Abstract
Introduction
Osteoarthritis (OA) is the most common type of arthritis and proinflammatory cytokines have been considered as the main etiologic factor in the pathogenesis of the disease. Serum levels of cytokines, that are associated with innate immunity and TH1 cells, have been analyzed in OA patients, however, there is limited research that profiles cytokines associated with Th17 cells and their relation to vitamin D3 and pain.
Material and methods
The sera from 131 patients with OA and 262 healthy controls were evaluated for serum levels of IL-17A, IL-21, IL-23 and vitamin D3 using ELISA.
Results
Serum levels of IL-17A, and IL-23 were statistically higher in OA patients than in healthy controls, while IL-21 and vitamin D3 were significantly lower in OA patients when compared to controls. A significant positive correlation was found between the serum levels of IL-17A and IL-23 using WOMAC pain scores and vitamin D3 serum levels.
Discussion
The results suggest that IL-17A plays a significant role in OA pathogenesis and the induction of pain. Decreased serum levels of vitamin D3 may reflect a positive role played by the factor in the regulation of immune responses in OA patients.
Introduction
Osteoarthritis (OA), which is the most common type of arthritis, is a chronic and degenerative disease of synovial joints. In addition to articular cartilage breakdown, other joint tissues such as synovial membranes and subchondral bone are also involved in the progression of the disease [1, 2]. Pain control and improvement of joint function is the main goal in the treatment of osteoarthritis. The usual drug treatments for this disease are nonsteroidal anti-inflammatory drugs (NSAID), analgesics, topical corticosteroids and injection of hyaluronic acid into the affected joint [3]. The exact etiology of osteoarthritis is yet to be clarified, however, several studies have shown that inflammation at the early stages of the disease appears to play a role in the development and progression of the disease [3]. Not surprisingly, proinflammatory cytokines like IL-1β, TNF and IL-6, play key roles as the main mediators of joint tissue catabolism in OA [4–6].
Among the proinflammatory cytokines, IL-17A, IL-21 and IL-23 are considered as those which play crucial roles in the induction of local inflammation [7] and cartilage destruction diseases such as rheumatoid arthritis (RA) [8]. Moreover, the in vitro effect of IL-17A on chondrocytes from OA patients and subsequent cytokine release and cartilage destruction has also been reported [9]. There are similarities between RA and OA with regards to inflammation and joint destruction [8], hence, it may be hypothesized that proinflammatory cytokines may play a role in the pathogenesis of both diseases.
Vitamin D3 has significant effects on bones through its role in calcium and phosphor metabolism but it also has immunomodulatory properties which enable vitamin D3 to regulate expression of several immune related molecules [10]. Therefore, vitamin D3 has the potential to regulate immune responses that can attenuate proinflammatory diseases including autoimmune diseases. In accord with this, decreased serum levels of vitamin D3 in the proinflammatory based diseases, including autoimmune diseases, have been reported in several studies [11, 12]. However, information regarding the status of vitamin D3 in OA is a controversy [13]. For example, increased and decreased expression of vitamin D3 in OA has been reported by several investigators [13–15].
The aim of the present study was to determine the serum levels of IL-17A, IL-21, IL-23 and vitamin D3 in a population of Iranian OA patients and their correlation with clinical manifestations of OA. The second aim of this study was to examine the relationship between serum levels of vitamin D3 and the proinflammatory cytokines.
Material and Method
Subjects
This cross-sectional study was performed from the 1st of January 2015 to the 31st of December 2015 on 131 patients (24 males and 107 females) with knee OA who were referred to the Orthopedic Department of Hamza Clinic, Fasa, Iran. All patients fulfilled the American College of Rheumatology (ACR) criteria for OA diagnosis [16]. Patients with symptoms of other chronic inflammatory diseases, diabetes, a history of corticosteroid treatment within the previous month prior to recruitment to the study and patients with other forms of arthritis were excluded from the study. The control group contained 262 sex matched healthy subjects without any sign of arthritis and other orthopedic disease. This study was approved by the Human Ethics Committee of the Fasa University of Medical Sciences. All participants were informed about the study procedure and all patients signed an inform consent before blood sampling. Blood samples were taken from the participants and sera was separated immediately and then kept at -20°C for further analysis.
Function and pain assessment
To assess pain in patients with knee OA, the Western Ontario McMaster University Osteoarthritis Index (WOMAC) questionnaire was used. This questionnaire involves functional activities and consists of questions regarding pain during horizontal motion, walking up and down stairs, sitting, standing, lying down, and pain at night (http://www.rheumatology.org/I-Am-A/Rheumatologist/Research/Clinician-Researchers/Western-Ontario-McMaster-Universities-Osteoarthritis-Index-WOMAC). A scale of 0–4 is used for scoring, where 0 means no pain and higher scores represent more severe pain. The total range of the WOMAC pain score is from 0 to 20.
Cytokine assays
The sera were tested for the presence of IL-17A, IL-21 and IL-23 using a commercial ELISA kit (eBioscience, USA) according to the manufacturer's recommendations. The sensitivity of the ELISA kit for the detection of IL-17A, IL-21 and IL-23 was 4pg/ml, 8pg/ml and 15pg/ml, respectively.
Vitamin D3 assay
Serum levels of vitamin D3 were evaluated using a commercial enzyme linked fluorescent assay (ELFA) kit from Vidas Company (Paris, France) according to the manufacturer's guidelines.
Statistical analysis
All statistical analyses were performed using SPSS 20 software (Chicago, IL). Age, BMI, serum levels of IL-21, IL-23, IL-17A and vitamin D3 were compared between groups using independent sample t tests. The Chi square test was used for comparisons of gender between the two groups. Correlation was computed by use of a Pearson test. A logistic regression was used to determined predictor(s) of OA. In this analysis OA served as the dependent variable, and cytokines, Vitamin D3, BMI, age and gender were considered as the independent variables. Data was presented showing the median and mean ± standard deviation and P-values less than 0.05 were considered significant.
Results
Statistical analysis revealed that there were statistically significant differences in age, BMI and WOMAC pain scores between the OA patients and healthy controls, while the two groups were matched regarding sex (Table 1).
Table 1. Status of sex, age, BMI and WOMAC in OA patients and healthy controls.
| OA (131) | Control (262) | P.value | |
|---|---|---|---|
| Age | 52 ± 8 | 55 ± 9 | 0.007 |
| Sex (m/f) | 24/107 | 48/214 | 0.551 |
| BMI | 28.24 ± 2.19 | 25.97 ± 2.38 | < 0.001 |
| WOMAC | 15.11 ± 3.24 | 5.50 ± 2.80 | < 0.001 |
Table illustrates that OA patients and healthy controls were significantly differ regarding age, BMI and WOMAC, while sex were match between groups.
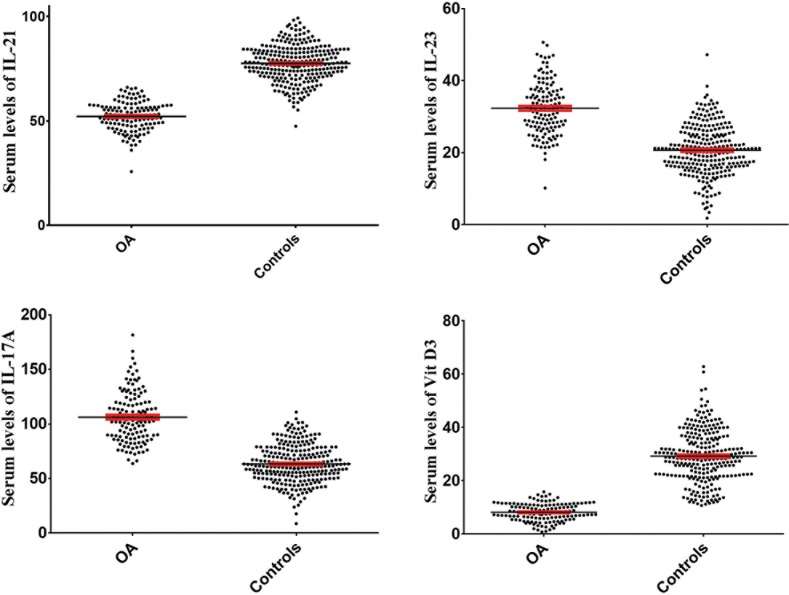
The results also revealed that serum levels of IL-23 (P< 0.001) and IL-17A (P< 0.001) were significantly increased in OA patients (32.49 ± 7.12 and 106.24 ± 23.03 pg/mL, respectively) when compared with healthy controls (20.62 ± 6.86 and 63.46 ± 17.40 pg/mL, respectively). Serum levels of IL-21 in OA patients and healthy controls were 52.14 ± 7.04 and 77.58 ± 8.80 pg/mL, respectively, which indicates that IL-21 was significantly decreased in OA patients (P< 0.001). Vitamin D3 levels were also significantly (P< 0.001) decreased in OA patients (8.14 ± 3.33) in comparison to healthy controls (29.21 ± 9.75). Logistic regression modelling which excluded the interfering factors, age and BMI, also confirmed these results (Table 2). The Fig 1 illustrates the serum levels of IL-21, IL-23, IL-17A and vitamin D3 in OA patients and healthy controls.
Table 2. Analysis of variables using logistic regression model.
| IL-17 (Adj. R2 = 0.556) | IL-21 (Adj. R2 = 0.722) | IL-23 (Adj. R2 = 0.464) | ||||
|---|---|---|---|---|---|---|
| B* | P-value | B* | P-value | B* | P-value | |
| State (OA) | 36.948 | < 0.001 | -24.198 | < 0.001 | 9.780 | < 0.001 |
| Gender (female) | 4.671 | 0.077 | 0.949 | 0.381 | 0.035 | 0.970 |
| Age | 0.101 | 0.424 | -0.036 | 0.489 | 0.054 | 0.223 |
| BMI | 2.077 | < 0.001 | -1.301 | < 0.001 | 0.956 | < 0.001 |
| Vitamin D | - 0.065 | 0.578 | -0.077 | 0.113 | -0.003 | 0.947 |
Table illustrates that the differences regarding serum levels of the cytokines and vitamin D3 are significant too after exclusion of age and BMI as interfered factors.
B*: Unstandardized Regression Coefficients beta.
Fig 1. Serum levels of IL-21, IL-23, IL-17A and vitamin D3 in OA patients in comparison to healthy controls.
Statistical analysis revealed that serum levels of IL-23 and IL-17A were significantly increased, while, IL-21 and vitamin D3 were decreased in OA patients in comparison to healthy controls.
Evaluation of correlation between WOMAC pain scores and serum levels of IL-21, IL-23, IL-17A and vitamin D3 using a Pearson correlation test revealed that WOMAC pain scores have a significant positive correlation with IL-23 and IL-17A and a significant negative correlation with IL-21 and vitamin D3 (Table 3). The data presented in Table 3 also demonstrated that vitamin D3 had a positive correlation with IL-23 and IL-17A.
Table 3. Correlation between WOMAC pain scores, IL-21, IL-23, IL-17A and vitamin D3 in OA patients.
| WOMAC pain scores | IL-21 | IL-23 | IL-17A | Vitamin D3 | ||
|---|---|---|---|---|---|---|
| WOMAC pain scores | Pearson correlation | 1 | -0.175 | 0.306 | 0.315 | -0.219 |
| P value | 0.045 | <0.001 | <0.001 | 0.012 | ||
| Number | 131 | 131 | 131 | 131 | 131 | |
| Vitamin D3 | Pearson correlation | -0.219 | 0.064 | -0.441 | -0.406 | 1 |
| P value | 0.012 | 0.469 | <0.001 | <0.001 | ||
| Number | 131 | 131 | 131 | 131 | 131 | |
Table illustrates that WOMAC pain scores have significant positive correlations with IL-23 and IL-17A and negative correlations with IL-21 and vitamin D3. Vitamin D3 also has significant negative correlations IL-23 and IL-17A.
Discussion
It has been proposed that Th17 plays crucial roles in the pathogenesis of proinflammatory disorders including autoimmune diseases via the production of IL-17A [17, 18]. IL-21 and IL-23 are important cytokines which play pivotal roles in development and maintenance of Th17, respectively [19]. Thus, it has been hypothesized that the cytokines may participate in the pathogenesis of OA, as a proinflammatory disease. The results presented here showed that serum levels of IL-17A and IL-23 significantly increased, while IL-21 significantly decreased in OA patients in comparison to healthy controls. Based on the fact that OA is an inflammatory disease, the results demonstrate that IL-17A significantly participates in the pathogenesis of OA. Interestingly, IL-23 the main cytokine required for the maintenance of Th17 lymphocytes, also increased in the patients, hence, it seems that survival and function of these lymphocytes are increased in OA patients. Based on the fact that IL-21 plays key roles in the development of Th17 lymphocytes, and according to the decreased serum levels of the cytokine in OA patients, it could be possible that the increased functions and survival of Th17 (by IL-17A and IL-23) lead to a negative feedback to decrease IL-21 and development of new clones of Th17 lymphocytes.
Previous investigations demonstrated the reactivity of T cells against chondrocyte antigens in OA [20] and also showed that IL-17A induces production of IL-1β, TNF-α and IL-6, the main pro-inflammatory cytokines, in OA. Moreover, the role of these proinflammatory cytokines in production of NO and MMP (matrix metalloproteinase) and reduction of proteoglycan levels has been shown in OA pathogenesis [21–24]. Furthermore, it has been suggested that IL-17A can inhibit proteoglycan synthesis in chondrocytes through NO induction [24]. Based on the aforementioned investigations and the results of the current study, it seems that IL-17A and its maintenance factor (IL-23) may be considered as key factors for induction of inflammation and osteoarthritis in the OA patients. The roles of IL-21, as the inducer of Th17 differentiation, need to be evaluated by additional studies in the context of OA. To the best of our knowledge, serum levels of IL-21 and IL-23 have not evaluated in OA patients, hence, this is the first report regarding the status of IL-21 and IL-23 in OA patients.
Vitamin D3 plays is an immunomodulator which has a significant effects on immune cells via interactions with its intracytoplasmic receptor. Results of the current study demonstrated that serum levels of vitamin D3 were significantly decreased in OA patients in comparison to healthy controls. Based on the immunomodulatory effects of vitamin D3, it seems that declined vitamin D3 is an important factor that may induce inflammation in OA patients. Statistical analysis also confirmed this hypothesis and revealed that serum levels of vitamin D3 have a negative correlation with serum levels of IL-23 and IL-17A in OA patients. Thus, it may be proposed that using vitamin D3 as therapeutic agent in OA patients may reduce immune responses and inflammation. However, previous investigations reported that administration of vitamin D3 is not effective for knee OA [14], but based on our results it may be effective against the Iranian genetic background.
The results also demonstrated that IL-17A and IL-23 have a significant positive correlation while vitamin D3 and IL-21 have a significant negative correlation with WOMAC pain scores in OA patients. It has been documented that IL-17A induces expression of cyclooxygenase 2 (COX2) and prostaglandin E2 (PGE2) production via the activation of MAPKs pathway [25]. It may be hypothesized that IL-17A induces pain in OA patients through the same pathway but this needs to be examined further. Additionally, based on the fact that serum levels of IL-21 and vitamin D3 decreased in OA patients it is possible that this also influences pain. Thus, it seems that more investigations regarding the roles played by these cytokines in the induction of pain in OA patients would shed light on the pathogenesis and clinical symptoms of this disease.
Conclusions
According to our data, it seems that the IL-17A may play a major role in the pathogenesis of OA and decreased serum levels of vitamin D3 in these patients may be a reason for the increased functions of Th17. Additionally, based on the positive correlation between serum levels of IL-17A and WOMAC pain score, this may open a door for new approaches in the treatment of pain and OA complications. Furthermore, the data suggests that the serum levels of IL-17A can reflect OA development and potentially be considered as a new biomarker for the disease.
Acknowledgments
The authors would like to thank the Non-communicable Diseases Research Center for their technical help.
Data Availability
All data that constitute the minimal dataset underlying the findings of our paper are within the manuscript itself.
Funding Statement
This study has been financially supported by grant number 93165 from the Fasa University of Medical Sciences.
References
- 1.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nature reviews Rheumatology. 2011;7(1):33–42. 10.1038/nrrheum.2010.196 . [DOI] [PubMed] [Google Scholar]
- 2.Lories RJ, Luyten FP. The bone–cartilage unit in osteoarthritis. Nature Reviews Rheumatology. 2011;7(1):43–9. 10.1038/nrrheum.2010.197 [DOI] [PubMed] [Google Scholar]
- 3.Felson DT. Clinical practice. Osteoarthritis of the knee. The New England journal of medicine. 2006;354(8):841–8. 10.1056/NEJMcp051726 . [DOI] [PubMed] [Google Scholar]
- 4.Massicotte F, Lajeunesse D, Benderdour M, Pelletier J-P, Hilal G, Duval N, et al. Can altered production of interleukin-1β, interleukin-6, transforming growth factor-β and prostaglandin E 2 by isolated human subchondral osteoblasts identify two subgroups of osteoarthritic patients. Osteoarthritis and Cartilage. 2002;10(6):491–500. 10.1053/joca.2002.0528 [DOI] [PubMed] [Google Scholar]
- 5.Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators of inflammation. 2014;2014:561459 10.1155/2014/561459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imamura M, Ezquerro F, Marcon Alfieri F, Vilas Boas L, Tozetto-Mendoza TR, Chen J, et al. Serum levels of proinflammatory cytokines in painful knee osteoarthritis and sensitization. International journal of inflammation. 2015;2015:329792 10.1155/2015/329792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MohammadKazemi A, AliAkbar P, Abdollah J, Gholamhossein H. Serum levels of IL-10 and IL-17A in occult HBV-infected South-East Iranian patients. Hepat Mont. 2010;2010(1, Winter):31–5. [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Kang S, Kim J, Kwon G, Koo S. Elevated levels of T helper 17 cells are associated with disease activity in patients with rheumatoid arthritis. Annals of laboratory medicine. 2013;33(1):52–9. 10.3343/alm.2013.33.1.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honorati M, Bovara M, Cattini L, Piacentini A, Facchini A. Contribution of interleukin 17 to human cartilage degradation and synovial inflammation in osteoarthritis. Osteoarthritis and Cartilage. 2002;10(10):799–807. [DOI] [PubMed] [Google Scholar]
- 10.Jamali Z, Asadikaram G, Mahmoodi M, Sayadi A, Jamalizadeh A, Saleh-Moghadam M, et al. Vitamin D status in female students and its relation to calcium metabolism markers, lifestyles, and polymorphism in vitamin D receptor. Clin Lab. 2012;59(3–4):407–13. [DOI] [PubMed] [Google Scholar]
- 11.Upala S, Sanguankeo A. Low 25-Hydroxyvitamin D Levels are Associated with Vitiligo: a Systematic Review and Meta-Analysis. Photodermatol Photoimmunol Photomed. 2016;Article in press. Epub 2016/03/24. 10.1111/phpp.12241 . [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Zynat J, Guo Y, Osiman R, Tuhuti A, Zhao H, et al. Low Serum Vitamin D Is Associated with Anti-Thyroid-Globulin Antibody in Female Individuals. Internat J Endocrin. 2015;2015:285290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goula T, Kouskoukis A, Drosos G, Tselepis AS, Ververidis A, Valkanis C, et al. Vitamin D status in patients with knee or hip osteoarthritis in a Mediterranean country. Journal of orthopaedics and traumatology: official journal of the Italian Society of Orthopaedics and Traumatology. 2015;16(1):35–9. Epub 2015/03/05. 10.1007/s10195-014-0322-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onuora S. Osteoarthritis: Vitamin D not effective for knee OA. Nature Reviews Rheumatology. 2016;12(5):252–. [Google Scholar]
- 15.Laslett LL, Quinn S, Burgess JR, Parameswaran V, Winzenberg TM, Jones G, et al. Moderate vitamin D deficiency is associated with changes in knee and hip pain in older adults: a 5-year longitudinal study. Annal Rheumatic Dis. 2014;73(4):697–703. [DOI] [PubMed] [Google Scholar]
- 16.Wu CW, Morrell MR, Heinze E, Concoff AL, Wollaston SJ, Arnold EL, et al. , editors. Validation of American College of Rheumatology classification criteria for knee osteoarthritis using arthroscopically defined cartilage damage scores. Seminars in arthritis and rheumatism; 2005: Elsevier. [DOI] [PubMed] [Google Scholar]
- 17.Koga T, Ichinose K, Tsokos GC. T cells and IL-17 in lupus nephritis. Clin Immunol. 2016;Article in press. 10.1016/j.clim.2016.04.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu S, Qian Y. IL-17/IL-17 receptor system in autoimmune disease: mechanisms and therapeutic potential. Clin Sci (Lond). 2012;122(11):487–511. 10.1042/CS20110496 . [DOI] [PubMed] [Google Scholar]
- 19.Arababadi MK, Bidaki MZ, Kennedy D. IL-17A in hepatitis B infection: friend or foe? Archives of virology. 2014;159(8):1883–8. 10.1007/s00705-014-2002-x . [DOI] [PubMed] [Google Scholar]
- 20.Alsalameh S, Mollenhauer J, Hain N, Stock KP, Kalden JR, Burmester GR. Cellular immune response toward human articular chondrocytes. T cell reactivities against chondrocyte and fibroblast membranes in destructive joint diseases. Arthritis & Rheumatism. 1990;33(10):1477–86. [DOI] [PubMed] [Google Scholar]
- 21.Benderdour M, Tardif G, Pelletier J-P, Di Battista JA, Reboul P, Ranger P, et al. Interleukin 17 (IL-17) induces collagenase-3 production in human osteoarthritic chondrocytes via AP-1 dependent activation: differential activation of AP-1 members by IL-17 and IL-1beta. The Journal of rheumatology. 2002;29(6):1262–72. [PubMed] [Google Scholar]
- 22.Pacquelet S, Presle N, Boileau C, Dumond H, Netter P, Martel-Pelletier J, et al. Interleukin 17, a nitric oxide-producing cytokine with a peroxynitrite-independent inhibitory effect on proteoglycan synthesis. The Journal of rheumatology. 2002;29(12):2602–10. [PubMed] [Google Scholar]
- 23.Martel‐Pelletier J, Mineau F, Jovanovic D, Di Battista JA, Pelletier JP. Mitogen‐activated protein kinase and nuclear factor κB together regulate interleukin‐17–induced nitric oxide production in human osteoarthritic chondrocytes: Possible role of transactivating factor mitogen–activated protein kinase–activated protein kinase (MAPKAPK). Arthritis & Rheumatism. 1999;42(11):2399–409. [DOI] [PubMed] [Google Scholar]
- 24.Lubberts E, Joosten LA, Van De Loo FA, Van Den Bersselaar LA, Van Den Berg WB. Reduction of interleukin‐17–induced inhibition of chondrocyte proteoglycan synthesis in intact murine articular cartilage by interleukin‐4. Arthritis & Rheumatism. 2000;43(6):1300–6. [DOI] [PubMed] [Google Scholar]
- 25.Li JK, Nie L, Zhao YP, Zhang YQ, Wang X, Wang SS, et al. IL-17 mediates inflammatory reactions via p38/c-Fos and JNK/c-Jun activation in an AP-1-dependent manner in human nucleus pulposus cells. J Transl Med. 2016;14(1):77 10.1186/s12967-016-0833-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that constitute the minimal dataset underlying the findings of our paper are within the manuscript itself.