixture 2 (AnaSpec), and CHCA matrix. The spectra were collected in linear mode with the pulsed extraction set to m/z 5200.
High-Resolution Electrospray Ionization Liquid Chromatography–Mass Spectrometry.
A Thermo Scientific EASY-Spray source and column (PepMap, C18, 3 µm, 100A, 75 µm × 15 µm) was used with a Waters Nano Acquity HPLC system and a Thermo Scientific LTQ-Orbitrap XL mass spectrometer for LCMS analysis. Zip-Tipped samples were diluted as appropriate in 0.1% formic acid in water and injected onto the column, which was maintained at 45 °C. All modified peptides eluted between 38 and 39.5% B (A = 0.1% formic acid in water, B = 0.1% formic acid in ACN) during a 30-min gradient of 30–45% B (total run time of method = 55 min).
Supplementary Material
Acknowledgments
We thank T. Acker for the sample of purified TEV protease. This work was supported by a grant from the Brigham Young University (BYU) College of Life Sciences Vaccine Royalties Fund (to R.A.R.), a BYU Graduate Studies Fellowship Award (to P.R.B.), private donations through the University of California, San Francisco (UCSF) Foundation (to S.M.M.), NIH P41 Grant P41GM103481 (LTQ-Orbitrap XL LCMS, UCSF Mass Spectrometry Facility), and UCSF Research Resource Program Shared Equipment Award funded by the Chancellor (AXIMA MALDI-TOF).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612161113/-/DCSupplemental.
References
- 1.Arnison PG, et al. Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nat Prod Rep. 2013;30(1):108–160. doi: 10.1039/c2np20085f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortega MA, van der Donk WA. New insights into the biosynthetic logic of ribosomally synthesized and post-translationally modified peptide natural products. Cell Chem Biol. 2016;23(1):31–44. doi: 10.1016/j.chembiol.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Just-Baringo X, Albericio F, Álvarez M. Thiopeptide engineering: A multidisciplinary effort towards future drugs. Angew Chem Int Ed Engl. 2014;53(26):6602–6616. doi: 10.1002/anie.201307288. [DOI] [PubMed] [Google Scholar]
- 4.Bowers AA, Acker MG, Koglin A, Walsh CT. Manipulation of thiocillin variants by prepeptide gene replacement: structure, conformation, and activity of heterocycle substitution mutants. J Am Chem Soc. 2010;132(21):7519–7527. doi: 10.1021/ja102339q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowers AA, Walsh CT, Acker MG. Genetic interception and structural characterization of thiopeptide cyclization precursors from Bacillus cereus. J Am Chem Soc. 2010;132(35):12182–12184. doi: 10.1021/ja104524q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelhardt K, Degnes KF, Zotchev SB. Isolation and characterization of the gene cluster for biosynthesis of the thiopeptide antibiotic TP-1161. Appl Environ Microbiol. 2010;76(21):7093–7101. doi: 10.1128/AEM.01442-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Zhang F, Kelly WL. Heterologous production of thiostrepton A and biosynthetic engineering of thiostrepton analogs. Mol Biosyst. 2011;7(1):82–90. doi: 10.1039/c0mb00129e. [DOI] [PubMed] [Google Scholar]
- 8.Yu Y, et al. NosA catalyzing carboxyl-terminal amide formation in nosiheptide maturation via an enamine dealkylation on the serine-extended precursor peptide. J Am Chem Soc. 2010;132(46):16324–16326. doi: 10.1021/ja106571g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tocchetti A, et al. Capturing linear intermediates and C-terminal variants during maturation of the thiopeptide GE2270. Chem Biol. 2013;20(8):1067–1077. doi: 10.1016/j.chembiol.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi S, et al. Genome mining reveals a minimum gene set for the biosynthesis of 32-membered macrocyclic thiopeptides lactazoles. Chem Biol. 2014;21(5):679–688. doi: 10.1016/j.chembiol.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Hudson GA, Zhang Z, Tietz JI, Mitchell DA, van der Donk WA. In vitro biosynthesis of the core scaffold of the thiopeptide thiomuracin. J Am Chem Soc. 2015;137(51):16012–16015. doi: 10.1021/jacs.5b10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wever WJ, et al. Chemoenzymatic synthesis of thiazolyl peptide natural products featuring an enzyme-catalyzed formal [4 + 2] cycloaddition. J Am Chem Soc. 2015;137(10):3494–3497. doi: 10.1021/jacs.5b00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennallack PR, et al. Reconstitution and minimization of a micrococcin biosynthetic pathway in Bacillus subtilis. J Bacteriol. 2016;198(18):2431–2438. doi: 10.1128/JB.00396-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennallack PR, Burt SR, Heder MJ, Robison RA, Griffitts JS. Characterization of a novel plasmid-borne thiopeptide gene cluster in Staphylococcus epidermidis strain 115. J Bacteriol. 2014;196(24):4344–4350. doi: 10.1128/JB.02243-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wieland Brown LC, Acker MG, Clardy J, Walsh CT, Fischbach MA. Thirteen posttranslational modifications convert a 14-residue peptide into the antibiotic thiocillin. Proc Natl Acad Sci USA. 2009;106(8):2549–2553. doi: 10.1073/pnas.0900008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkhart BJ, Hudson GA, Dunbar KL, Mitchell DA. A prevalent peptide-binding domain guides ribosomal natural product biosynthesis. Nat Chem Biol. 2015;11(8):564–570. doi: 10.1038/nchembio.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortega MA, et al. Structure and mechanism of the tRNA-dependent lantibiotic dehydratase NisB. Nature. 2015;517(7535):509–512. doi: 10.1038/nature13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Söding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33(suppl 2):W244-8. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Šali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234(3):779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 20.Dunbar KL, et al. Discovery of a new ATP-binding motif involved in peptidic azoline biosynthesis. Nat Chem Biol. 2014;10(10):823–829. doi: 10.1038/nchembio.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koehnke J, et al. Structural analysis of leader peptide binding enables leader-free cyanobactin processing. Nat Chem Biol. 2015;11(8):558–563. doi: 10.1038/nchembio.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg N, Salazar-Ocampo LMA, van der Donk WA. In vitro activity of the nisin dehydratase NisB. Proc Natl Acad Sci USA. 2013;110(18):7258–7263. doi: 10.1073/pnas.1222488110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnakumar AM, Nocek BP, Clark DD, Ensign SA, Peters JW. Structural basis for stereoselectivity in the (R)- and (S)-hydroxypropylthioethanesulfonate dehydrogenases. Biochemistry. 2006;45(29):8831–8840. doi: 10.1021/bi0603569. [DOI] [PubMed] [Google Scholar]
- 24.Filling C, et al. Critical residues for structure and catalysis in short-chain dehydrogenases/reductases. J Biol Chem. 2002;277(28):25677–25684. doi: 10.1074/jbc.M202160200. [DOI] [PubMed] [Google Scholar]
- 25.Ozaki T, et al. Insights into the biosynthesis of dehydroalanines in goadsporin. ChemBioChem. 2016;17(3):218–223. doi: 10.1002/cbic.201500541. [DOI] [PubMed] [Google Scholar]
- 26.Just-Baringo X, Albericio F, Álvarez M. Thiopeptide antibiotics: Retrospective and recent advances. Mar Drugs. 2014;12(1):317–351. doi: 10.3390/md12010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melby JO, Li X, Mitchell DA. Orchestration of enzymatic processing by thiazole/oxazole-modified microcin dehydrogenases. Biochemistry. 2014;53(2):413–422. doi: 10.1021/bi401529y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milne JC, et al. Cofactor requirements and reconstitution of microcin B17 synthetase: A multienzyme complex that catalyzes the formation of oxazoles and thiazoles in the antibiotic microcin B17. Biochemistry. 1999;38(15):4768–4781. doi: 10.1021/bi982975q. [DOI] [PubMed] [Google Scholar]
- 29.Tropea JE, Cherry S, Waugh DS. Expression and purification of soluble His6-tagged TEV protease. In: Doyle SA, editor. High Throughput Protein Expression and Purification: Methods and Protocols. Humana Press; Totowa, NJ: 2009. pp. 297–307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.