Abstract
OBJECTIVE:
Delayed onset of independent walking is common in intellectual disability (ID). However, in children with autism spectrum disorders (ASD), delayed walking has not been reported as frequently, despite the high rate of concurrent ID in ASD. This study directly examined the relationship between delayed walking and severity of ID in children with ASD versus other non-ASD diagnoses.
METHOD
Participants were 1185 individuals (ASD, n = 903; non-ASD, n = 282) who received an assessment at age 4 to 12 years (6.89 ± 2.25) that yielded an estimate of nonverbal IQ (NVIQ) and retrospectively reported age of walking from the Autism Diagnostic Interview–Revised. The relationship between diagnostic group and delayed walking (defined as occurring at ≥16 months) as a function of NVIQ was explored using the Cox proportional hazards model.
RESULTS:
Children with ASD were less likely to exhibit delayed walking than those with non-ASD diagnoses, and this difference was larger at lower levels of NVIQ (P = .002). For example, rates of delayed walking for ASD and non-ASD were 13% and 19%, respectively, in those with NVIQ >85 but 31% and 60% in children with NVIQ <70.
CONCLUSIONS:
Although lower IQ scores were associated with increased rates of late walking in both ASD and non-ASD groups, children with low IQ were more likely to show delayed walking in the absence of ASD. This raises the possibility of separate etiological pathways to ID in children with and without ASD.
What’s Known on This Subject:
Very early reports from small samples suggested that age of walking may be relatively preserved in autism spectrum disorders with intellectual disability compared to intellectual disability without autism spectrum disorders (ASD), but this has never been confirmed in a contemporary sample of systematically diagnosed children.
What This Study Adds:
Results indicated that later nonverbal IQ is indeed less closely associated with delayed walking in children with ASD compared with children without ASD, with discrepancies between nonverbal IQ and age of walking in children with versus without ASD becoming more pronounced at lower levels of IQ.
Delay in independent walking, defined as walking at or after 16 months, has been established as a marker of atypical development.1 According to the World Health Organization, 97% of both male and female children begin to walk before the age of 16 months, and ∼95% of children walk within the time frame of 9 to 16 months.2 Thus, for clinicians and parents, onset of independent ambulation signifies an important milestone. Compared with other developmental events, such as age of first words, age of walking is also a particularly reliable parent-reported milestone.3,4
Many influences on the attainment of gross motor milestones such as independent walking have been identified, including prenatal, perinatal, and postnatal environmental factors (eg, nutrition, cultural practices).5 One consistently identified association is between delayed walking and later intellectual disability (ID). Based on clinical reports of delayed walking in children later diagnosed with ID, early studies hypothesized a negative linear relationship between age of walking and IQ.6 However, studies in both typically developing and developmentally delayed populations have indicated no clear linear relationship, as children with high IQ do not necessarily walk early,7 and children with ID do not always walk late.8
On the other hand, individuals with ID are more likely to experience delays in walking compared with individuals in the general population.9 In 1 study of 185 consecutive referrals with ID, half or fewer of children with moderate (47%), severe (51%), and profound (35%) ID walked by the age of 15 months.6 Another study of 87 children with profound ID reported that fewer than a quarter walked by the age of 14 months, and only two-thirds started walking before their second birthdays.10 The likelihood of delayed walking is also associated with degree of cognitive delay within several specific neurologic disorders (eg, Duchenne muscular dystrophy).11 Studies of specific genetic syndromes, including Phelan-McDermid syndrome,12 Prader-Willi syndrome,13 and Angelman syndrome,14 highlight the extent of gross motor delay, where rates of walking late are very high, and many children at the most profound levels of ID do not ever walk independently.
Delayed walking has also been reported in other specific developmental disabilities, including autism spectrum disorder (ASD). In fact, Kanner’s first accounts of autism included descriptions of the children as having some gross motor clumsiness, including 1 child who “began to walk [at age 2 years] without any preliminary crawling or assistance by chairs.”15 Since then, numerous studies have pointed more generally to the potential role of the motor system in the development of ASD.16–18 In addition, retrospective studies have suggested a higher rate of late walking and other gross motor milestone delays in children with ASD compared with the general population,18 although these rates appear to be lower than in other disorders associated with ID.19
To date, very little has been done to explicitly investigate delayed walking in relation to ID within ASD. Existing research dates back to the 1970s and 1980s and includes samples that are small by today’s standards. Nevertheless, the apparently lower rate of late walking in ASD compared with ID reported in these early studies is both perplexing and intriguing, given that a high proportion of children with ASD are also eventually determined to have ID.20 One of the many questions this dissociation raises is what other features co-occur when gross motor milestones (such as walking) are abnormal in ASD.18
Using a large sample of children with neurodevelopmental disorders, we asked the following research questions: (1) Is there a difference in rates of delayed walking between children with ASD and non-ASD diagnoses, and does this change as a function of IQ? (2) What characteristics are associated with delayed walking in ASD versus non-ASD? and (3) In particular, is there a relationship between delayed walking and factors such as gender and epilepsy, which have been previously found to be relevant in biological investigations of ASD?
Methods
Participants were drawn from an existing database of children assessed ≥1 times in 3 autism specialty clinics (54%) or as part of research projects conducted at 4 universities. Data from 1 assessment, including both the behavioral evaluation and parent-reported historical information, were used for each participant. Participants were eligible for the current analyses only if their assessment had included the Autism Diagnostic Interview–Revised (ADI-R),21 a comprehensive parent interview designed to elicit information about ASD-related symptoms and behaviors; the Autism Diagnostic Observation Schedule (ADOS),22 a direct observation measure of social-communication impairments and restricted and repetitive behaviors associated with ASD; and a nonverbal IQ (NVIQ) test. Information about age of walking was obtained from question 5 on the ADI-R, which asks “At what age did [subject] walk without holding on?” For participants who were assessed multiple times, we selected the first assessment that occurred between the ages of 4 and 12 years. The lower age limit was selected to allow adequate time for the child to begin walking. The upper age limit was selected to avoid the confounding variable of ratio IQ at older ages.23 NVIQ, used in this study as a trait variable reflecting severity of ID, was used instead of verbal or full-scale IQ to minimize the effects of language in measuring intellectual abilities in this population. NVIQ was estimated using ratio IQ from the Mullen Scales of Early Learning or standard scores from the Differential Ability Scales or the Wechsler Intelligence Scale for Children, depending on the ability level of the child.
Diagnoses of ASD (defined as Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition24 diagnoses of autistic disorder, pervasive developmental disorder not otherwise specified, or Asperger disorder) were made by expert clinicians using information from the ADI-R, ADOS, and cognitive tests, in combination with other validated measures of language and behavior. For some studies, children with known genetic syndromes were eligible or were specifically recruited as non-ASD controls, but given the high rate of motor abnormalities associated with these syndromes, participants with known genetic syndromes (31 with Down syndrome, 8 with Williams syndrome, and 1 with fragile X syndrome) were excluded from the current study. Children with a known diagnosis of cerebral palsy were also excluded. Children who did not receive any Diagnostic and Statistical Manual of Mental Disorders diagnosis at the time of the assessment (eg, typically developing controls) were also excluded. As shown in Table 1, the final sample comprised 903 children with primary clinical diagnoses of ASD, with varying IQ levels, and 282 with non-ASD primary diagnoses, such as ADHD, language disorders, or ID.
TABLE 1.
Participant Characteristics
| Characteristic | ASD (n = 903) | Non-ASD (n = 282) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Value | n | Value | n | All | Value | n | Value | n | All | |
| Age at walking, mo | 14.83 ± 5.66 | 498 | 12.99 ± 2.93 | 405 | 14.00 ± 4.73 | 16.52 ± 5.75 | 102 | 13.43 ± 4.51 | 180 | 14.55 ± 5.2 |
| Late walker (≥16 M) | 148 (30) | 53 (13) | 201 (22) | 50 (49) | 102 | 35 (19) | 180 | 85 (30) | ||
| Seizuresa | 59 (14) | 26 (7) | 85 (11) | 16 (16) | 102 | 9 (5) | 180 | 25 (9) | ||
| Primary diagnosis | 102 | 180 | ||||||||
| ASD | 498 (100) | 405 (100) | 903 (100) | 0 | 0 | |||||
| FASD | 0 | 0 | 0 | 9 (8) | 11 (6) | 20 (7) | ||||
| ID of unknown etiology | 0 | 0 | 0 | 52 (51) | 0 | 52 (18) | ||||
| Mood/anxiety disorder | 0 | 0 | 0 | 9 (9) | 40 (22) | 49 (17) | ||||
| ADHD/ODD | 0 | 0 | 0 | 14 (14) | 78 (43) | 92 (33) | ||||
| Language disorder | 0 | 0 | 0 | 15 (15) | 46 (26) | 61 (22) | ||||
| Other or missing | 0 | 0 | 0 | 3 (3) | 5 (3) | 8 (3) | ||||
| Age at NVIQ test, y | 6.47 ± 2.15 | 498 | 7.20 ± 2.27 | 405 | 6.80 ± 2.24 | 7.25 ± 2.42 | 102 | 7.17 ± 2.18 | 180 | 7.20 ± 2.26 |
| NVIQb | 55.39 ± 18.69 | 498 | 105.36 ± 14.44 | 405 | 77.80 ± 30.07 | 67.34 ± 15.82 | 102 | 103.01 ± 11.49 | 180 | 90.11 ± 21.65 |
| ADOS CSS | 7.48 ± 1.97 | 478 | 6.79 ± 2.29 | 401 | 7.17 ± 2.15 | 2.95 ± 1.91 | 102 | 2.62 ± 1.92 | 179 | 2.74 ± 1.92 |
| ADOS Social Affect CSS | 7.38 ± 1.98 | 478 | 6.74 ± 2.34 | 401 | 7.09 ± 2.18 | 3.18 ± 1.88 | 102 | 3.01 ± 2.10 | 179 | 3.07 ± 2.03 |
| ADOS RRB CSS | 7.52 ± 2.14 | 478 | 7.03 ± 2.35 | 401 | 7.30 ± 2.25 | 4.36 ± 2.85 | 102 | 3.61 ± 2.61 | 179 | 3.88 ± 2.71 |
| Vineland ABC | 55.28 ± 13.76 | 476 | 74.97 ± 14.86 | 385 | 64.08 ± 17.3 | 69.00 ± 14.78 | 99 | 84.28 ± 14.29 | 172 | 78.7 ± 16.22 |
| Vineland Communication | 58.07 ± 16.43 | 479 | 83.74 ± 17.8 | 384 | 69.49 ± 21.3 | 72.52 ± 14.64 | 99 | 88.47 ± 15.73 | 172 | 82.64 ± 17.14 |
| Vineland Daily Living | 56.52 ± 17.21 | 476 | 77.73 ± 17.34 | 384 | 65.99 ± 20.23 | 71.86 ± 18.53 | 98 | 88.94 ± 15.62 | 171 | 82.72 ± 18.63 |
| Vineland Social | 59.50 ± 11.85 | 478 | 72.50 ± 14.55 | 384 | 65.29 ± 14.62 | 73.24 ± 13.82 | 99 | 83.45 ± 14.17 | 172 | 79.72 ± 14.86 |
Data are expressed as mean ± SD or n (%). ABC, Adaptive Behavior Composite; CSS, Calibrated Severity Score; FASD, fetal alcohol spectrum disorder; RRB, Restricted and Repetitive Behavior. Participants with known genetic syndromes were excluded.
Missing parent-reported seizure data on n = 105 (12%) participants with ASD and n = 10 (3%) non-ASD participants. Of the ASD participants with no seizure data, n = 79 had NVIQ <85. Of the non-ASD participants, n = 7 had NVIQ <85. The denominator for the reported proportion in this table excludes those with missing data.
NVIQ was obtained from various versions of the Mullen Scales of Early Learning, the Differential Ability Scale, or the Weschler Intelligence Scale for Children.
Analysis
We used survival analysis to evaluate the relationship between age of walking and diagnosis, as a function of severity of ID. In this case, the survival event modeled was parent-reported age of walking. We then examined whether ID severity, indexed by current NVIQ, was associated with age of walking attainment (eg, different survival experiences) and whether this relationship differed by ASD versus non-ASD diagnostic status. These variables, as well as the individual’s gender and parent-reported history of seizures, were entered into a Cox proportional hazards model. A hazard is the instantaneous event rate for an individual who has survived (ie, not walked) up to a given time. The Cox proportional hazards model is nonparametric, but with noncensored data such as these, it does assume proportional hazards, which were evaluated and satisfied. Relative model fit was assessed by using the likelihood ratio, which follows a χ2 distribution.
All analyses were completed by using SAS/STAT software, version 9.3 for Windows.25 α was set to 0.05.
Results
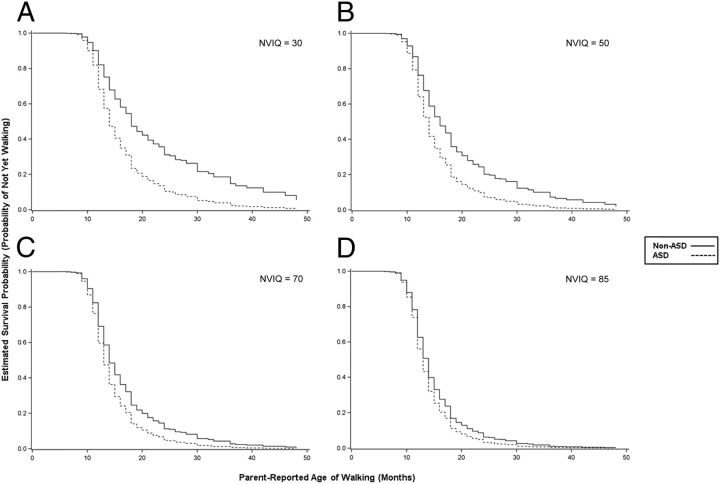
Participant characteristics are presented in Table 1. The rate of late walking (≥16 months) differed significantly between the ASD group (n = 201, 22%) and the non-ASD group (n = 85, 30%) (P = .007). Age of walking was modeled using survival analysis; diagnostic group, NVIQ, and their interaction were simultaneously entered into the model (Table 2). This model had a significantly better fit than the null model according to the likelihood ratio test (P < .001). The interaction between group and NVIQ was statistically significant (P < .001) and was characterized by a greater likelihood of walking in the ASD group relative to the non-ASD group, especially when the level of functioning was low (see Fig 1). For example, Fig 1D shows that the predicted probability of having achieved independent walking by 18 months was the same for ASD and non-ASD (∼85%) when NVIQ was held constant at 85. However, when NVIQ was held constant at 50 (Fig 1B), the predicted probability of having achieved independent walking by 18 months was ∼80% for ASD compared with ∼60% for non-ASD.
TABLE 2.
Results of Survival Analyses
| Model | Wald χ2 | P | −2LL |
|---|---|---|---|
| Model 0 (null) | 14 673.57 | ||
| Model 1 | 14 592.99 | ||
| Group | 11.25 | .001 | |
| NVIQ | 32.30 | <.001 | |
| Group × NVIQ | 7.63 | .006 | |
| Model 2 | 14 583.71 | ||
| Gender | 8.96 | .003 | |
| Group | 11.68 | .001 | |
| NVIQ | 32.64 | <.001 | |
| Group × NVIQ | 8.37 | .004 | |
| Model 3 | 14 581.68 | ||
| Seizure | 1.96 | .16 | |
| Gender | 8.52 | .004 | |
| Group | 10.73 | .001 | |
| NVIQ | 29.73 | <.001 | |
| Group × NVIQ | 7.52 | .006 |
Parent-reported seizure data were missing for n = 105 (12%) participants with ASD and n = 10 (3%) non-ASD participants. For the purposes of these analyses, missing data were coded as “no seizures” so that the full sample could be retained for Model 3. Sensitivity analyses were performed to ensure that the results were identical when Models 1 to 3 were evaluated in the sample with the full complement of data (n = 1070).
FIGURE 1.
Significant interaction between diagnostic group and later NVIQ in Cox proportional hazards model (n = 1185). Estimated survival probability (y axis) reflects the probability of having not walked by a given time, controlling for NVIQ. The interaction of diagnostic group (ASD versus non-ASD) and later NVIQ was statistically significant: χ2 = 7.63, P = .0006. Shown are the survival curves for each diagnostic group, held constant at various levels of NVIQ. The successively smaller distance between the curves in A through D illustrate the convergence in hazards between groups as NVIQ increases. The point estimates of the hazard ratios for ASD versus non-ASD (with 95% confidence interval) were as follows: A, 1.94 (1.35–2.79); B, 1.65 (1.27–2.14); C, 1.40 (1.18–1.66); and D, 1.24 (1.08–1.42). Higher hazard ratios indicate greater hazard of the event (walking) for an individual in the ASD group, relative to the non-ASD group, at any given time.
Two additional correlates were added to the model (Table 2): gender was entered first and significantly improved model fit (P < .005), followed by history of seizures, which did not improve model fit (P > 0.10) and was discarded. Males were more likely to have walked at a given time point than females (hazard ratio 1.23, 95% confidence interval 1.07–1.44). No significant interactions with gender were observed.
Discussion
The results of this study build on data from much earlier studies of small samples suggesting that the relationship between delayed walking and later ID differs between children with and without ASD. For children with IQs in the average range, rates of delayed walking were similar between those with ASD and those with other neurodevelopmental disorders (eg, attention deficit hyperactivity disorder, language disorder), with rates in both groups moderately elevated (13% and 19%, respectively) compared with general population samples of children with average IQ. On the other hand, for children with below-average IQ, and especially for those in the range of ID, the rate of delayed walking was lower in those with ASD. We found that as NVIQ decreased, the gap between the survival curves for ASD and non-ASD widened, reflecting earlier attainment of walking in ASD compared with non-ASD.
This difference in delayed walking between children with ASD and ID relative to ID alone raises the question of whether some children with ASD arrive at their ID via a different route than other children with ID with or without accompanying ASD. Given that late walking in ASD appears to be relatively less common than what would be expected at comparable levels of non-ASD ID, how significant is late walking when it does occur? A specific hypothesis that can be explored is whether delayed walking in ASD is associated with a greater likelihood of identifiable genetic mutations. In line with this hypothesis is the finding that in the overall group, females showed higher rates of delayed walking than males, and that there was a trend for this effect to be stronger in ASD than in non-ASD. Given that recent genetics studies in ASD have reported higher rates of de novo mutations26,27 and more penetrant copy number variations28 in ascertained females than in males, these findings add to the broader differential liability hypothesis that suggests phenotypic differences may relate to sex (female)-specific protective factors.29,30
Studies of genetic abnormalities in ASD have increasingly implicated IQ as an important stratification variable.27 The weaker association between age of walking and ID in ASD compared with non-ASD in this study suggests that markers of abnormal gross motor development such as late walking may be useful in further stratifying individuals with ASD into more etiologically similar subgroups. Precedence for this stratification exists in the ID literature, in which delayed walkers with ID are characterized by a higher rate of known etiology, other dysmorphic features, and medical comorbidities.8 These factors, which may be influenced by both genetics and the environment,31 are often not known or identified in individuals diagnosed with ID or ASD. Thus, as more detailed neurologic evaluations of individuals with ASD and ID are now being conducted clinically and through research, associations with delayed walking (which may differ based on sex) may become clearer.
Researchers in ASD are faced with many challenges in developing consistent and replicable phenotypes. Recent research has been less fruitful than hoped in the pursuit of phenotypes that link behavior with etiology. There are many potential explanations for the lack of identified correlates to date, most notably the reliance on behavioral measures that change within and across individuals over time.32 For this reason, an early developmental marker of neurobiological abnormality, such as delayed walking, is an attractive construct. Late walking can be accurately assessed at a single point in time and may link more readily to etiologic subgroups than other phenotypic variables that are more susceptible to retrospective reporting effects (eg, age of first words, regression)4,33 or that change over time (eg, social-communication symptoms, restricted and repetitive behaviors). Indeed, it is precisely that late walking is not specifically found to be associated with autism per se that it may be akin to one of the minor physical anomalies enumerated in complex autism, which was recently linked to a higher rate of genetic abnormalities.34
Other recent investigations suggest that measures of multiple motor skills, rather than 1 milestone, may be more powerful in predicting outcomes.35 In the current study, we were not focused on predicting developmental trajectories, but rather on whether we can conceptualize a single, important milestone as having differential relationships with cognitive functioning in different diagnostic groups. Eventually, considering both early motor development and behavioral trajectories (including later motor development) together will likely be most helpful in elucidating more biologically meaningful phenotypes.
Although retrospective parent report of age of walking is reliably recalled in both general population and ASD samples,3,4 the use of a single motor milestone may be a limitation, especially compared with detailed prospective analysis of motor problems in infant sibling studies36 or retrospective analysis of videorecorded motor behaviors.37 These detailed evaluations have revealed significant motor delays and differences in infants who later develop autism, with more subtle delays not necessarily reflective of a lower IQ later.38,39 On the other hand, because the ASD sample in the current study was large and was recruited for various purposes, our results may be more generalizable to the broad ASD population than smaller targeted prospective infant studies. Also, whereas infant sibling studies and other studies of high-risk samples use “low-risk” controls, our control group is more relevant in terms of understanding issues related to differential diagnosis and etiology of ASD and ID.
Nevertheless, because the sample was not obtained with epidemiologic methods, there are a number of potential sampling/referral biases that should be considered, including potential bias in how children are diagnosed with specific neurodevelopmental problems based on their early motor delays. Motor delays are often the first discernible sign of more global developmental problems,40 so it is possible that children with significant early motor delays were routed toward medical professionals (ie, neurology or genetics) for first evaluation, rather than ASD specialty clinics. Given that a substantial number of the ASD children in this study were ascertained from such clinics, late walkers with ASD may be underrepresented. Similarly, children with very significant motor delays may have been flagged with other dysmorphic features and, as a result, received genetic testing and subsequent genetic syndrome diagnoses. At the same time, although children with known genetic conditions were excluded from this study, genetic testing was not part of most clinic or research protocols, so it is likely that some members of both the ASD and non-ASD groups had unidentified specific genetic abnormalities. Future studies that use more epidemiologically diverse data from Centers for Disease Control and Prevention prevalence studies41 or from birth cohort studies that specifically monitor ASD and other developmental disorders42 would be useful in providing a more complete understanding of how delayed walking relates to NVIQ in children with and without ASD, as well as in children with and without identifiable genetic abnormalities.
Conclusions
Our findings suggest that at least 1 major motor milestone, on-time walking, is conserved more often than expected in children with ASD. Among children with ID, a greater proportion of those with ASD began walking within the normal time frame compared with those without ASD. These findings are important because they provide a relatively straightforward avenue for beginning to explore the relationship between early motor delays and later functioning, within the broader population of neurodevelopmental disorders. Further exploration of this topic, incorporating genetic and neurobiologic approaches, is warranted to determine whether on-time and delayed walkers with ASD and ID differ from one another in etiologically significant ways.
Acknowledgments
The authors are extremely grateful to all the families who participated in the research, as well as the clinicians and researchers who assisted in the collection and preparation of data included in the current study. We also thank Cherry Youn for her assistance in the preparation of this manuscript.
Glossary
- ADI-R
Autism Diagnostic Interview–Revised
- ADOS
Autism Diagnostic Observation Schedule
- ASD
autism spectrum disorder
- ID
intellectual disability
- NVIQ
nonverbal IQ
Footnotes
Dr Bishop conceptualized and designed the study and drafted the initial manuscript; Drs Bishop and Thurm interpreted the analyses; Drs Thurm and Farmer conceptualized the analyses; Dr Thurm worked on the draft of the initial manuscript; Dr Farmer conducted the analyses and collaborated in the interpretation of the results; Dr Lord coordinated and supervised data collection, approved the analytic plan, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: This project was supported by grants from the Health Resources and Services Administration (HRSA; R40MC28145) and National Institute of Child Health and Human Development (NICHD; R01HD065277) to Dr Bishop and the National Institute of Mental Health (R01MH081873-01A1 and R01-MH066496) and NICHD (U19-HD 35482) to Dr Lord.
References
- 1.Johnson A, Goddard O, Ashurst H. Is late walking a marker of morbidity? Steering Committee, Oxford Region Child Development Project. Arch Dis Child. 1990;65(5):486–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Multicentre Growth Reference Study Group . WHO Motor Development Study: windows of achievement for six gross motor development milestones. Acta Paediatr Suppl. 2006;95(S450):86–95 [DOI] [PubMed] [Google Scholar]
- 3.Bodnarchuk JL, Eaton WO. Can parent reports be trusted? Validity of daily checklists of gross motor milestone attainment. J Appl Dev Psychol. 2004;25(4):481–490 [Google Scholar]
- 4.Hus V, Taylor A, Lord C. Telescoping of caregiver report on the Autism Diagnostic Interview—Revised. J Child Psychol Psychiatry. 2011;52(7):753–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angulo-Barroso RM, Schapiro L, Liang W, et al. Motor development in 9-month-old infants in relation to cultural differences and iron status. Dev Psychobiol. 2011;53(2):196–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hreidarsson SJ, Shapiro BK, Capute AJ. Age of walking in the cognitively impaired. Clin Pediatr (Phila). 1983;22(4):248–250 [DOI] [PubMed] [Google Scholar]
- 7.Jenni OG, Chaouch A, Caflisch J, Rousson V. Infant motor milestones: poor predictive value for outcome of healthy children. Acta Paediatr. 2013;102(4):e181–e184 [DOI] [PubMed] [Google Scholar]
- 8.Kaminer RK, Jedrysek E. Age of walking and mental retardation. Am J Public Health. 1983;73(9):1094–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almuhtaseb S, Oppewal A, Hilgenkamp TI. Gait characteristics in individuals with intellectual disabilities: a literature review. Res Dev Disabil. 2014;35(11):2858–2883 [DOI] [PubMed] [Google Scholar]
- 10.Shapiro BK, Accardo PJ, Capute AJ. Factors affecting walking in a profoundly retarded population. Dev Med Child Neurol. 1979;21(3):369–373 [DOI] [PubMed] [Google Scholar]
- 11.Mirski KT, Crawford TO. Motor and cognitive delay in Duchenne muscular dystrophy: implication for early diagnosis. J Pediatr. 2014;165(5):1008–1010 [DOI] [PubMed] [Google Scholar]
- 12.Sarasua SM, Dwivedi A, Boccuto L, et al. Association between deletion size and important phenotypes expands the genomic region of interest in Phelan-McDermid syndrome (22q13 deletion syndrome). J Med Genet. 2011;48(11):761–766 [DOI] [PubMed] [Google Scholar]
- 13.Butler MG, Meaney FJ, Palmer CG. Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet. 1986;23(3):793–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Artigas-Pallarés J, Brun-Gasca C, Gabau-Vila E, Guitart-Feliubadaló M, Camprubí-Sánchez C. Medical and behavioural aspects of Angelman syndrome [in Spanish]. Rev Neurol. 2005;41(11):649–656 [PubMed] [Google Scholar]
- 15.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250 [PubMed] [Google Scholar]
- 16.Trevarthen C, Delafield-Butt JT. Autism as a developmental disorder in intentional movement and affective engagement. Front Integr Neurosci. 2013(Jun):1–31. [DOI] [PMC free article] [PubMed]
- 17.Chukoskie L, Townsend J, Westerfield M. Motor skill in autism spectrum disorders: a subcortical view. Int Rev Neurobiol. 2013;113:207–249 [DOI] [PubMed] [Google Scholar]
- 18.Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain Dev. 2007;29(9):565–570 [DOI] [PubMed] [Google Scholar]
- 19.Kokubun M, Haishi K, Okuzumi H, Hosobuchi T. Factors affecting age of walking by children with mental retardation. Percept Mot Skills. 1995;80(2):547–552 [DOI] [PubMed] [Google Scholar]
- 20.Dykens EM, Lense M. Intellectual disabliities and autism spectrum disorder: A cautionary note. In: Amaral DG, Dawson G, Geschwind DH, eds. Autism Spectrum Disorders. Oxford, UK: Oxford University Press; 2012:263–284 [Google Scholar]
- 21.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685 [DOI] [PubMed] [Google Scholar]
- 22.Lord C, Rutter M, Goode S, et al. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19(2):185–212 [DOI] [PubMed] [Google Scholar]
- 23.Bishop SL, Farmer C, Thurm A. Measurement of nonverbal IQ in autism spectrum disorder: scores in young adulthood compared to early childhood. J Autism Dev Disord. 2015;45(4):966–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth ed. Washington, DC: 1994
- 25.SAS II . SAS System for Windows. Cary, NC: 2011
- 26.Gamsiz ED, Viscidi EW, Frederick AM, et al. ; Simons Simplex Collection Genetics Consortium . Intellectual disability is associated with increased runs of homozygosity in simplex autism. Am J Hum Genet. 2013;93(1):103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iossifov I, O’Roak BJ, Sanders SJ, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515(7526):216–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto D, Delaby E, Merico D, et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 2014;94(5):677–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frazier TW, Georgiades S, Bishop SL, Hardan AY. Behavioral and cognitive characteristics of females and males with autism in the Simons simplex collection. J Am Acad Child Adolesc Psychiatr. 2014;53(3):329–340 [DOI] [PMC free article] [PubMed]
- 30.Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013;26(2):146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goetghebuer T, Ota MO, Kebbeh B, et al. Delay in motor development of twins in Africa: a prospective cohort study. Twin Res. 2003;6(4):279–284 [DOI] [PubMed] [Google Scholar]
- 32.Lord C, Bishop S, Anderson D. Developmental trajectories as autism phenotypes. Am J Med Genet B Neuropsychiatr Genet. 2015;169(2):198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozonoff S, Iosif AM, Young GS, et al. Onset patterns in autism: correspondence between home video and parent report. J Am Acad Child Adolesc Psychiatry. 2011;50(8):796–806 [DOI] [PMC free article] [PubMed]
- 34.Tammimies K, Marshall CR, Walker S, et al. Molecular diagnostic yield of chromosomal microarray analysis and whole-exome sequencing in children with autism spectrum disorder. JAMA. 2015;314(9):895–903 [DOI] [PubMed] [Google Scholar]
- 35.Bedford R, Pickles A, Lord C. Early gross motor skills predict the subsequent development of language in children with autism spectrum disorder [published online ahead of print December 22, 2015]. Autism Res. doi:10.1002/aur.1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brian J, Bryson SE, Garon N, et al. Clinical assessment of autism in high-risk 18-month-olds. Autism. 2008;12(5):433–456 [DOI] [PubMed] [Google Scholar]
- 37.Ozonoff S, Young GS, Goldring S, et al. Gross motor development, movement abnormalities, and early identification of autism. J Autism Dev Disord. 2008;38(4):644–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flanagan JE, Landa R, Bhat A, Bauman M. Head lag in infants at risk for autism: a preliminary study. Am J Occup Ther. 2012;66(5):577–585 [DOI] [PubMed] [Google Scholar]
- 39.Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry. 2006;47(6):629–638 [DOI] [PubMed] [Google Scholar]
- 40.Noritz GH, Murphy NA, Neuromotor Screening Expert P. Motor delays: early identification and evaluation. Pediatrics. 2013;131(6). Available at: www.pediatrics.org/cgi/content/full/131/6/e2016 [DOI] [PubMed]
- 41.Rice CE, Baio J, Van Naarden Braun K, Doernberg N, Meaney FJ, Kirby RS; ADDM Network . A public health collaboration for the surveillance of autism spectrum disorders. Paediatr Perinat Epidemiol. 2007;21(2):179–190 [DOI] [PubMed] [Google Scholar]
- 42.Stenberg N, Bresnahan M, Gunnes N, et al. Identifying children with autism spectrum disorder at 18 months in a general population sample. Paediatr Perinat Epidemiol. 2014;28(3):255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]