Abstract
Background
Endoscopically placed, temporary gastric electrical stimulation (tGES) may relieve symptoms of gastroparesis (Gp) and predict permanent gastric electrical stimulation (GES) outcomes.
Objective
To measure effects of 72 hours of temporary GES on Gp symptoms.
Design, Setting, and Patients
From 2005 to 2006, we conducted a hospital-based, randomized, placebo-controlled, crossover trial of two consecutive, 4-day sessions (session 1 and session 2), enrolling 58 patients (11 males, 47 females; mean age 46 years) with GP symptom histories of three etiologies (idiopathic, 38; diabetes mellitus, 13; postsurgical, 7).
Intervention
72 continuous hours temporary GES was provided for group A during session 1, and for group B during session 2.
Main Outcome Measurements
Symptoms measured daily; gastric emptying, electrogastrography, and quality of life measured at baseline and session close.
Results
In session 1, vomiting decreased in both groups, but was greater with stimulation, resulting in a day 3 difference of −1.02 (95% CI, −1.62 to −0.42; P < .001). Scores did not return to baseline during washout; on day 4, the difference persisted at −1.08 (95% CI, −1.81 to −0.35; P = .005). In session 2, vomiting slightly decreased with stimulation and slightly increased without it; at day 8, the nonactivated group had nonsignificantly greater vomiting, 0.12 (−0.68 to 0.92; P = .762). An overall treatment effect of a slight, nonsignificant daily decrease in average vomiting scores, −0.12 (−0.26 to 0.03; P = .116), was observed by pooling stimulation effects across sessions.
Limitations
Missing data; potential physiological imbalances between groups.
Conclusions
Although overall treatment effects were not significant, differences in favor of stimulation were suggested. Barriers to observing treatment effects included a decrease in vomiting for both groups during session 1, insufficient washout, and the absence of baseline vomiting for some patients. Future studies should better define inclusion criteria, use longer washout periods, randomize by etiology and baseline physiological findings, and pursue alternative designs. (Clinical trial registration number: 00432835.)
Gastroparesis (Gp) may stem from neuromuscular dysfunction; it certainly and severely compromises patients’ ability to manage nutrition, health, and social interactions. Incidence estimates for Gp remain a matter of controversy, but from 1995 to 2004, the disorder was reported as a primary diagnosis for some 10,000 hospitalizations and as a secondary one for another 134,000 admissions.1 The symptoms of Gp—nausea, vomiting, bloating, early satiety, dehydration, and abdominal pain—can lead to permanent disability, vulnerability to thrombosis,2,3 and greater mortality. Although increasing interest has led to dramatic increases in the characterization and diagnosis of Gp, the disease remains underrecognized,1,2 with diabetic and postsurgical etiologies more readily identified than idiopathic Gp, which occurs as frequently.1 When Gp is appropriately diagnosed, pharmacotherapies can now relieve some of its symptoms and types3–5; however, others, drug-refractory, exert profound, adverse effects on patients’ lives.
High-frequency, low-energy gastric electrical stimulation (GES) has been reported to reduce symptoms of drug-refractory Gp, with no deaths or injuries related to the stimulator or stimulation.4,6–10 In 1 decade-long study, patients with permanent GES remained symptom free or experienced significant symptom reductions throughout the study duration.6 In 2005, a tGES technique that delivers gastric stimulation through temporary leads attached (via the esophagus) to an external pacemaker demonstrated rapid, significant, and sustained symptom improvements, similar to permanent GES.2 More recently, a 2009 meta-analysis of all published findings reported that pooled study outcomes support GES’s effectiveness for Gp symptom relief.11
Nonetheless, GES remains controversial, at least in part owing to difficulties in assessing placebo effect and post-surgical gastric remodeling. Promising new electrophysiological tools that produce high-resolution gastric maps11 have been recently used to identify regions of disordered motility in Gp patients, as well as comparisons between normal and gastroparetic stomachs. These maps may eventually yield new insights into GES and symptom relief in Gp.
From July 2005 to October 2007, we studied the effects of tGES on 58 drug-refractory Gp patients to determine whether a 50% improvement in baseline symptom values could be demonstrated. Ancillary study outcomes included any measurable effect of tGES on gastric emptying, electrogastrography, and health-related quality of life.
METHODS
The Institutional Review Board at the University of Mississippi Medical Center approved all study protocols. An investigative device exemption was received from the Food and Drug Administration, and the study, conducted from July 2005 through October 2006, with long-term follow-up through November 2007, was registered as Clinical Trial 00432835.
Experimental design
This study was a University of Mississippi Medical Center hospital-based, double-masked, randomized, crossover trial in which each patient was observed and evaluated across two 4-day sessions for a total of 8 days. A schematic for this design, including the data collection schedule, is shown in Figure 1.
Figure 1.
Study flow diagrams. A, This study design and allocation schematic provides the data collection schedule for our double-masked, randomized, controlled trial from enrollment through evaluation across two 4-day sessions, for a total of 8 study days. B, The randomization process and study allocation for each group in our trial, along with reasons for which some patients did not complete the study, are provided. EGG, electrogastrogram; GET, gastric emptying time; QOL, quality of life.
Before enrollment, historical data were obtained through patient interviews with the evaluating physician. Consent was obtained by the physician or clinical study staff during the final enrollment visit, day 0, and each patient’s baseline symptoms and health-related quality of life was assessed.
For each patient, the day of endoscopic lead/electrode implantation was day 1. Just after the electrode had been endoscopically placed and just before the lead’s attachment to an external stimulator, the patient’s mucosal electrogastrogram (EGG) was recorded. After the patient’s recovery from endoscopy, the study’s unmasked computer technician, on a separate floor, randomized the stimulator (and thus the patient) to group A or B, masked to the patient and all other study staff, by following a prepared, computer-generated, randomization schedule. The technician programmed the device to activate for 72 continuous hours during the appropriate session, beginning with previously standardized parameters, as follows: frequency, 14 Hz; amplitude, 5 to 10 mA; pulse width, 330 μs; cycle ON, 0.1 to 1.0 seconds; cycle OFF, 5.0 to 4.0 seconds. Modification of parameters was permitted to alleviate any sensation of shock or similar discomfort.
On day 1, stimulators for patients in group A were activated for 72 continuous hours (session 1, days 1–3), but were not for patients in group B.
On day 4, a 24-hour period of no stimulation ensued for both groups (washout). On day 5, group B stimulators were activated for 72 continuous hours (session 2, days 5–7), but were not for patients in group A. On day 8, no stimulator was activated.
Symptoms were measured daily (days 0–8). Gastric emptying, electrogastrography, and health-related quality of life were assessed at baseline (day 0) and at each session’s close (days 4 and 8).
Study design, allocation, and adverse events
Fifty-eight patients were enrolled from the University of Mississippi Medical Center’s outpatient practices. The numbers of enrolled patients, discontinued participants, and patient completers by group are provided in Figure 1B. The dislodgment of orally-placed electrodes after day 3, with no further adverse event, accounted for discontinued observation in 13 of the 58 enrolled patients (24%; 6 group A; 7 group B). After these lead dislodgments, our protocol was amended to permit enrollment of up to 32 patients in each group, so as to obtain a total of 40 patients (20 per group) with complete data. During our study 58 patients were enrolled (group A = 30; group B = 28).
Patients of American Society of Anesthesiologists classes I and II were given standard sedation with fentanyl and mida-zolam for the electrode placement procedures. Patients of American Society of Anesthesiologists class III or higher were given monitored anesthesia care with propofol and/or general anesthesia. To minimize any effects from sedation on study outcomes, patients in group A receiving general anesthesia had an extra recovery day before activation. At the end of the 8-day study, all temporary, endoscopic GES electrodes were removed.
Inclusion criteria
Male and female patients between the ages of 18 and 70 years, inclusive, with a 1-year history or longer of symptoms of Gp of diabetic, postsurgical, or idiopathic etiology were eligible for this study. Patients refractory or intolerant to antiemetic drug classes (antihistamines and phenothiazines, serotonin receptor antagonists, dopamine receptor antagonists) and experiencing 7 or more episodes of chronic vomiting and/or nausea per week, irrespective of gastric emptying time (GET) values, were sought for inclusion in the study. To participate, patients had to be willing and able both to provide informed consent and to return for required follow-up visits.
Exclusion criteria
Patients who had active infections of any kind, were currently enrolled in another medical device or drug study, were pregnant, or were determined by the investigator to be unsuitable for endoscopy were ineligible for inclusion, as were patients unwilling or unable to provide informed consent or to return for required follow-up visits.
Endoscopic GES electrode placement
A standard, 140-cm-long endoscope with a 7F accessory channel was used to select an area as close as possible to the junction of the antrum and the body of the stomach. A temporary cardiac pacing lead (model 6414-200; Medtronic, Minneapolis, Minn) equipped with an inner bipolar electrode pacing lead and an outer 120-cm covering sheath was inserted through the accessory channel. Excessive coiling during insertion was avoided to facilitate clip attachment. The long inner lead was passed through the accessory channel and screwed into the stomach mucosa by a clockwise corkscrew motion. With the inner lead secured, the outer sheath was removed and the endoscope withdrawn while advancing an extra length (≈10 cm) of the lead into the stomach to help maintain the lead’s position.2
The endoscope was next reintroduced to the stomach, and an endoscopic clipping device (QuickClip 2; Olympus America Corp, Melville, NY, and/or Resolution Clip, Boston Scientific Corp, Natick, Mass) passed through the accessory channel. Three to 5 clips were applied within the stomach to hold the lead in place. At least 1 clip was placed near the distal metallic terminal part of the lead to achieve the desired electrical impedance. The lead itself was not attached to the external stimulator (Enterra Neurostimulator; Medtronic) until the initial mucosal EGG had been obtained. After this, the lead was connected to the stimulator and impedance determined to guide current delivery. The external stimulator could fit within a shirt pocket or cardiac telemetry pouch.
Symptom score
A structured, simple patient diary provided an easy method for recording the daily absence or presence and severity of vomiting, nausea, anorexia/early satiety, bloating/distention, and abdominal pain. This diary, a Likert scale, patient-reported outcome tool, permits the self-rating of each symptom from 0 to 4 (low to high). Study staff totaled the 5 daily ratings to calculate each patient’s total symptom score for each study day. Patient symptoms were also subclassified as chronic (noncyclic) or episodic (cyclic).12
Gastric emptying test
A radionuclide gastric emptying test was performed before endoscopic lead placement at the day 1 visit (baseline for GET) and during the day 4 and day 8 evaluations, following standard clinical practice.13 That day’s measures were totaled to calculate the total GET, respectively. GET findings were subcategorized as delayed (>10% remaining at 4 hours) or nondelayed.
Electrogastrogram
On days 1, 4, and 8, a 2-channel, cutaneous EGG was obtained and analyzed by signal-averaging techniques to determine the average frequency and amplitude. Mucosal EGGs were measured through the endoscopic lead on day 1, as described previously, and at the day 4 and 8 evaluations. Electrogastrographic frequency was subcategorized as low (<3.3 cycles per minute) or high (≥3.3 cycles per minute), as previously described.14
Health-related quality of life scores
Health-related quality of life was self-assessed on a −3 to +3 Likert scale, worst to best, on day 1 before lead placement and during the day 4 and 8 evaluations.15
Sample size and power
We amended our protocol to increase total study enrollment from the original power calculations of n = 40 to the IRB approved n = 58 in order to account for the 13 patients who had experienced lead dislodgment after the first session of stimulation. No changes were made in the plans for statistical analysis, and all patients enrolled were included in the analysis, both those for whom complete data was obtained and those who had missing data. Our increased sample size permitted us to exceed the required n = 20 in each group, originally calculated for appropriate power, with 30 patients enrolled in group A and 28 enrolled in group B.
Data analysis
A generalized linear mixed-models approach16 was used to estimate differences between periods of electrical stimulation and no stimulation while accounting for within-subject correlations arising from the crossover design. We examined unadjusted and adjusted models, both with and without inclusion of potential period effects. In addition to the primary efficacy comparisons, we conducted additional binary data analyses, examining outcomes as severe symptoms versus nonsevere symptoms (severe vomiting was defined as vomiting ≥3; severe nausea as nausea ≥3). Random intercepts were used for the association models, and robust variance estimates were used to calculate standard errors.
We conducted sensitivity analyses to examine the potential effects of the missing at random assumptions of the mixed models as well as to assess what effect our inclusion of patients who had no initial vomiting may have had on outcomes.
RESULTS
Table 1 provides a summary of patient baseline symptoms, demographics, and physiology for all study participants. Most study participants were female and white, had idiopathic Gp, and ranged in age from 23 to 77 years. Randomization produced a reasonably good balance among baseline inclusion characteristics. Nevertheless, the baseline vomiting score was 1.82 for group A and 2.68 for group B (differential = −0.86; 95% CI, −1.70 to −0.03; P = .043), implying some difference in the primary outcome measure at study outset despite randomization. Additionally, we observed some differences in baseline mucosal frequency/amplitude ratio (differential = −15.3; 95% CI, −27.5 to −3; P = .016), potentially representing differential physiologies. Not presented in Table 1, but of interest, is that more than half the study participants represented their baseline quality of life as the lowest possible score (−3).
TABLE 1.
Baseline symptoms, demographics, and physiology
| Group A (on/off), n = 28 | Group B (off/on), n = 30 | |
|---|---|---|
| Symptoms | ||
| Vomiting | ||
| Any | 19 (68%) | 23 (77%) |
| Score | 1.82 (1.55) | 2.68 (1.61) |
| Nausea | ||
| Any | 28 (100%) | 29 (96.67%) |
| Score | 3.27 (0.92) | 3.33 (1.03) |
| Total symptom score | ||
| Any | 28 (100%) | 29 (96.67%) |
| Score | 12.77 (4.95) | 14.57 (3.78) |
| Demographics | ||
| QOL | −2.14 (1.42) | −2.50 (0.84) |
| Body mass index | 29.42 (7.35) | 27.47 (7.74) |
| Ethnicity, white | 22 (78.57%) | 20 (66.67%) |
| Diabetic | 6 (21.43%) | 7 (23.33%) |
| Age, y | 46.75 (12.64) | 45.07 (16.08) |
| Sex, male) | 7 (28%) | 4 (13%) |
| Physiology | ||
| Cutaneous frequency | 4.53 (1.66) | 4.27 (1.42) |
| Cutaneous amplitude | 0.11 (0.13) | 0.10 (0.14) |
| Cutaneous ratio | 92.94 (75.95) | 77.11 (54.50) |
| Mucosal frequency | 4.04 (1.19) | 4.37 (2.38) |
| Mucosal amplitude | 0.80 (0.51) | 0.60 (0.49) |
| Mucosal ratio | 6.12 (4.31) | 21.37 (30.25) |
| Gastric emptying, h | ||
| 1 | 67.5 (21.6) | 65.7 (23.5) |
| 2 | 45.5 (24.1) | 38.7 (26.2) |
| 4 | 24.5 (26.5) | 19.4 (25.4) |
| Total | 136.6 (67.1) | 127.2 (70.2) |
Values in table are no. (%) for categorical data and means (standard deviations) for continuous data. Summaries for symptoms are means (standard deviations) for symptom scores and no. (%) for any reported symptoms (score >0).
QOL, Quality of life.
Our study utilized orally placed electrodes, which either remained orally positioned throughout the study, or, if dislodged, resulted in termination of the patient’s study participation. Other than this dislodgment, the leads were well-tolerated and not associated with any other adverse event. During washout (day 4) or the second study session (days 5 to 7), electrodes for 6 patients in group A and 7 in group B became dislodged. The between-group difference in relative frequency of dislodgement was not significant (P = 0.86).
Symptom summaries for groups A and B for days 1 to 8
The overall estimate for treatment effect across sessions 1 and 2 of this crossover study was a slight, nonsignificant decrease in average vomiting score of −0.12 per day (95% CI, −0.68 to 0.92; P = .762), during stimulator activation (Table 2, Fig. 2; please see additional models with differential session effects and alternative adjustment specifications in Online Appendix Table, available online at www.giejournal.org). Nonetheless, some important differences in symptom improvement over time were noted.
TABLE 2.
Outcome summaries within and between groups across study days
| Day | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Group A1 (on/off) n = 28 | |||||||||
| Vomiting | 1.82 (1.55) | 1.22 (1.55) | 0.64 (1.16) | 0.17 (0.67) | 0.29 (0.90) | 0.72 (1.34) | 0.65 (1.27) | 0.91 (1.44) | 0.77 (1.48) |
| Nausea | 3.27 (0.92) | 2.74 (2.52) | 1.43 (1.35) | 1.46 (1.29) | 1.73 (1.39) | 1.86 (1.43) | 2.02 (1.47) | 2.26 (1.18) | 1.95 (1.59) |
| TSS | 12.77 (4.95) | 8.11 (5.60) | 6.00 (5.56) | 5.32 (4.41) | 6.2 (5.19) | 6.64 (5.85) | 7.22 (5.20) | 8.13 (5.35) | 7.00 (5.32) |
| GET, h | |||||||||
| 1 | 67.07 (20.23) | 69.96 (23.79) | 64.71 (21.10) | ||||||
| 2 | 46.74 (23.27) | 46.85 (25.30) | 42.19 (24.37) | ||||||
| 4 | 24.70 (28.91) | 24.11 (23.76) | 24.71 (27.94) | ||||||
| Group B1 (off/on) n = 30 | |||||||||
| Vomiting | 2.68 (1.61) | 1.3 (1.47) | 0.97 (1.38) | 1.2 (1.47) | 1.37 (1.75) | 0.77 (1.27) | 0.72 (1.06) | 0.72 (1.17) | 0.65 (1.15) |
| Nausea | 3.33 (1.03) | 1.93 (1.51) | 1.62 (1.36) | 1.67 (1.49) | 1.82 (1.58) | 1.46 (1.37) | 1.76 (1.27) | 1.4 (1.38) | 1.48 (1.41) |
| TSS | 14.57 (3.78) | 8.35 (6.30) | 7.22 (5.63) | 7.93 (6.17) | 8.13 (6.68) | 6.81 (5.63) | 7.64 (5.31) | 5.64 (5.76) | 6.26 (5.71) |
| GET, h | |||||||||
| 1 | 66.13 (25.37) | 62.35 (24.75) | 68.67 (19.92) | ||||||
| 2 | 37.41 (28.06) | 38.00 (24.64) | 40.92 (26.50) | ||||||
| 4 | 16.43 (23.00) | 21.85 (28.51) | 20.25 (25.26) | ||||||
| Differences2,3 | |||||||||
| Vomiting | −0.86, P = .043 (−1.69 to −0.03) | −0.08, P = .847 (−0.88 to 0.73) | −0.32, P = .336 (−0.99 to 0.34) | −1.02, P ≤ .001 (−1.62 to −0.42) | −1.08, P = .00 (−1.8 to −0.35) | −0.05, P = .893 (−0.79 to 0.69) | −0.07, P = .842 (−0.75 to 0.62) | 0.19, P = .615 (−0.58 to 0.96) | 0.12, P = .762 (−0.68 to 0.92) |
| Nausea | −0.07, P = .799 (−0.58 to 0.45) | 0.44, P = .273 (−0.35 to 1.23) | −0.19, P = .599 (−0.90 to 0.52) | −0.20, P = .582 (−0.94 to 0.53) | −0.08, P = .829 (−0.87 to 0.70) | 0.40, P = .318 (−0.40 to 1.19) | 0.26, P = .513 (−0.54 to 1.06) | 0.86, P = .024 (0.11–1.60) | 0.48, P = .294 (−0.43 to 1.38) |
| TSS | −0.80, P = .127 (−4.13 to 0.53) | −0.24, P = .88 (−3.40 to 2.92) | −1.22, P = .411 (−4.16 to 1.73) | −2.61, P = .068 (−5.42 to 0.20) | −1.94, P = .22 (−5.07 to 1.20) | −0.17, P = .917 (−3.40 to 3.07) | −0.42, P = .781 (−3.48 to 2.63) | 2.50, P = .127 (−0.74 to 5.72) | 0.74, P = .655 (−2.58 to 4.06) |
| GET, h | |||||||||
| 1 | 0.94, P = .877 (−11.18 to 13.07) | 7.62, P = .259 (−5.78 to 21.01) | −3.95, P = .524 (−16.36 to 8.45) | ||||||
| 2 | 9.33, P = .180 (−4.45 to 23.10) | 8.85, P = .203 (−4.92 to 22.62) | 1.27, P = .867 (−14.02 to 16.57) | ||||||
| 4 | 8.28, P = .247 (−5.91 to 22.46) | 2.26, P = .755 (−12.25 to 16.78) | 4.46, P = .579 (−11.66 to 20.59) | ||||||
On days 1 through 3, negative differences indicate lower symptoms with stimulation, whereas on days 5 through 7, positive differences indicate lower symptoms with stimulation.
TSS, Total symptom score; GET, gastric emptying time.
Values in table are estimates (standard error) for group A and group B outcomes.
Values in table are estimates, P values (95% CIs) for differences (A–B).
Group A received stimulation on days 1 to 3, group B on days 5 to 7. Differences are presented throughout in terms of group A − group B.
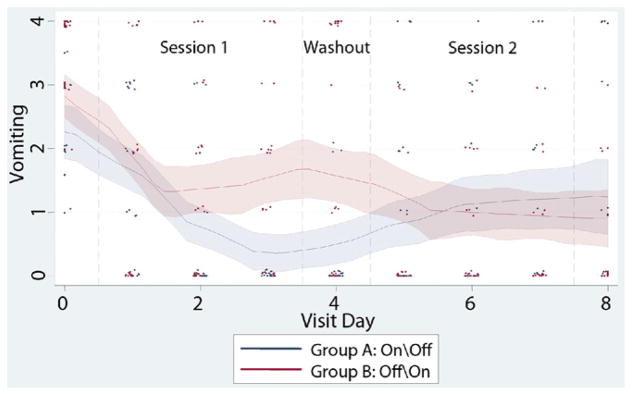
Figure 2.
Vomiting scores across the study. Vomiting scores over the course of the study are provided for both groups, with individual patient scores, daily means and smoothed average response trajectories along with 95% confidence bands. During session 1, a slight, non-significant decrease in average vomiting score was observed; however, the decrease with stimulation (group A) was greater. Scores did not return to baseline for either group during the washout period. During session 2, vomiting scores slightly decreased with stimulation (group B) and slightly increased without it (group A) (see Table 2).
Appendix Table.
Primary Results across different modeling specifications.
| Vomiting
|
Nausea
|
Total Sympom Score (TSS)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1: Full Model |
Model 2: Pooled trt-effect |
Model 3: Parsimonious Model |
Model 1: Full Model |
Model 2: Pooled trt-effect model |
Model 3: Parsimonious Model |
Model 1: Full Model |
Model 2: Pooled trt-effect model |
Model 3: Parsimonious Model |
|
| period 1 | |||||||||
|
| |||||||||
| slope (daily change) | |||||||||
|
| |||||||||
| Group A (Stim on) | −0.41 (−0.54,−0.29) p<0.001 | −0.40 (−0.51, −0.29) p<0.001 | −0.40 (−0.51, −0.29) p<0.001 | −0.33 (−0.46, −0.20) p<0.001 | −0.33 (−0.45, −0.22) p<0.001 | −0.33 (−0.45, −0.22) p<0.001 | −1.33 (−1.82, −0.84) p<0.001 | −1.30 (−1.76, −0.85) p<0.001 | −1.30 (01.76, −0.85) p<0.001 |
|
| |||||||||
| Group B (Stim off) | −0.27 (−0.39, −0.15) p<0.001 | −0.28 (−0.40, −0.17) p<0.001 | −0.28 (−0.40, −0.17) p<0.001 | −0.40 (−0.53, −0.27) p<0.001 | −0.40 (−0.52, −0.28) p<0.001 | −0.40 (−0.51, −0.28) p<0.001 | −1.61 (−2.12, −1.10) p<0.001 | −1.63 (−2.10, −1.16) p<0.001 | −1.63 (−2.10, −1.16) p<0.001 |
|
| |||||||||
| Trt effect: (Diffs in slopes) | |||||||||
|
| |||||||||
| A (on) - B (off) ** | −0.14 (−0.31, 0.32) p=0.112 | −0.07 (−0.25, 0.11) p=0.449 | −0.28 (−0.99, 0.43) p=0.441 | ||||||
|
| |||||||||
| period 2 | |||||||||
|
| |||||||||
| slope (daily change) | |||||||||
|
| |||||||||
| Group A (Stim off) | 0.05 (−0.15, 0.24) p=0.632 | 0.08 (−0.08, 0.23) p=0.329 | 0.08 (−0.08, 0.23) p=0.329 | −0.01 (−0.20, 0.19) p=0.948 | −0.02 (−0.18, 0.14) p=0.839 | −0.02 (−0.18, 0.14) p=0.841 | −0.29 (−1.04, 0.50) p=0.494 | −0.21 (−0.83, 0.42) p=0.514 | −0.20 (−0.83, 0.42) p=0.516 |
|
| |||||||||
| Group B (Stim on) | −0.01 (−0.20, 0.18) p=0.899 | −0.04 (−0.19, 0.11) p=0.607 | −0.04 (−0.19, 0.11) p=0.606 | 0.34 (−0.17, 0.24) p=0.775 | 0.04 (−0.12, 0.21) p=0.583 | 0.04 (−0.12, 0.21) p=0.585 | 0.19 (−0.60, 0.98) p=0.641 | 0.12 (−0.51, 0.75) p=0.701 | 0.12 (−0.51, 0.75) p=0.700 |
|
| |||||||||
| Trt effect: (Diffs in slopes) | |||||||||
|
| |||||||||
| B (on) - A (off)** | −0.06 (−0.33, 0.21) p=0.666 | −0.04 (−0.32, 0.24) p=0.775 | −0.46 (−1.56, 0.65) p=0.417 | ||||||
|
| |||||||||
| Treatment effect difference between periods | |||||||||
|
| |||||||||
| slope diff Pd2 - slope diff Pd1 | 0.08 (−0.24, 0.40) p=0.616 | 0.03 (−0.31, 0.36) p=0.866 | −0.18 (−1.49, 1.13) p=0.790 | ||||||
|
| |||||||||
| Pooled trt effect: | −0.12 (−0.26, 0.03) p=0.116 | −0.12 (−0.26, 0.03) p=0.116 | −0.06 (−0.21, 0.09) p=0.428 | −0.06 (−0.21, 0.09) p=0.431 | −0.33 (−0.92, 0.26) p=0.277 | −0.33 (−0.92, 0.27) p=0.277 | |||
Model 1, Full Model: Different Effects of Stimulation by Session, adjusted for age gender ethnicity.
Model 2, Pooled Treatment Effect: Effects of Stimulation are pooled across Sessions, adjusted for age gender ethnicity.
Model 3, Parsimonious Model: Effects of Stimulation are pooled across Sessions, adjusted for age.
Negative values indicate (daily) decreases in Symptoms (Stimulation - No Stimulation).
In session 1, vomiting decreased for both groups, but the decrease was greater with stimulation (group A), resulting in a day 3 difference in vomiting scores of −1.02 (95% CI, −1.62 to −0.42; P < .001). Scores did not return to baseline for either group during the washout period, resulting in a continued day 4 difference of −1.08 (95% CI, −1.81 to −0.35; P = .005 (Table 2, Fig. 2). In session 1, results for nausea and total symptom scores were similar to results for vomiting (Table 3), but no discernable changes were noted for GETs.
TABLE 3.
Primary model results
| Vomiting | Nausea | Total symptom score | |||
|---|---|---|---|---|---|
| Session 1 | Slope (daily change) | Group A (Stim on) | −0.40 (95% CI, −0.51 to −0.29); P < .001 | −0.33 (95% CI, −0.45 to −0.22); P < .001 | −1.30 (95% CI, 01.76 to −0.85); P < .001 |
| Group B (Stim off) | −0.28 (95% CI, −0.40 to −0.17); P < .001 | −0.40 (95% CI, −0.51 to −0.28); P < .001 | −1.63 (95% CI, −2.10 to −1.16); P < .001 | ||
| Session 2 | Slope (daily change) | Group A (Stim off) | 0.08 (95% CI, −0.08 to 0.23) P < .329 | −0.02 (95% CI, −0.18 to 0.14); P < .841 | −0.20 (95% CI, −0.83 to 0.42); P < .516 |
| Group B (Stim on) | −0.04 (95% CI, −0.19 to 0.11); P = .606 | 0.04 (95% CI, −0.12 to 0.21); P < .585 | 0.12 (95% CI, −0.51 to 0.75); P < .700 | ||
| Treatment effect pooled across periods | −0.12 (95% CI, −0.26 to 0.03); P = .116 | −0.06 (95% CI, −0.21 to 0.09); P = .431 | −0.33 (95% CI, −0.92 to 0.27); P = .277 | ||
Model: treatment effect pooled over period, adjusted for age and sex.
Negative values indicate decreases in symptoms.
See Online Appendix Table for additional models with treatment effect by period examinations.
Stim, Stimulation.
During session 2, vomiting scores decreased slightly with stimulation (group B) and increased slightly without it (group A). This resulted in nonsignificantly higher vomiting scores for group A (no stimulation) at day 7 of 0.19 (95% CI, 0.58 to −0.96; P = .615) and at day 8 of 0.12 (95% CI, −0.68 to 0.92; P = .762) (Table 2, Fig. 2). Total symptom score results were similar to vomiting in session 2 (Table 3), and no gastric emptying time differences were noted. We did observe more nausea at day 7 for the nonstimulated group (differential = 0.86; 95% CI, 0.11–1.60; P = .024).
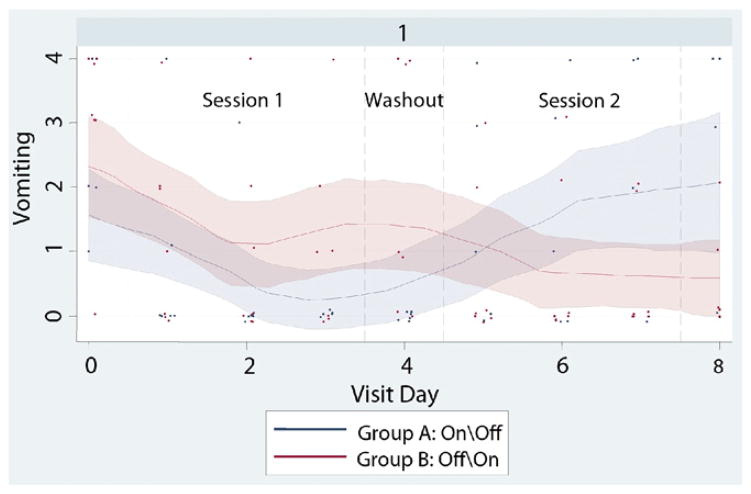
We conducted additional analyses to examine the effects of missing data, baseline vomiting (Online Appendix Fig. 3, available at www.giejournal.org), and subgroup effects for diabetic/nondiabetic etiology and cyclic/noncyclic symptom pattern (Online Appendix Figs. 4 and 5, available online at www.giejournal.org). Participant characteristics did not vary significantly for completers versus noncompleters. Treatment effects for vomiting symptoms were similar, but accentuated and statistically significant for those with baseline vomiting present (n = 42), at −0.23 units per day with stimulation (−0.42, −0.04) P = 0.019. Similarly, patients with diabetic etiology (n = 13) had stronger treatment effects for vomiting scores, at −0.31 units per day with stimulation (−0.64, 0.02) P = 0.069; additional sensitivity and subgroup results available upon request.
Figure 3.
Patients who had at least some initial vomiting at baseline reported improved symptoms with stimulation. Mean vomiting scores decreased for both groups with 72-hours active stimulation.
Figure 4.
For patients with diabetic gastroparesis in both Groups A and B, improved symptoms were seen with stimulation. Mean vomiting scores decreased in Group A with 72-hours active stimulation, and then approached baseline levels when stimulation was terminated.
Figure 5.
Vomiting symptoms experienced by group B patients with a cyclic symptom pattern were reduced with temporary GES ON. When treatment with temporary GES was withdrawn by switching OFF the device, vomiting symptoms worsened in this group.
DISCUSSION
The primary aim of this masked, crossover study was to measure the reduction of patient-reported GI symptoms during 72-hours of endoscopically implanted, tGES. Vomiting decreased for both stimulated and nonstimulated groups in session 1, with a larger decrease for the stimulated group (90%) than for the nonstimulated group (55%). In session 2, changes in symptoms were more moderate for both groups, leading to a nonsignificant overall treatment effect. Treatment effects appeared somewhat stronger for patients with diabetes and cyclic pattern symptom onset.
All 58 patients enrolled in the EndoStim study elected to have a permanent gastric electrical stimulator placed. However, insurance denial or delay resulted in only 37 of the original 58 patients (group A, 21; group B, 16) receiving one. Of these 37 patients, follow-up data were collected on average at 451 days following permanent stimulator placement for 36 patients (20 of 21 patients from group A, and 16 of 16 from group B), in effect a self-selected group, as follow-up studies were requested for all patients. Post-hoc analyses on these 36 patients suggested associations between symptom scores recorded at the final day of temporary stimulation and symptom scores recorded at the latest follow-up date after permanent stimulation, with potentially stronger associations for patients whose baseline electrophysiology was more normal. We consider these post hoc findings preliminary and require further follow up.
We previously reported that tGES provides a nearly immediate, antiemetic effect.17 The findings here strengthen that argument, but also suggest that endoscopic device placement itself may produce unexpected beneficial changes in upper GI symptoms, potentially because of some type of placebo effect. Our observations further suggest that the effects of GES, in general, may maximize in 3 to 4 days. GES may also produce a remodeling effect, as shown by group A’s persistent decrease in reported symptoms, even after stimulation ceased; this may help explain results from another recent randomized, crossover trial.18–21
Study limitations
The number of patients evaluated was small and electrode dislodgment occurred in 13 patients (22% of the 58 patients enrolled). Some results that were not statistically significant here might become so with a larger sample size. Our tGES protocol now uses trans-nasal rather than orally placed leads, which have greatly reduced dislodgment rates.
The 24-hour washout period may have been insufficient to eliminate the effects of stimulation. Potential carryover effects should be addressed by the use of alternative study designs (eg, parallel groups, longer study/washout periods, stepped-wedge designs).
We observed symptom and physiologic differences between groups A and B at baseline in spite of randomization, which can happen by chance alone in any given study. The imbalances we observed may impede a clear examination of the underlying questions of interest. Post-hoc analyses that controlled additionally for baseline variables (baseline vomiting, nausea, mucosal EGG frequency and amplitude) provided similar results, with reported primary pooled treatment effect on vomiting = −0.12 (−0.26, 0.03) P = 0.116, and pooled treatment effect with additional baseline variable adjustment = −0.10 (−.23, 0.06) P = 0.253. To avoid similar difficulties in future studies, we recommend that baseline symptom status and electrophysiology be taken into account when constructing the randomization protocol, and particularly baseline mucosal EGG recorded after mucosal lead placement, but prior to stimulator activation. Additionally, mucosal EGG should be evaluated for its potential utility as an indicator of permanent stimulator outcomes.
Finally, we elected in this study to leave all our patients on the at-home medications for nausea or pain that they were using at the time of baseline assessment. In group A (n = 28), 17 patients (60.71%) were on prokinetics, and 16 (57.14%) were on anti-emetics. In group B (n = 30), 23 patients (76.67%) were on prokinetics, and 18 (60%) were on anti-emetics.
CONCLUSION
This double-masked, randomized, crossover study showed that endoscopically placed, tGES may reduce symptoms such as vomiting and nausea. tGES may help indicate whether a patient is likely to benefit from permanent stimulation, sparing both cost and invasive surgery in patients in whom tGES does not provide relief. Unexpected difficulties that arose during the study impede our ability to make conclusive therapeutic statements; however, we did note important differences between stimulation groups with improvements for patients on stimulation. The results from this trial should also assist the development of better designs for evaluating the effects of GES.
Take-home Message.
Endoscopically placed, temporary gastric electric stimulation (GES) may reduce symptoms such as vomiting and nausea.
These findings should assist in the development of better designs for evaluating the effects of GES on symptoms associated with gastroparesis. The authors recommend that baseline symptom status and electrophysiology, particularly baseline mucosal electrogastrography performed before device activation, be taken into account when constructing the randomization protocol.
Acknowledgments
The authors thank Edy Sofer, MD, and Warren Starkebaum, PhD, for their suggestions on trial design. We also thank Greg O’Grady, MD, for his review of the manuscript. Finally, we thank the staff of the GI Division, GI Laboratory, and Department of Nuclear Medicine at the University of Mississippi Medical Center for their help with this study, and Jo Anne Fordham for her assistance with manuscript production.
Abbreviations
- EGG
electrogastrogram
- Gp
gastroparesis
- GES
gastric electrical stimulation
- GET
gastric emptying time
- tGES
temporary gastric electrical stimulation
APPENDIX
Etiological and Cyclic/Non Cyclic Symptom Pattern Subgroups
Post hoc analyses of our study findings revealed vomiting score differences between Groups A and B which corresponded to etiological and cyclic/non cyclic symptom subgroup. Patients in Groups A and B who had diabetic gastroparesis (DM GP) appeared to derive the greatest benefit from GES stimulation. Mean vomiting scores for DM GP patients in Group A decreased with three days of active stimulation. Moreover, when stimulation for Group A was terminated at Day 5, symptom relief for these patients deteriorated, and their vomiting scores approached baseline levels (See Figure 4).
Patients who, during their clinical history assessment, were assessed to have a chronic or noncyclic (n = 14) Gp symptom pattern also reported greater symptom relief than those who were diagnosed with a cyclical or episodic (n = 31) Gp symptom pattern. In contrast to patients with DM Gp, however, these patients reported only a slight deterioration in Gp symptoms upon termination of treatment with temporary GES. Only completer patient data was evaluated in this graph (See Figure 5).
Gastric Emptying (GET)
Both 1 and 4 hour GET measures were recorded for all patients at baseline and on Days 4 and 8. With stimulation, patients in Group B whose baseline GET had been delayed at baseline experienced a slight improvement at 4 hr GET that was not statistically significant. The change in 1 hr GET for the non-delayed cohort of Group B, however, approached significance (p=0.12).
Electrophysiology
For Groups A and B, baseline mucosal frequency and amplitude values were both higher than normal (3.0 frequency; 0.40 amplitude) literature controls, possibly representing abnormal electrophysiology. Baseline EGG values, particularly mucosal EGG, should thus be explored to determine whether or not they may help indicate the presence of underlying, neuromuscular abnormalities. If they can, temporary gastric electrical technique may also help clinicians determine when a full thickness biopsy with neuromuscular staining of the gastrointestinal tract is in order.(21)
Footnotes
DISCLOSURE: The following author disclosed a financial relationship relevant to this publication: Dr. Abell: a licensee, consultant, and investigator for Medtronic, Inc. The other authors disclosed no financial relationships relevant to this publication. Supported in part by Medtronic, Inc. The University of Mississippi has filed an Intellectual Property claim regarding aspects of the technology used in this study.
References
- 1.Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes. 1995–2004. Am J Gastroenterol. 2008;103:313–22. doi: 10.1111/j.1572-0241.2007.01658.x. [DOI] [PubMed] [Google Scholar]
- 2.Ayinala S, Batista O, Goyal A, et al. Temporary gastric electrical stimulation with orally or PEG-placed electrodes in patients with drug refractory gastroparesis. Gastrointest Endosc. 2005;61:455–61. doi: 10.1016/s0016-5107(05)00076-3. [DOI] [PubMed] [Google Scholar]
- 3.Lobrano A, Blanchard K, Rock W, et al. Assessing thrombosis risk in patients with idiopathic, diabetic, and postsurgical gastroparesis. Adv Ther. 2006;23:750–68. doi: 10.1007/BF02850315. [DOI] [PubMed] [Google Scholar]
- 4.Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35–43. doi: 10.1111/j.1365-2982.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 5.Abell TL, Bernstein RK, Cutts T, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–83. doi: 10.1111/j.1365-2982.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 6.Anand C, Al-Juburi A, Familoni B, et al. Gastric electrical stimulation is safe and effective: a long-term study in patients with drug-refractory gastroparesis in three regional centers. Digestion. 2007;75:83–9. doi: 10.1159/000102961. [DOI] [PubMed] [Google Scholar]
- 7.Abell TL, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277–81. doi: 10.1177/0148607103027004277. [DOI] [PubMed] [Google Scholar]
- 8.Abell TL, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–8. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 9.Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204–12. doi: 10.1159/000068359. [DOI] [PubMed] [Google Scholar]
- 10.Forster J, Sarosiek I, Lin Z, et al. Further experience with gastric stimulation to treat drug refractory gastroparesis. Am J Surg. 2003;186:690–5. doi: 10.1016/j.amjsurg.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 11.O’Grady G, Angeli TR, Lahr C, et al. High-resolution mapping of slow wave activity in the gastroparetic stomach: initial results. Presented at: Neurogastroenterology and Motility Joint International Meeting; Boston, MA. August 26–29, 2010. [Google Scholar]
- 12.Christensen CJ, Johnson WD, Abell TL. Patients with cyclic vomiting pattern and diabetic gastropathy have more migraines, abnormal electrogastrograms, and gastric emptying. Scand J Gastroenterol. 2008;43:1066–75. doi: 10.1080/00365520802085411. [DOI] [PubMed] [Google Scholar]
- 13.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–62. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim CH, Hanson BS, Abell TL, et al. Effect of inhibition of prostaglandin synthesis on epinephrine-induced gastroduodenal electromechanical changes in humans. Mayo Clin Proc. 1989;63:149–57. doi: 10.1016/s0025-6196(12)65668-7. [DOI] [PubMed] [Google Scholar]
- 15.Cutts TF, Abell TL, Karas JG, et al. Symptom improvement from prokinetic therapy corresponds to improved quality of life in patients with severe dyspepsia. Dig Dis Sci. 1996;41:1369–78. doi: 10.1007/BF02088561. [DOI] [PubMed] [Google Scholar]
- 16.Diggle PJ, Heagerty P, Liang KY, et al. The analysis of longitudinal data. 2. Oxford (UK): Oxford University Press; 2002. [Google Scholar]
- 17.Familoni B, Abell TL, Bhaskar SK, et al. Gastric electrical stimulation has an immediate antiemetic effect in patients with gastroparesis. Eng IEEE Trans Biomed. 2006;53:1038–46. doi: 10.1109/TBME.2006.873395. [DOI] [PubMed] [Google Scholar]
- 18.Abell TL. Gastric electric stimulation is a viable option in gastroparesis treatment. Nat Clin Pract Gastronenterol Hepatol. 2009;6:E8–13. doi: 10.1038/ncpgasthep1370. [DOI] [PubMed] [Google Scholar]
- 19.McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947–54. doi: 10.1016/j.cgh.2010.05.020. quiz e116. [DOI] [PubMed] [Google Scholar]
- 20.Koch KL. Gastric electrical stimulation and the “eye of the beholder. Clin Gastroenterol Hepatol. 2010;8:908–9. doi: 10.1016/j.cgh.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Abell TL, Familoni B, Voeller G, et al. Electrophysiologic, morphologic, and serologic features of chronic unexplained nausea and vomiting: lessons learned from 121 consecutive patients. Surgery. 2009;145:476–85. doi: 10.1016/j.surg.2008.12.006. [DOI] [PubMed] [Google Scholar]