Significance
Chronic helminth infections are accompanied by profound immune regulation. In humans, helminth-induced immune reactivity has not been thoroughly investigated in trial settings. We assessed the effect of anthelmintic treatment on immune responses in a whole community in a placebo-controlled randomized controlled trial. We show increased immune responses to helminth-specific as well as unrelated antigens, in parallel with decreased expression of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), which is a molecule involved in putting the brake on immune activation. Deworming seems to lead to decreased immunoregulation and increased immune responsiveness. These findings are of importance regarding the suboptimal vaccine responses in helminth-endemic areas and also in anticipating the future rise in inflammatory diseases when helminth infections are increasingly controlled.
Keywords: helminths, albendazole, cytokine responses, Indonesia, deworming
Abstract
In cross-sectional studies, chronic helminth infections have been associated with immunological hyporesponsiveness that can affect responses to unrelated antigens. To study the immunological effects of deworming, we conducted a cluster-randomized, double-blind, placebo-controlled trial in Indonesia and assigned 954 households to receive albendazole or placebo once every 3 mo for 2 y. Helminth-specific and nonspecific whole-blood cytokine responses were assessed in 1,059 subjects of all ages, whereas phenotyping of regulatory molecules was undertaken in 121 school-aged children. All measurements were performed before and at 9 and 21 mo after initiation of treatment. Anthelmintic treatment resulted in significant increases in proinflammatory cytokine responses to Plasmodium falciparum-infected red blood cells (PfRBCs) and mitogen, with the largest effect on TNF responses to PfRBCs at 9 mo—estimate [95% confidence interval], 0.37 [0.21–0.53], P value over time (Ptime) < 0.0001. Although the frequency of regulatory T cells did not change after treatment, there was a significant decline in the expression of the inhibitory molecule cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) on CD4+ T cells of albendazole-treated individuals, –0.060 [–0.107 to –0.013] and –0.057 [–0.105 to –0.008] at 9 and 21 mo, respectively; Ptime = 0.017. This trial shows the capacity of helminths to up-regulate inhibitory molecules and to suppress proinflammatory immune responses in humans. This could help to explain the inferior immunological responses to vaccines and lower prevalence of inflammatory diseases in low- compared with high-income countries.
Soil-transmitted helminths (STHs) represent the most common infectious disease worldwide (1). In addition to specific worm-associated morbidities, it has been argued that chronic STH infections may magnify health-related burdens in communities remote from health care facilities, exacerbating anemia, poor nutritional status, and possibly poor cognitive development (1). However, this was not fully supported by the latest analysis of the Cochrane database (2).
Immunologically, cellular immune hyporesponsiveness is a hallmark of chronic helminth infections that may allow parasites’ long-term survival (3). The consequences of immunosuppression are manifold with potentially major public health relevance. Immune hyporesponsiveness could curtail effective immune responses, thereby increasing susceptibility to pathogens, and helminths are associated with suboptimal vaccine responses (4–6). The helminth-related dampened immune responses might nevertheless help to prevent immunopathology during coinfections and, possibly, aberrant reactivity to environmental or self-antigens (7). With respect to the latter, there is currently much interest in the use of helminth infections to treat allergies and autoimmune diseases, exploiting their ability to induce immune hyporesponsiveness (8).
Suppressed lymphocyte responses were described in the 1970s (9), but the evidence base has not moved much beyond animal models and cross-sectional studies in humans (10). The cellular mechanisms associated with helminth-related immune hyporesponsiveness are not fully understood. Several regulatory cells and molecules are thought to play an important role in the regulatory network (3). Within T-cell responses, expansion of T-regulatory cells (Tregs) is reported in both animal models (10) and some human studies (11, 12). Tregs suppress helminth-specific and bystander proliferative and proinflammatory responses. Expression of T-cell–associated molecules, including cytotoxic T lymphocyte-associated antigen 4 (CTLA) and programmed death-1 (PD-1), may also be involved in helminth-induced hyporesponsiveness and spillover suppression (13).
Longitudinal studies assessing the effect of anthelmintic treatment on cellular immune responsiveness are rare and either lack placebo controls, target children only, or measure immune responses at one time point posttreatment (14–16). Moreover, none have examined the changes in regulatory cells or molecules. No large-scale community-based intervention studies to establish whether helminth infections lead to immune hyporesponsiveness in humans have been reported.
To disentangle the impact of helminths on the immune system from other influences, we conducted a household cluster-randomized, double-blind, placebo-controlled trial of albendazole once every 3 mo in communities with high STH prevalence on Flores island, Indonesia. Here we present results concerning the effects of anthelmintic treatment on cellular immune responses.
Results
Albendazole Treatment Reduces but Does Not Eliminate Helminth Infections.
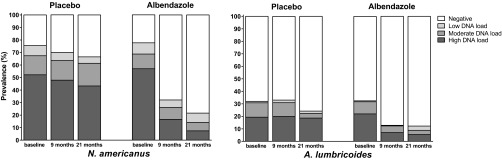
Characteristics of the study participants (n = 1,059) are shown in Table 1. At baseline, one or more helminth species were found in 88.7% of individuals, with hookworm being the most prevalent (77.1% of total). The trial consort diagram with follow-up data can be found in Fig. S1. Albendazole treatment reduced the prevalence of geohelminths after 9 (51.9% vs. 84.1% for placebo) and 21 mo (39.2% vs. 80% for placebo) (Table S1). In the whole immunological study within Scientific Programme Indonesia–Netherlands (IMMUNOSPIN) trial, the prevalence of geohelminth infection was 87.3%, and albendazole treatment reduced prevalence of geohelminths after 9 (51.4% vs. 82.8% for placebo) and 21 mo (41.9% vs. 78.8% for placebo). As for the whole IMMUNOSPIN trial, the greatest effect was on hookworm followed by Ascaris, whereas the effect on Trichuris infections was less pronounced. Albendazole also reduced intensities of hookworm and Ascaris infections, as assessed by PCR (Fig. S2).
Table 1.
Baseline characteristics of the study population
| Parameter | N | Placebo | N | Albendazole |
| Age, mean in years (SD) | 572 | 25.7 (18.5) | 487 | 24.9 (18.4) |
| Sex, female, n (%)* | 572 | 328 (57.3) | 487 | 279 (57.3) |
| Area, rural, n (%)* | 572 | 114 (19.9) | 487 | 106 (21.8) |
| BMI >19 y old, mean (SD) | 264 | 22.1 (4.1) | 220 | 22.1 (3.8) |
| z score of BMI ≤19 y old, mean (SD) | 194 | −1.15 (1.11) | 386 | −1.14(1.15) |
| Parasite infection, n (%)* | ||||
| Helminth, any spp. | 322 | 286 (88.8) | 237 | 210 (88.6) |
| Hookworm† | 335 | 255 (76.1) | 245 | 192 (78.4) |
| N. americanus† | 335 | 252 (75.2) | 245 | 188 (76.7) |
| A. duodenale† | 335 | 25 (7.5) | 245 | 17 (6.9) |
| A. lumbricoides† | 335 | 105 (31.3) | 245 | 80 (32.7) |
| S. stercoralis† | 335 | 3 (0.9) | 245 | 14 (5.7) |
| T. trichiura† | 415 | 106 (25.5) | 310 | 62 (20.0) |
| Malarial parasitaemia, any spp.‡ | 567 | 24 (4.2) | 483 | 24 (5.0) |
| P. falciparum | 567 | 16 (2.8) | 483 | 11 (2.3) |
| Plasmodium vivax | 567 | 8 (1.4) | 483 | 10 (2.1) |
| Plasmodium malariae | 567 | 0 (0.0) | 483 | 4 (0.8) |
| Cytokine production, pg/mL, median [IQR] | ||||
| LPS | ||||
| TNF-α | 554 | 743 [368−1,293] | 468 | 769 [339−1,318] |
| IL-10 | 554 | 271 [163−441] | 468 | 256 [158−406] |
| PHA | ||||
| TNF-α | 516 | 100 [50−222] | 435 | 103 [50−214] |
| IL-10 | 515 | 76 [41−129] | 435 | 70 [37−116] |
| IFN-γ | 516 | 1,625 [584−3,983] | 435 | 1,270 [538−4,340] |
| IL-2 | 516 | 23 [0−101] | 432 | 23 [0−92] |
| IL-5 | 516 | 563 [309−840] | 435 | 520 [317−829] |
| PfRBCs | ||||
| TNF-α | 299 | 18 [4−42] | 237 | 14 [3−38] |
| IL-10 | 300 | 10 [5−19] | 238 | 10 [5−20] |
| IFN-γ | 300 | 163 [75−388] | 239 | 176 [70−376] |
| IL-2 | 300 | 50 [5−125] | 239 | 40 [5−112] |
| IL-5 | 300 | 14 [5−26] | 239 | 12 [4−23] |
| AscAg | ||||
| TNF-α | 517 | 5 [0−15] | 438 | 6 [0−14] |
| IL-10 | 516 | 7 [2−15] | 438 | 7 [1−14] |
| IFN-γ | 516 | 19 [6−47] | 441 | 21 [7−47] |
| IL-2 | 497 | 38 [4−114] | 426 | 36 [0−107] |
| IL-5 | 515 | 24 [9−68] | 440 | 24 [9−63] |
IQR, interquartile range.
The number of positives (n) of the total population examined (N).
Diagnosed by PCR.
Diagnosed by microscopy.
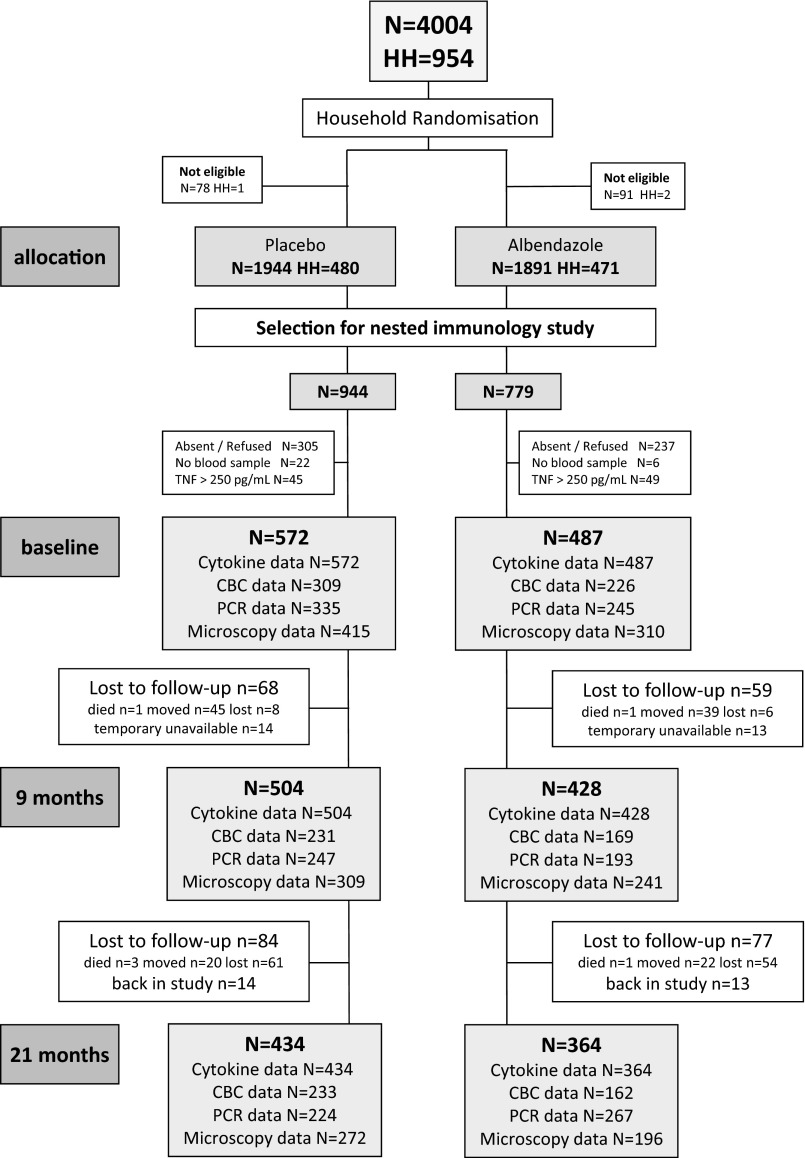
Fig. S1.
Trial consort diagram. The current study is nested within the ImmunoSPIN trial, with a total of 4,004 individuals in two participating villages who were allocated to placebo and albendazole treatment. For the immunological studies, a random selection was made. Cytokines were assessed for 1,059 subjects, of which 572 were in the placebo and 487 in the albendazole arm. After 9 mo, 504 and 428 and, after 21 mo, 434 and 364 individuals were analyzed in the placebo and albendazole group, respectively. Availability of CBCs and parasitological data are indicated at the different time points for both groups.
Table S1.
Prevalence of helminth infections at posttreatment time points
| Study group | N | Any spp., n (%) | Hookworm, n (%)† | A. lumbricoides, n (%)† | Trichuris trichiuria, n (%)‡ |
| 9 mo | |||||
| Placebo | 227 | 191 (84.1) | 160 (70.5) | 76 (33.5) | 72 (31.7) |
| Albendazole | 181 | 94 (51.9)** | 59 (32.6)** | 24 (13.3)** | 38 (21.0)** |
| 21 mo | |||||
| Placebo | 215 | 171 (80.0) | 144 (67.0) | 52 (24.2) | 62 (28.8) |
| Albendazole | 148 | 58 (39.2)** | 32 (21.6)** | 18 (12.2)** | 24 (16.2)** |
**P values were generated from the modeled data for combined effect of albendazole treatment over time and were significant (P < 0.001) for any helminth and for each of the species separately. N, total number per group; n, number of infected subjects.
Diagnosed by PCR.
Diagnosed by microscopy.
Fig. S2.
Prevalence and intensity infections at posttreatment time points. Helminth prevalence was assessed at baseline and 9 and 21 mo posttreatment by PCR (Ascaris and hookworms) or microscopy (Trichuris). Helminth infection intensity is given for baseline, 9-mo, and 21-mo time points, based on cycle threshold values derived from PCR analysis of stool samples. Positive Ct values were grouped into three categories—Ct < 30.0, 30.0 ≤ Ct < 35.0, and ≥35.0—representing a high (dark gray bars), moderate (medium gray bars), and low (light gray bars) DNA load, respectively. The white bars represent the uninfected. (Left) Data for N. americanus infection; (Right) A. lumbricoides infections. The P values were generated from linear mixed models of the combined effect of albendazole treatment over time, which were significant (P < 0.001) for any helminth and for each of the species separately.
Helminth-Specific and Nonspecific Whole-Blood Cytokine Responses Are Increased After Albendazole Treatment.
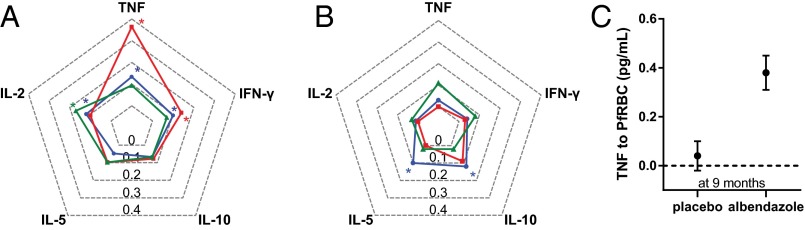
Fig. 1 A and B shows the effect of treatment on cytokine responses at 9 mo and 21 mo, respectively. Regarding helminth-specific cytokines, Ascaris antigen (AscAg)-induced interleukin-2 (IL-2) production was significantly enhanced by treatment over the study period (Ptime = 0.018), with a significant increase in the treated group at 9 mo—estimate [95% confidence interval (CI)], 0.17 [0.05–0.28] (Fig. 1A).
Fig. 1.
The effect of anthelminthic treatment on cytokine responses to AscAg, PfRBCs, and PHA. TNF, IFN-γ, IL-2, IL-5, and IL-10 concentrations were assessed in supernatants of 72 h-stimulated whole-blood cultures. The values on the y axis (the spider web lines) represent the estimated outcome (beta) of the effect of albendazole treatment on cytokine responses to PHA (blue), PfRBCs (red), and AscAg (green). By comparing the responses in the albendazole versus placebo group, the estimates of the treatment effect in the whole study population after 9 (A) and 21 (B) mo of albendazole treatment were obtained using linear mixed models, and positive values were plotted in a spider chart. Statistically significant estimates at 9 mo were IL-2 responses to AscAg (estimated effect of treatment [95% CI], 0.17 [0.05–0.28]), TNF (0.37 [0.21–0.53]), and IFN-γ (0.14 [0.03–0.24]) responses to PfRBCs and TNF (0.14 [0.05–0.24]), IFN-γ (0.10 [0.01–0.19]), and IL-2 (0.12 [0.01–0.23]) responses to PHA. At 21 mo posttreatment, PHA-induced IL-5 (0.10 [0.01–0.19]) and IL-10 (0.12 [0.05–0.19]) were significantly enhanced. As an indication of the magnitude of change in level of cytokines that were significantly different between the placebo and albendazole group, geometric mean and SE for TNF to PfRBCs at 9 mo (C) are given as an example.
In response to plasmodial antigens (Plasmodium falciparum-infected red blood cells, PfRBCs), there was an increase over time in proinflammatory cytokine tumor necrosis factor (TNF; Ptime < 0.0001) and IFN-gamma (IFN-γ; Ptime = 0.036) after albendazole treatment. As shown in Fig. 1A, both TNF and IFN-γ were significantly higher in the albendazole compared with the placebo group at the 9-mo time point (0.37 [0.21–0.53] for TNF and 0.14 [0.03–0.24] for IFN-γ). To get an indication of the absolute changes in cytokine levels, TNF production to PfRBCs in the two groups at the 9-mo time point is shown in Fig. 1C. The differences in other statistically significant cytokine changes are shown in Fig. S3. None of the significant changes in antigen-specific responses were correlated with worm burden before treatment (Table S2).
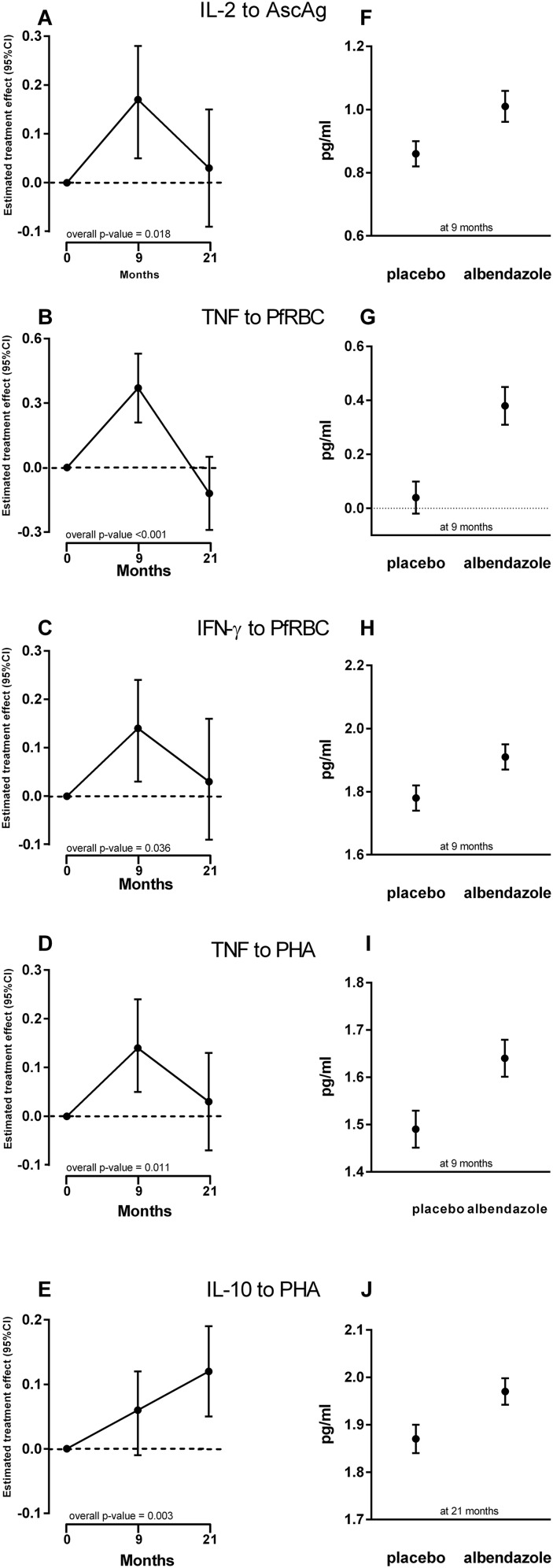
Fig. S3.
Effect of deworming on cytokine responses to AscAg, PfRBCs, and PHA. Cytokine concentrations were assessed in supernatants of 72 h-stimulated whole-blood cultures. The estimated effects of albendazole treatment on the production of the significant cytokines over the whole trial period [IL-2 to AscAg (A), TNF (B) and IFN-γ (C) to PfRBCs, and TNF (D) and IL-10 (E) to PHA] are shown (Left). The estimates are calculated based on the differences in treatment versus placebo data but taking into account the clustered design and missing at random. The estimate, beta value is the unit change in cytokine as a result of treatment. (Right) The corresponding geometric means and SE of these cytokine levels [IL-2 to AscAg (F), TNF (G) and IFN-γ (H) to PfRBCs, TNF (I) and IL-10 (J) to PHA] in pg/mL. It should be noted that these geometric means were calculated only in subjects for whom we have data at those follow-up time points. Interpreting these means is valid only under missing completely at random.
Table S2.
Associations of worm burden pretreatment with cytokine responses
| Helminth infection | Association at 9 mo | ||
| Cytokine level | Pearson’s coefficient | P value | |
| Ascaris infection | TNF to AscAg | −0.022 | 0.79 |
| IFN-γ to AscAg | 0.104 | 0.21 | |
| IL-2 to AscAg | 0.010 | 0.91 | |
| IL-5 to AscAg | 0.073 | 0.37 | |
| IL-10 to AscAg | −0.018 | 0.82 | |
| All helminth infections, highest intensity | TNF to PfRBCs | −0.076 | 0.25 |
| IFN-γ to PfRBCs | 0.097 | 0.14 | |
| IL-2 to PfRBCs | 0.078 | 0.23 | |
| IL-5 to PfRBCs | −0.029 | 0.66 | |
| IL-10 to PfRBCs | −0.015 | 0.82 | |
Cycle threshold (CT) values of the PCR results (the lower the CT value, the higher the load) pretreatment were correlated with cytokine levels at 9 mo posttreatment. For responses to AscAg, intensity of Ascaris infection was used for correlation, whereas for bystander responses (to PfRBCs and PHA), the intensity of any helminth infection was used. For the analysis of all helminths, the lowest CT value was used in the analysis.
Regarding the general adaptive response (cytokine responses to phytohemagglutinin, PHA), albendazole treatment significantly increased TNF and IL-10 secretion (Ptime = 0.011 and Ptime = 0.003, respectively) over the trial period; for TNF, albendazole treatment resulted in elevated responses at 9 mo, whereas for IL-10 the response was significantly higher after 21 mo (for TNF at 9 mo, 0.14 [0.05–0.24], Fig. 1A; for IL-10 at 21 mo, 0.12 [0.05–0.19], Fig. 1B). The IFN-γ and IL-2 responses to PHA were transiently increased at 9 mo posttreatment, and PHA-induced IL-5 was higher at the 21-mo time point, but this did not reach statistical significance over the whole trial time period (IFN-γ Ptime = 0.076, IL-2 Ptime = 0.11, IL-5 Ptime = 0.068; Fig. 1).
Albendazole did not affect responses to lipopolysaccharide (LPS) (Table S3). Cytokines in unstimulated blood revealed no treatment-related differences (Table S3). IFN-γ responses to uninfected red blood cells (uRBCs) were not significantly different between treatment arms (Ptime = 0.91), however TNF production was increased posttreatment (Ptime = 0.018). This was only significant at 9 mo (9-mo estimate, 0.13 [0.01–0.25]; at 21 mo, –0.13 [–0.26–0.003]), although to a much lesser extent than the response to PfRBCs.
Table S3.
Effect of albendazole treatment on innate and unstimulated cytokine responses
| Outcome | Effect of treatment at 9 mo, β [95% CI]* | Effect of treatment at 21 mo, β [95% CI]* | Ptime† |
| Effect of albendazole treatment on innate cytokine responses, to LPS | |||
| TNF | −0.03 [−0.09–0.04] | −0.01 [−0.08–0.07] | 0.77 |
| IL-10 | 0.05 [0.00–0.10] | 0.00 [−0.05–0.06] | 0.12 |
| Effect of albendazole treatment on unstimulated cytokine responses | |||
| TNF | 0.01 [−0.07–0.09] | 0.03 [−0.06–0.12] | 0.80 |
| IFN-γ | −0.03 [−0.07–0.02] | 0.00 [−0.05–0.05] | 0.49 |
| IL-2 | 0.04 [−0.02–0.11] | −0.01 [−0.08–0.05] | 0.35 |
| IL-5 | 0.00 [−0.10–0.10] | −0.06 [−0.17–0.05] | 0.55 |
| IL-10 | 0.04 [−0.03–0.11] | 0.03 [−0.05–0.10] | 0.46 |
β (beta) and 95% CI are based on linear mixed models (see Statistical Analysis of Cytokine Responses).
Overall P value for the effect of treatment over time.
The Enhancement of Cytokine Responses Is Not a Direct Albendazole Effect.
To rule out albendazole as a direct cause of enhanced immune responses, we stratified the analysis on STH infection status at baseline (Table 2). Enhanced PfRBC-induced TNF and AscAg-induced IL-2 by albendazole treatment were seen in helminth-infected (Ptime = 0.0004 and Ptime = 0.006, respectively; Table 2) but not in uninfected subjects (Table 2) at 9 mo posttreatment. The effect of anthelmintic treatment on PHA-stimulated TNF in the stratified analysis was seen at 9 mo posttreatment in the helminth-infected individuals, but over the trial period, this was not statistically significant (Ptime = 0.098; Table 2, helminth-infected individuals). Corresponding background (unstimulated and uRBC-induced) cytokine responses were not increased in either helminth-infected or -uninfected subjects (Table S4).
Table 2.
Effect of albendazole treatment on immune responses by helminth infection status at baseline
| Outcome | Effect of treatment at 9 mo | Effect of treatment at 21 mo | |||||
| Placebo N | Albendazole N | β [95%CI]* | Placebo N | Albendazole N | β [95%CI]* | Ptime | |
| Effect of albendazole on cytokine responses in helminth-infected individuals | |||||||
| PHA | |||||||
| TNF | 261 | 190 | 0.14 [0.01–0.26] | 228 | 152 | 0.03 [–0.11–0.17] | 0.098 |
| IL-10 | 260 | 190 | 0.08 [–0.00–0.16] | 227 | 152 | 0.06 [–0.03–0.15] | 0.12 |
| PfRBCs | |||||||
| TNF | 154 | 106 | 0.42 [0.20–0.64] | 133 | 84 | −0.10 [–0.33–0.14] | 0.0004 |
| IFN-γ | 155 | 108 | 0.12 [–0.02–0.26] | 134 | 86 | −0.01 [–0.19–0.16] | 0.18 |
| AscAg | |||||||
| IL-2 | 249 | 182 | 0.25 [0.10–0.41] | 215 | 146 | 0.04 [–0.12–0.20] | 0.006 |
| Effect of albendazole on cytokine responses in helminth-uninfected individuals | |||||||
| PHA | |||||||
| TNF | 31 | 19 | 0.02 [–0.40–0.43] | 28 | 19 | 0.20 [–0.21–0.62] | 0.63 |
| IL-10 | 31 | 19 | 0.03 [–0.25–0.31] | 28 | 19 | 0.31 [0.01–0.60] | 0.12 |
| PfRBCs | |||||||
| TNF | 26 | 17 | 0.33 [–0.13–0.78] | 22 | 15 | 0.15 [–0.38–0.67] | 0.35 |
| IFN-γ | 26 | 17 | 0.34 [–0.03–0.71] | 22 | 15 | −0.00 [–0.42–0.41] | 0.18 |
| AscAg | |||||||
| IL-2 | 31 | 20 | 0.08 [–0.36–0.53] | 28 | 19 | −0.08 [–0.54–0.39] | 0.83 |
The analysis of the effect of anthelmintic treatment was stratified based on helminth infection status at baseline. By comparing the responses in the albendazole versus placebo group, the estimated outcome (beta) of the treatment effect after 9 and 21 mo of albendazole treatment was obtained. N, the number of the total population examined (N). An overall P value (Ptime) is indicated for the effect of treatment over time. Statistically significant results (P < 0.05) are given in bold.
β (beta) and 95% CIs are based on linear mixed models.
Table S4.
Effect of albendazole treatment on background immune responses by helminth infection status at baseline
| Outcome | Effect of treatment at 9 mo, β [95% CI]* | Effect of treatment at 21 mo, β [95% CI]* | Ptime† |
| Effect of albendazole on cytokine responses in helminth-infected individuals | |||
| Unstimulated | |||
| TNF | 0.02 [−0.10–0.13] | 0.03 [−0.10–0.02] | 0.86 |
| IFN-γ | −0.05 [−0.11–0.02] | −0.00 [−0.07–0.06] | 0.32 |
| IL-2 | 0.06 [−0.02–0.15] | −0.01 [−0.10–0.07] | 0.34 |
| IL-5 | 0.02 [−0.12–0.17] | −0.03 [−0.17–0.12] | 0.89 |
| IL-10 | 0.07 [−0.02–0.17] | 0.05 [−0.05–0.15] | 0.22 |
| uRBCs | |||
| TNF | 0.11 [−0.05–0.28] | −0.11 [−0.29–0.06] | 0.18 |
| IFN-γ | 0.02 [−0.07–0.12] | −0.04 [−0.13–0.06] | 0.68 |
| Effect of albendazole on cytokine responses in helminth-uninfected individuals | |||
| Unstimulated | |||
| TNF | 0.16 [−0.25–0.57] | −0.16 [−0.58–0.26] | 0.57 |
| IFN-γ | −0.08 [−0.35–0.19] | −0.06 [−0.33–0.22] | 0.78 |
| IL-2 | −0.03 [−0.31–0.26] | −0.04 [−0.31–0.24] | 0.95 |
| IL-5 | 0.11 [−0.32–0.54] | −0.08 [−0.51–0.35] | 0.82 |
| IL-10 | −0.10 [−0.37–0.16] | −0.15 [−0.44–0.13] | 0.50 |
| uRBCs | |||
| TNF | 0.40 [−0.05–0.85] | −0.18 [−0.66–0.30] | 0.18 |
| IFN-γ | −0.07 [−0.29–0.16] | 0.01 [−0.22–0.23] | 0.84 |
β (beta) and 95% CI are based on linear mixed models (see Statistical Analysis of Cytokine Responses).
Overall P value for the effect of treatment over time.
Changes in Cell Counts After Albendazole Treatment Do Not Explain Changes in Cytokine Responses.
To determine whether increased cellular responses could be explained by higher cell numbers, we analyzed complete blood counts (CBCs) and sought associations with cytokine responses. Total leukocytes—most markedly monocytes—were increased in the albendazole group compared with placebo at 9 mo posttreatment but not subsequently (Table S5). Leukocyte counts were positively associated with IL-2 to AscAg, however the rest were mainly negative associations, of which the one with TNF responses to PfRBC was significant. No association was found between monocyte numbers and cytokine responses to any of the stimuli (Table S6). This indicates that increased leukocyte numbers did not account for the general enhancement of cytokine responses. Moreover, when analysis of the treatment effect on cytokine responses was adjusted for leukocyte or monocyte counts, similar effect sizes were observed. No treatment effect was noted on other hematological parameters (Table S5).
Table S5.
Effect of albendazole treatment on CBCs
| Outcome | Effect of treatment at 9 mo, β [95% CI]* | Effect of treatment at 21 mo, β [95% CI]* | Ptime† |
| Hemoglobin, g/dL | −0.25 [–0.63–0.13] | −0.27 [–0.64–0.09] | 0.25 |
| Hematocrit, % | −0.32 [–1.4–0.76] | −0.54 [–1.56–0.48] | 0.57 |
| Erythrocytes, •1012/L | −0.07 [–0.20–0.06] | −0.08 [–0.20–0.04] | 0.36 |
| Thrombocytes, •109/L | 4.71 [–12.0–21.5] | 4.13 [–10.4–18.7] | 0.79 |
| Leukocytes, •109/L | 0.65 [0.19–1.10] | 0.16 [–0.30–0.62] | 0.017 |
| % lymphocytes | 0.01 [–1.95–1.96] | −1.25 [–2.97–0.47] | 0.33 |
| N lymphocytes, •109/L | 0.16 [–0.07–0.40] | −0.05 [–0.29–0.19] | 0.12 |
| % neutrophils | −1.76 [–3.89–0.37] | 0.79 [–1.05–2.63] | 0.18 |
| N neutrophils, •109/L | 0.06 [–0.27–0.39] | 0.10 [–0.24–0.43] | 0.85 |
| % monocytes | 1.20 [0.60–1.80] | −0.08 [–0.55–0.40] | <0.001 |
| N monocytes, •109/L | 0.15 [0.09–0.22] | 0·003 [–0.05–0.05] | <0.001 |
N, absolute number of cells within leukocyte differentiation; %, percentage of cells within leukocyte compartment. Statistically significant results (P < 0.05) are given in bold.
β (beta) and 95% CI are based on linear mixed models (see Statistical Analysis of Cytokine Responses).
Overall P value for the effect of treatment over time.
Table S6.
Associations of cell counts with cytokine responses
| CBC parameter | Cytokine parameter | Association at 9 mo | Association at 21 mo | ||
| Pearson’s coefficient | P | Pearson’s coefficient | P | ||
| N leukocytes | TNF to PfRBCs | –0.141 | 0.011 | −0.077 | 0.165 |
| IFN-γ to PfRBCs | −0.102 | 0.066 | −0.073 | 0.190 | |
| IL-2 to AscAg | 0.173 | 0.001 | 0.100 | 0.062 | |
| TNF to PHA | −0.103 | 0.051 | –0.179 | 0.001 | |
| IL-10 to PHA | −0.005 | 0.925 | −0.067 | 0.216 | |
| % monocytes | TNF to PfRBCs | 0.047 | 0.410 | −0.072 | 0.211 |
| IFN-γ to PfRBCs | 0.011 | 0.849 | −0.039 | 0.501 | |
| IL-2 to AscAg | −0.056 | 0.290 | 0.048 | 0.388 | |
| TNF to PHA | 0.046 | 0.391 | −0.024 | 0.667 | |
| IL-10 to PHA | 0.023 | 0.670 | 0.087 | 0.118 | |
| N monocytes | TNF to PfRBCs | −0.003 | 0.960 | −0.085 | 0.140 |
| IFN-γ to PfRBCs | −0.027 | 0.630 | −0.064 | 0.263 | |
| IL-2 to AscAg | 0.010 | 0.846 | 0.055 | 0.320 | |
| TNF to PHA | −0.032 | 0.546 | –0.113 | 0.042 | |
| IL-10 to PHA | 0.011 | 0.854 | 0.025 | 0.659 | |
N, absolute number of cells; %, percentage of cells within leukocyte compartment. Statistically significant results (P < 0.05) are given in bold.
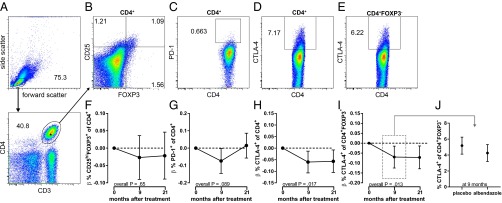
Albendazole Does Not Affect Treg Frequencies but Does Expand CTLA-4–Expressing CD4+ T Cells.
To identify potential mechanisms of immune hyporesponsiveness and their reversal by anthelmintics, we examined Treg (defined as CD4+CD25hiFOXP3+ T cells) as well as CD4+ cells expressing the suppressive molecules PD-1 and CTLA-4 in CD4+ T cells (Fig. 2). The frequency of Tregs did not change in the albendazole group compared with placebo (estimates [95% CI] at 9 mo, –0.027 [–0.090–0.036]; at 21 mo, –0.022 [–0.089–0.046]; Ptime = 0.65; Fig. 2 B and F). Similarly, treatment did not alter the expression of PD-1–expressing CD4+ T cells over the whole trial period, although at 9 mo there was a significant decrease (–0.074 [–0.145 to –0.002] and 0.015 [–0.057–0.086]; Ptime = 0.089, Fig. 2 C and G). However, the proportion of CTLA-4–expressing CD4+ T cells decreased after treatment and was significantly lower in the albendazole group at both time points posttreatment (–0.060 [–0.107 to –0.013] and –0.057 [–0.105 to –0.008], respectively; Ptime = 0.017; Fig. 2 D and H). Similar to total CD4+ T cells, the frequency of CTLA-4–expressing CD4+FOXP3– effector T cells decreased significantly after treatment with albendazole (–0.07 [–0.125 to –0.015] and –0.072 [–0.129 to –0.014], respectively; Ptime = 0.013; Fig. 2 E and I). The absolute change in CTLA-4 expression on effector T cells is shown in Fig. 2J.
Fig. 2.
Effect of deworming on cell subsets and marker expression. Flow cytometry was performed on PBMCs from a subset of school children. Gating strategy is shown for (A) lymphocytes and CD4+ T cells, from which (B) CD25hiFOXP3+ Treg cell, (C) PD-1, and (D) CTLA-4 expressions on CD4+ T cells were derived. (E) CTLA-4 expression on CD25hiFOXP3− cells was gated from B. The estimated effect of albendazole treatment is shown for the time points 9 and 21 mo after the start of treatment for percentages of CD25hiFOXP3+ (F), PD-1+ (G), and CTLA-4+ (H) of CD4+ T cells and CTLA-4+ of CD4+FOXP3– cells (I). Estimates and β (beta) were obtained by linear mixed models; 95% CIs and overall P values over time (Ptime) are indicated. As an indication of magnitude of change, the actual percentage of CTLA-4+ of CD4+FOXP3– cells in placebo and albendazole groups is shown at 9 mo (J).
Discussion
This is the report of a unique trial analyzing cytokine responses as well as regulatory molecules in a community before and after long-term anthelmintic treatment. We show that treatment of STH infections ablates their immunosuppressive effects, enhancing immune responses to helminth and unrelated antigens as well as to mitogen. Most pronounced were elevated proinflammatory cytokine responses after stimulation with plasmodial antigens and mitogen. In addition, we observed a reduction in CTLA-4–expressing CD4+ T cells in albendazole-treated children, indicating that immuno-inhibitory mechanisms could be affected by deworming.
The strongest effect of anthelmintic treatment was on antiplasmodial responses. These had not been specifically investigated in anthelmintic treatment RCTs. However, in cross-sectional studies examining the effect of helminths on malaria-specific cytokine responses, results are inconsistent (17–19). The increase in response to malaria antigens could be due to a concurrent increase in malarial parasitemia in the albendazole-treated group 6 mo after initiation of treatment (20), coincident with peak transmission season. By performing the analysis without malaria-positive subjects, we ruled out that this could explain the enhanced plasmodial-specific cytokine responses.
With regard to immune regulation, no treatment-related change in Treg frequencies was seen, consistent with the finding of similar Treg frequencies in STH-infected and -uninfected children reported from the same study area (12). The proportion of PD-1–expressing CD4+ T cells was not significantly altered by albendazole treatment over 2 y, although in the first year posttreatment this was significantly lower. This is consistent with studies that show increased PD-1 expression is associated with helminth infections (13, 18). The significant decrease in CTLA-4–expressing CD4+ T cells adds support to the important role of this molecule in suppression of immune responses in general and its suggested role in immune hyporesponsiveness induced by helminths (21). When put in the context of the blockade of CTLA-4 (as well as PD-1) in treatment of melanoma and other cancers (22), these findings lend further support to the suggested similarities between immunoregulation in chronic infectious diseases and cancers (23).
Three-monthly albendazole treatment over a 2-y period did not eliminate helminths. In earlier reports, the efficacy of one-time single or double doses of albendazole and/or mebendazole treatment has been low for Ascaris and Trichuris (24). Here we show that this is the case even after seven doses of albendazole at three-monthly intervals. By using a household-clustered randomization design, repeated treatments, and observed intake, we expected a more effective reduction in prevalence of STHs. For better deworming results, more intensive treatment or inclusion of environmental control would be needed. However, it is clear that even a 50% reduction in helminth infections in the community can start to reverse immune hyporesponsiveness and that more effective deworming might give even more pronounced immunological effects.
Subsequent to the increased proinflammatory responses after 9 mo, IL-5 and IL-10 responses increased 21 mo posttreatment. Stratified analyses revealed that the increased mitogen-stimulated IL-5 and IL-10 was not specific to helminth-infected subjects, suggesting that factors other than the elimination of helminths may be responsible. This increased IL-10 response after 2 y of treatment might account for the fact that immune responses are not higher in the albendazole versus placebo at this time point.
Enhanced cytokine responses could also be the result of a boosted immune response due to the release of antigens from dying or dead worms. However, the strongest increases in responses were not to worm antigen but to the unrelated malarial antigen. Moreover, using pretreatment worm burden as a proxy for antigen release, the modest increase seen to AscAg was not correlated with burden of Ascaris lumbricoides at pretreatment, nor were responses to nonrelated antigens correlated with baseline worm burden. These findings argue that observed boosted immune responses would not be due to release of antigens from dying worms, which has been shown to account for part of the increase in immune responses after treatment in schistosomiasis (25), but rather be due to the decrease in immune regulation.
A number of factors other than reduction in helminths could contribute to the findings of this study, such as a direct effect of albendazole, alterations in immune cell counts, or changes in nutrients. Albendazole has been shown to affect cytokine responses in vitro (26). The higher effect sizes in the stratified analysis of helminth-positives than those in the total group indicate that the enhancement of proinflammatory cytokine responses is unlikely to be due to albendazole directly affecting the immune system. Immune hyporesponsiveness could stem from alteration in cell counts and changes in nutrients essential to functioning of the immune system (27). Although cell counts were affected by treatment, cell numbers did not account for cytokine responses. Because improved energy resources can enhance immune responses, we assessed body mass index (BMI) and fasting glucose level as proxies for nutritional status, but these parameters were not affected by deworming (20).
Our study shows significant effects of deworming on the immune system. The effects could lead to enhanced immune responses to other pathogens and vaccines. With respect to vaccines, there is increasing concern regarding poor immunogenicity in rural areas of developing countries (28, 29); therefore, any measure to alleviate hyporesponsiveness would have a major public health impact. It is also important to consider the long-standing evolutionary coexistence between humans and helminths, the disturbance of which might lead to the emergence of pathological conditions (30). However, for this outcome, long- rather than short-term treatment courses are expected to reveal any clinical impact (31). Considering this, it will be important to include immunological measurements in future deworming programs and anthelmintic therapy trials to better understand and predict clinical outcomes.
Methods
Study Design.
The study was nested within the ImmunoSPIN trial, a double-blind placebo-controlled trial conducted in two villages on Flores island, Indonesia (20). All households were randomized to receive either a single dose of 400 mg albendazole or placebo once every 3 mo for 2 y. Treatment was allocated to households to minimize the risk of reinfection and was provided to all household members older than 2 y, except for pregnant women, according to Indonesian guidelines. Intake was observed by field workers. Participants gave written informed or parental consent. The study was approved by the Ethics Committee of the Medical Faculty, University of Indonesia, Jakarta and was filed by the Ethics Committee of the Leiden University Medical Center, the Netherlands. The trial was registered as ISRCTN83830814.
Study Population.
Randomization was based on 954 households in total, comprising 2,022 (481 houses) and 1,982 (473 houses) subjects in placebo and albendazole groups, respectively. For immunological studies, 250 households in the main village were randomly selected, and individuals older than 4 y of age were invited for venous blood sampling and assessment of anthropometric parameters. Thereby 882 individuals were included, of which 858 provided sufficient blood for whole-blood cultures. In the other village, 250 children were randomly selected from the total population, and children from the same households were also included, giving 295 children in total with whole-blood cultures. After cleaning the data (see Whole-Blood Culture and Cytokine Measurements), at baseline 839 and 220 subjects were included from the two areas, comprising 572 placebo- and 487 albendazole-treated individuals.
Because STH infection and associated immunological changes were anticipated to be most prevalent in school-age children, detailed analyses of regulatory components were only performed in this age group (4–12 y old). From a randomized selection separate from the previously mentioned subset, 145 children were included (71 randomized for placebo; 74 for albendazole), of which 121 (61 and 60, respectively) had sufficient numbers of cells. After 9 and 21 mo, 116 (56/60) and 107 (52/55) were followed up, respectively.
Whole-Blood Culture and Cytokine Measurements.
Whole-blood was stimulated in vitro as described before (32) for 24 h (LPS stimulation) and 72 h [A. lumbricoides antigen (AscAg), PfRBCs, uRBCs, and PHA stimulations]. PfRBCs and uRBCs were prepared according to a standardized procedure (32). AscAg was a homogenate of adult worm A. lumbricoides obtained from infected humans. Supernatants were stored at –20 °C until quantification using Luminex kits (Biosource) on a Liquichip 200 Workstation (Qiagen). TNF and IL-10 were quantified in all supernatants, whereas IFN-γ, IL-2, and IL-5 were quantified only in 72-h supernatants. Samples with TNF levels ≥250 pg/mL in unstimulated blood were excluded from the analyses, as they were considered possibly contaminated. This cutoff value was derived from outliers in the data distribution. Cytokine concentrations below the assay’s detectable range were replaced by half the detection limit provided by the manufacturer.
Stool Examination by Microscopy and PCR.
Stool samples were collected annually. Trichuris trichiura was detected by microscopy after formol-ether concentration, whereas multiplex real-time PCR detected hookworm (Ancylostoma duodenale, Necator americanus), A. lumbricoides, and Strongyloides stercoralis DNA, as described previously (32).
CBCs.
CBCs before and 1 y posttreatment were determined using heparinized blood on a cell counter (Coulter Ac-T diff Analyzer, Beckman Coulter Inc.), whereas CBCs 2 y posttreatment were determined on a Sysmex KX-21N hematology analyzer (PT Sysmex Indonesia). Because both heparinized and EDTA blood samples were used at the last time point, 325 samples were tested in parallel analysis. All outcomes were highly comparable except for thrombocyte counts; thus, the data of all parameters but thrombocyte counts were pooled.
Flow Cytometry.
Peripheral blood mononuclear cells (PBMCs) from 121 school children were isolated by Ficoll gradient centrifugation. PBMCs were fixed with FOXP3 Staining Buffer (eBioscience Inc.) and cryopreserved until further analysis. After thawing, cells were permeabilized and stained with anti-CD3, anti-CD4, anti-FOXP3, anti-CD25, anti–CTLA-4, anti–PD-1, and anti-Ki67 antibodies (Table S7). Data were acquired on a FACSCanto (BD Biosciences) and analyzed using FlowJo software (Treestar Inc.).
Table S7.
Specifics of flow antibodies
| Marker | Conjugate | Clone | Company |
| CD3 | APC-eFluor780 | UCHT1 | eBioscience |
| CD4 | PE-Cy7 | SK3 | BD Biosciences |
| CD4 | APC-eFluor780 | RPA-T4 | eBioscience |
| CD25 | PE-Cy7 | 2A3 | BD Biosciences |
| FOXP3 | eFluor450 | PCH101 | eBioscience |
| CTLA-4 | PE-Cy5 | BNI3 | BD Biosciences |
| PD-1 | PerCP-eFluor710 | eBioJ105 | eBioscience |
Statistical Analysis.
Log transformation was used for cytokines [log10(concentration + 1)] and most flow cytometry [log10(value)] data to obtain normally distributed variables. For children’s BMI, age-standardized z scores were calculated according to WHO references (33). To assess treatment effects, linear mixed models were used; these are described in more detail in Statistical Analysis of Cytokine Responses. Parameter estimates and 95% CIs for treatment effects at 9 and 21 mo are reported. The analysis was intention-to-treat and involved all participants as assigned randomly at the start of the trial.
Statistical Analysis of Cytokine Responses
Three random effects were added to the linear mixed models: namely, a random household-specific intercept to model clustering within households and a random subject-specific intercept and slope-to-model correlation within subjects. The treatment effect was assessed at 9 and 21 mo, and P values were obtained using likelihood ratio tests by comparing the model with and without the treatment effect, which is a 2 degrees of freedom test (Ptime). Unless stated otherwise, all outcomes were adjusted for area by using area as a covariate in the model. All models were fitted using the lme4 package.
Acknowledgments
The authors thank the research team, the health staff from the Puskesmas Primary Health Centers of Nangapanda and Welamosa, and most of all, the participants from Nangapanda and Anaranda.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604570113/-/DCSupplemental.
References
- 1.Hotez PJ, et al. Helminth infections: The great neglected tropical diseases. J Clin Invest. 2008;118(4):1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor-Robinson DC, Maayan N, Soares-Weiser K, Donegan S, Garner P. Deworming drugs for soil-transmitted intestinal worms in children: Effects on nutritional indicators, haemoglobin, and school performance. Cochrane Database Syst Rev. 2015;7(7):CD000371. doi: 10.1002/14651858.CD000371.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev. 2012;25(4):585–608. doi: 10.1128/CMR.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper PJ, et al. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J Infect Dis. 2000;182(4):1199–1206. doi: 10.1086/315837. [DOI] [PubMed] [Google Scholar]
- 5.Elias D, et al. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guérin (BCG) vaccination. Clin Exp Immunol. 2001;123(2):219–225. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esen M, et al. Reduced antibody responses against Plasmodium falciparum vaccine candidate antigens in the presence of Trichuris trichiura. Vaccine. 2012;30(52):7621–7624. doi: 10.1016/j.vaccine.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Rook GA. Review series on helminths, immune modulation and the hygiene hypothesis: The broader implications of the hygiene hypothesis. Immunology. 2009;126(1):3–11. doi: 10.1111/j.1365-2567.2008.03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M. Helminth therapy or elimination: Epidemiological, immunological, and clinical considerations. Lancet Infect Dis. 2014;14(11):1150–1162. doi: 10.1016/S1473-3099(14)70771-6. [DOI] [PubMed] [Google Scholar]
- 9.Ottesen EA, Hiatt RA, Cheever AW, Sotomayor ZR, Neva FA. The acquisition and loss of antigen-specific cellular immune responsiveness in acute and chronic schistosomiasis in man. Clin Exp Immunol. 1978;33(1):37–47. [PMC free article] [PubMed] [Google Scholar]
- 10.Daniłowicz-Luebert E, O’Regan NL, Steinfelder S, Hartmann S. Modulation of specific and allergy-related immune responses by helminths. J Biomed Biotechnol. 2011;2011:821578. doi: 10.1155/2011/821578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricci ND, et al. Induction of CD4(+)CD25(+)FOXP3(+) regulatory T cells during human hookworm infection modulates antigen-mediated lymphocyte proliferation. PLoS Negl Trop Dis. 2011;5(11):e1383. doi: 10.1371/journal.pntd.0001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wammes LJ, et al. Regulatory T cells in human geohelminth infection suppress immune responses to BCG and Plasmodium falciparum. Eur J Immunol. 2010;40(2):437–442. doi: 10.1002/eji.200939699. [DOI] [PubMed] [Google Scholar]
- 13.Babu S, et al. Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen-4 and programmed death-1. J Infect Dis. 2009;200(2):288–298. doi: 10.1086/599797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper PJ, et al. Repeated treatments with albendazole enhance Th2 responses to Ascaris Lumbricoides, but not to aeroallergens, in children from rural communities in the Tropics. J Infect Dis. 2008;198(8):1237–1242. doi: 10.1086/591945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flohr C, et al. Reduced helminth burden increases allergen skin sensitization but not clinical allergy: A randomized, double-blind, placebo-controlled trial in Vietnam. Clin Exp Allergy. 2010;40(1):131–142. doi: 10.1111/j.1365-2222.2009.03346.x. [DOI] [PubMed] [Google Scholar]
- 16.Wright VJ, et al. Early exposure of infants to GI nematodes induces Th2 dominant immune responses which are unaffected by periodic anthelminthic treatment. PLoS Negl Trop Dis. 2009;3(5):e433. doi: 10.1371/journal.pntd.0000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diallo TO, et al. Schistosomiasis coinfection in children influences acquired immune response against Plasmodium falciparum malaria antigens. PLoS One. 2010;5(9):e12764. doi: 10.1371/journal.pone.0012764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartgers FC, et al. Responses to malarial antigens are altered in helminth-infected children. J Infect Dis. 2009;199(10):1528–1535. doi: 10.1086/598687. [DOI] [PubMed] [Google Scholar]
- 19.Metenou S, et al. Filarial infection suppresses malaria-specific multifunctional Th1 and Th17 responses in malaria and filarial coinfections. J Immunol. 2011;186(8):4725–4733. doi: 10.4049/jimmunol.1003778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiria AE, et al. The effect of three-monthly albendazole treatment on malarial parasitemia and allergy: A household-based cluster-randomized, double-blind, placebo-controlled trial. PLoS One. 2013;8(3):e57899. doi: 10.1371/journal.pone.0057899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steel C, Nutman TB. CTLA-4 in filarial infections: Implications for a role in diminished T cell reactivity. J Immunol. 2003;170(4):1930–1938. doi: 10.4049/jimmunol.170.4.1930. [DOI] [PubMed] [Google Scholar]
- 22.Riley JL. Combination checkpoint blockade--Taking melanoma immunotherapy to the next level. N Engl J Med. 2013;369(2):187–189. doi: 10.1056/NEJMe1305484. [DOI] [PubMed] [Google Scholar]
- 23.Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med. 2014;371(4):380–383. doi: 10.1056/NEJMcibr1404664. [DOI] [PubMed] [Google Scholar]
- 24.Namwanje H, Kabatereine NB, Olsen A. Efficacy of single and double doses of albendazole and mebendazole alone and in combination in the treatment of Trichuris trichiura in school-age children in Uganda. Trans R Soc Trop Med Hyg. 2011;105(10):586–590. doi: 10.1016/j.trstmh.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Wilson S, et al. Posttreatment changes in cytokines induced by Schistosoma mansoni egg and worm antigens: Dissociation of immunity- and morbidity-associated type 2 responses. J Infect Dis. 2014;209(11):1792–1800. doi: 10.1093/infdis/jit826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizuno K, Toyoda Y, Fukami T, Nakajima M, Yokoi T. Stimulation of pro-inflammatory responses by mebendazole in human monocytic THP-1 cells through an ERK signaling pathway. Arch Toxicol. 2011;85(3):199–207. doi: 10.1007/s00204-010-0584-y. [DOI] [PubMed] [Google Scholar]
- 27.Chandra RK. Nutrition and the immune system from birth to old age. Eur J Clin Nutr. 2002;56(Suppl 3):S73–S76. doi: 10.1038/sj.ejcn.1601492. [DOI] [PubMed] [Google Scholar]
- 28.Muyanja E, et al. Immune activation alters cellular and humoral responses to yellow fever 17D vaccine. J Clin Invest. 2014;124(7):3147–3158. doi: 10.1172/JCI75429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obiero JM, et al. Impact of malaria preexposure on antiparasite cellular and humoral immune responses after controlled human malaria infection. Infect Immun. 2015;83(5):2185–2196. doi: 10.1128/IAI.03069-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott DE, Weinstock JV. Helminth-host immunological interactions: Prevention and control of immune-mediated diseases. Ann N Y Acad Sci. 2012;1247:83–96. doi: 10.1111/j.1749-6632.2011.06292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endara P, et al. Long-term periodic anthelmintic treatments are associated with increased allergen skin reactivity. Clin Exp Allergy. 2010;40(11):1669–1677. doi: 10.1111/j.1365-2222.2010.03559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiria AE, et al. Does treatment of intestinal helminth infections influence malaria? Background and methodology of a longitudinal study of clinical, parasitological and immunological parameters in Nangapanda, Flores, Indonesia (ImmunoSPIN Study) BMC Infect Dis. 2010;10:77. doi: 10.1186/1471-2334-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO . WHO Child Growth Standards: Lenght/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Method and Development. World Health Organization; Geneva: 2006. [Google Scholar]