Significance
Neurons that innervate the inner ear originate as neuroblasts in the otic vesicle, the epithelial precursor of the inner ear. Neuroblasts subsequently delaminate from the otic epithelium to complete differentiation near the hindbrain. Despite growing understanding of otic neurogenesis, the mechanism by which neuroblasts delaminate from the otic vesicle is unknown. Here we show that delamination is triggered by Goosecoid (Gsc), a homeobox gene famously discovered as the first known regulator of the “Spemann” embryonic organizer. Gsc is expressed in the otic vesicle in a region overlapping with neuroblasts, inducing localized epithelial-to-mesenchymal transition (EMT). Hence, regulation of cellular dynamics appears to be a general function of Gsc during otic neurogenesis as well as in the embryonic organizer.
Keywords: inner ear, neurogenesis, EMT, Gsc, Pax2
Abstract
Neurons of the Statoacoustic Ganglion (SAG), which innervate the inner ear, originate as neuroblasts in the floor of the otic vesicle and subsequently delaminate and migrate toward the hindbrain before completing differentiation. In all vertebrates, locally expressed Fgf initiates SAG development by inducing expression of Neurogenin1 (Ngn1) in the floor of the otic vesicle. However, not all Ngn1-positive cells undergo delamination, nor has the mechanism controlling SAG delamination been elucidated. Here we report that Goosecoid (Gsc), best known for regulating cellular dynamics in the Spemann organizer, regulates delamination of neuroblasts in the otic vesicle. In zebrafish, Fgf coregulates expression of Gsc and Ngn1 in partially overlapping domains, with delamination occurring primarily in the zone of overlap. Loss of Gsc severely inhibits delamination, whereas overexpression of Gsc greatly increases delamination. Comisexpression of Ngn1 and Gsc induces ectopic delamination of some cells from the medial wall of the otic vesicle but with a low incidence, suggesting the action of a local inhibitor. The medial marker Pax2a is required to restrict the domain of gsc expression, and misexpression of Pax2a is sufficient to block delamination and fully suppress the effects of Gsc. The opposing activities of Gsc and Pax2a correlate with repression or up-regulation, respectively, of E-cadherin (cdh1). These data resolve a genetic mechanism controlling delamination of otic neuroblasts. The data also elucidate a developmental role for Gsc consistent with a general function in promoting epithelial-to-mesenchymal transition (EMT).
The Statoacoustic Ganglion (SAG) connects the inner ear to the brain and transmits hearing and balance information. SAG neurons are generated by a stepwise program that starts in the otic vesicle, the precursor of the inner ear. Initially, a subset of the cells in the otic epithelium is specified for neural fate by the up-regulation of the proneural gene neurogenin1 (ngn1) (1, 2). Otic expression of ngn1 is first detected by 16 hpf, peaks at around 24 hours postfertilization (hpf), and then gradually declines, ceasing entirely by 42 hpf (3). Throughout this period, a subset of newly specified neuroblasts undergoes epithelial-to-mesenchymal transition (EMT) and delaminates from the otic vesicle (4–6). In zebrafish, most neuroblasts lose ngn1 expression after leaving the otic vesicle and subsequently up-regulate the related proneural factor neurod (7, 8). neurod-expressing cells form a group of proliferating and migrating precursors called the transit-amplifying (TA) pool (3, 9). As TA cells differentiate into mature SAG neurons, they lose neurod expression and up-regulate mature neuronal markers such as Islet1 and Islet2b (10, 11). The first mature Isl1+ SAG neurons are detected by 20 hpf and subsequently accumulate at a linear rate through at least 72 hpf (3). At the same time, the TA pool is maintained as a stable population by proliferative renewal, assuring further growth of the SAG as larvae develop (3).
Specification of the neurogenic domain is established by a low threshold level of Fgf signaling (3, 12). However, nothing is known about the mechanisms regulating delamination of neuroblasts from the otic vesicle. Ngn1 is required for neuroblast fate specification (1, 2), but ngn1 is not sufficient to induce delamination. In mouse, many cells that initially express Ngn1 ultimately remain in the otic vesicle and contribute to developing sensory epithelia (13). In zebrafish too, delamination of cells within the ngn1 domain appears highly restricted. Clearly additional factors are required to initiate EMT in the otic epithelium during SAG development.
In addition to positive regulation, other factors appear to stabilize the otic epithelium and prevent inappropriate EMT. In zebrafish, chick, and mouse, Pax2 marks the nascent otic placode and is later restricted to the medial half of the otic vesicle (14–18). Loss of Pax2 and related factor Pax8 compromises epithelial integrity, leading to faulty morphogenesis of the otic vesicle and cell dispersal (17, 18).
EMT is characterized by loss of epithelial markers and up-regulation of mesenchymal genes, many of which confer the ability to migrate. This process is critical for establishment of the vertebrate body plan during gastrulation and is initiated by a unique group of cells originally described as Spemann’s organizer. Goosecoid (gsc) is the most abundantly expressed homeobox gene in the vertebrate organizer (19, 20). Ectopic expression of Gsc is sufficient to induce organizer activity (21) and promote cell migration (22). Gsc is also expressed in the tissues that undergo tissue remodeling at later stages, such as neural crest-derived mesenchymal tissues (23). Loss of Gsc function leads to craniofacial defects in mouse and humans (24–26). It has also been found that many aggressive metastatic cancers show strong up-regulation of Gsc, and experimental misexpression of Gsc strongly promotes EMT and enhances metastasis (27, 28). Interestingly, Gsc expression has been reported in the developing otic vesicle in mouse (23, 29), but its functional importance has never been investigated. Due to these widespread roles of Gsc in regulating epithelial dynamics, we examined whether Gsc regulates EMT during otic neurogenesis in zebrafish.
Here we describe a full time course for gsc expression in the zebrafish otic vesicle. Disruption of gsc impairs delamination of SAG neuroblasts, whereas misexpression of gsc strongly promotes neuroblast delamination. Although gsc is regulated by Fgf in a domain that partially overlaps with ngn1, gsc does not affect neural fate specification. Thus, ngn1 and gsc act in parallel downstream of Fgf to coordinate neural fate specification with morphogenesis. Further analysis revealed the transcription factor Pax2a functions as a strong epithelializing factor expressed in the nonneurogenic regions of the otic vesicle. Moreover, Pax2a represses gsc transcription and function, helping to restrict EMT to the neurogenic domain of the otic vesicle.
Results
Expression of gsc During Otic Neurogenesis.
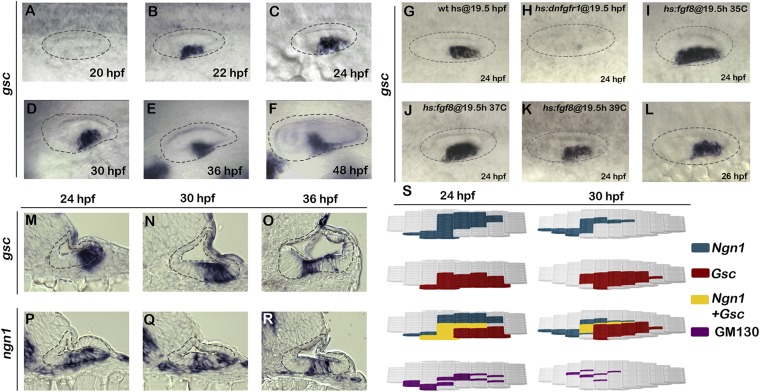
To assess the function of gsc during development of SAG neurons, we examined expression of gsc in the otic vesicle during relevant stages. gsc is first detected in a small number of ventral otic cells at 20 hpf and becomes strongly up-regulated in a ventrolateral domain by 22 hpf (Fig. 1 A and B). This domain lies close to the neurogenic domain of the otic vesicle (1). Ventrolateral expression of gsc is maintained in the otic vesicle through at least 48 hpf (Fig. 1 C–F), beyond the stage when neurogenesis normally ceases (3).
Fig. 1.
Expression and regulation of gsc during otic neurogenesis. (A–L) Whole-mount images (dorsal up, anterior left) show dorsolateral views of gsc expression in the otic vesicle (outlined) at the indicated times. (M–R) Cross-sections (dorsal up, medial left) passing through the widest part of the neurogenic domain showing expression of gsc or ngn1 at the indicated times. The otic epithelium is outlined in each image. (Magnification: F, 512×; all other images, 640×.) (S) Maps of regional markers in the floor of the otic vesicle (medial up, anterior left) generated from serial cross-sections of embryos stained for ngn1, gsc, or GM130 at 24 or 30 hpf. The location and number of cells expressing individual markers (four embryos each) was normalized and plotted accordingly.
Neurogenesis in the otic vesicle, marked by expression of ngn1, is initiated by a low level of Fgf signaling, whereas high-level Fgf signaling blocks expression of ngn1 (3). Expression of gsc shows similar regulation by Fgf. Specifically, blocking Fgf signaling by activation of hs:dnfgfr1 (dominant-negative Fgf receptor) completely eliminated gsc expression in the otic vesicle (Fig. 1 G and H). Additionally, low-level activation of hs:fgf8 at 35 °C expanded the domain of gsc expression, with a more modest expansion seen at 37 °C (Fig. 1 I and J). Thus, the requirement for Fgf and response to low-level Fgf appears highly similar for gsc and ngn1. However, expression of gsc does not require ngn1: High-level activation of hs:fgf8 at 39 °C represses ngn1 expression (3) but did not abolish gsc expression (Fig. 1K). Additionally, expression of gsc was normal in ngn1 morphants (Fig. 1L). Similarly, ngn1 expression does not require gsc (Fig. 2B). Thus, gsc and ngn1 are coinduced by low-level Fgf signaling but are not dependent on each other.
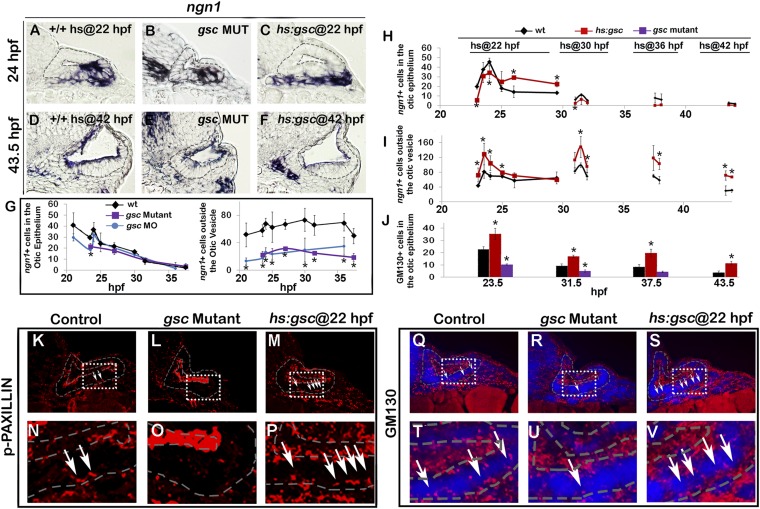
Fig. 2.
Gsc promotes EMT of otic neuroblasts. (A–F) Expression of ngn1 in cross-sections passing through the widest part of the neurogenic domain (dorsal up, medial left) just posterior to the utricular sensory epithelium expression in controls, gsc mutants, and hs:gsc embryos at 24 hpf (A–C) or 43.5 hpf (D–F). Control and transgenic embryos were heat shocked at 22 hpf (A and C) or 42 hpf (D and F). The otic epithelium is outlined in each image. (G) Mean and SD of the total number of ngn1+ cells in the otic epithelium and outside the otic vesicle for the genotypes indicated in the color key (counted from the serial sections, n = 3 or 4 otic vesicles per time point). The number of ngn1+ neuroblasts in the otic epithelium was normal in gsc mutants and morphants at all stages, except for a small but significant reduction seen in gsc morphants at 24 hpf (P < 0.05, asterisk). (H–J) For the genotypes indicated in the color key, embryos were heat shocked at the indicated times and fixed several hours later to stain for ngn1 (H and I) or basal relocalization of GM130 (J). Data show the mean and SD of the total number of stained cells in the otic epithelium or delaminated cells outside the otic vesicle (counted from the serial sections, n = 3 otic vesicles per time point). Asterisks indicate significant differences from control specimens (P < 0.05). (K–V) EMT markers in cross-sections (dorsal up, anterior left) passing through the neurogenic domain of control embryos, gsc mutants, or hs:gsc embryos immunostained for p-Paxillin (K–P) or GM130 (red) and DAPI (blue) (Q–V). Controls and transgenic embryos were heat shocked at 22 hpf. Boxed regions in K–M are magnified in N–P, and boxed regions in Q–S are magnified in T–V. White arrows indicate elevated basal staining in cells undergoing EMT. (Magnification: A, B, D, E, K–M, Q–S, 640×; C and F, 512×; N–P, T–V, 2,000×.)
We next compared the spatial patterns of gsc and ngn1 expression in serial sections. This confirmed that expression of gsc partially overlaps with ngn1 in the otic floor at least through 36 hpf (Fig. 1 M–R). gsc expression can also be detected in a small number of cells just ventral to the otic vesicle (Fig. 1M), presumably marking recently delaminated neuroblasts. After leaving the otic vesicle, these cells quickly lose gsc expression: Neither TA neuroblasts (marked by neurod) nor mature SAG neurons (marked by Isl1) show detectable expression of gsc (Fig. S1). To further examine the degree of overlap between gsc and ngn1 expression domains, we mapped the locations of cells expressing either gsc or ngn1 in the otic floor based on data from serial sections. ngn1 is expressed in the otic floor adjacent and lateral to the developing sensory epithelia (Fig. 1S). Expression of gsc overlaps with lateral portions of the ngn1 domain but extends to more lateral and posterior regions of the otic floor (Fig. 1S). Because Gsc is known to regulate EMT (22, 28), we also examined expression of GM130, a Golgi marker that undergoes a dramatic basal relocalization as cells undergo EMT (30, 31). The pattern of GM130 staining in the otic floor revealed that the highest rate of EMT occurs in the region where ngn1 and gsc are coexpressed (Fig. 1S).
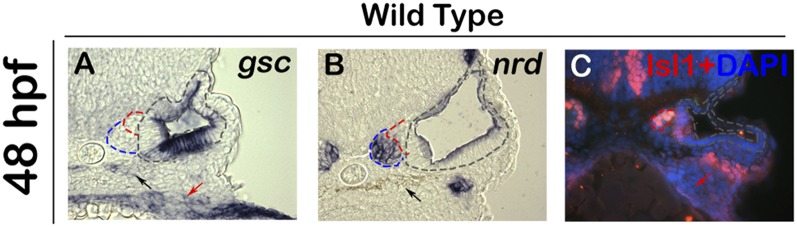
Fig. S1.
Expression of gsc during later stages of SAG development. (A–C) Images show expression of gsc (A), nrd (B), and immunodetection of Isl1 in DAPI-stained (C) cross-sections passing through the middle of the otic vesicle in wild-type embryos. The otic epithelium is outlined with black, TA cells are outlined with blue, and mature SAG neurons are outlined in red. Gsc expression is absent from the TA cells or mature SAG neurons. gsc is also expressed in the pharyngeal endoderm (red arrows) and neural crest derived pigment cells (black arrows).
Role of Gsc During Otic Neurogenesis.
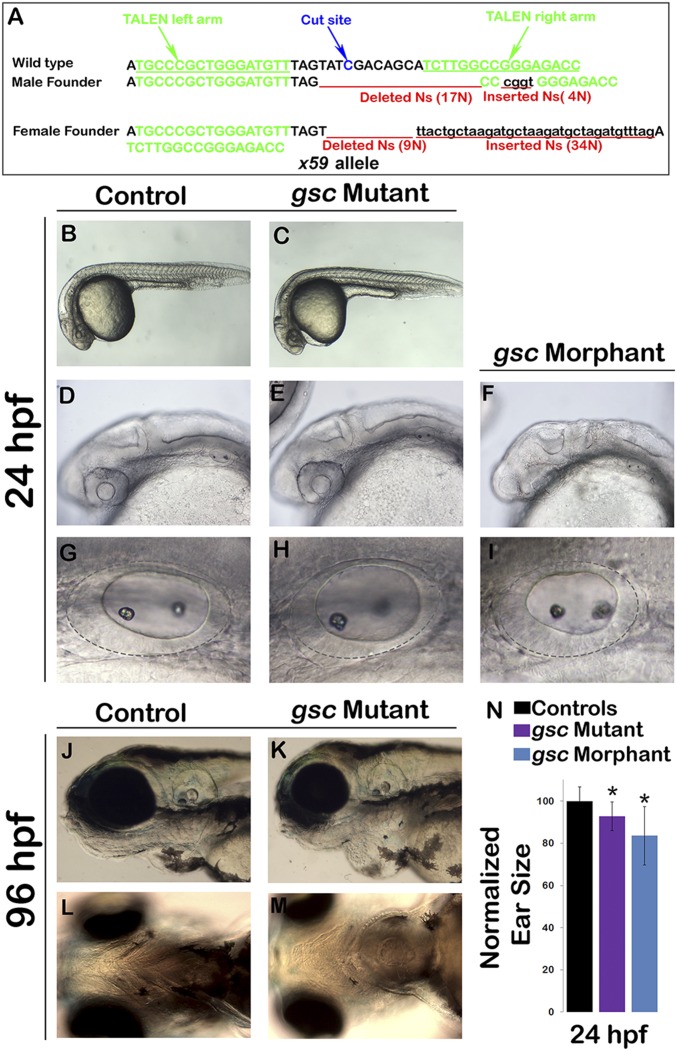
We next tested the effects of disrupting or misexpressing gsc on otic neurogenesis. Using transcription activator-like effector nuclease (TALEN)-mediated targeting, we recovered two lesions predicted to eliminate gsc function (Fig. S2A). Disruption of gsc did not cause axial defects or any overt changes in the gross morphology at 24 hpf (Fig. S2 B and C). However, gsc mutants did show a slight (∼7%) reduction in the size of the otic vesicle (Fig. S2 G–I and N) and impaired neural delamination (Fig. 2G). At later stages, gsc mutants also developed cardiac edema and a severe jaw defect (Fig. S2 J–M). Similar phenotypes were seen in gsc morphants, although gsc morphants also showed mild brain necrosis not observed in mutant embryos (Fig. S2 D–F).
Fig. S2.
Generation of gsc mutants via TALEN targeting. (A) A summary of the TALEN target sequences on the first exon of the gsc locus (marked in green) and the resulting lesions (marked in red) at the predicted cut site (marked in blue) are shown for the two F1 founders isolated during the screening process. (B–I) Live specimens at 24 hpf were viewed at low magnification to show general morphology (B and E) and at higher magnifications to show cranial development (D–F) and the otic vesicle (G–I) in controls, gsc morphants, and gsc mutants. (J–L) Live specimens at 96 hpf viewed from lateral (J and K) or ventral (L and M) reveal cardiac edema and jaw hypoplasia in gsc mutants. (N) Means and SD of surface area of the otic vesicles, normalized to control embryos, are indicated for gsc mutants and gsc morphants at 24 hpf. Asterisks indicate statistically significant differences from the control (P < 0.05).
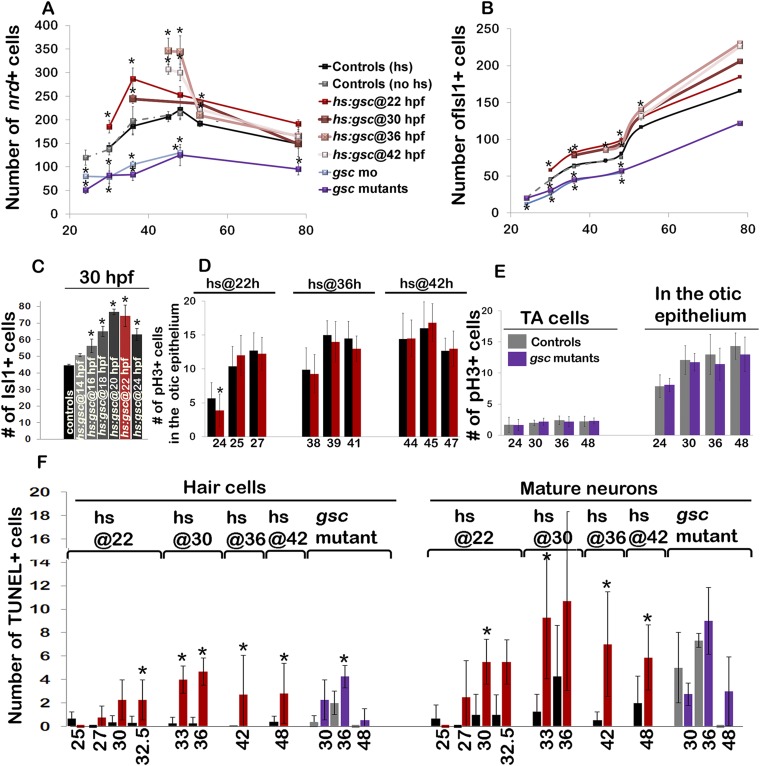
Despite the reduced size of the otic vesicle in gsc mutants, most aspects of otic patterning appeared normal, including expression of various regional markers and accumulation of sensory hair cells (Fig. S3). In addition, gsc mutants produced a normal number of the ngn1+ neuroblasts in the otic epithelium (Fig. 2 A, B, and G). However, the number of ngn1+ neuroblasts outside the otic vesicle was reduced by more than 50% at all stages of neurogenesis, suggesting a reduced rate of neuroblast delamination. A similar deficiency of recently delaminated ngn1+ neuroblasts was seen in gsc morphants (Fig. 2G).
Fig. S3.
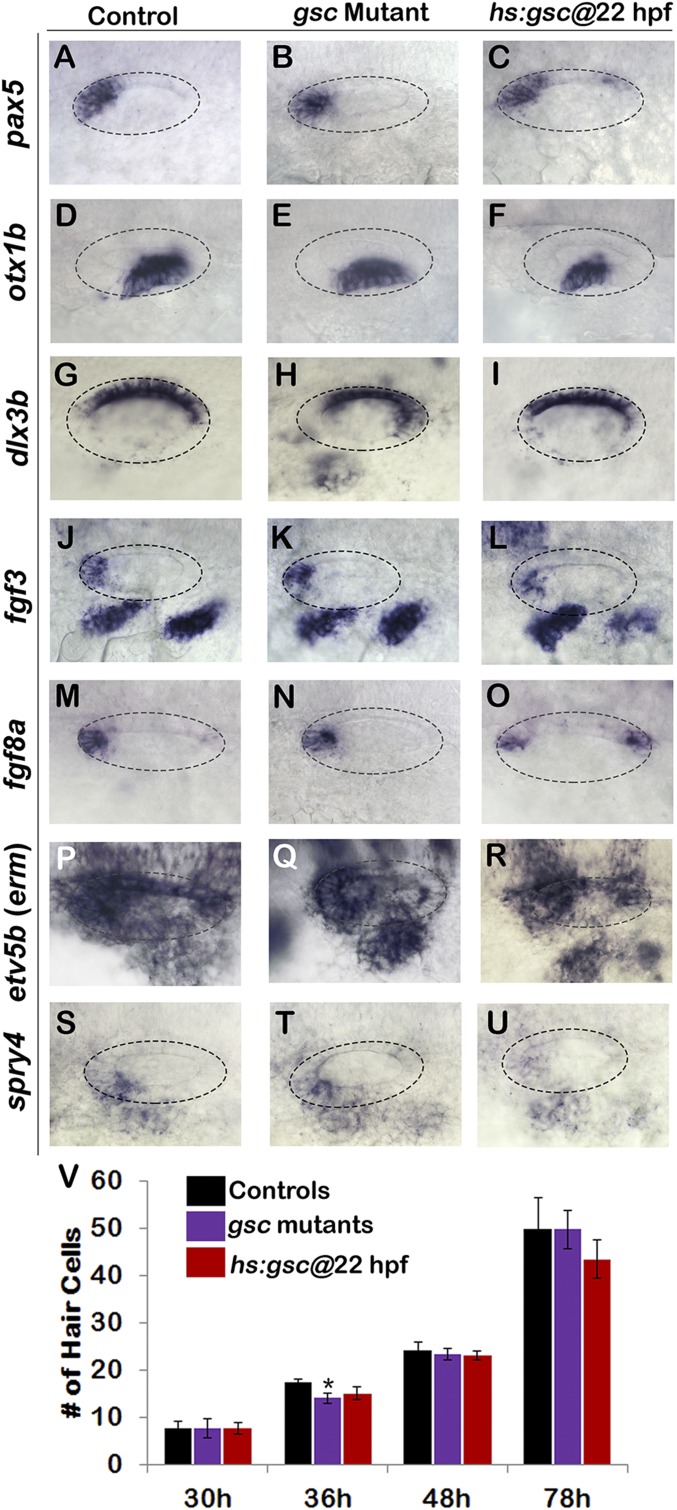
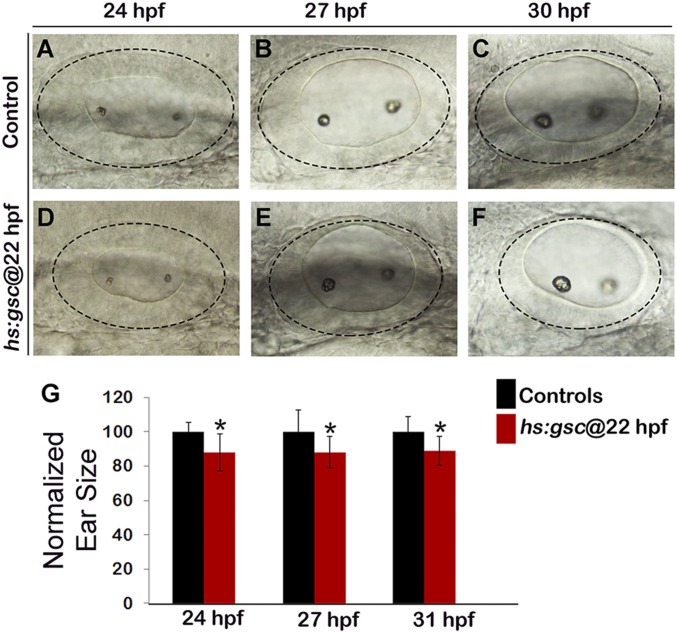
Effects of altering gsc function on otic vesicle patterning. (A–U) Whole-mount images (dorsal up, anterior left) show dorsolateral views of the otic vesicle (outlined) in controls, gsc mutants, and hs:gsc embryos for the indicated genes at 24 hpf. Patterning of the otic vesicle is not affected in gsc mutants. Hs:gsc embryos show a slight decrease in the expression of ventral-lateral otic marker otx1 (F) but otherwise appear normal. (V) Means and SD of the total number of hair cells in utricular and saccular maculae of control embryos, gsc mutants, and hs:gsc embryos at the indicated times (n = 3 embryos each). Significant differences (P < 0.05) from the control are indicated by asterisks. Data were obtained by counting hair cells in the serial sections. Accumulation of the hair cells was normal except that gsc mutants showed a small but significant decrease relative to the control at 36 hpf. There was also a slight decrease in the number of hair cells (though not statistically significant) in hs:gsc embryos at 78 hpf.
To overexpress gsc, we generated a heat shock-inducible transgenic line, hs:gsc (Fig. S4). Overexpression of gsc at 22 hpf led to a dramatic decrease in the number of ngn1+ neuroblasts in the otic epithelium within 60 min, with a concomitant increase in the number of ngn1+ cells outside the otic vesicle (Fig. 2 C, H, and I). The number of ngn1+ cells outside the ear remained elevated in hs:gsc embryos for several hours but then returned to control levels by 25 hpf (Fig. 2I), presumably reflecting the decline in transgene activity (Fig. S4). Activation of hs:gsc at 30 hpf or 36 hpf gave results similar to those observed following activation at 22 hpf (Fig. 2 H and I). Otic neurogenesis normally ends by 42 hpf (3), so we tested whether activation of hs:gsc at this stage could reinitiate neuroblast specification. Activation of hs:gsc at 42 hpf failed to reinitiate ngn1 expression in the otic vesicle but nevertheless caused a substantial increase in the number of ngn1+ cells outside the otic vesicle (Fig. 2 D, F, H, and I). This latter increase appears to result from a stage-specific effect on proliferation of TA neuroblasts (Fig. 3K). Together, these data suggest that Gsc does not affect neuroblast specification but instead enhances the ability of neuroblasts to leave the otic vesicle. The effect of Gsc on neuroblast delamination was highly specific, as other aspects of otic vesicle development appeared largely normal several hours after activating hs:gsc (Fig. S3).
Fig. S4.
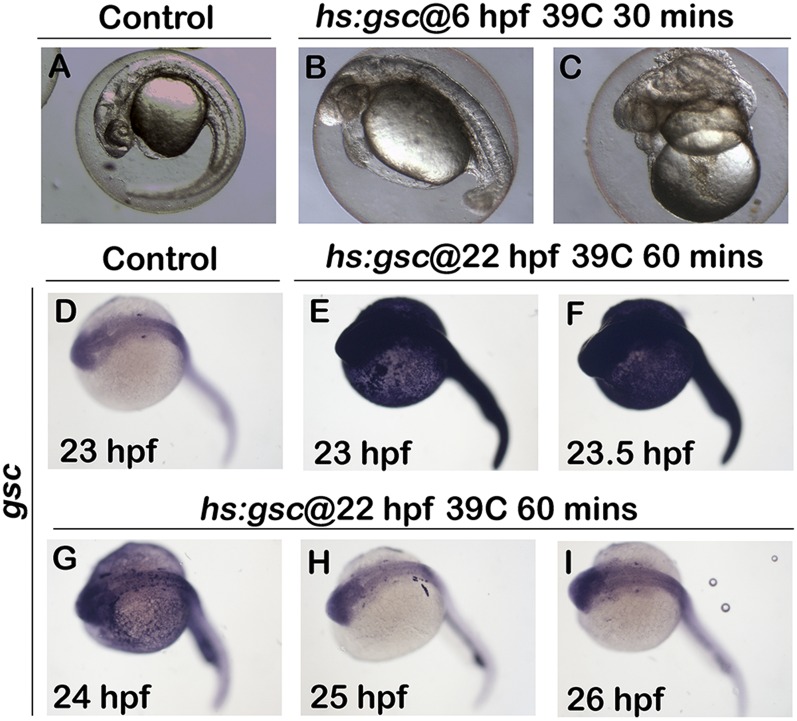
Heat-shock activation of hs:gsc during gastrulation or otic development. (A–C) Whole-mount live images at 24 hpf (dorsal up, anterior left) show the phenotypic effects of a 30-min, 39 °C heat shock initiated at 6 hpf in control and hs:gsc embryos. Activation of hs:gsc under these standard heat-shock conditions dorsalized the embryos to varying degrees (B and C). (D–I) Whole-mount images (dorsal up, anterior left) showing gsc expression in controls and hs:gsc embryos. Embryos were fixed and stained at indicated times after the end of a 60-min heat shock initiated at 22 hpf. Activation of hs:gsc at 22 hpf led to a strong increase in the gsc level by the end of the heat-shock period (E). The level of gsc expression was maximal at 23.5 hpf (F) but declined rapidly thereafter, returning to normal by 25 and 26 hpf (H and I).
Fig. 3.
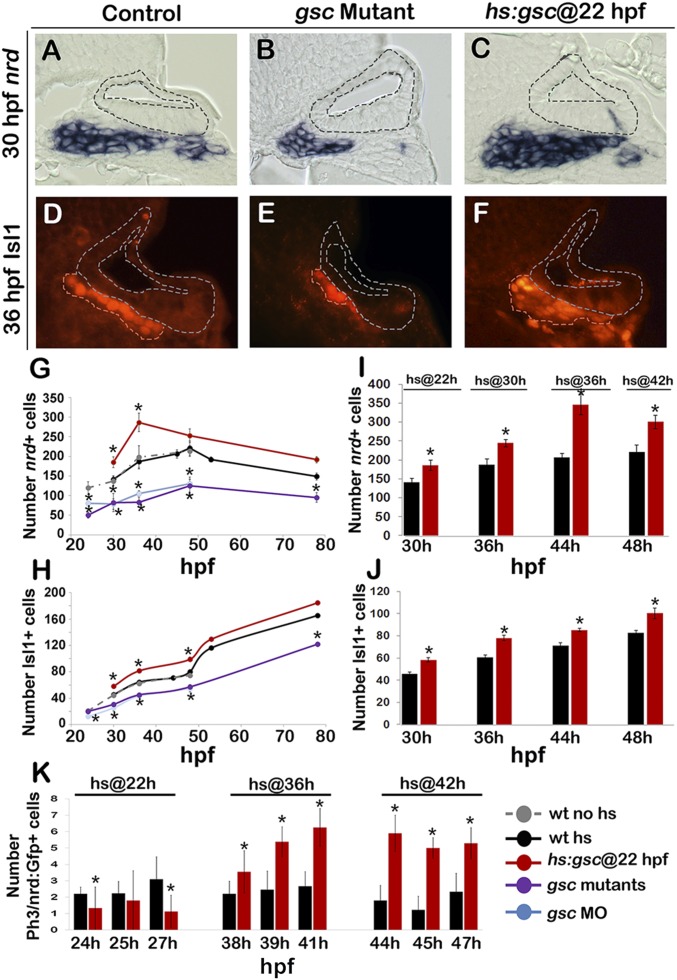
Effects of Gsc on later stages of SAG development. (A–F) Cross-sections passing through the utricular sensory epithelium (dorsal up, medial left) in controls, gsc mutants, and hs:gsc embryos showing expression of nrd (A–C) or Isl1 (outlined in orange, D–F). The otic epithelium is outlined in all images. (Magnification: A–F, 640×.) (G–J) Mean and SD of the total number of nrd+ (G and I) or Isl1+ (H and J) cells for the genotypes indicated in the color key at times presented on the x axes. nrd+ cells were counted on serial sections (n = 3–5), and Isl1+ cells were counted on whole mounts for time points between 30 and 48 hpf (n = 10–17) and on serial sections for 53 and 78 hpf (n = 2–3). (K) Means and SD of the total number of Phospho-Histone H3 (pH3)+ cells within the nrd:Gfp+ domain (which marks TA cells) at the indicated times in control and hs:gsc embryos. Embryos were heat shocked at the indicated times. Asterisks indicate statistically significant differences compared with control embryos (P < 0.05).
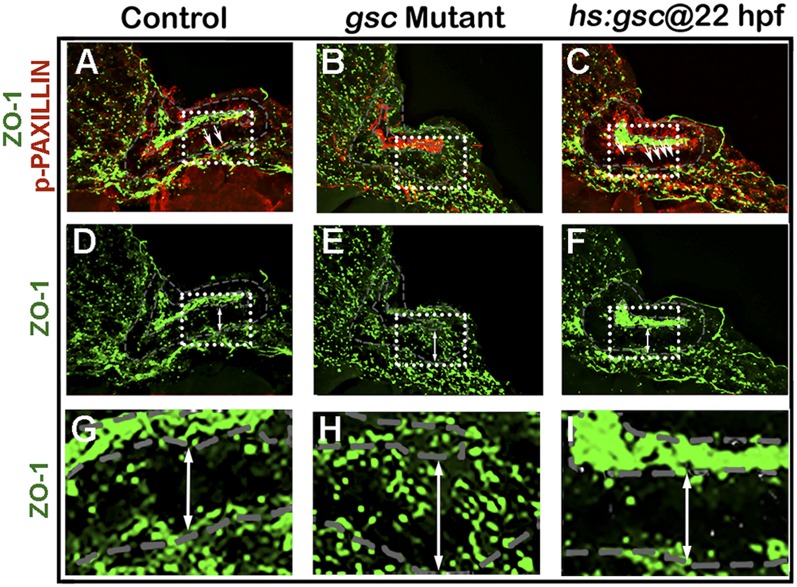
We next examined the effects of Gsc on the epithelial and mesenchymal cell markers. Zonula Occludens (ZO)-1 is expressed apically in epithelial otic cells but is lost upon transition to the mesenchymal state (Fig. S5 A, D, and G), whereas the transition is marked by activation of the focal adhesion protein p-Paxillin at the leading edge of migrating cells (Fig. 2 K and N and Fig. S5A). Delaminating otic cells also show dramatic redistribution of golgi marker GM130 to the basal surface as cells transition to the mesenchymal state (Fig. 2 Q and T). Gsc mutants showed more ZO-1 staining in the otic epithelium (Fig. S5 B, E, and H) and a loss of cells with p-Paxillin staining (Fig. 2 L and O). gsc mutants also showed a reduced number of cells with basal GM130 staining (Fig. 2 R and U). Conversely, activation of hs:gsc reduced the ZO-1 staining in the otic epithelium (Fig. S5 C, F, and I) and increased the number of cells with p-Paxillin staining (Fig. 2 M and P and Fig. S5C). Activation of hs:gsc also increased the number of cells with basal GM130 staining (Fig. 2 S and V). Overall, these results suggest that gsc stimulates EMT of neural progenitors in the otic vesicle without affecting cell fate specification. Consistent with this idea, we observed a ∼12% decrease in the size of the otic vesicle at 24 hpf following activation of hs:gsc at 22 hpf (Fig. S6 A and D), likely caused by the increased number of cells leaving the otic vesicle. This size reduction persisted through at least 31 hpf (Fig. S6), suggesting a limited capacity to compensate for earlier cell loss.
Fig. S5.
Effects of altering gsc function on ZO-1 and p-Paxillin. (A–I) Cross-sections through the neurogenic domain just posterior to the utricular macula in a control embryo (A, D, and G), gsc mutant (B, E, and H) and hs:gsc embryo (C, F, and I) at 24 hpf. These are the same sections depicted in Fig. 2 K–P but showing costaining for p-Paxillin (red) and ZO-1 (green) (A–C) or ZO-1 alone (D–I). The boxed regions in A–F are enlarged in G–I. White arrows (A–C) indicate cells undergoing EMT with elevated basal accumulation of p-Paxillin, whereas double-headed arrows (D–I) span the otic epithelium to highlight changes in ZO-1 staining.
Fig. S6.
The size of the otic vesicle declines following activation of hs:gsc. (A–F) Whole-mount live specimens show dorsolateral views of the otic vesicle in controls (A–C) and hs:gsc embryos (D–F). (G) Means and SD of surface area of the otic vesicle, normalized to control embryos, are indicated for hs:gsc embryos at the indicated time points. Significant differences (P < 0.05) from the control are indicated with asterisks.
Effects of Gsc on Later Stages of SAG Development.
Next, we examined whether altered delamination of neuroblasts affected later stages of neural development. Normally, newly delaminated neural progenitors quickly lose expression of ngn1 and up-regulate neurod, marking the TA stage of SAG development (3, 7–9). TA cells migrate toward the hindbrain as they proliferate and then differentiate into mature neurons, marked by Isl1 staining. The number of neurod+ TA cells and mature neurons was significantly reduced in gsc mutants and morphants at every time point examined (Fig. 3 B, E, G, and H). This is consistent with impairment of neuroblast delamination seen in these embryos. Conversely, activation of hs:gsc at 22 hpf led to a ∼30% increase in the number of neurod+ TA cells and mature neurons (Fig. 3 C and F–H). Furthermore, the number of mature SAG neurons remained elevated in these embryos through at least 50 hpf before returning to control levels (Fig. 3H). Overexpression of gsc during earlier placodal stages also increased accumulation of Isl1+ neurons at 30 hpf, although to a lesser degree than activation at 22 hpf (Fig. S7C). Activation of hs:gsc at 30 hpf gave results similar to activation at 22 hpf (Fig. 3 I and J and Fig. S7 A and B). Interestingly, activation of hs:gsc at 36 or 42 hpf caused a disproportionately greater increase in the number of neurod+ TA cells compared with earlier activation (Fig. 3I). This was unexpected because rates of neuroblast specification are very low at these later stages, suggesting another source of supernumerary neurod+ cells. Analysis of the cell proliferation revealed that activation of hs:gsc at 36 or 42 hpf dramatically increased the rate of mitosis in TA cells (Fig. 3K), likely accounting for increased numbers of TA cell expression of ngn1 and neurod (Figs. 2F and 3I). In contrast, activation of hs:gsc at 22 hpf reduced the rate of proliferation among TA cells (Fig. 3K). Thus, activation of hs:gsc increases the number of TA cells by different mechanisms at different stages: Gsc increases the rate of neuroblast delamination during early stages of neurogenesis, whereas it increases the rate of proliferation in TA cells during later stages of neurogenesis. Activation of hs:gsc did not alter the rate of proliferation in the otic epithelium (Fig. S7D). gsc mutants showed normal proliferation in the otic epithelium and in TA cells at all stages (Fig. S7E).
Fig. S7.
Effects of Gsc on development of SAG neurons and sensory epithelia. (A–C) Means and SD of the total number of nrd+ TA cells (A) or Isl1+ (B and C) SAG neurons for the genotypes indicated in the color key. Nrd+ cells were counted on serial sections, and Isl1+ cells were counted on whole mounts for time points between 30 and 48 hpf (n = 10–17) and on serial sections for 53 and 78 hpf (n = 2–3). (D and E) Means and SD for the number of pH3+ cells in the otic epithelium of hs:gsc embryos (D) or in the otic epithelium or TA cells in gsc mutants (E) at the indicated times. (F) Means and SD of the total number of TUNEL+ hair cells or SAG neurons at the indicated times in controls, gsc mutants, or hs:gsc embryos. TUNEL+ cells were counted from serial sections (n = 3 or 4 otic vesicles per time point) except at 42 and 48 hpf, when counts were performed on whole mounts for hs:gsc and control embryos (n = 10–15). Asterisks indicate significant differences compared with control embryos (P < 0.05).
Despite the initial surge in neurod+ cells following activation of hs:gsc at 36 or 42 hpf, the number of neurod+ TA cells subsequently returned to the level seen in control embryos by 78 hpf (Fig. S7A). The decline in TA cells occurred concomitantly with a corresponding increase in the number of mature Isl1+ neurons (Fig. S7B).
gsc loss of function and overexpression led to a modest increase in the rate of apoptosis among hair cells and mature neurons (Fig. S7F). The elevated cell death possibly reflects the detrimental effects of altering epithelial integrity or nonautonomous effects of gsc function (see Discussion).
Cooperation Between Gsc and Ngn1 in Regulating EMT.
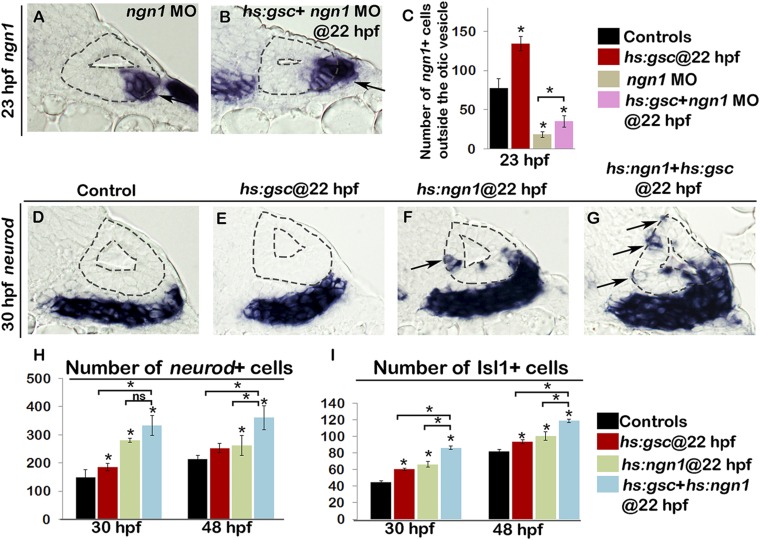
We noted that the ability of Gsc to promote EMT appeared to be restricted to the otic floor near the domain of ngn1 expression. This prompted us to examine the role of ngn1 in EMT. In ngn1 morphants, the number of ngn1+ cells that accumulated outside the otic vesicle was severely reduced (Fig. S8A), although a small number of delaminated cells was still observed. The fate of these cells is unclear, but they are unable to continue SAG development, as ngn1 morphants produce no TA cells or mature SAG neurons. The number of delaminated cells nearly doubled in ngn1 morphants following activation of hs:gsc but remained far below normal (Fig. S8 B and C). These data suggest that Gsc provides otic cells with a limited capacity for undergoing EMT in the absence of proper fate specification, but the capacity for delamination is strongly enhanced by ngn1. To test this further, we tested the effects of simultaneous overexpression of gsc and ngn1. Coactivation of hs:ngn1 and hs:gsc resulted in additive increases in the number of neurod+ TA cells and mature SAG neurons by 30 hpf, and these increases persisted through at least 48 hpf (Fig. S8 D–I). Interestingly, activation of hs:ngn1 induced ectopic neurod+ neuroblasts in the medial wall of the otic vesicle, but these neuroblasts were not observed to undergo delamination (Fig. S8F). In contrast, coactivation of hs:ngn1 and hs:gsc appeared to promote delamination of ectopic neurod+ neuroblasts from the medial wall (Fig. S8G). Thus, ngn1 and gsc synergize to promote EMT in the otic floor and to a lesser degree the medial wall. However, the ability to promote neurogenesis and delamination from ectopic sites was quite limited, suggesting that other regional factors act to oppose these functions in nonneurogenic regions.
Fig. S8.
Ngn1 and Gsc work in concert during otic neurogenesis. Transgenic embryos were heat shocked at 22 hpf. (A and B) Cross-sections passing through the neurogenic domain of the otic vesicle just posterior to the utricular macula show ngn1 expression in the indicated genotypes. Black arrows indicate delaminated cells outside the otic vesicle. (C) Means and SD for the total number of ngn1+ cells outside the otic vesicle. Genotype of the embryos is indicated in the color key. (D–G) Cross-sections show nrd+ cells in embryos with indicated phenotypes. Black arrows point to the ectopic expression of nrd in the medial otic vesicle (F and G) including delamination from the medial wall of the otic vesicle in hs:gsc embryos (G). (H and I) Means and SD for the total number of nrd+ cells (H) and Isl1+ mature SAG neurons (I) in embryos with genotypes indicated in the color key. Asterisks indicate statistically significant differences between groups indicated by brackets or compared with control embryos (P < 0.05).
Pax2a Opposes Gsc Function in the Otic Vesicle.
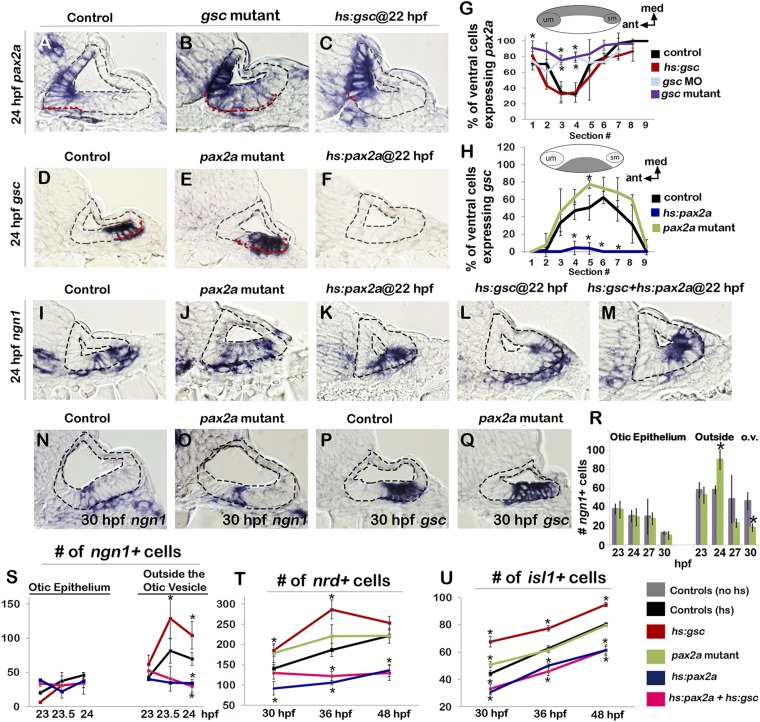
We hypothesized that pax2a, which is expressed in the medial half of the otic vesicle, acts to oppose gsc function and block EMT. Pax2 has been shown to stabilize the otic epithelium during placodal stages in zebrafish, chick, and mouse (17, 18, 32, 33), raising the possibility that this function persists after expression becomes restricted to the medial wall of the otic vesicle (Fig. 4A). We therefore examined the functional relationship between pax2a and gsc in the otic vesicle. Normally, pax2a expression abuts but does not overlap the neurogenic domain in the otic floor. In gsc mutants, pax2a expression expanded laterally into the neurogenic domain, albeit at a relatively low level (Fig. 4 B and G), whereas activation of hs:gsc caused the domain of pax2a to recede slightly from the otic floor in regions near the sensory maculae (Fig. 4 C and G). Conversely, in pax2a mutants, the domain of gsc expression showed a weak medial expansion, whereas activation of hs:pax2a completely eliminated gsc expression within 2 h (Fig. 4 D–F and H). These data suggest that gsc and pax2a mutually repress each other’s expression in the otic floor, with an especially prominent role of pax2a in repressing gsc. Next we examined whether pax2a function affects neurogenesis or EMT in the otic vesicle. Loss of pax2a function did not alter ngn1 expression in the otic epithelium yet transiently increased the number of delaminated ngn1+ neuroblasts at 24 hpf (Fig. 4 J and R), consistent with the observed expansion of gsc expression in these embryos (Fig. 4 E and H). However, the number of delaminating cells in pax2a mutants subsequently fell to less than half of normal at 27 and 30 hpf (Fig. 4R). Consistent with dynamic changes in delamination, accumulation of TA cells and mature neurons was initially elevated in pax2a mutants but subsequently returned to normal after 30 hpf (Fig. 4 T and U). The later decline probably reflects sporadic cell death in otic neurons and epithelia as previously noted in zebrafish and mouse mutants lacking Pax2 (33–35). In contrast to the effects of disrupting pax2a, activation of hs:pax2a at 22 hpf strongly suppressed delamination of ngn1+ neuroblasts by 23–24 hpf (Fig. 4 K and S), consistent with loss of gsc expression (Fig. 4F). Accumulation of TA cells and mature SAG neurons was also severely impaired following activation of hs:pax2a, and these deficiencies persisted through at least 48 hpf (Fig. 4 T and U). Importantly, coactivation of hs:gsc and hs:pax2a at 22 hpf completely masked the effects of hs:gsc, causing a phenotype similar to activation of hs:pax2a alone: Specifically, neuroblast delamination was strongly suppressed (Fig. 4 M and S), and there was a lasting deficit in accumulation of TA cells and mature SAG neurons (Fig. 4 T and U). Thus, in addition to repressing gsc transcription, Pax2a antagonizes transgenic Gsc activity.
Fig. 4.
Pax2a opposes the function of Gsc in the otic epithelium. (A–F) Cross-sections (dorsal up, medial left) passing through the widest part of the neurogenic domain of the otic vesicle just posterior to the utricular macula showing expression of pax2a or gsc at 24 hpf (outlined in red) in embryos with indicated genotypes. Control and transgenic embryos were heat shocked at 22 hpf. The otic epithelium is outlined black in each image. (G and H) Means and SD of the percentage of cells expressing pax2a or gsc in successive sections through the otic floor in the embryos with indicated genotypes. Data were obtained by counting the number of stained and unstained cells in each section (n = 3–4 specimens). Illustrations of typical domains of pax2a and gsc (medial up, anterior left) are provided above each graph to help clarify spatial patterns within each section of the otic floor. (I–M) Expression of ngn1 at 24 hpf in embryos with indicated genotypes. Transgenic embryos were heat shocked at 22 hpf. (N–Q) Expression of ngn1 (N and O) and gsc (P and Q) in controls and pax2a mutants at 30 hpf. (R–U) Means and SD of the total number of ngn1+ cells inside the otic epithelium or outside the otic vesicle (R and S; counted on serial sections, n = 3–4), nrd+ TA cells (T; counted on serial sections, n = 3–4) and Isl1+ cells (U; counted on whole mounts, n = 6–12). Control (hs) and transgenic embryos were heat shocked at 22 hpf and fixed at time points indicated. Asterisks indicate significant differences compared with control embryos (P < 0.05). Hs:gsc+hs:pax2a embryos were significantly different from hs:gsc embryos at all time points but showed no statistical difference compared with hs:pax2a embryos. (Magnification: All images, 640×.)
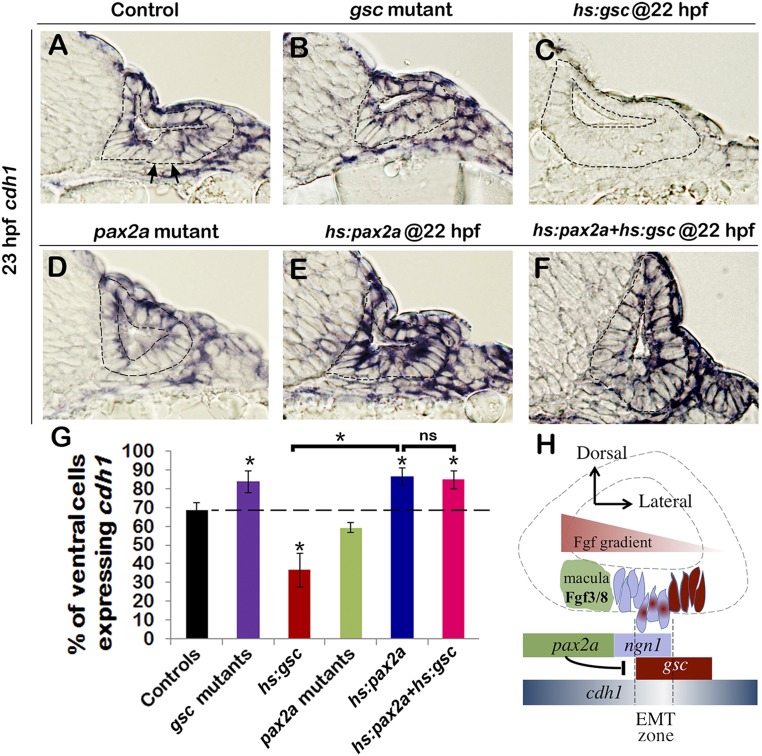
EMT is typically induced by repression of genes encoding Cadherins. We therefore surveyed expression of various cadherin genes in relation to gsc and pax2a function in the otic vesicle. During normal development, the E-cadherin gene cdh1 is expressed throughout the otic vesicle, but expression levels varied markedly in the otic floor, with low-expressing cells potentially corresponding to cells undergoing EMT (Fig. 5A). In gsc mutants, cdh1 was expressed at uniformly high levels throughout the otic floor (Fig. 5B), whereas activation of hs:gsc at 22 hpf caused global down-regulation of cdh1 in the otic epithelium (Fig. 5C). The opposite relationship was seen with regard to Pax2a function: pax2a mutants showed little change in cdh1 expression, although levels appeared slightly reduced in the otic floor (Fig. 5D). Activation of hs:pax2a caused substantial up-regulation of cdh1 throughout the otic vesicle, including uniformly high expression in the otic floor (Fig. 5E). Coactivation of hs:pax2a and hs:gsc led to uniformly high expression of cdh1 throughout the otic vesicle, similar to activation of hs:pax2a alone (Fig. 5 E and F). Changes in the percentage of cells in the otic floor expressing cdh1+ cells, determined by counting cells in serial sections, confirmed the above trends (Fig. 5G). Overall, these results suggest that Gsc and Pax2a have opposing effects on tissue architecture mediated in part by differential regulation of cdh1 transcription.
Fig. 5.
Gsc and Pax2a differentially regulate cdh1. (A–F) Cross-sections passing through the neurogenic domain of the otic vesicle just posterior to the utricular macula showing cdh1 expression at 23 hpf in embryos with indicated genotypes. The otic epithelium is outlined in each image. Arrows in A indicate cells with very low cdh1 expression interspersed with cells showing high cdh1 expression. (Magnification: A–F, 640×.) (G) Means and SD of the percentage of cells in the otic floor expressing cdh1 in the embryos with indicated genotypes. Data were obtained by counting the number of stained and unstained cells in serial sections (n = 3–4). Transgenic embryos were heat shocked at 22 hpf. Asterisks indicate significant differences between groups indicated by brackets or compared with control embryos (P < 0.05). (H) A model for regulation of epithelial tissue dynamics during otic neurogenesis. See Discussion for details.
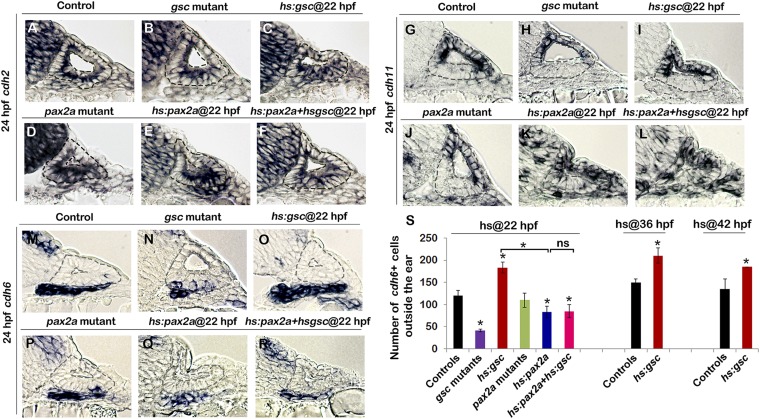
Global loss of E-cadherin transcription does not lead to widespread cell dispersal in hs:gsc embryos. This prompted us to analyze the expression of other cadherin genes that might have redundant functions in the otic vesicle. Indeed, expression of cdh2 remained unaffected in the otic vesicle upon loss of function or overexpression of gsc and/or pax2a (Fig. S9 A–F). Cdh11 is expressed in the nonneurogenic regions of the otic vesicle such as the medial and lateral walls (Fig. S9G) and did not show any changes in gsc mutants or hs:gsc embryos (Fig. S9 H and I). However, cdh11 transcript was lost from part of the medial wall in pax2a mutants, whereas activation of hs:pax2a induced cdh11 expression in ectopic locations including the otic floor (Fig. S9 J–L). Conceivably, cdh11 also helps to mediate Pax2a’s role in stabilizing epithelial integrity. Cdh6 is predominantly expressed in the delaminated otic neuroblasts in the TA pool (Fig. S9M). In keeping with the effects of gsc on delamination, gsc mutants had fewer cdh6+ cells outside the otic vesicle and gsc overexpression increased accumulation of cdh6+ cells in the TA pool (Fig. S9 N, O, and S). In contrast, overexpression of pax2a reduced the number of cdh6+ otic neuroblasts outside the ear and suppressed the effects of activating hs:gsc (Fig. S9 Q–S). pax2a mutants showed a statistically normal number of cdh6+ neuroblasts at 24 hpf (Fig. S9 P and S), possibly because elevated cell death counterbalances the transient spike in neuroblast delamination seen at 24 hpf in these embryos (Fig. 4R).
Fig. S9.
Expression of cadherin genes in relation to Pax2a or Gsc function in the otic vesicle. Transgenic embryos were heat shocked at the indicated times. (A–R) Cross-sections (dorsal up, medial left) passing through the middle of the neurogenic domain showing expression of cdh2 (A–F), cdh11 (G–L), and cdh6 (M–R) in embryos with indicated phenotypes. (S) Means and SD of the total number of cdh6-expressing cells outside the otic vesicle. Asterisks indicate significant differences between groups indicated by brackets or compared with control embryos (P < 0.05).
Discussion
Delamination from the otic vesicle is a vital step in otic neurogenesis that has heretofore been described only at the morphological level. Here we elucidate a molecular mechanism for this process (Fig. 5H). First, we describe a role for the organizer gene gsc in promoting delamination of neuroblasts from the otic vesicle. Loss of gsc function impairs delamination of SAG neuroblasts and leads to a significant loss of mature SAG neurons, whereas misexpression of gsc enhances neuroblast delamination and increases the size of the mature SAG. Second, Gsc’s ability to promote EMT requires coexpression of ngn1 as a parallel output of Fgf signaling. Comisexpression of gsc and ngn1 stimulates neuroblast delamination from ectopic sites within the otic vesicle. Third, we document a role for Pax2a in stabilizing the otic epithelium in opposition to Gsc. Pax2a not only represses gsc transcription, but it also suppresses Gsc protein function and blocks EMT. The opposing activities of Pax2a and Gsc correlate with their differential regulation of the cdh1, which is down-regulated in delaminating neuroblasts in zebrafish as well as mouse (36).
In this model, distinct regulation of ngn1 and gsc assures orderly delamination coupled with ongoing renewal of neuroblasts within the otic epithelium: As gsc+ neuroblasts delaminate, adjacent neuroblasts presumably move into the gsc domain in preparation for their own EMT. In early stages of neurogenesis, the domain of ngn1 expands, allowing replacement of cells lost through EMT. Eventually Fgf levels rise to block further ngn1 induction (3), terminating the ability to replenish neuroblasts as they delaminate. This mechanism is reminiscent of Gsc’s role in driving cellular dynamics and replenishment in the vertebrate organizer (19–22). An important goal of future research will be to conduct detailed cell-labeling experiments to elucidate patterns of epithelial rearrangement implied in our current study.
In addition to promoting EMT, overexpression of gsc had unexpected stage-specific effects on proliferation of TA cells. When activated at 22 hpf, hs:gsc caused a slight decrease in proliferation in the TA pool. This is understandable, as hs:gsc causes virtually all ngn1+ neuroblasts to delaminate at once, temporarily disrupting the normal steady flow of cycling progenitors into the TA pool. The sudden bolus of TA cells would then continue to develop synchronously and shift the population mean toward a postmitotic state, reducing the overall rate of proliferation. In contrast, activation of hs:gsc at 36 hpf or later caused a dramatic increase in proliferation of TA cells (Fig. 3K). The mechanistic basis for this is unclear but could reflect the changing status of Fgf signaling during successive stages of SAG neurogenesis (3, 12). During early stages of otic vesicle development, the level of Fgf signaling is relatively low, which stimulates specification of neuroblasts in the otic epithelium but is not sufficient to affect development of TA cells. As development proceeds, mature SAG neurons begin to accumulate and express fgf5, which eventually exceeds an upper threshold to terminate specification of neuroblasts in the otic epithelium. Elevated Fgf5 also delays terminal differentiation and promotes proliferation of TA cells. We speculate that forced expression of gsc in TA cells reinforces this effect of Fgf5, thereby increasing the TA pool disproportionately at later stages. This does not reflect a normal function of gsc, as it is not normally expressed in TA cells. Indeed, proliferation of TA cells was normal at all stages in gsc mutants (Fig. S7E). Further studies will be required to rigorously test the relationship between gsc and fgf5 during later stages of otic development.
Conservation and Diversity of Gsc Function.
The function of gsc in the zebrafish inner ear is likely to be conserved in other vertebrates. In mouse, Gsc is expressed in the developing otocyst in a pattern similar to that in zebrafish (23, 29), although its function has not been investigated. Loss of Gsc function in humans causes SAMS syndrome (syndrome of short stature, auditory canal atresia, mandibular hypoplasia, and skeletal abnormalities), characterized by mandibular hypoplasia similar to that seen in zebrafish gsc mutants, as well as loss of the auditory canal. These defects reflect deficiencies in neural crest-derived pharyngeal arches 1 and 2, known sites of Gsc expression, but it is unknown whether auditory neurons are also affected. Although a recently reported human chromosomal deficiency spanning Gsc causes auditory neuropathy, the deficiency also removes other genes that potentially affect the trait (37). Thus, additional studies are needed to clarify the role of Gsc in otic neurogenesis in mammals.
Interestingly, the role of Gsc in otic neurogenesis in zebrafish appears similar to the role of Gsc in regulating the stomatogastric nervous system (SNS) in Drosophila. SNS neuroblasts in fly initially form in the foregut epithelium and subsequently delaminate and migrate significant distances to form the equivalent of vertebrate enteric neurons. Epithelial SNS neuroblasts express Gsc, and delamination is strongly impaired in Gsc mutants (38, 39). Additionally, delamination requires Egfr and the RAS–MAPK pathway (40, 41). Together, these findings suggest that a broadly conserved pathway, acting through RAS–MAPK and Gsc, functions to localize neuroblast delamination in these widely divergent species.
In addition to gsc, it is likely that additional factors regulate EMT in the otic vesicle. We note that neuroblasts normally begin to delaminate from the otic epithelium by 17 hpf, several hours before the onset of gsc expression (Fig. 1), and delamination is not completely lost in gsc mutants (Fig. 2G). A number of transcription factors known to regulate EMT in other tissues, including Snail and Zeb proteins, are also expressed in the otic vesicle at appropriate stages (42–44). These might help promote delamination from the otic vesicle, but functional studies are yet to be reported.
Pax2 as an Epithelial Stabilizer.
Pax2 appears to coordinate cell fate specification and epithelial integrity in several contexts. In zebrafish, combinatorial knockdown of redundant genes pax2a, pax2b, and pax8 leads to progressive dispersal of otic cells soon after formation of the otic vesicle (18). Similarly, loss of both Pax2 and Pax8 in mouse impairs placode invagination and severely reduces otic vesicle size, apparently due to abnormal cell migration (33). Studies in chick show that Pax2 is required for proper expression of NCAM and N-cadherin to stabilize epithelial integrity during placode invagination (17). A continuing role in epithelial maintenance at later stages of otic development might explain why mouse and zebrafish embryos lacking Pax2 or Pax5 function show elevated cell death in the otic vesicle, especially in sensory epithelia (34, 35). Similarly, Pax2 plays a role in epithelial maintenance during kidney development. Mouse Pax2 mutants display severe renal defects resulting from loss of epithelial structure in the nephric duct, accompanied by formation of irregular outgrowths and increased cell motility (45, 46).
Regulation of Cadherin Dynamics.
The functions of Gsc and Pax2a counter each other in regulating the level of E-cadherin (cdh1) transcription (Fig. 5 A–F). E-cadherin is classically associated with epithelia, and its down-regulation is a common signature of EMT. Hence the ability of Pax2a to totally suppress the effects of Gsc can be explained partly through its ability to maintain cdh1 expression. However, this mechanism is likely not sufficient. Activation of hs:gsc down-regulates cdh1 throughout the otic vesicle yet does not induce widespread dispersal of the otic epithelium. This is probably because other cell adhesion molecules like cdh2 and cdh11 are coexpressed in the otic epithelium and are not affected by Gsc activity. The physical arrangement and functional relationships between coexpressed cell adhesion molecules remain poorly understood aspects of epithelial structure, but partial redundancy likely explains the limited effects of Gsc activity.
Another factor limiting Gsc’s ability to promote EMT is the requirement for coexpression of Ngn1. These factors probably regulate different subsets of genes to facilitate EMT. Gsc acts as a transcriptional repressor (47–50), likely explaining its ability to down-regulate cdh1 in the otic vesicle (Fig. 5C) as well as in a diverse array of aggressive metastatic cancers in which Gsc promotes EMT (27, 28, 51). In contrast, Ngn1 acts predominantly as a transcriptional activator. Relevant targets of Ngn1 might include factors that regulate f-actin dynamics or proteases that degrade basement membrane. All of these processes are potentially coordinated under the combinatorial control of Gsc and Ngn1 to ensure a robust EMT response.
EMT is typically associated with Cadherin switching. For example, cdh6 is up-regulated in neuroblasts after delamination, with weak expression first appearing in scattered cells within the otic epithelium (Fig. S9). Such switching may help weaken epithelial junctions and/or inhibit re-epithelialization of neuroblasts after delamination, while facilitating collective migration. Interestingly, the role of specific Cadherins often differs according to context. For example, cdh11 is often associated with mesenchymal cells (52, 53) yet is expressed in the most stable parts of the otic epithelium in zebrafish (Fig. S9). Conversely, cdh6 is expressed in migrating otic neuroblasts in zebrafish, whereas it marks premigratory neural crest in chick ectoderm and must be down-regulated to allow neural crest delamination (54, 55). The otic vesicle promises to be a useful model for future functional studies to determine how diverse cell adhesion molecules interact and contribute to tissue architecture and dynamics.
Materials and Methods
Fish Strains and Developmental Conditions.
All adult fish were maintained in a facility inspected and approved by the Institutional Animal Care and Use Committee (IACUC). Wild-type embryos were derived from the AB line. Transgenic lines used in this study include Tg(hsp70:fgf8a)x17 (56), Tg(hsp70I:dnfgfr1-EGFP)pd1 (57), TgBAC(neurod:EGFP) (58), Tg (hsp70:pax2a)x23 (59), and (lines produced for this report) Tg(hsp70:gsc)x58 and Tg(hsp70:ngn1)x28. Transgenic lines are named in the text as hs:fgf8, hs:dnfgfr1, nrd:GFP, hs:pax2a, hs:gsc, and hs:ngn1, respectively. Mutant lines gscx59 and pax2a tu29a (60) were used for loss of function analysis. Homozygous mutants were identified by characteristic morphological changes. Embryos were maintained at 28.5 °C (except where noted) and staged accordingly to standard protocols (61). PTU (1-phenyl 2-thiourea, 0.3 mg/mL; Sigma) was added to block pigment formation.
Gene Misexpression and Morpholino Injections.
To activate the heat shock transgenes, heterozygous carriers were incubated in a water bath at 39 °C for 60 min (except where noted). After heat shock, embryos were kept at 33 °C until the fixation. At least 15 embryos were observed for each time point. Transgenic carriers were identified by characteristic phenotypes when available or by PCR genotyping as previously described (62). Primer sequences are as follows (5′–3′): hs:gsc, GCAATGAACAGACGGGCATTTA (forward, F), GAATACACGGACACTGTTGCG (reverse, R); hs:pax2a, GCAATGAACAGACGGGCATTTA (F), TCTGCTTTGCAGTGAATATCCA (R). In some experiments, ngn1 or gsc were knocked down by injecting embryos at the one-cell stage with 5 ng of morpholino oligomer (MO) using previously published MO sequences (1, 63).
In Situ Hybridization and Immunohistochemistry.
Whole-mount in situ hybridization and antibody labeling were performed as previously described (64, 65). The primary and secondary antibodies used in this study are as follows: Anti-Islet1/2 (Developmental Studies Hybridoma Bank 39.4D5, 1:100), anti-GM130 (BD Transduction Laboratories 610822, 1:100), anti-ZO1 (ThermoFisher Scientific 33–9100, 1:150), anti–phospho-Paxillin pTyr118 (ThermoFisher Scientific PA5-17828, 1:50), anti–phospho-Histone H3 (EMD MILLIPORE 06–570, 1:350), and Alexa 546 goat anti-mouse or anti-rabbit IgG (ThermoFisher Scientific A-11003/A-11010, 1:50). TUNEL assay was performed by using Promega terminal deoxynucleotidyl transferase (M1871) according to the manufacturer’s protocol. Whole-mount stained embryos were prepared for cryosectioning as previously described (3) and cut serially into 10-μm sections.
Statistics.
Quantitation of cells expressing genes of interest was performed either in whole mounts (n = 6–20 specimens each) or by counting cells in serial sections (n = 2–4 specimens each). In experiments to test the effects of altering gene function, homozygous mutants and transgenic embryos were identified by characteristic morphological changes or PCR genotyping. Student’s t test was used for pairwise comparisons. Comparisons between three or more samples were analyzed by one way ANOVA and Tukey post hoc HSD (honest significant difference) test.
Acknowledgments
This work was supported by NIH/NIDCD Grant R01-DC03806.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609146113/-/DCSupplemental.
References
- 1.Andermann P, Ungos J, Raible DW. Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev Biol. 2002;251(1):45–58. doi: 10.1006/dbio.2002.0820. [DOI] [PubMed] [Google Scholar]
- 2.Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20(3):469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- 3.Vemaraju S, Kantarci H, Padanad MS, Riley BB. A spatial and temporal gradient of Fgf differentially regulates distinct stages of neural development in the zebrafish inner ear. PLoS Genet. 2012;8(11):e1003068. doi: 10.1371/journal.pgen.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddon C, Lewis J. Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol. 1996;365(1):113–128. doi: 10.1002/(SICI)1096-9861(19960129)365:1<113::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Hemond SG, Morest DK. Ganglion formation from the otic placode and the otic crest in the chick embryo: Mitosis, migration, and the basal lamina. Anat Embryol (Berl) 1991;184(1):1–13. doi: 10.1007/BF01744256. [DOI] [PubMed] [Google Scholar]
- 6.Carney PR, Silver J. Studies on cell migration and axon guidance in the developing distal auditory system of the mouse. J Comp Neurol. 1983;215(4):359–369. doi: 10.1002/cne.902150402. [DOI] [PubMed] [Google Scholar]
- 7.Kim WY, et al. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128(3):417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jahan I, Kersigo J, Pan N, Fritzsch B. Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell Tissue Res. 2010;341(1):95–110. doi: 10.1007/s00441-010-0984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Amico-Martel A. Temporal patterns of neurogenesis in avian cranial sensory and autonomic ganglia. Am J Anat. 1982;163(4):351–372. doi: 10.1002/aja.1001630407. [DOI] [PubMed] [Google Scholar]
- 10.Tsuchida T, et al. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79(6):957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 11.Gong Z, Hui CC, Hew CL. Presence of isl-1-related LIM domain homeobox genes in teleost and their similar patterns of expression in brain and spinal cord. J Biol Chem. 1995;270(7):3335–3345. doi: 10.1074/jbc.270.7.3335. [DOI] [PubMed] [Google Scholar]
- 12.Kantarci H, Edlund RK, Groves AK, Riley BB. Tfap2a promotes specification and maturation of neurons in the inner ear through modulation of Bmp, Fgf and notch signaling. PLoS Genet. 2015;11(3):e1005037. doi: 10.1371/journal.pgen.1005037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raft S, et al. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134(24):4405–4415. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- 14.Riley BB, Phillips BT. Ringing in the new ear: Resolution of cell interactions in otic development. Dev Biol. 2003;261(2):289–312. doi: 10.1016/s0012-1606(03)00245-8. [DOI] [PubMed] [Google Scholar]
- 15.Padanad MS, Riley BB. Pax2/8 proteins coordinate sequential induction of otic and epibranchial placodes through differential regulation of foxi1, sox3 and fgf24. Dev Biol. 2011;351(1):90–98. doi: 10.1016/j.ydbio.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hans S, Liu D, Westerfield M. Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors. Development. 2004;131(20):5091–5102. doi: 10.1242/dev.01346. [DOI] [PubMed] [Google Scholar]
- 17.Christophorou NA, Mende M, Lleras-Forero L, Grocott T, Streit A. Pax2 coordinates epithelial morphogenesis and cell fate in the inner ear. Dev Biol. 2010;345(2):180–190. doi: 10.1016/j.ydbio.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackereth MD, Kwak SJ, Fritz A, Riley BB. Zebrafish pax8 is required for otic placode induction and plays a redundant role with Pax2 genes in the maintenance of the otic placode. Development. 2005;132(2):371–382. doi: 10.1242/dev.01587. [DOI] [PubMed] [Google Scholar]
- 19.Blumberg B, Wright CV, De Robertis EM, Cho KW. Organizer-specific homeobox genes in Xenopus laevis embryos. Science. 1991;253(5016):194–196. doi: 10.1126/science.1677215. [DOI] [PubMed] [Google Scholar]
- 20.Blum M, et al. Gastrulation in the mouse: The role of the homeobox gene goosecoid. Cell. 1992;69(7):1097–1106. doi: 10.1016/0092-8674(92)90632-m. [DOI] [PubMed] [Google Scholar]
- 21.Cho KW, Blumberg B, Steinbeisser H, De Robertis EM. Molecular nature of Spemann’s organizer: The role of the Xenopus homeobox gene goosecoid. Cell. 1991;67(6):1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niehrs C, Keller R, Cho KW, De Robertis EM. The homeobox gene goosecoid controls cell migration in Xenopus embryos. Cell. 1993;72(4):491–503. doi: 10.1016/0092-8674(93)90069-3. [DOI] [PubMed] [Google Scholar]
- 23.Gaunt SJ, Blum M, De Robertis EM. Expression of the mouse goosecoid gene during mid-embryogenesis may mark mesenchymal cell lineages in the developing head, limbs and body wall. Development. 1993;117(2):769–778. doi: 10.1242/dev.117.2.769. [DOI] [PubMed] [Google Scholar]
- 24.Yamada G, et al. Targeted mutation of the murine goosecoid gene results in craniofacial defects and neonatal death. Development. 1995;121(9):2917–2922. doi: 10.1242/dev.121.9.2917. [DOI] [PubMed] [Google Scholar]
- 25.Rivera-Pérez JA, Mallo M, Gendron-Maguire M, Gridley T, Behringer RR. Goosecoid is not an essential component of the mouse gastrula organizer but is required for craniofacial and rib development. Development. 1995;121(9):3005–3012. doi: 10.1242/dev.121.9.3005. [DOI] [PubMed] [Google Scholar]
- 26.Parry DA, et al. SAMS, a syndrome of short stature, auditory-canal atresia, mandibular hypoplasia, and skeletal abnormalities is a unique neurocristopathy caused by mutations in Goosecoid. Am J Hum Genet. 2013;93(6):1135–1142. doi: 10.1016/j.ajhg.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartwell KA, et al. The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc Natl Acad Sci USA. 2006;103(50):18969–18974. doi: 10.1073/pnas.0608636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue TC, et al. Goosecoid promotes the metastasis of hepatocellular carcinoma by modulating the epithelial-mesenchymal transition. PLoS One. 2014;9(10):e109695. doi: 10.1371/journal.pone.0109695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vitelli F, et al. TBX1 is required for inner ear morphogenesis. Hum Mol Genet. 2003;12(16):2041–2048. doi: 10.1093/hmg/ddg216. [DOI] [PubMed] [Google Scholar]
- 30.Zhao J, Yang C, Guo S, Wu Y. GM130 regulates epithelial-to-mesenchymal transition and invasion of gastric cancer cells via snail. Int J Clin Exp Pathol. 2015;8(9):10784–10791. [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura N. Emerging new roles of GM130, a cis-Golgi matrix protein, in higher order cell functions. J Pharmacol Sci. 2010;112(3):255–264. doi: 10.1254/jphs.09r03cr. [DOI] [PubMed] [Google Scholar]
- 32.Padanad MS, Bhat N, Guo B, Riley BB. Conditions that influence the response to Fgf during otic placode induction. Dev Biol. 2012;364(1):1–10. doi: 10.1016/j.ydbio.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouchard M, de Caprona D, Busslinger M, Xu P, Fritzsch B. Pax2 and Pax8 cooperate in mouse inner ear morphogenesis and innervation. BMC Dev Biol. 2010;10:89. doi: 10.1186/1471-213X-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwak SJ, et al. Zebrafish pax5 regulates development of the utricular macula and vestibular function. Dev Dyn. 2006;235(11):3026–3038. doi: 10.1002/dvdy.20961. [DOI] [PubMed] [Google Scholar]
- 35.Burton Q, Cole LK, Mulheisen M, Chang W, Wu DK. The role of Pax2 in mouse inner ear development. Dev Biol. 2004;272(1):161–175. doi: 10.1016/j.ydbio.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 36.Davies D. Cell-extracellular matrix versus cell-cell interactions during the development of the cochlear-vestibular ganglion. J Neurosci Res. 2011;89(9):1375–1387. doi: 10.1002/jnr.22664. [DOI] [PubMed] [Google Scholar]
- 37.Schlade-Bartusiak K, Macintyre G, Zunich J, Cox DW. A child with deletion (14)(q24.3q32.13) and auditory neuropathy. Am J Med Genet A. 2008;146A(1):117–123. doi: 10.1002/ajmg.a.32064. [DOI] [PubMed] [Google Scholar]
- 38.Hahn M, Jäckle H. Drosophila goosecoid participates in neural development but not in body axis formation. EMBO J. 1996;15(12):3077–3084. [PMC free article] [PubMed] [Google Scholar]
- 39.Hernández K, Myers LG, Bowser M, Kidd T. Genetic tools for the analysis of Drosophila stomatogastric nervous system development. PLoS One. 2015;10(6):e0128290. doi: 10.1371/journal.pone.0128290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forjanic JP, Chen CK, Jäckle H, González Gaitán M. Genetic analysis of stomatogastric nervous system development in Drosophila using enhancer trap lines. Dev Biol. 1997;186(2):139–154. doi: 10.1006/dbio.1997.8590. [DOI] [PubMed] [Google Scholar]
- 41.González-Gaitán M, Jäckle H. Tip cell-derived RTK signaling initiates cell movements in the Drosophila stomatogastric nervous system anlage. EMBO Rep. 2000;1(4):366–371. doi: 10.1093/embo-reports/kvd064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119(4):1203–1215. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- 43.Thisse C, Thisse B, Postlethwait JH. Expression of snail2, a second member of the zebrafish snail family, in cephalic mesendoderm and presumptive neural crest of wild-type and spadetail mutant embryos. Dev Biol. 1995;172(1):86–99. doi: 10.1006/dbio.1995.0007. [DOI] [PubMed] [Google Scholar]
- 44.Delalande JM, Guyote ME, Smith CM, Shepherd IT. Zebrafish sip1a and sip1b are essential for normal axial and neural patterning. Dev Dyn. 2008;237(4):1060–1069. doi: 10.1002/dvdy.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soofi A, Levitan I, Dressler GR. Two novel EGFP insertion alleles reveal unique aspects of Pax2 function in embryonic and adult kidneys. Dev Biol. 2012;365(1):241–250. doi: 10.1016/j.ydbio.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouchard M, Souabni A, Mandler M, Neubüser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16(22):2958–2970. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danilov V, Blum M, Schweickert A, Campione M, Steinbeisser H. Negative autoregulation of the organizer-specific homeobox gene goosecoid. J Biol Chem. 1998;273(1):627–635. doi: 10.1074/jbc.273.1.627. [DOI] [PubMed] [Google Scholar]
- 48.Latinkic BV, Smith JC. Goosecoid and mix.1 repress Brachyury expression and are required for head formation in Xenopus. Development. 1999;126(8):1769–1779. doi: 10.1242/dev.126.8.1769. [DOI] [PubMed] [Google Scholar]
- 49.Yao J, Kessler DS. Goosecoid promotes head organizer activity by direct repression of Xwnt8 in Spemann’s organizer. Development. 2001;128(15):2975–2987. doi: 10.1242/dev.128.15.2975. [DOI] [PubMed] [Google Scholar]
- 50.Izzi L, et al. Foxh1 recruits Gsc to negatively regulate Mixl1 expression during early mouse development. EMBO J. 2007;26(13):3132–3143. doi: 10.1038/sj.emboj.7601753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taube JH, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci USA. 2010;107(35):15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimura Y, et al. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev Biol. 1995;169(1):347–358. doi: 10.1006/dbio.1995.1149. [DOI] [PubMed] [Google Scholar]
- 53.Hadeball B, Borchers A, Wedlich D. Xenopus cadherin-11 (Xcadherin-11) expression requires the Wg/Wnt signal. Mech Dev. 1998;72(1-2):101–113. doi: 10.1016/s0925-4773(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 54.Coles EG, Taneyhill LA, Bronner-Fraser M. A critical role for Cadherin6B in regulating avian neural crest emigration. Dev Biol. 2007;312(2):533–544. doi: 10.1016/j.ydbio.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taneyhill LA, Coles EG, Bronner-Fraser M. Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development. 2007;134(8):1481–1490. doi: 10.1242/dev.02834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Millimaki BB, Sweet EM, Riley BB. Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev Biol. 2010;338(2):262–269. doi: 10.1016/j.ydbio.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee Y, Grill S, Sanchez A, Murphy-Ryan M, Poss KD. Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development. 2005;132(23):5173–5183. doi: 10.1242/dev.02101. [DOI] [PubMed] [Google Scholar]
- 58.Obholzer N, et al. Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J Neurosci. 2008;28(9):2110–2118. doi: 10.1523/JNEUROSCI.5230-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sweet EM, Vemaraju S, Riley BB. Sox2 and Fgf interact with Atoh1 to promote sensory competence throughout the zebrafish inner ear. Dev Biol. 2011;358(1):113–121. doi: 10.1016/j.ydbio.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brand M, et al. Mutations in zebrafish genes affecting the formation of the boundary between midbrain and hindbrain. Development. 1996;123:179–190. doi: 10.1242/dev.123.1.179. [DOI] [PubMed] [Google Scholar]
- 61.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 62.Westerfield M. 1993. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio) (University of Oregon Press, Eugene, OR)
- 63.Seiliez I, Thisse B, Thisse C. FoxA3 and goosecoid promote anterior neural fate through inhibition of Wnt8a activity before the onset of gastrulation. Dev Biol. 2006;290(1):152–163. doi: 10.1016/j.ydbio.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 64.Phillips BT, Bolding K, Riley BB. Zebrafish fgf3 and fgf8 encode redundant functions required for otic placode induction. Dev Biol. 2001;235(2):351–365. doi: 10.1006/dbio.2001.0297. [DOI] [PubMed] [Google Scholar]
- 65.Riley BB, Chiang M, Farmer L, Heck R. The deltaA gene of zebrafish mediates lateral inhibition of hair cells in the inner ear and is regulated by pax2.1. Development. 1999;126(24):5669–5678. doi: 10.1242/dev.126.24.5669. [DOI] [PubMed] [Google Scholar]