Abstract
Marfan syndrome (MFS) is a rare, severe, chronic, life-threatening disease with multiorgan involvement that requires optimal multidisciplinary care to normalize both prognosis and quality of life. In this article, each key team member of all the medical disciplines of a multidisciplinary health care team at the Hamburg Marfan center gives a personal account of his or her contribution in the management of patients with MFS. The authors show how, with the support of health care managers, key team members organize themselves in an organizational structure to create a common meaning, to maximize therapeutic success for patients with MFS. First, we show how the initiative and collaboration of patient representatives, scientists, and physicians resulted in the foundation of Marfan centers, initially in the US and later in Germany, and how and why such centers evolved over time. Then, we elucidate the three main structural elements; a team of coordinators, core disciplines, and auxiliary disciplines of health care. Moreover, we explain how a multidisciplinary health care team integrates into many other health care structures of a university medical center, including external quality assurance; quality management system; clinical risk management; center for rare diseases; aorta center; health care teams for pregnancy, for neonates, and for rehabilitation; and in structures for patient centeredness. We provide accounts of medical goals and standards for each core discipline, including pediatricians, pediatric cardiologists, cardiologists, human geneticists, heart surgeons, vascular surgeons, vascular interventionists, orthopedic surgeons, ophthalmologists, and nurses; and of auxiliary disciplines including forensic pathologists, radiologists, rhythmologists, pulmonologists, sleep specialists, orthodontists, dentists, neurologists, obstetric surgeons, psychiatrist/psychologist, and rehabilitation specialists. We conclude that a multidisciplinary health care team is a means to maximize therapeutic success.
Keywords: multidisciplinary, Marfan syndrome, health care, team, profession, sociology, management
Introduction
Marfan syndrome (MFS) is a rare, severe, chronic, life-threatening disease with multiorgan involvement that requires optimal multidisciplinary care to normalize both prognosis and quality of life. In this article, each key team member of all medical disciplines of a multidisciplinary health care team gives a personal account of his or her contribution in the management of patients with MFS. We show how with the support of health care managers self-determined team members organize themselves in an organizational structure to create a common meaning, to maximize therapeutic success of patients with MFS.
How to read this article
The article is quite long and readers may not have the time to go through the entire text. However, this article serves two purposes. First, to provide a detailed knowledge on what each discipline can contribute to maximize the therapeutic success of multidisciplinary health care for MFS. Second, to provide a groundwork on how multidisciplinary health care can be organized in such a way that therapeutic success can be maximized, where we present what we call “the strategy paradigm of multidisciplinary care”. Accordingly, readers who focus on the first end are referred to the following sections: “Marfan syndrome and the spectrum of genetic aortic diseases”, “Medical standards”, “Core disciplines”, and “Auxiliary disciplines”. Readers who focus on the second are referred to the following sections: “The strategy paradigm of multidisciplinary health care: team member, structure, meaning”, “Method and overview of the article”, “History and presence of multidisciplinary health care for MFS”, “Organizational structures”, and “Meaning”.
MFS and the spectrum of genetic aortic diseases
MFS is a disorder of the connective tissue that is inherited in an autosomal dominant fashion and is caused by mutations in the gene coding for fibrillin-1 (FBN1). MFS has a prevalence of 1.5 to 17.2 per 100,000 individuals in the general population with similar frequency in both sexes and in all countries and races.1 MFS is a typical example of a rare disease: It has a prevalence of <1 per 5,000; it is a severe, chronic, life-threatening disease with multiorgan involvement.2 Moreover, MFS is associated with chronic fatigue, chronic pain, and psychological despair that compromise the quality of life and impose restrictions on the autonomy of affected persons.3–8
Until the early 1970s, medical treatment was not available for patients with MFS. Accordingly, 50% of affected men and women died by the age of 40 years and 48 years, respectively, which corresponded to a reduction in life expectancy by 30%–40% as compared to the normal population.9 Since then, however, 30 years of research has brought ~30 years of increase in the average life expectancy.10 In this article, we show how physicians organize themselves into multidisciplinary health care teams to maximize therapeutic success for patients with MFS. When we speak of MFS in this article, we include a seemingly ever-increasing list of Marfan-like disorders, which are all genetically heritable diseases that affect the aorta, where the vascular Ehlers-Danlos syndrome, and Loeys-Dietz syndrome (LDS) are the most prominent variants.11,12 However, our focus is MFS and we do not detail all aspects of health care that are specific to these alternative syndromes.
The strategy paradigm of multidisciplinary health care: team member, structure, and meaning
In its broadest sense, strategy is social action that uses means for defined goals.13 More specifically, we design multidisciplinary care as a means to maximize therapeutic success for patients with MFS (Figure 1).13 The authors prefer the term “multidisciplinary” instead of other terminologies, such as “interdisciplinary” or “multiprofessional” health care.14 The term “multidisciplinary” best describes our view that it is team members who join a collaborative structure to maximize overall therapeutic success. Therefore, the strategy paradigm designs multidisciplinary health care as a strategy to join key team members in a multidisciplinary team structure to maximize therapeutic success.13,15
Figure 1.
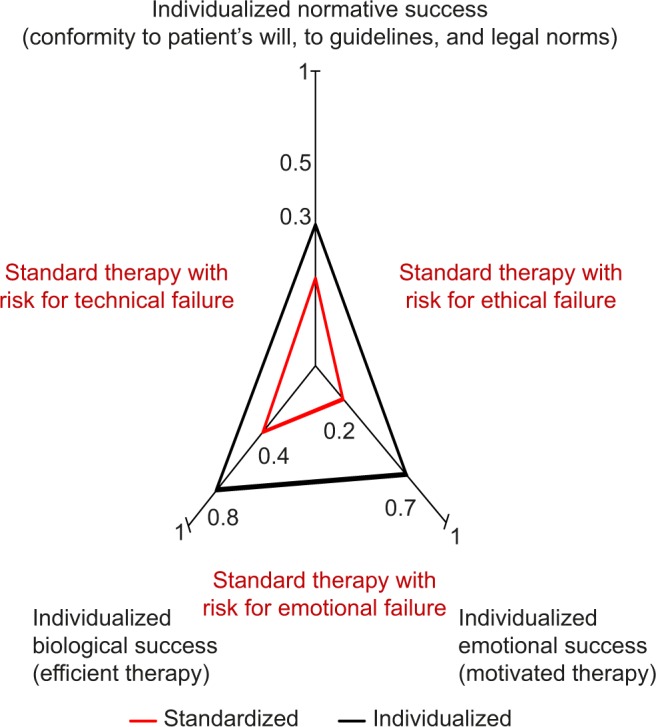
Maximizing therapeutic success requires maximizing success in three dimensions comprising i) biology of disease and patient’s physical make-up, ii) norms with conformity of therapy with patient’s autonomy, with medical guidelines, and laws, and iii) emotions including the patient’s motivational support of therapy. The extent to which therapy is maximized in an individual patient corresponds to the areas of the red and black triangles in the graph. Usually, therapy according to standard is unable to maximize therapeutic success because it fails to accommodate biological individuality such as comorbidity, or the patient’s autonomy by neglecting his or her will, or because it fails to obtain the patient’s motivational support. Information from previous studies.13,23,115
Method and overview of the article
This article is a narrative that illustrates how we implemented, and currently perform multidisciplinary health care in a Marfan center. We organize the article in three sections according to team member, structure, and meaning. In every section, key team members of our center provide their authentic voice to give a personal account of their view and contribution to multidisciplinary health care for MFS. We provide the initials provided in the list of authors to identify the personal account.
In the “Structure” section, we focus on structure, where we first provide accounts of the history and presence of Marfan centers: we show how pioneering team members in the US introduced multidisciplinary health care, how we used this experience to establish and continuously develop a Marfan Center for adults in Hamburg, followed by a center for children, and how representatives of the Marfan Hilfe Deutschland made this Marfan center possible and how they keep on doing so. Then, we explain the formal structures of multidisciplinary health care for Marfan patients (Table 1; GO, SN, and CW-N), and finally we present medical standards as part of health care structures (Table 2; all authors).
Table 1.
Organizational structures of the Hamburg multidisciplinary center for patients with Marfan syndrome and other genetic aortic diseases
| Structure element | Criteria and demands | Current practice |
|---|---|---|
| Structures of the University Medical Center Hamburg-Eppendorf (UKE) | ||
| External quality assurance (EQA) | German federal law requires monitoring and reporting of procedural results and complications of inpatient care (§ 135a SGB V) for several tracer diagnosis and procedures | Our university medical center assesses, analyzes, and publishes external quality assurance data |
| Quality management (QM) system | German law demands hospitals to introduce and maintain quality management systems (§ 135a SGB V)101 | Our university medical center has a quality management system certified according to DIN ISO 9001,116 where we 1) define all core processes by writing and updating SOPs, 2) define quality goals, 3) monitor quality indicators such as satisfaction of staff, patients, and referring physicians, and 4) have regular quality management board meetings including all professional groups of our heart center |
| Clinical risk management (CRM)101 | German law demands hospitals to introduce and maintain CRM (§ 135 a SGB V)101 | At our university medical center 1) we have a critical incident reporting system (CIRS), 2) report and analyze adverse events (AE) in mortality and morbidity conferences, 3) reflect all reported AE’s in a high-level expert commission in the sense of a peer review, 4) have an advanced praise and complaint management, and 5) proactively identify, analyze and manage risks for patient safety and quality |
| Martin Zeitz Center for Rare Diseases (MZCSE)36 | The University Medical Center of Hamburg Eppendorf is an accredited A-center for Rare Diseases, where the Hamburg Marfan Center is a B-center. Nationaler Aktionsplan für Menschen mit Seltenen Erkrankungen [National plan of action for people with rare disease] (NAMSE)37 base their criteria on the following: 1) high-level expertise for the specific rare disease, 2) coordinator to navigate patients, 3) SOP to define patient pathways, 4) SOP to define diagnostic criteria, 5) availability of a multidisciplinary team as defined by guidelines, 6) identification of responsible physicians in each medical discipline including SOP for case conferences and documentation of regular team meetings, 7) concepts for psychosocial care, 8) concepts for transition, 9) SOP defining structured cooperation with patient organization, 10) SOP defining cooperation with other disease-specific B-centers including case conferences and quality circles, 11) SOP defining cooperation with other disease-specific C-centers, GP and non-medical service-providers, including case conferences and quality circles, SOP defining case-conferences for external patients, IT-based and tele-medical networks, 12) access to specialized diagnostic modalities, 13) SOP that describes how to participate in the development of medical information material, 14) obligation to contribute information to the mapping of the service landscape, 15) concepts for a structured medical education program, and 16) SOPs about participation in research projects, clinical studies, health care research and registers, IT-structure for data exchange, and criteria for research quality | Our MFS-B-center fulfills NAMSE-criteria 1–8, and 12. 6) Marfan-board meetings take place every 3 months; all other conferences take place by phone, or directly together with the patient, 9) We cooperate intensively with the Marfan organization, 10) with other B-centers, and 11) with GPs, although formal C-centers are not established, and 13) we provide a plethora of disease information material, but we do not have SOPs for these issues, 14) Our center enlists in the Orphanet database (http://www.orpha.net/national/DE-DE/index/startseite/) with annual up-date of information, 15) we do not have a structured educational program on MFS, but we provide training in our case conferences almost on a daily basis. Our center participates in national and international research and clinical programs, but there is no IT-based data exchange, 16) Our university center has spent thousands of Euros on an IT-based register infrastructure, but data protection regulations impose rigid barriers to its actual use. |
| Structures of University Heart Center (UHZ) | ||
| German Aorta Center Hamburg (DAZH)13 | Case conferences including decision making and morbidity and mortality analysis103 | We use our aorta center board meetings for multidisciplinary discussion of complex aortic pathologies and also for updating outcomes of decisions and procedures23 |
| Generic structural elements | ||
| Structures for a multidisciplinary program according to the framework of Meguid et al104 | Establish/obtain/maintain 1) a business model, 2) physician/administrative buy-in, 3) administrative and hospital support, 4) hire a multidisciplinary clinic coordinator, 5) scheduling logistics, 6) rotating schedule from all participating specialists, 7) support services, 8) patient flow templates, 9) current summary reports of diagnosis and treatment, 10) provide welcome folder with letter describing all appointments for that day, 11) mock days to minimize obstacles and delays, 12) flag patients to capture clinic volumes in the EMR, 13) marketing, 14) community outreach, and 15) data collection for research projects | 1) German legal directives regulate the financing of MFS care,25 2 and 3) the hospital provides staffing, administration, infrastructure, 4) coordinating physicians are employees of the hospital with many additional professional obligations, 5) a coordinating nurse has a “Marfan-telephone” to organize individualized appointments in an electronic scheduling system, 6) interns can rotate voluntarily into our Marfan center and get trained by experienced colleagues, 7) nutrition councilors, social works, pharmacists, microbiologists are available as part of the hospital structure, 8) patient flow templates are constantly revised along changing routines and patient requests, 9) we keep clinical information on each patient current in our EMR, where every MFS-physician has access to summary records, original imaging recordings, laboratory findings and all other clinical information, 10) we use standard invitation letters that describe our schedules, 11) mocking is part of our culture of criticism and we have a formalized praise and complaint management system, 12) our university controlling department uses SAP-based IT-systems to monitor MFS-patient volumes and costs, 13) a flyer and a website presents our Marfan clinic, which provides contact information, scope of care, and educational content on MFS, 14) we communicate continuously with the referring physicians via dismissal letters, phone and email about specific patients, and we communicate constantly with local and national representatives of the German Marfan organization, 15) prospective data collection from clinical routines for research severely restricted by privacy and data protection laws |
| Structures for patient- centered care based upon the framework of Bergeson and Dean117 | Bergeson and Dean identify four evidence-based design criteria to support patient-centered care:118 A) ensure access and continuity of care: 1) open access or advanced access patient scheduling, 2) continuity of care with a clinician, 3) multiple routes of practice access including access to non-physician members, contact methods can be by telephone, email, or drop-in visit, or patients access to EMRs. B) Provide opportunities for patients to participate in the care process: 1) designing office visits specifically to address patients’ concerns, eg, using tools like encouraging the use of patient lists of concerns, agenda cards, 2) involving families in the design of care, 3) (web-based) tools for self-assessment of health status, and 4) providing patients with information about the care they should be receiving. C) Provide self-management support: 1) collaborative goal setting and action planning, 2) action planning (web-based) tools, 3) clear and agreed-upon follow-up plans, 4) use of printed post-visit summary, 5) peer support by patient-to-patient mentorship, advocacy roles of individuals, and group education in self-management skills. D) Coordinating care between settings: 1) establish a specific care coordinator role within the primary care team, 2) providing standardized referral and hand-off information, 3) patient-held records |
Our MFS center has adopted the following measures from the list of Bergeson and Dean: A) access and continuity of care: 1) we alleviate scheduling of appointments and access to our center by using a “Marfan-phone” for direct contact to our “Marfan-nurses”, 2) we provide continuity of care both with nurses and physicians, 3) we provide access to our nurses, we have our phone-numbers and email addresses publically assessable on the web, and we permit drop-in visits for brief counseling. B) Opportunities for patients to participate in the care process: 1) we schedule one hour for each initial patient interview, and we encourage patients to use lists of concerns and to 2) have their family members or other confidant with them in the interview, 3) we developed screening-devices for clinical MFS-self-assessment,111 4) we provide patients with written instructions about clinical management C) Provide self-management support: 1) we use shard-decision approaches for action-planning, 2) we do not use formalized tools, 3) we communicate clear follow-up plans, 4) including written summary reports, and 5) we encourage contact to the German Marfan Organization D) Coordinating care between settings: 1) our center coordinators and social workers help patients to navigate in the health care system, 2) we use letters of invitation to inform patients prior to visiting our center, and we 3) send our medical reports to the GP and to the patient, where we try to use generic rather than medical language |
Abbreviations: EMR, electronic medical record; GP, general practitioners; MFS, Marfan syndrome; SOP, standard operating procedure.
Table 2.
Multidisciplinary management of Marfan syndrome according to the aim and method of care in each discipline
| Discipline | Aim of care | Method of care |
|---|---|---|
| Core disciplines | ||
| Pediatrician/pediatric cardiologist | To diagnose MFS and other GAS as early in life as possible To prevent cardiovascular, ophthalmic, or orthopedic complications To achieve full integration into school, physical education, and support early career choices To achieve a successful transition from adolescence to adulthood |
Periodical evaluation of cardiovascular features (ECG, TTE), growth, skeletal features, ocular symptoms, pulmonary airway, integument, dural ectasia, and dental features Review diagnosis periodically in unclear cases Provide prophylactic medication including BAB, ARB, or ACEi and endocarditis prophylaxis Review physical activity restrictions/lifestyle modifications |
| Human geneticist | To inform patients and families about implications of GAS To confirm the clinical diagnosis by identifying the disease-causing mutation To molecularly differentiate MFS from other GAS |
Complete family history and analysis of the pedigree Genetic counseling Arrange appropriate molecular genetic testing Interpret and counsel regarding the results |
| Cardiologist | To establish a correct diagnosis of MFS or of other GAS To predict, prevent, retard, or treat aortic aneurysm, mitral or aortic valve regurgitation or IE, or myocardial dysfunction To protect women from aortic complications when they plan pregnancy |
Inform patients about life-style modifications including some restrictions in adults such as no contact sports, no isometric exertion, no exertion at maximal capacity Treat with BAB, ARB, or ACEi Perform baseline NT-pro-BNP serum levels, 12-lead resting ECG, 24-hour blood pressure measurements, TTE, MRI of entire aorta (in Germany, not in the US) Perform annual follow-up visits including NT-pro-BNP serum levels, 12-lead resting ECG, blood pressure measurements, TTE, and MRI of the aorta if indicated |
| Heart surgeon | To rescue life when aortic dissection or rupture occurs To normalize life-expectancy by performing prophylactic aortic root replacement for growing aneurysm or reconstructive surgery of other heart valves To protect women from aortic complications when they plan pregnancy To improve life quality by avoiding anticoagulation, artificial prosthetic noise, or unnecessary cosmetic impairment |
Emergency replacement of the aortic root using composite- graft replacement with a mechanical valve or valve-sparing root replacement techniques (favor for David technique119) when aortic dissection or rupture occurs Prophylactic aortic root replacement using the reimplantation technique according to David118 Mitral valve repair surgery Participate in cardiologic post-surgical follow-up visits |
| Vascular surgeon/vascular interventionist | To rescue life when aortic dissection or rupture occurs (type B) To protect against rupture of the descending thoracic or abdominal aorta, or both To protect against organ malperfusion from aortic dissection or vascular embolism |
Open surgery or endovascular treatment of aortic aneurysm (prophylactic), dissection (acute and chronic), or rupture of the thoracic or abdominal aorta Treatment of vascular complications of main aortic branches |
| Orthopedic surgeon | To enable and support professional and private living arrangements To convince parents of the importance of regular sporting activities To support and instruct orthopedic colleagues, who less frequently are confronted with MFS patients |
Imaging of the spine Podiatrist care, “short foot exercise” Preparticipation evaluation (PPE) before athletic participation Conservative and operative treatment options to both children and adults with scoliosis or with protrusive hip arthritis |
| Ophthalmologist | To assess ophthalmic diagnostic criteria of MFS To provide a reliable statement on the ophthalmic prognosis and treatment options To implement and improve new ophthalmic screening techniques |
Basic ophthalmological examinations: distance corrected visual acuity, intraocular pressure measurement, documentation of pupil centration (miosis), slit-lamp examination to determine iris stromal atrophy and dilated funduscopy of the retina and thorough lens position and zonular status determination New techniques: corneal topography, tomography, and dynamic in vivo curve analysis |
| Nurse | To provide whole-person-perspective-care comprising five dimensions, ie, physical, psychological, sociocultural, development based, and spiritual dimension | Strengthen the individual patient’s daily self-care activities Networking between specialized departments to provide individualized care Education to make patients experts of their own disease |
| Auxiliary disciplines | ||
| Forensic pathologist | To determine the cause of death in persons who died outside the clinical setting To identify possible treatment failures including misdiagnosis and malpractice To identify genetic causes of death such as GAS To initiate family member support |
Autopsy of all persons who die of unclear cause outside a hospital setting Perform genetic testing in deceased persons with aortic disease and a risk for GAS Genetic counseling of family members of the deceased person |
| Radiologist | To assess diagnostic criteria of MFS To identify aortic and vascular complications of MFS or other GAS including aneurysms of cerebral-, carotid-, visceral, and peripheral arteries. To specify chronic aortic and vascular pathology as aneurysm, tortuosity, dissection To identify acute aortic syndromes (AAD, IMH, PAU) and vascular complication including the rupture and organ malperfusion |
Tomographic imaging of the entire aorta (index and follow-up CT or MRI scans) Tomographic imaging of the dura as diagnostic criteria of MFS Cranial radiographs (craniofacial characteristics), Conventional chest radiography and CT (lung emphysema and pneumothorax) |
| Pulmonologist and sleep specialist | To identify emphysema, pneumothorax, and restrictive lung disease (from skeletal deformities) and to prevent or treat pulmonary complication To identify and treat individuals with sleep apnea to improve cardiovascular prognosis |
Counseling on potential restriction in physical activities Pulmonary function testing Chest radiography or CT Polygraphy to screen for OSA/CSA CPAP therapy |
| Rhythmologist | To identify patients at risk for SCD, to stratify such risk, and to initiate preventive therapy, where indicated | Methods for risk stratification of SCD: TTE: myocardial dysfunction? Aortic regurgitation? Mitral valve prolapse? Mitral valve regurgitation? 12-lead resting ECG: PVCs? 24-hour-Holter ECG: PVCs >10/h, non-sustained and sustained VTs, abnormal heart rate turbulence (TS and TO abnormal) NT-proBNP serum levels: elevated (>200–600 pg/mL) Genetics: FBN1-mutations within exon 24–32? |
| Orthodontist/dentist | To identify dental and skeletal class II configurations, joint hypermobility To prevent temporomandibular joint dysfunction and condylar resorption To identify and prevent periodontal inflammation to reduce the risk of IE To prevent reduced chewing efficiency, lip incompetence and craniofacial dysmorphology |
Bite correction and regulation of craniofacial growth in childhood and in adolescence Diagnosis of temporomandibular joint dysfunction If required prescribe physiotherapy Professional tooth cleaning at regular intervals to reduce periodontal inflammation Prescribe myofunctional therapy to achieve lip competence and to strengthen the orofacial muscle |
| Neurologist | To assess neurologic diagnostic criteria to establish the diagnosis of MFS To prevent or identify cardioembolic stroke, cervical and vertebral artery dissections, and intracranial aneurysms subarachnoid hemorrhage, especially in LDS To consider GAS in young individuals with stroke and cervical artery dissection |
Neurological examination Regular neurovascular imaging in LDS Interpretation of accentuated vertebral and carotid artery tortuosity Neurosurgical and endovascular treatment of cerebral aneurysms Acute treatment and secondary prevention of stroke |
| Obstetric surgeon | To allow mothers and families to make an autonomous decision on family planning and pregnancy To prevent or to manage complications of pregnancy in mother and child successfully |
Counseling for family planning and pregnancy in terms of risks of mother and child TTE prior to, during and until 3 months after pregnancy Obstetric board meetings during pregnancy Emergency planning in high-risk pregnancies |
| Psychologist | To reduce the burden of anxiety, trauma, feeling of stigmatization To improve coping with MFS by strengthening the patients’ self-confidence |
Establish a solid, trust-based patient-therapist-alliance Identify body image disorders, family conflicts, accidental risk behavior, sex-specific aspects |
| Rehabilitation specialist | To achieve the best possible support of the patient’s capacities with respect to biological, psychological, and social aspects | Formulation of patient’s individual rehabilitation goals to make rehabilitation plan Provide specialized education Daily bicycle ergometry, gymnastics, fitness training, and nordic walking units to overcome patients’ uncertainty regarding their physical abilities Psychological counseling Relaxation training Counseling for job-related issues and dietary counseling |
Abbreviations: AAD, acute aortic dissection; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin-receptor blockers, BAB, beta-adrenergic blockers; CPAP, continuous positive airway pressure; CSA, central sleep apnea; CT, computed tomography; ECG, electrocardiography; GAS, genetic aortic syndromes; IE, infective endocarditis; IMH, intramural hematoma; LDS, Loeys-Dietz syndrome; MFS, Marfan syndrome; MRI, magnetic resonance imaging, NT-pro-BNP, N-terminal probrain natriuretic peptide; OSA, obstructive sleep apnea; PAU, penetrating therosclerotic ulcer; PVCs, premature ventricular contractions; SCD, sudden cardiac death; TTE, transthoracic echocardiography.
In the “Team member” section, we focus on team members, where we provide the personal accounts of key team members from each core discipline comprising the team of coordinators, pediatrician/pediatric cardiologist, cardiologists, human geneticist, heart surgeons, vascular surgeons, orthopedic surgeons, ophthalmologists, and nurses and from ancillary disciplines comprising forensic pathologists, radiologist, rhythmologist, pulmonology/sleep medicine, orthodontist/dentists, neurologist, obstetrician, psychologist, and rehabilitation specialist.
In the “Meaning” section, we focus on meaning, where we elucidate the interdependency of team member, structure, and meaning and its significance for medical strategies that seek to maximize therapeutic success for patients with MFS.
Structure
History and presence of multidisciplinary health care for MFS
The American model
By Reed E Pyeritz
Comprehensive care for individuals and families with rare disorders has diverse origins, compositions, and financial support. Most will have their origin within an academic medical center, and research and education will be important components. Before considering the typical characteristics of programs for MFS and related disorders, it will be instructive to consider how clinics for other rare disorders arose and are supported.
Cystic fibrosis (CF) and muscular dystrophy are two disorders that exemplify how to organize, accredit, support, and sustain comprehensive clinical services. The CF Foundation in the US supports >120 care centers nationwide, includinĝ100 that include services for adults living with CF.16 Each CF care center must adhere to diagnostic and management guidelines established by the professional advisors of the foundation and must provide data that can be utilized for research. Annual accreditation occurs and periodic site visits insure that high standards of service are maintained. For the diagnosis and management of a wide range of neuromuscular conditions, >150 Muscular Dystrophy Association (MDA) Care Centers exist in the US, each supported financially to some extent by the Muscular Dystrophy Association. Each has a cadre of health professionals, including physicians of various specialties, dieticians, genetic counselors, physiatrists, physical and occupational therapists, speech pathologists, and social workers. A health care service coordinator is a central figure in each clinic.17
Most other disorders have not had the benefit of such organizational support. For example, the Hereditary Hemorrhagic Telangiectasia (HHT) Foundation was a grass-roots organization with a strong medical director who established the criteria for a comprehensive center in the late 1980s. Subsequently, any medical center anywhere in the world that was interested in establishing a center with the imprimatur of the HHT Foundation needed to invite the medical director for a site visit. He would meet with the individual specialists (interventional radiology, cardiology, gastroenterology, otorhinolaryngology, medical genetics, and hematology) before giving his blessing that the center could be established and advertised on the HHT Foundation (now called Cure hht) website. More than 40 such centers now exist internationally.18 However, there was never any intention to provide monetary support for the centers nor has there been any ongoing attempt to insure that a center has persisted in providing both comprehensive and quality care.
Organizations devoted to a rare disorder continue to be needed and typically arise through the efforts of one or a small group of committed individuals. A recent example is the disease research organization for Castleman’s disease, a localized or systemic disorder of lymphadenopathy. One serious form, involving systemic inflammation and termed idiopathic multicentric Castleman’s disease, affects one of my colleagues. Largely through his efforts, the Castleman Disease Collaborative Network was established to foster an international effort to both understand the pathophysiology of the condition and develop targeted therapies.15
The first comprehensive clinic for MFS arose at the Johns Hopkins Hospital under the direction of Dr Victor McKusick in the late 1960s. Because of the large number of patients who traveled to Hopkins for diagnosis and management, such as they were in those days, a diverse group of medical specialists became involved, conducted clinical research, and to a large measure established their academic careers based on MFS. Collaborations among the members of the Hopkins clinic and colleagues elsewhere produced clinical trials that validated both medical (beta-adrenergic blockade) and surgical (prophylactic aortic root repair) therapies that have directly led to increased life expectancy. Furthermore, these collaborators discovered the first mutations in FBN1 in patients with classic MFS.19 We now recognize that the patients defined as having MFS in the 1960s and 1970s actually included many with a variety of conditions that can be differentiated today on both clinical and genetic grounds. The National Marfan Foundation was established in the late 1970s in Baltimore and eventually became established in Port Washington, Long Island, New York. It also was a grass-roots organization with a medical advisory board that strongly contributed to the development and implementation of its tripartite mission of medical care, stimulating and sponsoring research, and support of patients and their families. As conditions such as LDS, Shprintzen-Goldberg syndrome, the many forms of familial thoracic aortic aneurysm and dissection, and other disorders could be differentiated from MFS, the National Marfan Foundation (now called simply the Marfan Foundation)20 and its equivalent societies internationally broadened their missions to include these related disorders. For example, the Canadian Marfan Association is now called Genetic Aortic Disorder Association Canada21 with seven comprehensive clinics spread across that country.
Hamburg Marfan center for adults
By Yskert von Kodolitsch
The 4th International Symposium on the Marfan syndrome in Davos in 1996 marked the starting point for German clinics to adopt the Hopkins model of multidisciplinary care patients with MFS. The story started with a stroll through Davos with Professor Dr Yskert von Kodolitsch, Dr Victor McKusick, a dinner with Dr Reed Pyeritz resulting in a postdoctoral research project in his clinic for MFS in Pittsburgh, a conversation with Dr Michael Raghunath who did basic research on connective tissue diseases in Münster, and a train ride from Davos back to Hamburg with Marina Vogler from the patient self-support group Marfan Hilfe Deutschland. This group of people founded the Marfan Center in Hamburg. Two years after Davos, the Hamburg Marfan center published the first German report on strategies of multidisciplinary care for MFS.22
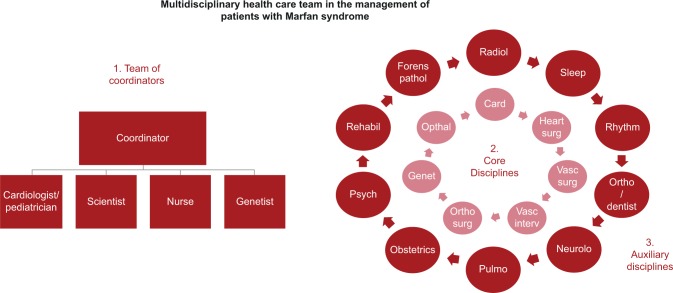
Figure 2 provides a sketch of the structure of a typical multidisciplinary care team, that still has a similar structure as in 1998. The Hamburg Marfan center consists of three components: 1) the team of coordinators, where a cardiologist, a scientist, a nurse, and a geneticist work together. Their task is to coordinate the actions of all other members of the team; 2) those physicians who are involved in the diagnostics and therapy of cardiovascular disease manifestations represent the core disciplines of care, whom all MFS patients consult in their ambulatory visits. Usually, we make decisions on overall diagnosis of MFS and on therapy jointly with the patient and physicians form these core disciplines. We only discuss complex diagnostic questions in our Marfan board and complex therapeutic questions in our aorta board;23 3) we designate as auxiliary disciplines those physicians who do not participate in decision making directly, like the radiologists, or who stand in only when special clinical problems occur, like obstetric surgeons and neonatologists. In our routines, many of these colleagues collaborate intensively with the core team to assess the diagnostic criteria of MFS, but they provide their specialized care more or less autonomously of the core team when it comes to specialized organ treatment, like the ophthalmologists or orthopedic surgeons.
Figure 2.
The structure of the Hamburg Marfan center.
Abbreviations: Card, cardiology; Forens pathol, forensic pathology; Genet, genetics; Heart surg, heart surgery; Neurolo, neurology; Opthal, opthalmology; Ortho, orthodontology; Ortho surg, orthontic surgery; Psych, psychology; Pulmo, pulmonology; Radiol, radiology; Rehabil, rehabilitation; Vasc interv, vascular intervention; Vasc surg, vascular surgery.
Together with the “Marfan Hilfe Deutschland” (German Marfan Organization), we supported colleagues in other German cities to set up their own multidisciplinary health care teams. Through the years, we refined our basic model along with new insights from science and technology, with new needs promoted by our patients, and with new regulations. Usually we publish about important changes that affect the structure of our center, such as the new role of molecular genetics for clinical decision making,24 the effects of new legal directives on health care that affect our patients with MFS (§116b SGBV),25 the shift from treating complications to preventing complications,26 and the shift from improving survival to improving quality of life.27
Hamburg Marfan center for children
By Thomas S Mir
In 2008, we established a pediatric outpatient department in Hamburg dedicated to children with MFS and related disorders. Whenever our patients reach adulthood, they are led over to the well-established Marfan consultation, in a structured fashion. A timely confirmation of the disease is of significant importance not only for medical reasons but also for the patients’ further life planning. We arrange ophthalmological examinations, treatment by pediatric experts and orthopedic examinations and treatment that account for the specific dynamics of the various organ manifestations of MFS in childhood. There is a lively exchange between our two clinics for MFS so that we can ensure optimal support not only for the individual but for whole families.
In our experience, specialized care for pediatric MFS is important for six reasons. First, the extensive initial examination and consultation with the aim to establish a definitive diagnosis requires pediatric expertise that can account for the age-specific presentation of various organ manifestations. Second, periodical evaluation with timely indication and dosing of medical prophylaxis requires specific insight in the age-dependent dynamics of organ growth, and dosage of medication. Third, specialized heart and blood vessel ultrasound requires the experience of a pediatric cardiologists, and children can undergo magnetic resonance imaging (MRI) or computed tomography (CT) only with the support and surveillance of pediatric experts. Fourth, the pediatrician is able to account for the specific psychological and social situation of children and of the specific guidelines, regarding physical activity restrictions and lifestyle modifications in children as compared to adults.28 Fifth, the assessment of motor milestones, cognitive, and social skills and how they develop in time are essential for appropriate management of children with MFS. Sixth, specialized care for neonates is important in severe variants of MFS or LDS.
Consultation of individuals with MFS exemplifies how rare and complex diseases can be cared for through the participation of various medical disciplines. This has an extremely positive effect on the adherence, prognosis, quality of life and, eventually the future of the children.29,30
Marfan Hilfe Deutschland (German Marfan Organization)
By Marina Vogler
The development of Marfan Centers in Germany, as well as in other European countries, began in the late 1990s, in Germany in the aftermath of the International Marfan Symposium in Switzerland 1996. Patients and scientist began to discuss their needs, aims, and special views on MFS. Improvement in the cooperation with experts was a main goal, as described for several other patient organizations in the SHILD study recently.31 Marfan organizations were arguing for a multidisciplinary care for patients with MFS, and the first step was to create a strategy to bring medical experts together in a network.22 The second step was to convince people that even a long journey is worth the effort, when medical competence is available. This was the duty of patient organizations and is an ongoing process.
The main needs of patients with MFS today remain similar to those that we faced at the end of the 1990s. They want a clear diagnosis, competent care in routine checkups as well as in surgery, and a competent estimate for the personal health future.32 In addition, nowadays the priorities of patients include the wish to discuss their condition on equal footing with their health care specialists. Patients are well educated about medical treatment and scientific innovation. Many of them have become experts on their condition. Although aortic disease is of course the main point, people are very interested in issues influencing their quality of life. This explains why affected people are attracted to seminars like “Healthiness with Marfan syndrome” or “40-Years-Plus – Aging in Marfan syndrome” much more than by pure medical information. Setting up the first rehabilitation course for people with MFS in Germany in 2014 is more evidence for the development toward practical help in daily life. For the future of Marfan Centers it could assist patients with MFS in many ways. This seems nearly as utopic today, as the idea to establish Marfan Centers in Germany seemed 20 years ago.
A brief email questionnaire in 2016 asked 402 patients what their most important aspect concerning a Marfan Center was. Of these patients, 370 had MFS, 15 LDS, six related diseases, and eleven had suspected MFS. A total of 77 individuals answered (19%), including 21 men and 56 women. The most appreciated items were competence of the medical team (33.8%), multidisciplinary care (29.9%), and trusting the doctor, overcoming fear, explanations (15.6%). There was a difference between men and women in competence (47.6% of men and 28.6% of women) and multidisciplinary care (14.3% of men and 35.8% of women).33 The answers corroborated the long-term experience of the Marfan Hilfe team.34
Organizational structures
By Yskert von Kodolitsch
Most persons who participate in the multidisciplinary health care of patients with MFS are employees of the university clinic, working in clinics such as cardiology or heart surgery, and they have many professional obligations other than caring for patients with MFS. Moreover, our center for patients with MFS is part of other organizational structures of the clinic. Most importantly, our center is integrated into the German Aorta Center Hamburg (DAZH), which was founded in 2012 to provide multidisciplinary care for persons with complex thoracic and abdominal aortic disease.15,35 In this center, we hold weekly board meetings where heart surgeons (CD and AMB), vascular surgeons (SD and AL-A), endovascular specialists (TK), and Marfan specialists (YvK, HS, and MR) discuss therapeutic strategies for patients with complex aortic conditions including patients with MFS.23
Of similar importance, in 2013 the university board of directors integrated Marfan care into the Martin Zeitz Center for Rare Diseases (MZCSE).35 Our former director of the university clinic, Dr Martin Zeitz founded the MZCSE in compliance with criteria formulated in the German Nationaler Aktionsplan für Menschen mit Seltenen Erkrankungen [National plan of action for people with rare disease] (NAMSE).36 According to these criteria, German centers for rare diseases have a three-staged center structure, where A-centers are located at a university clinic and coordinate the action of B-centers that are also located at the university and that provide specialized care for certain diseases such as MFS. A-centers network with rare disease centers on a national and European level, they have patient coordinators (CW-N) who direct individuals with uncertain diagnosis to specialists in B-centers, and the B-center doctors train physicians for health care for patients with rare diseases. It has been proposed that B-centers could collaborate in a network of C-centers that provide local care for single rare diseases or rare disease groups. Such C-centers comprise specialized medical private practices, medical care centers, or regional hospitals.
We involve other multidisciplinary health care teams for pregnancy,37 care for the neonate with MFS and for rehabilitation of MFS (DB). Beyond these organizational structures, there are a couple of managerial structures and practices, which include external quality assurance, quality management systems, and structures for patient-centered care. We list and briefly explain these in Table 1.
Medical standards
By Yskert von Kodolitsch
Medical strategy aligns interventions to maximize the overall therapeutic success, whereas medical tactics deal primarily with the proper delivery of specific interventions.13 Accordingly, although strategy requires an openness to different choices and individualization according to patients’ utilities and values, tactics require standardization with clearly-defined routines, to overcome variation that hinders successful delivery of diagnostic or therapeutic interventions. Therefore, clear medical standards including current knowledge on the evidence basis of health care practices for MFS and a clear standardization of medical procedures are indispensable groundworks to maximize therapeutic success (Figure 1).38 In Table 2, we list the medical goals for each discipline according to the suggestion of colleagues of each discipline. In addition, the major medical methods and procedures needed to achieve these goals are listed (Table 2).
Team members
Core disciplines
Team of coordinators
By Yskert von Kodolitsch, Meike Rybczynski, Kerstin Kutsche, and Georg Rosenberger
In general, the task of coordinators is to organize and coordinate the actions of persons in the structures of the Marfan center to maximize the overall therapeutic success for the patient. More specifically, coordination requires action on three different levels.
First, on the level of each medical discipline, one or two physicians coordinate actions in their respective medical expertise and they take responsibility for communication with the centers patient care coordinator.
Second, patient care coordinators take responsibility for the entire treatment for individual patients with MFS, usually as partner and counselor, over many years. The coordinating physicians (MR, HS, and TSM) have an integrative understanding of both the medical standards of care for patients with MFS in all medical disciplines, and knowledge of all three dimensions of the patient’s individual character comprising physical, sociological, and psychological aspects.13 The Marfan center does not have certified training schedules. However, it provides personal mentoring, continuous feedback, and training on the job. In addition, personal expertise is augmented by contribution to scientific talks and papers, especially by writing review articles, where each author studies all available scientific literature. These coordinators integrate multidisciplinary care among specialists both within the center and outside the center including the actions of general practitioners and other outpatient caregivers. The coordinating nurse usually knows all individual patients best, with knowledge of many aspects of their overall life (BN). She plays an important role in the organization of appointments and in supporting patients in the myriad of challenges of daily life, and she therefore breathes life into the aspect of “whole patient care”.
Third, at the level of the Marfan center, corresponding to the level of B-center coordination within MZCSE, coordinators take responsibility for team members, structure, and meaning of the center as a whole. This includes recruitment of team members for medical disciplines, the integrity and continuous adaption of organizational structures of the center and medical standards of care, the smooth interaction between disciplines, levels of care, other health care structures inside and outside the university, outpatient health care structures, and coordination of research activities; cooperating closely with the university departments of strategic management (GO) and quality management (SN) to keep abreast with latest managerial technology.
Pediatrics/pediatric cardiology
By Thomas S Mir
Mutations in FBN1 are associated with a wide phenotypic spectrum ranging from classic features of MFS presenting in childhood and early adulthood to severe neonatal presentation with rapidly progressive disease. Tinkle et al have pointed out that crucial signs of Marfan syndrome including ectopia lentis, aortic dilation and dural ectasia manifested in an age-dependent manner, where many children and adolescents therefore do not fulfil formal diagnostic criteria and are often described as having “potential” Marfan syndrome.39
In pediatrics, specific attention is given to the monitoring and follow-up of growth and development, skeletal features such as the dynamics of pectus deformity, ocular symptoms, pulmonary airway, integument, dural ectasia, dental features, and physical activity. All children with the suspicion or diagnosis of MFS should be followed by a pediatric cardiologist familiar with MFS due to the wide phenotypic spectrum regarding the cardiovascular features.29,30,40,41
Because MFS can affect the very young and continues to manifest and progress throughout adolescence and adulthood, it is important that pediatric patients with MFS are recognized as people and that they can find a medical “home”. The care needs to be coordinated among the various specialities, especially during the period of transition from adolescence to adulthood.29 During the process of transition, changes in the role of patients from child to adult have to be managed, but continuity of care and familiar reference persons are equally important. Therefore, in the period of transition, the pediatric and adult cardiologist talks jointly with the patient and his or her family.
As early as in childhood, extensive therapeutic decisions may be necessary. This particularly concerns orthopedic and ophthalmologic issues from an operational viewpoint, and also pharmacological issues. Early diagnosis and rigorous follow-up can help to prevent ocular and cardiac complications in pediatric MFS. Typical findings of the aorta, and the heart valves are detectable from early childhood. Enlargement of the aorta is present in 85% of children with MFS, with consecutive regurgitation of the aortic valve in many children. Most of these children are medically treated with beta-adrenergic blockers (BABs) or angiotensin-receptor blockers to delay the progression of the disease, since the principle applies here, that the sooner the treatment begins, the longer threatening complications can be delayed.40
Most importantly: the multidisciplinary approach provides more precise data for diagnosis and possible phenotype–genotype correlations.41 Integration into school, physical education, and early career choices have to be considered and navigated by the pediatrician. Which sports should we recommend to individuals with MFS, how about their capacity in school and work life? MFS affects each individual differently and has a significant effect on daily activities and perceived quality of life in a different amount.29,41
Molecular genetic examination and human genetic consultation should be carried out early whenever indicated. This is especially important when clinical manifestations of MFS remain unclear. In individuals with “potential MFS”, genetic tests may provide early confirmation of the disease. In situations where testing children or other persons who are not able to give informed consent is considered, those individuals should be involved in genetic counseling and in the decision-making process, according to their capacities.
Cardiology
By Yskert von Kodolitsch and Helke Schüler
The cardiologist takes responsibility for the Marfan patients’ entire scope of cardiovascular health. Therefore, the cardiologist integrates information form diagnostic disciplines such as genetics and radiology into a comprehensive clinical syndrome diagnosis and estimates the prognosis of cardiovascular manifestations. Moreover, the cardiologist supervises and coordinates the actions of colleagues from heart surgery, vascular surgery, cardiac rhythmology, pulmonology, sleep medicine, and rehabilitation to integrate these into a comprehensive therapeutic strategy. The cardiologist also plays a central role in the counseling and management of cardiovascular risks of pregnancy. The overarching goal of the cardiologists’ actions is to predict, prevent, retard, or treat aortic aneurysm, mitral or aortic valve regurgitation or endocarditis, and myocardial dysfunction. In addition, the cardiologist counsels the patient in the difficult balance of cardiovascular risks with personal needs including family, leisure time, and professional plans and activities.42
Human genetics
By Kerstin Kutsche
MFS is an autosomal dominant disorder with 50% recurrence risk. The majority of patients inherited the causative mutation from a parent, yet 25% have a de novo mutation. Genetic testing of individuals with suspected MFS is important to molecularly confirm the clinical diagnosis by identifying the disease-causing mutation.43 Genetic diagnostics significantly contributes to the classification and individualized care of patients as MFS belongs to a group of connective tissue disorders associated with cardiovascular manifestations including LDS, familial thoracic aortic aneurysms and aortic dissection (TAAD), and the vascular type of Ehlers–Danlos syndrome (type IV).44–46 Initial genetic counseling is indicated to inform, eg, about the purpose of the test, the inheritance pattern, and recurrence risk, as well as the reliability and limitations of the test. It seems of specific importance to inform that 1) genetic screening of an index case does not allow to exclude a genetic form of disease and 2) the identification of a causative mutation does not allow to exactly predict the individual degree and onset of symptoms. After adequate counseling, the patient must give written consent to the analysis; authorization for genetic testing of children is required from their parents or legal representatives.
In a patient with strong clinical suspicion for MFS, mutation scanning of the FBN1 gene, including direct sequencing of all coding exons and deletion/duplication analysis of single/multiple exon(s) by, eg, multiplex ligation-dependent probe amplification (MLPA), is recommended.41 In cases without such clear suspicion, a next-generation sequencing-based approach should be applied for simultaneous testing of “core genes” and “additional genes”. The recent core gene list for hereditary nonsyndromic and syndromic TAAD has been established by Arslan-Kirchner et al47 and includes ACTA2, COL3A1, FBN1, FLNA, MAT2A, MFAP5, MYH11, MYLK, NOTCH1, PRKG1, SMAD3, TGFB2, TGFB3, TGFBR1, and TGFBR2. Identified variants should be evaluated for their presence in mutation databases, such as Human Gene Mutation Database (http://www.hgmd.org/) or ClinVar (http://www.ncbi.nlm.nih.gov/clinvar/) and single-nucleotide polymorphism databases, such as the dbSNP Database (http://www.ncbi.nlm.nih.gov/snp/) or the Exome Aggregation Consortium (http://exac.broadinstitute.org/). If no disease-causing mutation is identified, MLPA of genes for which a commercial kit is available, should be performed. By simultaneous sequencing of the 15 core and possible additional genes and/or MLPA, a faster diagnosis has been demonstrated.48–50 This is of particular importance as clinical differentiation between the overlapping phenotypes is challenging. Molecular diagnostics is important to classify the TAAD condition and adjust the follow-up and therapeutic scheme accordingly. For example, in young individuals with a causative TGFBR1, TGFBR2, or COL3A1 mutation early prophylactic surgery and pharmacological therapy is recommended.51,52 However, a positive test result does not allow a statement on the individual prognosis due to the high intra- and interfamilial clinical variabilities. In case of mutation identification, genetic recounseling must be offered. The identification of a disease-causing mutation in the index patient allows testing of affected and unaffected (predictive testing) family members. If the known family mutation is absent in a clinically unaffected relative, he or she can be released from diagnostic monitoring unless otherwise indicated. Finally, although prenatal diagnostics are available, the Hamburg Marfan center multidisciplinary care team did not receive any request on prenatal diagnostics to date.
Heart surgery
By Christian Detter and Alexander M Bernhardt
It is well known that poor life expectancy in MFS is mainly triggered by cardiovascular complications such as acute aortic dissection or rupture. In most cases, aortic root dilatation is the predominant aortic manifestation.42 Thus, careful serial imaging of the aorta by TTE and tomographic imaging is important to evaluate aortic root diameter and descending aorta as well as aortic and mitral valve function.
To avoid acute aortic syndromes in MFS, prophylactic surgery of the aortic root should be performed when indicated according to the recommendation of the American Heart Association and European Society of Cardiology guidelines.53 Furthermore, the operative risk of a planned procedure is very low in this young patient group. Compared to high early mortality in an acute setting, both early and late mortalities improve significantly with prophylactic intervention. With prophylactic aortic surgery and customized multidisciplinary expert care, MFS patients have a close-to-normal life-expectancy.54
Although composite-graft replacement with a mechanical valve is still recommended, most studies show excellent results using valve-sparing root replacement techniques such as the reimplantation procedure according to David and the remodeling technique according to Yacoub. David et al51 reported excellent freedom from reoperation at 15 years and 18 years of 94.8%. Also both techniques show good long-term results, the David procedure demonstrates higher freedom from significant long-term aortic insufficiency in MFS because the aortic root and annulus are more stabilized. Thus, current evidence is in favor of the David rather than Yacoub technique in pathologies such as MFS. Due to the excellent durability and valve function of aortic valve sparing in MFS, the David technique is the Hamburg Marfan center’s preferred technique and the main surgical goal in this young patient cohort.52 Further improvements are much higher patient satisfaction because anticoagulation therapy is not required and valve-related complications are rare. This is particularly important for younger women, who are planning to be pregnant. As prophylactic aortic surgery is a planned procedure, detailed informed consent is required in this young patient cohort. Usually, patients with MFS are well informed and connected. Thus, the dialog should be very informative and comprehensive to alleviate the fears about the surgical procedure, and to support the MFS patients and their families.
Vascular surgery/vascular intervention
By Axel Lerena-Avellaneda, Tilo Kölbel, and E Sebastian Debus
While arterial aneurysms and aortic dissection play a major role in morbidity of patients with MFS, peripheral occlusive disease is practically nonexistent in these cases. Therefore, vascular repair in MFS is focused on aortic surgery, which has undergone a dramatic technical change since the early 1990s. Generally, nowadays, most patients are treated with endovascular procedures. While the typical aneurysm patient will profit from this minimally invasive operation in regard to mortality and morbidity, this may not apply for patients with MFS.23,55
While in the beginning, stent-grafting was advocated in patients with MFS, it soon became evident that the endoleak and secondary intervention rates were much higher than in patients without MFS.56,57 The reason is presumably the fixation of the vascular prostheses in the aortic vessel. While in open surgery, the alloplastic material is sewn to the artery, the endoprostheses are attached by a stent with radial force. Due to the connective tissue weakness, the stents tend to dilate the artery, and besides dilation and insufficiency of the anastomosis, the stents may rupture the vessel.
Since patients with MFS are considerably younger than the average patient with aortic aneurysm, the results of the open procedures are remarkably good, even in thoracoabdominal aneurysms.58 In acute type B dissection, stent-grafting remains the procedure of choice, as the open operation remains difficult because of the fragility of the arterial wall and the risk of paraplegia. The risk for type B dissection in MFS is considerable (6%–34%), with increasing risk after surgery in the proximal thoracic aorta and dilation of the descending thoracic aorta.59
MFS is a rare disease, and most vascular surgeons are not used to finding themselves confronted with these challenging patients. Furthermore, as these patients can be followed over years, the vascular situation after dissection may get very complex over time, and individual approaches regarding all open and endovascular techniques are necessary. Especially thoracoabdominal or arch chronic false lumen aneurysms in patients after operation for acute type A dissection are challenging.
Standard vascular techniques may not always be applied, so a special strategy seems to be mandatory to obtain good long-term results. Therefore, in participating in the MFS center, vascular surgeons had to adopt our treatment pathways to the characteristics of vascular connective tissue disorders. The availability of a weekly “aortic conference”, in which an experienced team of cardiologists, heart surgeons, and vascular specialists attends is the perfect modality to tailor the ideal strategy.23 Staged hybrid procedures may be planned: to achieve stable landing zones for subsequent minimal invasive stent-graft implantation, the aortic arch may be repaired using the “frozen elephant technique”. The infrarenal and iliac aneurysms are operated in open manner to obtain a good distal landing zone. If the patient bears a very low general risk, total open repair is planned.
Orthopedic surgery
By Malte Schroeder
Although MFS-related musculoskeletal symptoms in general would not be life-limiting, they have a great impact on professional and private living arrangements, participation in sporting activities, and the self-perception of the patient’s body. The multiple manifestations of the disease of the thorax (pectus excavatum and carinatum), the spine (scoliosis or kyphosis), the joints (hyperlaxity), and deformities of the foot require continuous orthopedic treatment. Thus, the idealized requirements of an orthopedic surgeon as part of a multidisciplinary Marfan network include being an extensively trained podiatrist who routinely treats different clinical stages of protrusion hip arthritis, who is familiar with sports performance diagnostics in patients with cardiovascular diseases, and who is able to offer a wide array of conservative and operative treatment options to both children and adults with scoliosis. A single individual can certainly not fully cover this idealized demand profile. Furthermore, the idealized orthopedic surgeon should support and instruct orthopedic colleagues, who are confronted with MFS patients less frequently.
Regarding the commonly occurring spinal deformities in MFS, special attention is required in times of accelerated skeletal growth where clinical, and possibly also radiological controls, are mandatory to monitor potentially relevant changes of scoliosis or kyphosis. Particularly during puberty, the possibly impaired self-perception of the body could prevent young patients from taking part in physical activities. Disruption of body image also affects emotional and relational aspects including restrictions in the participation in physical activity. The treating orthopedic surgeon should try to convince patients and their parents of the importance of regular sporting activities. Recommended physical activities should be of low or medium intensity and without, or with limited, physical contact. Activities that could lead to an increase in systolic blood pressure >160 mmHg or recurrent valsalva maneuvers must be avoided in patients with MFS, weightlifting, contact sports, and basketball and soccer at performance level, should not be performed. Recommended sports include hiking, cycling, golf, roller-skating, snorkeling, and youth soccer.
Regular sporting activities can not only improve muscular strength and the cardiopulmonary system but also should in particular be considered as an essential part of normal life. Preparticipation evaluation should be performed before athletic participation is permitted in patients with MFS, and should be repeated at least annually.60
Stabilizing orthotics should only be prescribed when pronounced deformities or instabilities, especially hind foot deformities, are diagnosed. The importance of the intrinsic foot muscle function can hardly be overstated. The recently described foot core system has introduced a new paradigm for understanding the movement and stability of the foot arch.61 Parallels between the lumbopelvic core and the intrinsic foot muscles should be taken into account regarding normal foot and lower extremity function. Therefore, exercises such as the “short foot exercise” should be an integral part of the everyday routine in every patient with forefoot deformity and particularly in patients with MFS.61
Finally, some remarks should be made regarding the possibility of chiropractic treatments. Despite individual reports in the literature that cervical manipulation was performed without dramatic consequences in patients with MFS presenting aortic aneurysms, chiropractic manipulation techniques should be declined. Rather, the manual medicine repertoire offers a variety of other treatment options to successfully treat the manifold musculoskeletal manifestations in patients with MFS.
Ophthalmology
By Stephan J Linke and Bettina Fuisting
The role of the precise ocular status in diagnosing and defining progression of MFS has gained prominence with the revised Ghent criteria, as the presence of EL with aortic dilation (Z-score ≥2) is currently sufficient for diagnosis.56 How much help can the ophthalmologist be in diagnosing mild or atypical MFS, or evaluating its progression? The aim of the ophthalmologist’s clinical examination and diagnostic screening process should be threefold.
First, to guarantee a reliable clinical statement for the patient: can the vision be improved by spectacles or contact lenses? Is lens surgery indicated, and if yes which is the best way to perform intraocular surgery with stable lens implantation? Due to high myopia prevalence in MFS, a thorough funduscopy is warranted to detect subtle changes in the retina and prevent retinal detachment.
Second, to provide information on established nosologic criteria for diagnosing MFS including EL and myopia >−3D.
Third, to implement and improve new ophthalmic screening techniques in close cooperation with experts from the related subspecialities under guidance of the cardiologist. These include corneal topography and tomography detecting subtle changes in the cornea. In vivo confocal microscopy may help to reveal abnormal deposits in the pre-Descemet region of the cornea. Last, but not least, new dynamic in vivo curve analysis of the cornea to analyze biomechanical changes may broaden the screening armamentarium.
In the patients’ interests, a coordinated and straight forward clinical examination of all involved subspecialities is warranted. Regarding the eye and vision of the patient, a basic ophthalmological examination should include distance corrected visual acuity, intraocular pressure measurement, documentation of pupil centration (miosis), slit-lamp examination to determine iris stromal atrophy, dilated fundus examination of the retina, and thorough lens position and zonular status determination. The cardinal feature of EL is currently evaluated by slit-lamp examination under complete pupillary dilation (cyclopentolate 10 mg/mL and phenylephrine 100 mg/mL). EL was classified as subluxated when only a subtle posterior disclocation was seen. The EL was classified as luxated when the lens had any displacement (most commonly superotemporally). An estimated 40% of individuals with MFS do not have subluxated lenses.57
Submaximum pupil dilation in some patients with MFS and subjective EL estimation limits the discriminant capacity of this approach. The potential for ocular abnormalities to aid in diagnosis of MFS may be much greater than currently acknowledged due to aforementioned modern screening techniques. Investigation of biomechanical and dynamic changes in tissues that contain abnormal FBN1 is a reasonable next step in refining the approach to diagnosing MFS.62,63
Nursing
By Barbara Napp
Ultimately, the same is true in the nursing care of patients with MFS as for all patients, the care perspective must include the whole person, that is to say it contains five dimensions: physical, psychological, sociocultural, developmental, and spiritual. There are several international science-based nursing concepts that address these dimensions.64 For example, the “activities of daily living” that aim to strengthen peoples’ daily self-care activities. Focusing on patients with MFS this means to address their special needs. This rare, chronic illness with so many different phenotypic appearances and no standardized therapy, requires very patient-centered nursing with excellent knowledge of the impacts of this rare disease and corresponding therapies; as well as prevention of, and early detection of symptoms and complications. Best networking between the specialized departments in order to provide best individualized care is necessary.
The education of patients in order to be expert in their own disease is significant. But also the identification of psychological needs of the patients and their relatives is important, offering advice and guidance in order to strengthen their capability of self-maintenance. Often patients with MFS are long-term patients over decades, the relationship between patients and the therapeutic team of nurses, doctors, and others is very close. Thus, the concept of a relationship to address the patient’s special needs, but not to be too in the personal condition of the patient, is another key aspect of care.
Auxiliary disciplines
Forensic pathology
By Anna Lena Kammal and Klaus Püschel
In the field of pathology, in particular the study of forensic medicine, we often encounter cases of sudden unexpected death, usually of an internal nature. We have previously examined questions and problems pertaining to this issue in other studies and reviews.1,65,66 From a pathological point of view, the most frequently observed and positively identifiable causes of death are sudden cardiac arrest (50%), as well as aortic or vascular disease (1%–3% of autopsies). In 2015, our team of forensic pathologists identified an extremely high number of almost 100 fatal events from aortic dissection in a total of 1,300 autopsies performed at our institution (7.69%). In 85% of these cases, vascular pathology proved to be the primary cause of death.
It should be noted however, that in other cities the rate of dissections is considerably lower than that in Hamburg. At the University of Hamburg, the rate is ~8%, whereas other regions generally have a rate of only 1%–2%. Many cases of fatal connective tissue and vascular pathology are therefore likely to be missed or overlooked. The greatest portion of vascular pathology cases is seemingly based on arteriosclerotic changes, whereas connective tissue diseases such as MFS and vascular Ehlers-Danlos syndrome were present in 3% of these cases. The special challenge here lies in the cases with sudden death in young people, because certain questions are raised that reside outside the scope of forensic medicine. In particular, we face difficulties with the following tasks:
Precise determination of the cause of death.
Identifying possible treatment failures (misdiagnosis, malpractice).
Identifying genetic causes of death.
Initiating family member support.
Often the deceased have a long medical history of characteristic symptoms, and all too often their records show long odysseys of unclear or erroneous diagnoses. It often becomes obvious that there are severe problems in the proper establishment of a diagnosis. The misdiagnoses primarily pertain to the fields of internal medicine as well as orthopedic medicine. It would be important to actively consider aortic dissection in young patients with acute chest pain and to search for stigmata such as marfanoid habitus, or arachnodactyly in these individuals. Screening tools like the “seven-signs” instrument may support identification of young individuals at risk for such fatal aortic events.1,67
The Institute of Forensic Medicine in Hamburg has cooperated with the multidisciplinary MFS center on this matter for some time now. Once the diagnosis has been made within the scope of the dissection or the autopsy report, and further diagnostic evidence suggests such an illness, it is of particular importance to run all corresponding diagnostics, ie, on the bicuspid aortic valve. After these steps have been taken, the consultation and consolation of family members should take the highest priority, especially with regard to the prevention of future events of sudden death in the family (ie, genetic screening). Afflicted families should be carefully informed about possible screening and treatment options and should be sent to a geneticist for family counseling. The geneticist may consider molecular testing in family members. This demands a certain degree of multidisciplinary care and supervision. For this reason, we consider the close proximity to a center of multidisciplinary care to be a critical factor in a successful prevention and treatment outcome.
Radiology
By Peter Bannas
The indication for surgical prophylactic aortic root replacement in MFS is highly dependent on accurate and precise measurements of the aortic root. Therefore, radiological imaging of the aortic root plays a key role for the diagnosis of MFS according to the Ghent-2 nosology68 and is a core element of annual follow-up examinations to detect aortic root aneurysms. Only excellent multidisciplinary communication between radiologist, cardiologists, and vascular or cardiac surgeons enables appropriate timing for surgery. Also, after aortic root replacement, annual imaging of the entire aorta is recommended in patients with MFS. Hence, patients with MFS experience lifelong annual radiological imaging of the aorta, which is a key component of their lifelong multidisciplinary monitoring.
From a radiological perspective, echocardiography, CT, and MRI are available for noninvasive imaging of the aortic root. Echocardiography is widely available, but cannot assess the entire aorta and its accuracy is operator dependent. CT allows exact measurements but uses ionizing radiation and requires the application of iodinated contrast. MRI does not require ionizing radiation or iodinated contrast. Therefore, MRI is recommended for imaging of the entire thoracic aorta in patients with MFS.69
Since annual lifelong imaging is performed in patients with MFS, noncontrast MRI techniques should be preferred over contrast-enhanced techniques. Noncontrast techniques allow for electrocardiography (ECG) triggering, which allows for sharp delineating of the aortic root, resulting in accurate and precise aortic diameter measurements.70–72
Moreover, MRI allows not only for imaging of the aortic root but also of the thoracic and abdominal aorta and peripheral vessels with excellent image quality in an observer-independent fashion. Thus, MRI enables detection of arterial aneurysms other than of the aortic root, eg, of intracranial aneurysms that have a high prevalence of 14% and grading of vessels tortuosity, which is associated with a more severe aortic phenotype in MFS.73,74
MRI is also the preferred imaging method for detection and grading of dural ectasia.75,76 Other imaging methods such as conventional radiography allow for detection of skeletal malformations and craniofacial dysmorphology.67 Conventional radiography and CT allow for detection of lung emphysema and pneumothorax. CT in particular, allows detection and monitoring of pulmonary abnormalities before they aggravate.77
An experienced and skillful team is needed for lifelong image-based monitoring and management of patients with MFS. In a multidisciplinary team approach, radiologists, cardiologists, and vascular and cardiac surgeons can best perform this management of vital importance for patients with MFS.
Rhythmology
By Boris A Hoffmann
Consequences of aneurysms and dissections of the thoracic aorta are the major cause of death in patients with MFS.78 In the past 15 years, there is increasing evidence indicating an elevated risk for ventricular arrhythmias (VA) and sudden cardiac death (SCD).78–81
Chen et al82 reported episodes of VA in 33% of children with MFS including one survived SCD. This patient required resuscitation and was found to have multiform premature ventricular contractions (PVCs) and ventricular tachycardia (VT). Of all children with VA, 75% had mitral valve prolapse, whereas only two children (8%) had severe mitral valve regurgitation. The authors found that serious VA in children with MFS can occur with or without substantial valve disease. In our center, we identified that 14% of adult MFS patients suffer giddiness or syncope and that PVCs occur in up to 13% of adults with MFS suffer giddiness or syncope and PVCs were observed in 13% in this group.75,82–84 In contrast, Yetman et al75 reported that none of the patients died from aortic dissection, while 4% died from VA over a follow-up period of up to 24 years. VAs are identified in 21% of these patients and were associated with an increased left ventricular (LV) size, mitral valve prolapse, and repolarization abnormalities.76
Reduced LV ejection fraction in patients with MFS has been described previously; Hetzer et al85 demonstrated that heart failure is the leading cause of death, especially in neonatal and juvenile patients with MFS. In the majority of cases, heart failure is caused by heart valve incompetence rather than primary myocardial dysfunction.75 In contrast, Yetman et al76 found that, even in the absence of aortic valve regurgitation, LV dilation occurs commonly in patients with MFS and is associated with altered repolarization that may subsequently lead to ventricular arrhythmia. In this study, 11% of patients with MFS had LV systolic dysfunction.76
Among different risk stratification parameters, eg, ECG and heart rate variability, the serum N-terminal probrain natriuretic peptide (NT-proBNP) as a correlate of LV dysfunction remained the exclusive predictor for the composite end point comprising SCD, VT, ventricular fibrillation, or arrhythmogenic syncope in our group of patients with MFS.75 NT-proBNP showed an optimal cutoff point at 214.3 pg/mL and predicted the composite primary end point accurately (sensitivity 100% and specificity 79.0%). In other observations of our group, NT-proBNP was repeatedly correlated with arrhythmogenic events, and the optimal cutoff was calculated with 465 pg/mL and 618 pg/mL, respectively.80,86
Recently, LV diastolic abnormalities and RV and LV systolic function in patients with MFS were described by conventional echocardiography and tissue Doppler imaging.87,88 The precise mechanism of LV dysfunction is unclear. Aortic wall stiffness leading to an increased LV afterload and subsequently to LV dilation and an altered LV filling in the absence of significant aortic or mitral regurgitation due to defective microfibrils and elastic fibers in the cardiac cytoskeleton are discussed.89 The inadequate amount of microfibrils, constituted of FBN1-rich microfibrils, may result in loss of extracellular matrix structural integrity which in turn impairs the coordination of contractile and elastic tensions.90 Abnormal ventricular diastolic relaxation and impaired systolic contractility observed in patients with MFS could be explained by extracellular matrix remodeling and an abnormal TGF-β biological pathway due to a FBN1-deficient environment.90
Conventional ECG parameters and heart rate variability failed to stratify the risk of SCD.79 In our recent publication, an altered heart rate turbulence (combination of abnormal turbulence slope and turbulence onset) was found to be an independent risk predictor of arrhythmogenic events in patients with MFS.80 Genetic factors may elucidate the SCD risk in the future. Recently, published data suggest that VT events were significantly elevated in patients with FBN1 mutations in exon 24–32.86
Patients with MFS are at increased risk for SCD that is caused by ventricular arrythmnia rather than aortic rupture or dissection. Risk factors include the presence of VA, eg, PVCs, VTs, impaired LV systolic function, elevated NT-proBNP serum levels, abnormal heart rhythm turbulence (HRT), and FBN1 mutations within exon 24–32. Patients with MFS who are at risk probably benefit from a close follow-up of a multidisciplinary health care team.
Pulmonology/sleep medicine
By Nele Gessler
MFS can lead to several pulmonary manifestations as well as sleep-related breathing disorders. These can result directly from the connective tissue changes themselves or from skeletal abnormalities like pectus excavatum or scoliosis, which can cause restrictive lung disease or may increase the risk of spontaneous pneumothorax. The reported prevalence of pneumothorax in MFS is 4% to 11%.91 It occurs especially young adults and is seen more often in males than in females. It was found that the presence of blebs and bullae, which frequently occur in MFS, is associated with a greater risk of pneumothorax.92 Because of this risk, patients are advised to avoid activities with high pressure changes like skydiving, playing wind instruments, or scuba diving.
Additionally to classic pulmonary manifestations, there is a strong association between sleep-related breathing disorders like sleep apnea and MFS. Approximately 30% of patients with MFS have evidence of sleep apnea. While it is mostly described as obstructive, central sleep apnea is also seen in our patients.83 Although central sleep apnea is mainly associated with heart failure, obstructive sleep apnea is known to be associated with multiple cardiovascular diseases such as atrial fibrillation, VA, heart failure, and stroke and is an independent risk factor for arterial hypertension. Due to the mechanism of forced breathing against the closed upper airway, intrathoracic pressure changes can be as high as −65 mmHg, which is probably the cause for the increased risk of aortic dissection in individuals with obstructive sleep apnea.84
Particularly in patients with MFS, it is necessary to identify and treat obstructive sleep apnea, which is easily done with continuous positive airway pressure (CPAP) therapy. Unfortunately, clinical signs of sleep apnea like excessive daytime sleepiness did not relate to sleep apnea in MFS patients. Thus, we suggest screening patients with MFS for sleep apnea even when not clinically suspected.
Orthodontology/dentistry
By Eva Vahle-Hinz and Bärbel Kahl-Nieke
Apart from cardinal manifestations involving the ocular, skeletal, and cardiovascular systems, various craniofacial and oral abnormalities occur in patients with MFS. Of particular interest from an orthodontics point of view are: dental and skeletal class II configurations with maxillary and mandibular retrognathia, and dolichocephaly as well as maxillary constriction with an increased palatal depth including dental crowding. The prevalence of joint hypermobility is another common finding that leads to temporomandibular joint dysfunction and possibly causes condylar resorption. According to this finding, physiotherapy should be one of the mainstays of the therapy of temporomandibular joint dysfunction disorders. This includes joint stabilization in patients with MFS at the beginning of every orthodontic treatment.
Concluding from a clinical periodontal examination, patients with MFS do not show more periodontal damage, eg, severe forms of periodontitis, but a tendency toward more inflammation signs caused by crowding.93 Therefore, patients with MFS should receive professional teeth cleaning at regular intervals to reduce periodontal inflammation and consequently their increased risk of endocarditis.
Connective tissue disorder in patients with MFS is associated with reduced lip strength as a result of orofacial muscular hypotonia and increased lower facial height. This correlates with increased clockwise rotation of the mandible, resulting in an increased gonial angle. A reduced chewing efficiency for hard foods has been described as an additional syndromal characteristic caused by weak ligaments and muscular hypotonia. Systemic muscular hypotonia in patients with MFS may affect the craniofacial morphology in adolescent patients. Therefore, early myofunctional therapy to achieve lip competence as well as to strengthen the orofacial muscle is recommended for patients suffering from a connective tissue disorder in childhood, and in adolescence. During the time of growth, the aim of orthodontic treatment should be bite correction and regulation of the craniofacial growth with functional appliances.
Neurology
By Götz Thomalla
Neurological manifestations or complications are not key factors for MFS, neither in clinical practice nor as part of diagnostic algorithms. Dural ectasia is the only neurological manifestation of MFS that is used as a diagnostic criterion. Dural ectasia has been related to chronic lumbosacral pain syndrome, and it may give rise to complications in rare circumstances. For example, spontaneous cerebrospinal fluid leaks can result in intracranial hypotension presenting with postural headache; and giant anterior or lateral meningocele may cause constipation, urinary symptoms including retention or incontinence, or dysmenorrhea.94,95
There are, however, conditions in MFS and LDS that may require neurological management. These are mainly cerebrovascular events either resulting from alterations of vascular structures in these disorders of connective tissue or being complications of cardiovascular manifestation of the disease in these patients.
Ischemic stroke with cerebral or spinal ischemia may occur resulting from valve disease of atrial fibrillation or after dissection of cervical arteries within the context of aortic dissection. In a large retrospective case series, 2.9% of patients with MFS suffered from transient ischemic attack or ischemic stroke, mostly from a cardioembolic source.87 Thus, oral anticoagulation is usually indicated in conditions with increased risk of cardioembolism in MFS or LDS such as prosthetic heart valves or atrial fibrillation.
Dissections of cervical arteries are the most frequent cause of stroke in young patients, and both MFS and LDS are associated with arterial dissections. Cervical artery dissections may occur together with or independent from aortic disease. However, given the overall low incidence of MFS and LDS, both disorders are rather rare findings in cohorts of young stroke patients.88
There has been some debate about an increased risk of intracranial hemorrhage with MFS, and previous reports suggesting increased frequency of intracranial aneurysms in MFS.96 Neither of these were confirmed by a more recent and larger case series.96 Conversely, LDS is characterized by a high incidence of intracranial arterial aneurysms that may be associated with a risk of subarachnoid hemorrhage.97 Both neurosurgical and endovascular treatment of these aneurysms may be challenging given the sometimes extreme arterial tortuosity, which is a typical feature of LDS. Regular neurovascular imaging is recommended in patients with LDS.
Dural ectasia, ie, dilatation of the dural sac, is common in MFS and LDS but is usually asymptomatic. It may also lead to pain or spinal nerve root compression. A rather rare complication together with dural ectasia is intracranial hypotension with position-dependent headache.
Neurologists should look for clinical signs of connective tissue disorders in young patients with stroke and cervical artery dissections and consider testing for MFS or LDS if clinical signs are present. If cerebral ischemic events occur in patients with MFS or LDS, acute treatment does not differ from usual stroke treatment. Potential sources of cardiac or aortic embolism should be carefully looked for, and further management including oral anticoagulation should be coordinated together with cardiologists at a center for genetic aortic disease. In patients with known MFS or LDS, neurovascular imaging should be performed as screening, and should be performed at regular intervals, especially in LDS. In cases of intracranial aneurysm, treatment decision making should be multidisciplinary, involving neurologists, neurosurgeons, and interventional neuroradiologists.
Obstetric surgery
By Meike Rybczynski
As a genetic disorder, MFS influences every patients’ life-planning decisions, particularly with respect to family planning, there are specific aspects to consider. In daily practice, many practitioners lack the knowledge necessary in estimating the risk of pregnancy in MFS patients with respect to aortic enlargement as well as the potential disease risk to the child.98
Therefore, caregivers should discuss family planning and pregnancy with affected couples, to ensure that every woman with MFS is aware of her own personal risk. The transition from pediatric care to adult cardiology represents an ideal opportunity to introduce special aspects of pregnancy in MFS.
Expert consensus considers an aortic diameter of 40 mm or less safe for vaginal delivery; therefore, every female patient should undergo echocardiography before getting pregnant.99 It is recommended that patients with larger diameters undergo elective aortic replacement prior to planned pregnancy.100,101 Even in low-risk pregnancy, the physicians at the Hamburg Marfan center perform routine visits with echocardiography in every trimester to ensure stable aortic status. In general, pregnancy leads to an aortic enlargement of ~2 mm even in healthy women.102,103
Family planning is a highly individual and personal part of life and decisions are made in an individual, and on an emotional basis. It is extremely challenging for medical professionals to respect these deeply personal aspects and to ensure that all disease-specific considerations are understood and taken into account. Specific aspects that should be addressed include counseling of the mother, and comprise multidisciplinary counseling during pregnancy, regular evaluation for changes in medical therapy, consideration of referral to a Marfan center for delivery, potential risks of vaginal delivery, and problems related to breastfeeding. Whenever the life of the mother is in danger due to a pronounced aortic aneurysm enlargement, abortion may be considered.
Counseling for the parents in respect of the child includes, that if the child is at risk of MFS (50% chance), high intrafamiliar variability of clinical symptoms is possible, that early diagnosis and presymptomatic gene testing is warranted to initiate preventive therapy, and that there is no increased risk of preterm delivery of other pregnancy-related complications
BAB can be continued during pregnancy and lactation. Angiotensin converting enzyme inhibitors must be discontinued as soon as possible due to teratogenic effects (consider switching to BAB). In case of arterial hypertension, methyldopa may be an option.99
In regards to breastfeeding, the role of oxytocin especially during the lactation period as a destabilizing factor of the aortic wall and potential risk factor of aortic dissection remains unclear and requires further evaluation.12 Since breastfeeding is strongly recommended for the health of the newborn and since there is still a lack of evidence, we perform shared decision making for this issue.
Trust-based and professional communication is essential in providing a high level of quality in medical care in this specific patient collective. In Hamburg Marfan center physicians’ experience, joining obstetric board meetings is a good option to discuss further procedures, if needed. In addition, a multidisciplinary team should be involved in planning pregnancy for women with MFS. Planning should include the mode of delivery, and clear communication of a plan in case of emergency, comprising an alert plan in case of aortic dissection to the team comprising members of disciplines such as cardiac surgery, cardiology, obstetrics, anesthesiology, and pediatric intensive care. Such planning comprises facility planning (which operating room–obstetrics or cardiac surgery?), equipment availability (incubator, etc?), postpartum planning (which setting? Monitoring required? Rooming-in possible? When is discharge possible?), and planning of follow-up concepts in an outpatient setting.
Psychology
By Meike Rybczynski and Dieter Benninghoven
Patients with chronic illnesses are frequently subject to psychological distress. Problems can either manifest acutely as a result of a specific illness-related event or they can develop parallel to the chronic illness as comorbid psychopathology. There is some evidence that patients with MFS show a lower quality of life and a higher level of psychopathology, mainly anxiety and depression, than comparable healthy subjects. In addition, some limited evidence exists showing that patients with MFS may also have neuropsychological deficits.3,5,7,104,105 But these findings need further confirmation through studies with larger sample sizes. In the absence of robust empirical scientific evidence the following representative collection of clinical impressions is based on many interactions with MFS patients in daily clinical practice.
Traumatic experiences: an acute aortic dissection and its treatment represent an imminent life-threatening situation. It is a very painful event associated with fear of death. Additionally, this event is often the distinct incident that leads to the diagnosis of MFS. The initial diagnosis of a chronic illness can be a burden per se, and may be perceived as another traumatic stressor. As a result some patients may show symptoms of an adjustment disorder or of a posttraumatic stress disorder.
Body image: some patients with MFS feel stigmatized by their appearance (“I am a walking diagnosis”). Young adults are especially sensitive due to physical malformations such as thorax deformities, scoliosis, or large body size, which distinguish them from their peers. These characteristics may lead to reduced therapy compliance or a strong desire to undergo operative correction of these perceived malformations. On the other hand, many patients often report that their “big and tall” stature provokes expectations from others that they are unable to meet (“strong, resilient”). If then the disease is not communicated at the workplace, for example, misunderstandings rapidly arise (“he is such a big strong guy and he is always avoiding work”).
Family life: the majority of patients with MFS have parents who are also affected by the disease. In conflict situations that often arise with young adults, genetics, and parental responsibility can become a heated topic of discussion (“how could you choose to have a child with this disease when you knew exactly what it would go through”). This type of situation is often very difficult for parents. Similarly, the decision for or against parenthood is complicated and MFS patients sometimes require counseling regarding this question.
Autonomy development: another challenge for adolescents is gaining independence from parents and autonomy with respect to disease management and therapy. From a medical perspective, the child should be involved in disease management as early as possible. In practice, many parents with “sick” and therefore “helpless” children have difficulties letting go.
Accidental risk behavior: if the patient is unable to make a successful transition from a pediatric to an adult setting, they often fall into the pattern of accidental risk behavior. As a result of the desire to assimilate into one’s peer group as far as possible, denial of the disease is the result, which translates into noncompliance with respect to medication and check-ups. The preparation and guidance of this transition into personal responsibility for one’s health and disease management requires a solid relationship and good communication among health care givers.
Sex-specific aspects: hard data on quality of life of patients with MFS is lacking. Data from self-help groups show that women report a lower quality of life and higher burden related to the disease coupled with managing the household, working, and holding the majority of the responsibility for childcare.
Work environment: negative experiences sometimes also arise at work places. Many job-related activities are not recommended for patients with MFS. This may lead to frustration. Bullying and teasing are not as prominent at work as they are for children at school, but may nevertheless occur.
General: a solid and trust-based doctor–patient relationship is the key in the treatment of patients with MFS. Furthermore, the awareness of psychological burdens and their exploration at regular intervals must be a self-evident part of the routine treatment. A systematic screening for frequent psychopathological symptoms, mainly anxiety and depression, through an adequate screening questionnaire, for instance, the hospital anxiety and depression scale (HADS)106 is recommended. Ideally, a clinical psychologist is a member of the multidisciplinary team and can initiate further diagnostics and psychotherapeutic interventions if necessary. Similarly, attention should be paid to neurospsychological deficits and special training should be provided if needed.
It is important to strengthen the patients’ self-confidence in dealing with MFS and in informing other medical professionals about important background information related to the disease. This type of behavior helps patients process their disease and eases situations with outsiders. Self-help groups for patients with MFS are extremely well organized and allow patients not only to find others with the disease, but also to become politically active.
Rehabilitation
By Dieter Benninghoven
Rehabilitation programs specifically for MFS patients are rare, and scientifically evaluated programs do not exist. In 2014, the first inpatient rehabilitation program in Germany for people with MFS was established based on experiences from the National Resource Center for Rare Disorders in Norway. It is now offered annually to patients with MFS in medically safe and stable condition in order to ensure or improve participation in all areas of life.
The MFS rehabilitation program lasts 3 weeks and is based on regular German inpatient cardiac rehabilitation.107 The main goal is to achieve the best possible support of the patient’s capacities with respect to biological, psychological, and social aspects. Besides physicians, the rehabilitation team includes nursing staff, exercise therapists, physiotherapists, psychologists, nutritionists, and social workers. After an extensive initial diagnosis, the patient’s individual somatic, educative, psychological, and social rehabilitation goals are formulated. Based on these, a rehabilitation plan is determined, which is modified and adjusted to the success of rehabilitation. People with MFS are admitted to the program in groups of ten patients, on average. They receive special education about MFS, such as talks by experts about current treatment options, new research findings, and behavior recommendations. Based on exercise ECG performed at the beginning of the rehabilitation, the appropriate target heart rate for training is defined (with a systolic blood pressure ≥160 mmHg as a stop criterion). Throughout the course of rehabilitation, the training combines daily bicycle ergometry, gymnastics, fitness training, and nordic walking adding up to at least three sport units per day. The main goals here are to overcome patients’ uncertainty regarding their physical abilities and, thereby, to improve patients’ body image. Twice a week, patients take part in a psychological group therapy focusing on coping with MFS.105 Additionally, a psychologist trains patients in a relaxation technique. One-to-one psychotherapeutic interventions are important and offered according to demand. Counseling for job-related issues and dietary counseling is offered, and patients get introduced to a self-help organization for MFS patients. As a core element, the rehabilitation process of every patient is reviewed weekly in multidisciplinary team conferences. Finally, an extensive examination is performed at the end of the rehabilitation to evaluate individual therapy success with regard to predefined therapy goals.
Meaning
The magnet metaphor of team member, structure, meaning
Patients, physicians, nurses, and managers, just as all human beings seek meaning in life. About meaning, McKee says in screenwriting: “Values, the positive/negative charges of life, are at the soul of our art. The writer shapes story around a perception of what’s worth living for, what’s worth dying for, what’s foolish to pursue, the meaning of justice, truth – the essential values”.108 The brief essays of our expert physicians, patient representatives, nurses, and managers give us a vivid idea of competence, commitment, positive charges of value, and enthusiasm. A multidisciplinary health care team consists of team members who are individual and autonomous, but who share similar professional life experiences, and common professional values. Well-designed structures allow team members to join for action to maximize therapeutic success as the ultimate value of our professions.
Importantly, there is a dynamic relationship between team member, structure, and meaning. The Chicago sociologist Eliot Freidson (1920–2005) provided a landmark study of the medical profession,109 he analyzed the social structures of the medical profession as according to data that was available up to 1970, including the profession’s structures for self-regulation in the medical societies, and the work-settings in hospitals and private practices. He concluded that the medical profession was organized in such a way that it was virtually impossible for physicians to perform in accord to their own professional claims of high medical and ethical standards. Freidson disclosed the dominance of informal structures for professional self-regulation. Such informal structures promoted a limited sense of medical responsibility, predominance of prestige and authority, and worked through personal networks of referral relations, bureaucratized reward systems, personal privilege systems, personal boycotts, colleague careers, internal fraternities, sponsorship, and patronage.110 In summary, Freidson’s110 study provides a terrifying example of how distorted structures of the medical profession can result in a system of health professionals who prefer playing political games rather than maximizing therapeutic success.111
Almost 50 years after Freidson published his seminal work, ideas on hospital organization appear to make a U-turn. Medical professionals get designed as “bureaucratized professionals”, who are supposed to align strictly into managerial structures, instructions, and interests – such bureaucratized professionals can probably only exist by design.112 However, if they exist, they are unlikely to share the same spirit of enterprise and enthusiasm as the professionals in our paper on multidisciplinary care today.
Structures align professionals to create meaning, like a magnet aligns iron shavings by the power of the magnet field (Figure 3). Structures that are unable to provide clear purpose and direction will distort professional performance and yield a meaning of medicine as a personal game of team members. Conversely, structures that are too rigid will immobilize team members, paralyze their agility, suffocate their sense of excellence, innovation, and initiative, and will create the meaning of medicine as the disengaged execution of bureaucratic rules. Only a well-weighted balance of professionals and structures will yield a meaning of medicine as a team effort for maximizing therapeutic success. Meaning organizes professionals with the lowest financial and organizational effort, it is invisible like a magnet field, but it works for team members like a compass for navigation for difficult tasks in complex environments.
Figure 3.
The magnet metaphor of team member, structure, and meaning. (A) In the absence of instructive structures team members scatter like iron shavings in a loose pile. (B) Structures align team members by power of meaning like a magnet aligns iron shavings by the power of its magnet field.
Teams as means to maximize therapeutic success
For the strategy paradigm, a multidisciplinary health care team is a means to maximize therapeutic success. In this sense, the success of a multidisciplinary health care team is to maximize therapeutic success in each individual patient. Team member, structure, and meaning of such teams are designed to serve this objective. Clearly, every human organization, a hospital, or a multidisciplinary health care team has an end in itself, which is an ethical demand but also a functional demand. We will address this big and important issue in a later paper dealing with “organizational medical strategies (OMS)”.
In contrast to many other approaches to assessing the “effectiveness of multidisciplinary teamwork”,113 the strategy paradigm of multidisciplinary health care exclusively accepts outcome as the “effectiveness measure”, which is to maximize therapeutic success. In our strategy paradigm, therapeutic success has three dimensions: 1) efficiency by achieving biological goals such as diagnose, prevent, cure, rehabilitate, or palliate disease (“do therapy right”); 2) effectiveness by achieving social goals such as satisfying guidelines, values, or ethical demands (“do right therapy”); and 3) motivation by achieving psychological goals such as emotional support, or patients’ identification with therapy (“do therapy that feels right”).13
The strategy paradigm assesses the quality of teams by means of a fitness matrix of multidisciplinary health care teams for its capability of maximizing therapeutic success. In this matrix, we identify team capacities to maximize therapeutic success according to the three dimensions separately, for team members, structures, and meaning of the team. We present our strategic fitness matrix as a 3×3 contingency table in Table 3.
Table 3.
The strategic fitness matrix of multidisciplinary health care teams for maximizing therapeutic success
| Three dimensions of therapeutic success
|
|||
|---|---|---|---|
| Team capacities that contribute to maximizing therapeutic success: | (1) Efficiency by achieving biological goals, such as diagnose, prevent, cure, rehabilitate, or palliate disease (“Do therapy right”) |
(2) Effectiveness by achieving social goals such as satisfying guidelines, values, or ethical demands (“Do right therapy”) |
(3) Motivation by achieving psychological goals such as emotional support, or patients’ identification with therapy (“Do therapy that feels right”) |
| Team member | Team of professionals who share a common purpose and who understand their own and the others’ role, function, and responsibility | Team of professionals with high communicative, social, legal, and ethical competence, and respect for other team members, and democratic style of decision making | Team of professionals who have a strong own motivation, have team spirit, confidence, trust, to motivate the entire team |
| Structure | Organizational structures that provide team with resources, equipment, physical environment to reduce risks and errors, and to support the team’s medical performance | Organizational structures that support trustful team relationships, that teach, train, and support communicative, legal and ethical standards for all team members | Organizational structures such as human resource management and leadership that support intrinsic motivation of team members in the team |
| Meaning | A shared enthusiasm for medical excellence through team effort | A shared focus on patients and on ethical values | A team with high motivation that enkindles their patients |
Strategizing from inside-out rather than from outside-in
Myriads of fascinating business engineering instruments, management change strategies, and controlling tools are ready for use to implement a new organizational culture, including clinical governance; lifelong learning; new performance frameworks; new attitudes of innovation, process, patient, or stakeholder orientation; accountability, service orientation; market discipline; and transparency, to mention only some culture components listed in a single paper on organizational culture.114 Imagine a health care manger launching a new initiative every now and then. Approaching a plethora of problems with a plethora of concepts corresponds to what McKee calls writing from the outside-in: “We could, by working from the outside-in, render a surface of character that’s genuine, even fascinating. But the one crucial dimension we would not create is emotional truth”.108
Conversely, strategizing from the inside-out means to start with a clear premise and to work from there into the complexity of the problem. The premise works a controlling idea for the entire endeavor. It has to be a simple, strong, and deeply felt emotional truth that all members of the team can share. Table 4 presents statements of such emotional truths as written down quickly upon telephone or email request for a statement of meaning as felt by some of our team members (Table 4). A premise such as “we build-up a multidisciplinary health care team to maximize therapeutic success for patients with MFS” is a strong and simple starting point for strategizing from the inside-out. When strategizing from the inside of a core idea of team members, we can identify structural requirements with ease, we do what is needed without squandering efforts that go in the wrong direction, we avoid using “alien instruments” that do not fit our beliefs, and we do not implement more structures than we really need to maximize therapeutic success.
Table 4.
Statements of meaning
| Patient representative | For patients and their families multidisciplinary care means a way out of fear and uncertainty, a way toward a normalized life expectancy and the highest possible quality of life. This is what Marfan Hilfe is fighting for. Marina Vogler, Marfan Hilfe Deutschland |
| Patient coordinator | Multidisciplinary teams are crucial for providing excellent care to patients with complex disorders. Apart from the reward that each team member receives through improved patient well-being, being part of such a team is extremely rewarding to the team members themselves by means of emotional, medical, and personal support they receive by participating in these teams. Christina Weiler-Normann, patient coordinator of the Martin Zeitz center for rare diseases |
| Pediatric cardiologist | Just one sentence from the pediatric Marfan view: The patient is the family. Thomas S Mir, coordinator of the pediatric Marfan clinic |
| Human geneticist | I want to provide optimal whole-person care for our patients with Marfan syndrome in a multidisciplinary team of experts that allows us all to learn from each other to continuously optimize the quality of our high-standard care. And I want to have fun doing my work as a professional. Kerstin Kutsche, human geneticist |
| Heart surgeon | The key challenge is to identify the individualized best therapeutic strategy for patients with Marfan syndrome, who present with complex medical problems resulting from multiorgan disease. Getting to the best achievable quality, and maximizing therapeutic success gets me the highest possible satisfaction as a medical professional. Alexander M Bernhardt, heart surgeon |
| Vascular surgeon | Multidisciplinary teams serve as gear wheals within a dedicated framework of diverse functions, knowledge, and skills. Hereditary soft tissue disorders specifically need a distinct multifaceted approach due to their manifold nature. Only intransigent dedication and highest level of expertise in every aspect of these demanding diseases will achieve a contented and healthy individual patient, which should be the primary goal of any multidisciplinary approach. E Sebastian Debus, director of the Clinic of Vascular Medicine |
| Vascular interventionist | Multidisciplinarity in treatment of aortic diseases is a key-factor to clinical success. This approach requires excellent communication skills and may be time-consuming. Besides the advantage of combining the best knowledge of many specialists, it allows us to grow by gaining insight into each other’s background, way of thinking, and clinical decision making. Tilo Kölbel, coordinator of the Aorta Center |
| Orthopedic surgeon | The main benefit in working in a multidisciplinary team for the care of patients with MFS is the combination of being able to use the physicians’ expertise in a subspecialty with the possibility to rely on and learn from the detailed knowledge of other team members and thereby improve patient care. Malte Schroeder, orthopedic surgeon |
| Ophthalmology | As ophthalmologist I want to contribute to the following five important goals: 1. Early diagnosis of Marfan syndrome to avoid multiple unnecessary examinations, 2. to maximize the individual therapeutic success, 3. to avoid blindness due to early examination especially in children but also in adults, 4. to reduce the psychiological stress reaction for the patient and his family, and 5. to keep in close contact to specialized colleagues to enable new therapeutic strategies. Bettina Fuisting, Clinic of Ophthalmology |
| Nursing | The comprehensive cross-linked treatment of our patients according to best quality standards and individual sight on our patients. Working on a same eye-level of the entire therapeutic team. Putting our clinic’s mission statement into use: Our success is the patients’ satisfaction Barbara Napp, chief of Nursing |
| Forensic pathologist | In the context of legal medicine it is essential to learn from the dead, ie, to serve the living, especially family members. Moreover, diagnostic errors and wrong tracks can be avoided by quality assurance concerning the cause of death. Klaus Püschel, director of the Institute of Forensic Medicine and Anna L Kammal, forensic pathologist |
| Rhythmologist | My dedication as rhythmologist is to bring results of electrophysiological tests in a knowledgeable and experienced multidisciplinary care team and to identify patients with Marfan syndrome who are at risk for sudden cardiac death. Prevention of this fatal outcome is the significant driver of my work. Boris A Hoffmann, rhythmologist |
| Sleep specialist | Sleep-related breathing disorders can result from different conditions (eg, heart failure, craniofacial abnormalities) and at the same time have influence on others (eg, hypertension, aortic dilatation). So to get the optimal results for the individual patient, it is highly beneficial to work multidisciplinary. Nele Gessler, cardiologist and sleep specialist |
| Orthodontist | A close multidisciplinary cooperation improves the early and efficient orthodontic and orthopedic treatment among patients with Marfan syndrome suffering from various craniofacial and oral abnormalities. Eva Vahle-Hinz, orthodontist |
| Neurologist | Neurologists rather rarely see patients with Marfan syndrome but if so, they usually present with severe conditions and challenging treatment decisions. Discussing these cases in a team of experts helps to better understand the patients’ specific problems and make the right treatment decisions. Götz Thomalla, neurologist |
| Strategic business development | The best medical treatment of the patient comes first. To facilitate local multidisciplinary team work, and national and international cooperation it requires more administrative support. For the future I hope that the complex treatment of patients with rare diseases will receive improved refunding. Gunda Ohm, chief of Strategic Business Development |
| Rehabilitation specialist | There is empirical evidence that multidisciplinary rehabilitation team care effectively improves rehabilitation outcome for different health problems. And we have the personal experience that it is very helpful for individuals with Marfan syndrome. Dieter Benninghoven, rehabilitation specialist |
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.von Kodolitsch Y, De Backer J, Schüler H, et al. Perspectives on the revised Ghent criteria for the diagnosis of Marfan syndrome. Appl Clin Genet. 2015;8:137–155. doi: 10.2147/TACG.S60472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eidt D, Frank M, Reimann A, Wagner TOF, Mittendorf T, Graf von der Schulenburg JM. Maßnahmen zur Verbesserung der gesundheitlichen Situation von Menschen mit Seltenen Erkrankungen in Deutschland. Forschungsbericht [Actions to improve the health situation of people with rare diseases in Germany. A research report. Study on behalf of the federal ministry of health] Studie im Auftrag des Bundesministeriums für Gesundheit. 2009. [Accessed February 23, 2010]. Available from: http://www.bmg.bund.de/themen/praevention/gesundheitsgefahren/seltene-erkrankungen.html. German.
- 3.Rand-Hendriksen S, Sorensen I, Holmstrom H, Andersson S, Finset A. Fatigue, cognitive functioning and psychological distress in Marfan syndrome, a pilot study. Psychol Health Med. 2007;12(3):305–313. doi: 10.1080/13548500600580824. [DOI] [PubMed] [Google Scholar]
- 4.Bathen T, Velvin G, Rand-Hendriksen S, Robinson HS. Fatigue in adults with Marfan syndrome, occurrence and associations to pain and other factors. Am J Med Genet A. 2014;164a(8):1931–1939. doi: 10.1002/ajmg.a.36574. [DOI] [PubMed] [Google Scholar]
- 5.Peters KF, Kong F, Horne R, Francomano CA, Biesecker BB. Living with Marfan syndrome I. Perceptions of the condition. Clin Genet. 2001;60(4):273–282. doi: 10.1034/j.1399-0004.2001.600405.x. [DOI] [PubMed] [Google Scholar]
- 6.Peters KF, Petrill SA. Comparison of the background, needs, and expectations for genetic counseling of adults with experience with Down syndrome, Marfan syndrome, and neurofibromatosis. Am J Med Genet A. 2011;155a(4):684–696. doi: 10.1002/ajmg.a.33863. [DOI] [PubMed] [Google Scholar]
- 7.Rand-Hendriksen S, Johansen H, Semb SO, Geiran O, Stanghelle JK, Finset A. Health-related quality of life in Marfan syndrome: a cross-sectional study of Short Form 36 in 84 adults with a verified diagnosis. Genet Med. 2010;12(8):517–524. doi: 10.1097/GIM.0b013e3181ea4c1c. [DOI] [PubMed] [Google Scholar]
- 8.Fusar-Poli P, Klersy C, Stramesi F, Callegari A, Arbustini E, Politi P. Determinants of quality of life in Marfan syndrome. Psychosomatics. 2008;49(3):243–248. doi: 10.1176/appi.psy.49.3.243. [DOI] [PubMed] [Google Scholar]
- 9.Murdoch JL, Walker BA, Halpern BL, Kuzma JW, McKusick VA. Life expectancy and causes of death in the Marfan syndrome. N Engl J Med. 1972;286(15):804–808. doi: 10.1056/NEJM197204132861502. [DOI] [PubMed] [Google Scholar]
- 10.Pyeritz RE. Marfan syndrome: 30 years of research equals 30 years of additional life expectancy. Heart. 2009;95(3):173–175. doi: 10.1136/hrt.2008.160515. [DOI] [PubMed] [Google Scholar]
- 11.De Backer J, Renard M, Campens L, et al. Marfan syndrome and related heritable thoracic aortic aneurysms and dissections. Curr Pharm Des. 2015;21(28):4061–4075. doi: 10.2174/1381612821666150826093152. [DOI] [PubMed] [Google Scholar]
- 12.Pyeritz RE. Recent progress in understanding the natural and clinical histories of the Marfan syndrome. Trends Cardiovasc Med. 2016;26(5):423–428. doi: 10.1016/j.tcm.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 13.von Kodolitsch Y, Bernhardt AM, Kölbel T, Detter C, Reichenspurner H, Debus ES. Maximizing therapeutic success: the key concepts of individualized medical strategy (IMS) Cogent Med. 2015;2(1):1109742. [Google Scholar]
- 14.Chamberlain-Salaun J, Mills J, Usher K. Terminology used to describe health care teams: an integrative review of the literature. J Multidiscip Healthc. 2013;6:65–74. doi: 10.2147/JMDH.S40676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fajgenbaum DC, Ruth JR, Kelleher D, Rubenstein AH. The collaborative network approach: a new framework to accelerate Castleman’s disease and other rare disease research. Lancet Haematol. 2016;3(4):e150–e152. doi: 10.1016/S2352-3026(16)00007-7. [DOI] [PubMed] [Google Scholar]
- 16.Cystic Fibrosis Foundation. [homepage on the Internet] [Accessed August 16 2016]. Available from https://www.cff.org/for-caregivers/cf-care-centers/
- 17.Muscular Dystrophy Assoication. [homepage on the Internet] [Accessed August 16 2016]. Available from https://www.mda.org/services/your-mda-care-center.
- 18.Cure hht. [homepage on the Internet] [Accessed August 16 2016]. Available from http://curehht.org/resources/hht-treatment-centers/
- 19.Pyeritz RE. Marfan syndrome: 30 years of research equals 30 years of additional life expectancy. Heart. 2009;95:173–175. doi: 10.1136/hrt.2008.160515. [DOI] [PubMed] [Google Scholar]
- 20.The Marfan Foundation. [homepage on the Internet] [Accessed August 16 2016]. Available from https://www.marfan.org/
- 21.Genetic Aortic Disorders Assciation Canada. [homepage on the Internet] [Accessed August 16 2016]. Available from http://www.gadacanada.ca/
- 22.von Kodolitsch Y, Raghunath M, Nienaber CA. Das Marfan Syndrom. Strategien einer interdisziplinären Betreuung. Marfan syndrome: strategies of interdisciplinary care. Dtsch Med Wochenschr. 1998;123(1–2):21–25. doi: 10.1055/s-2007-1023893. German. [DOI] [PubMed] [Google Scholar]
- 23.von Kodolitsch Y, Bernhardt AM, Robinson PN, et al. Analysis of strengths, weaknesses, opportunities, and threats as a tool for translating evidence into individualized medical strategies (I-SWOT) Aorta (Stamford) 2015;3(3):98–107. doi: 10.12945/j.aorta.2015.14.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Kodolitsch Y, Rybczynski M, Bernhardt A, et al. Marfan syndrome and the evolving spectrum of heritable thoracic aortic disease: do we need genetics for clinical decisions? Vasa. 2010;39(1):17–32. doi: 10.1024/0301-1526/a000002. [DOI] [PubMed] [Google Scholar]
- 25.Manow ML, Paulsen N, Rybczynski M, et al. Analyse der Erlössituation bei der ambulanten Behandlung nach § 116 b SGB V am Beispiel des Marfan-Syndroms [Analysis of costs and profits of ambulatory care of marfan patients after initiation of a novel German legal directive (section sign 116 b SGB V)] Med Klin (Munich) 2010;105(8):529–537. doi: 10.1007/s00063-010-1090-y. German. [DOI] [PubMed] [Google Scholar]
- 26.von Kodolitsch Y, Blankart CR, Vogler M, Kallenbach K, Robinson PN. Genetik und Prävention am Beispiel genetischer Aortensyndrome (GAS) und des Marfan-Syndroms [Genetics and prevention of genetic aortic syndromes (GAS) and of the Marfan syndrome] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58(2):146–153. doi: 10.1007/s00103-014-2093-2. German. [DOI] [PubMed] [Google Scholar]
- 27.von Kodolitsch Y, Rybczynski M, Trivic V, Hofmann T, Meinertz T. In Kompetenzzentren behandeln: lebensqualität und Lebenserwartung beim Marfan-Syndrom verbessern [Treatment in a center of competence: Improving quality of life and life expectancy in Marfan syndrome] Klinikarzt. 2002;31:201–206. German. [Google Scholar]
- 28.Braverman AC, Harris KM, Kovacs RJ, Maron BJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 7: aortic diseases, including Marfan syndrome: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66(21):2398–2405. doi: 10.1016/j.jacc.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 29.Mueller GC, Steiner K, Wild JM, Stark V, Kozlik-Feldmann R, Mir TS. Health-related quality of life is unimpaired in children and adolescents with Marfan syndrome despite its distinctive phenotype. Acta Paediatr. 2016;105(3):311–316. doi: 10.1111/apa.13264. [DOI] [PubMed] [Google Scholar]
- 30.Mueller GC, Stark V, Steiner K, et al. Impact of age and gender on cardiac pathology in children and adolescents with Marfan syndrome. Pediatr Cardiol. 2013;34(4):991–998. doi: 10.1007/s00246-012-0593-0. [DOI] [PubMed] [Google Scholar]
- 31.Trojan A. Kooperation von Selbsthilfegruppen mit Einrichtungen des Gesundheitswesens: die Ergebnisse der SHILD-Studie im Kontext von Praxis und Forschung [Cooperation of support groups with institutions of health service: The results of the SHILD-study in the context of practice and research] Medizinsoziologie: Selbsthilfe und Selbsthilfeunterstützung in Deutschland. 2016;24(4.3):277–301. German. [Google Scholar]
- 32.Marfan-Hilfe D. Marfan-Syndrom: Ein Ratgeber für Patienten, Angehörige und Betreuende [Marfan syndrome: A guide for patients, relatives and carers] 2007. (Marfan Support (Germany) eV). German. [Google Scholar]
- 33.Hilfe M [webpage on the Internet] Bedürfnisse von Patienten in Bezug auf Kliniken mit Marfan-Sprechstunde in Deutschland. 2016. [Accessed May 6, 2016]. Available from: http://www.marfan.de/images/stories/pdf/Wissenschaft/Umfrage_MarfanCenter_2016.pdf.
- 34.Marfan [webpage on the Internet] Umfrage Marfan-Sprechstunden 2016. 2016. [Accessed July 28, 2016]. Available from: http://www.marfan.de/weiterfuehrende-infos/336-umfrage-marfan-sprechstunden-2016.html.
- 35.UKE [webpage on the Internet] Welcome to the Martin Zeitz Center for Rare Diseases. [Accessed March 5, 2016]. Available from: http://www.uke.de/english/departments-institutes/centers/martin-zeitz-center-for-rare-diseases/index.html.
- 36.NAMSE Nationaler Aktionsplan für Menschen mit Seltenen Erkrankungen. Handlungsfelder, Empfehlungen und Maßnahmenvorschläge [National action plan for people with rare diseases. Fields of action, recommendations and suggestions of measures] 2013German. [Google Scholar]
- 37.Greutmann M, Pieper PG. Pregnancy in women with congenital heart disease. Eur Heart J. 2015;36(37):2491–2499. doi: 10.1093/eurheartj/ehv288. [DOI] [PubMed] [Google Scholar]
- 38.Ostermeyer J. Perfektion und Exzellenz. Erinnerungen an John W. Kirklin [Perfection and excellence. Memories of John W. Kirklin] Hamburger Ärzteblatt. 2011;12:12–17. German. [Google Scholar]
- 39.Tinkle BT, Saal HM, Committee on genetics Health supervision for children with Marfan syndrome. Pediatrics. 2013;132(4):e1059–e1072. doi: 10.1542/peds.2013-2063. [DOI] [PubMed] [Google Scholar]
- 40.Mueller GC, Stark V, Steiner K, Weil J, von Kodolitsch Y, Mir TS. The kid-short marfan score (Kid-SMS) – an easy executable risk score for suspected paediatric Marfan patients. Acta Paediatr. 2013;102(2):e84–e89. doi: 10.1111/apa.12072. [DOI] [PubMed] [Google Scholar]
- 41.Chevallier B, Oberkampf B, Stheneur C. Suivi et prise en charge multidisciplinaire du syndrome de Marfan de l’enfant [Multidisciplinary management and paediatric Marfan syndrome] Arch Pediatr. 2008;15(5):582–583. doi: 10.1016/S0929-693X(08)71840-2. French. [DOI] [PubMed] [Google Scholar]
- 42.von Kodolitsch Y, Robinson PN. Marfan syndrome: an update of genetics, medical and surgical management. Heart. 2007;93(6):755–760. doi: 10.1136/hrt.2006.098798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dietz HC. Marfan Syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews(R) Seattle, WA: 1993. [Google Scholar]
- 44.Loeys BL, Dietz HC. Loeys-Dietz Syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews(R) Seattle, WA: 1993. [Google Scholar]
- 45.Milewicz DM, Regalado E. Thoracic Aortic Aneurysms and Aortic Dissections. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews(R) Seattle, WA: 1993. [Google Scholar]
- 46.Pepin MG, Murray ML, Byers PH. Vascular Ehlers-Danlos Syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews(R) Seattle, WA: 1993. [Google Scholar]
- 47.Arslan-Kirchner M, Arbustini E, Boileau C, et al. Clinical utility gene card for: hereditary thoracic aortic aneurysm and dissection including next-generation sequencing-based approaches. Eur J Hum Genet. 2016;24(1):e1–e5. doi: 10.1038/ejhg.2015.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campens L, Callewaert B, Muino Mosquera L, et al. Gene panel sequencing in heritable thoracic aortic disorders and related entities – results of comprehensive testing in a cohort of 264 patients. Orphanet J Rare Dis. 2015;10:9. doi: 10.1186/s13023-014-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Proost D, Vandeweyer G, Meester JA, et al. Performant mutation identification using targeted next-generation sequencing of 14 thoracic aortic aneurysm genes. Hum Mutat. 2015;36(8):808–814. doi: 10.1002/humu.22802. [DOI] [PubMed] [Google Scholar]
- 50.Wooderchak-Donahue W, VanSant-Webb C, Tvrdik T, et al. Clinical utility of a next generation sequencing panel assay for Marfan and Marfan-like syndromes featuring aortopathy. Am J Med Genet A. 2015;167A(8):1747–1757. doi: 10.1002/ajmg.a.37085. [DOI] [PubMed] [Google Scholar]
- 51.David TE, Feindel CM, David CM, Manlhiot C. A quarter of a century of experience with aortic valve-sparing operations. J Thorac Cardiovasc Surg. 2014;148(3):872–879. doi: 10.1016/j.jtcvs.2014.04.048. discussion 879–880. [DOI] [PubMed] [Google Scholar]
- 52.Bernhardt AM, Treede H, Rybczynski M, et al. Comparison of aortic root replacement in patients with Marfan syndrome. Eur J Cardiothorac Surg. 2011;40(5):1052–1057. doi: 10.1016/j.ejcts.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 53.von Kodolitsch Y, Robinson P, Berger J. When should surgery be performed in marfan syndrome and other connective tissue disorders to protect against type a dissection? In: Bonser RS, Pagano D, Haverich A, Mascaro J, editors. Controversies in Aortic Dissection and Aneurysmal Disease. London: Springer; 2014. pp. 17–47. [Google Scholar]
- 54.Silverman DI, Burton KJ, Gray J, et al. Life expectancy in the Marfan syndrome. Am J Cardiol. 1995;75(2):157–160. doi: 10.1016/s0002-9149(00)80066-1. [DOI] [PubMed] [Google Scholar]
- 55.von Kodolitsch Y, Sachweh A, Bernhardt AM, et al. I-SWOT: ein Instrument zur Individualisierung medizinischer Entscheidungen [I-SWOT: an instrument to individualize medical decisions] Journal für angeborene Herzfehler. 2016;04:46–51. German. [Google Scholar]
- 56.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47(7):476–485. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 57.Drolsum L, Rand-Hendriksen S, Paus B, Geiran OR, Semb SO. Ocular findings in 87 adults with Ghent-1 verified Marfan syndrome. Acta Ophthalmol. 2015;93(1):46–53. doi: 10.1111/aos.12448. [DOI] [PubMed] [Google Scholar]
- 58.Coselli JS, Green SY, Price MD, et al. Results of open surgical repair in patients with marfan syndrome and distal aortic dissection. Ann Thorac Surg. 2016;101(6):2193–2201. doi: 10.1016/j.athoracsur.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 59.den Hartog AW, Franken R, Zwinderman AH, et al. The risk for type B aortic dissection in Marfan syndrome. J Am Coll Cardiol. 2015;65(3):246–254. doi: 10.1016/j.jacc.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 60.Miller DJ, Blum AB, Levine WN, Ahmad CS, Popkin CA. Preparticipation evaluation of the young athlete: what an orthopaedic surgeon needs to know. Am J Sports Med. 2016;44(6):1605–1615. doi: 10.1177/0363546515598994. [DOI] [PubMed] [Google Scholar]
- 61.McKeon PO, Hertel J, Bramble D, Davis I. The foot core system: a new paradigm for understanding intrinsic foot muscle function. Br J Sports Med. 2015;49(5):290. doi: 10.1136/bjsports-2013-092690. [DOI] [PubMed] [Google Scholar]
- 62.Beene LC, Traboulsi EI, Seven I, et al. Corneal deformation response and ocular geometry: a noninvasive diagnostic strategy in Marfan syndrome. Am J Ophthalmol. 2016;161(56–64):e51. doi: 10.1016/j.ajo.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steinberg J, Frings A, Mousli A, et al. New scheimpflug dynamic in vivo curve analyses to characterize biomechanical changes of the cornea after cross-linking for progressive keratoconus. J Refract Surg. 2016;32(1):34–39. doi: 10.3928/1081597X-20151207-09. [DOI] [PubMed] [Google Scholar]
- 64.Mariano C. Holistic nursing as a specialty: holistic nursing - scope and standards of practice. Nurs Clin North Am. 2007;42(2):165–188. v. doi: 10.1016/j.cnur.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 65.Brinkmann B, Madea B. Handbuch gerichtliche Medizin, Bd. 1 [ Handbook of Forensic Medicine] Heidelberg, New York, Tokyo: Springer; 2003. Auflage German. [Google Scholar]
- 66.Madea B. Rechtsmedizin –Befunderhebung, Rekonstruktion, Begutachtung. Heidelberg, New York, Tokyo: Springer; 2015. Auflage. [Google Scholar]
- 67.De Coster P, De Pauw G, Martens L, De Paepe A. Craniofacial structure in Marfan syndrome: a cephalometric study. Am J Med Genet A. 2004;131(3):240–248. doi: 10.1002/ajmg.a.30393. [DOI] [PubMed] [Google Scholar]
- 68.Bannas P, Groth M, Rybczynski M, et al. Assessment of aortic root dimensions in patients with suspected Marfan syndrome: intraindividual comparison of contrast-enhanced and non-contrast magnetic resonance angiography with echocardiography. Int J Cardiol. 2013;167(1):190–196. doi: 10.1016/j.ijcard.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 69.Russo V, Renzulli M, Buttazzi K, Fattori R. Acquired diseases of the thoracic aorta: role of MRI and MRA. Eur Radiol. 2006;16(4):852–865. doi: 10.1007/s00330-005-0028-x. [DOI] [PubMed] [Google Scholar]
- 70.Bannas P, Groth M, Rybczynski M, et al. Assessment of aortic root dimensions in patients with suspected Marfan syndrome: intraindividual comparison of contrast-enhanced and non-contrast magnetic resonance angiography with echocardiography. Int J Cardiol. 2013;167(1):190–196. doi: 10.1016/j.ijcard.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 71.Veldhoen S, Behzadi C, Derlin T, et al. Exact monitoring of aortic diameters in Marfan patients without gadolinium contrast: intraindividual comparison of 2D SSFP imaging with 3D CE-MRA and echocardiography. Eur Radiol. 2015;25(3):872–882. doi: 10.1007/s00330-014-3457-6. [DOI] [PubMed] [Google Scholar]
- 72.Groth M, Henes FO, Mullerleile K, Bannas P, Adam G, Regier M. Accuracy of thoracic aortic measurements assessed by contrast enhanced and unenhanced magnetic resonance imaging. Eur J Radiol. 2012;81(4):762–766. doi: 10.1016/j.ejrad.2011.01.071. [DOI] [PubMed] [Google Scholar]
- 73.Kim ST, Brinjikji W, Kallmes DF. Prevalence of intracranial aneurysms in patients with connective tissue diseases: a retrospective study. AJNR Am J Neuroradiol. 2016 Mar 8; doi: 10.3174/ajnr.A4718. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Franken R, El Morabit A, de Waard V, et al. Increased aortic tortuosity indicates a more severe aortic phenotype in adults with Marfan syndrome. Int J Cardiol. 2015;194:7–12. doi: 10.1016/j.ijcard.2015.05.072. [DOI] [PubMed] [Google Scholar]
- 75.von Kodolitsch Y, Raghunath M, Nienaber CA. Marfan syndrome: prevalence and natural course of cardiovascular manifestations. Z Kardiol. 1998;87(3):150–160. doi: 10.1007/s003920050167. [DOI] [PubMed] [Google Scholar]
- 76.Yetman AT, Bornemeier RA, McCrindle BW. Long-term outcome in patients with Marfan syndrome: is aortic dissection the only cause of sudden death? J Am Coll Cardiol. 2003;41(2):329–332. doi: 10.1016/s0735-1097(02)02699-2. [DOI] [PubMed] [Google Scholar]
- 77.Corsico AG, Grosso A, Tripon B, et al. Pulmonary involvement in patients with Marfan Syndrome. Panminerva Med. 2014;56(2):177–182. [PubMed] [Google Scholar]
- 78.Devereux RB, Roman MJ. Aortic disease in Marfan’s syndrome. N Engl J Med. 1999;340(17):1358–1359. doi: 10.1056/NEJM199904293401710. [DOI] [PubMed] [Google Scholar]
- 79.Hoffmann BA, Rybczynski M, Rostock T, et al. Prospective risk stratification of sudden cardiac death in Marfan’s syndrome. Int J Cardiol. 2012;167(6):2539–2545. doi: 10.1016/j.ijcard.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 80.Schaeffer BN, Rybczynski M, Sheikhzadeh S, et al. Heart rate turbulence and deceleration capacity for risk prediction of serious arrhythmic events in Marfan syndrome. Clin Res Cardiol. 2015;104(12):1054–1063. doi: 10.1007/s00392-015-0873-9. [DOI] [PubMed] [Google Scholar]
- 81.Savolainen A, Kupari M, Toivonen L, Kaitila I, Viitasalo M. Abnormal ambulatory electrocardiographic findings in patients with the Marfan syndrome. J Intern Med. 1997;241(3):221–226. doi: 10.1046/j.1365-2796.1997.115125000.x. [DOI] [PubMed] [Google Scholar]
- 82.Chen S, Fagan LF, Nouri S, Donahoe JL. Ventricular dysrhythmias in children with Marfan’s syndrome. Am J Dis Child. 1985;139(3):273–276. doi: 10.1001/archpedi.1985.02140050067024. [DOI] [PubMed] [Google Scholar]
- 83.Rybczynski M, Koschyk D, Karmeier A, et al. Frequency of sleep apnea in adults with the Marfan syndrome. Am J Cardiol. 2010;105(12):1836–1841. doi: 10.1016/j.amjcard.2010.01.369. [DOI] [PubMed] [Google Scholar]
- 84.Sampol G, Romero O, Salas A, et al. Obstructive sleep apnea and thoracic aorta dissection. Am J Respir Crit Care Med. 2003;168(12):1528–1531. doi: 10.1164/rccm.200304-566OC. [DOI] [PubMed] [Google Scholar]
- 85.Hetzer R, Gehle P, Ennker J. Results of cardiovascular surgery for Marfan syndrome in Berlin. In: Hetzer R, Gehle P, Ennker J, editors. Cardiovascular Aspects of Marfan Syndrome. Darmstadt: Steinkopff Verlag; 1995. pp. 109–118. [Google Scholar]
- 86.Aydin A, Adsay BA, Sheikhzadeh S, et al. Observational cohort study of ventricular arrhythmia in adults with Marfan syndrome caused by FBN1 mutations. PLoS One. 2013;8(12):e81281. doi: 10.1371/journal.pone.0081281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wityk RJ, Zanferrari C, Oppenheimer S. Neurovascular complications of Marfan syndrome: a retrospective, hospital-based study. Stroke. 2002;33(3):680–684. doi: 10.1161/hs0302.103816. [DOI] [PubMed] [Google Scholar]
- 88.Arnold M, Kappeler L, Georgiadis D, et al. Gender differences in spontaneous cervical artery dissection. Neurology. 2006;67(6):1050–1052. doi: 10.1212/01.wnl.0000237341.30854.6a. [DOI] [PubMed] [Google Scholar]
- 89.Savolainen A, Nisula L, Keto P, et al. Left ventricular function in children with the Marfan syndrome. Eur Heart J. 1994;15(5):625–630. doi: 10.1093/oxfordjournals.eurheartj.a060558. [DOI] [PubMed] [Google Scholar]
- 90.Kiotsekoglou A, Sutherland GR, Moggridge JC, Nassiri DK, Camm AJ, Child AH. The unravelling of primary myocardial impairment in Marfan syndrome by modern echocardiography. Heart. 2009;95(19):1561–1566. doi: 10.1136/hrt.2008.152934. [DOI] [PubMed] [Google Scholar]
- 91.Wood JR, Bellamy D, Child AH, Citron KM. Pulmonary disease in patients with Marfan syndrome. Thorax. 1984;39(10):780–784. doi: 10.1136/thx.39.10.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karpman C, Aughenbaugh GL, Ryu JH. Pneumothorax and bullae in Marfan syndrome. Respiration. 2011;82(3):219–224. doi: 10.1159/000322958. [DOI] [PubMed] [Google Scholar]
- 93.Staufenbiel I, Hauschild C, Kahl-Nieke B, et al. Periodontal conditions in patients with Marfan syndrome – a multicenter case control study. BMC Oral Health. 2013;13(1):59. doi: 10.1186/1472-6831-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bassani L, Graffeo CS, Behrooz N, et al. Noninvasive diagnosis and management of spontaneous intracranial hypotension in patients with Marfan syndrome: case Report and Review of the Literature. Surg Neurol Int. 2014;5:8. doi: 10.4103/2152-7806.125629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Voyvodic F, Scroop R, Sanders RR. Anterior sacral meningocele as a pelvic complication of Marfan syndrome. Aust N Z J Obstet Gynaecol. 1999;39(2):262–265. doi: 10.1111/j.1479-828x.1999.tb03390.x. [DOI] [PubMed] [Google Scholar]
- 96.Debette S, Germain DP. Neurologic manifestations of inherited disorders of connective tissue. Handb Clin Neurol. 2014;119:565–576. doi: 10.1016/B978-0-7020-4086-3.00037-0. [DOI] [PubMed] [Google Scholar]
- 97.Rodrigues VJ, Elsayed S, Loeys BL, Dietz HC, Yousem DM. Neuroradiologic manifestations of Loeys-Dietz syndrome type 1. AJNR Am J Neuroradiol. 2009;30(8):1614–1619. doi: 10.3174/ajnr.A1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rossiter JP, Repke JT, Morales AJ, Murphy EA, Pyeritz RE. A prospective longitudinal evaluation of pregnancy in the Marfan syndrome. Am J Obstet Gynecol. 1995;173(5):1599–1606. doi: 10.1016/0002-9378(95)90655-x. [DOI] [PubMed] [Google Scholar]
- 99.Westhoff-Bleck M, Hilfiker-Kleiner D. Marfan syndrome and pregnancy: monitoring and management. Eur Heart J. 2015;36(18):1066–1067. [PubMed] [Google Scholar]
- 100.G-BA [webpage on the Internet] Richtlinie über die grundsätzlichen Anforderungen an ein einrichtungsinternes Qualitätsmanagement für nach § 108 SGB V zugelassene Krankenhäuser; Gemeinsamer Bundesausschuss [Guideline on the basic requirements of an institutional quality management for hospitals with authorization according to directive § 108 SGB V] 2016. [Accessed March 2, 2016]. Available from: https://www.g-ba.de/informationen/richtlinien/40/ German.
- 101.Crema M, Verbano C. Future developments in health care performance management. J Multidiscip Healthc. 2013;6:415–421. doi: 10.2147/JMDH.S54561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Orlander JD, Barber TW, Fincke BG. The morbidity and mortality conference: the delicate nature of learning from error. Acad Med. 2002;77(10):1001–1006. doi: 10.1097/00001888-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 103.Meguid C, Ryan CE, Edil BH, et al. Establishing a framework for building multidisciplinary programs. J Multidiscip Healthc. 2015;8:519–526. doi: 10.2147/JMDH.S96415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gritti A, Pisano S, Catone G, Iuliano R, Salvati T, Gritti P. Psychiatric and neuropsychological issues in Marfan syndrome: a critical review of the literature. Int J Psychiatry Med. 2015;50(4):347–360. doi: 10.1177/0091217415612701. [DOI] [PubMed] [Google Scholar]
- 105.Velvin G, Bathen T, Rand-Hendriksen S, Geirdal AO. Systematic review of the psychosocial aspects of living with Marfan syndrome. Clin Genet. 2015;87(2):109–116. doi: 10.1111/cge.12422. [DOI] [PubMed] [Google Scholar]
- 106.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 107.Karoff M, Held K, Bjarnason-Wehrens B. Cardiac rehabilitation in Germany. Eur J Cardiovasc Prev Rehabil. 2007;14(1):18–27. doi: 10.1097/HJR.0b013e3280128bde. [DOI] [PubMed] [Google Scholar]
- 108.McKee R. Story: Style, Structure, Substance, and the Principles of Screenwriting. New York: Harper Collins; 2010. [Google Scholar]
- 109.Freidson E. Profession of Medicine: A Study of the Sociology of Applied Knowledge. Chicago, IL: University of Chicago Press; 1988. [Google Scholar]
- 110.Contributors W [webpage on the Internet] ‘Game of Thrones’, Wikipedia, The Free Encyclopedia, 9 May 2016, 15:05 UTC. [Accessed May 10, 2016]. Available from: https://en.wikipedia.org/w/index.php?title=Game_of_Thrones&oldid=719414408.
- 111.Mintzberg H. Mintzberg on Management: Inside our Strange World of Organizations. New York: Free Press; London: Collier Macmillan; 1989. [Google Scholar]
- 112.Lega F, DePietro C. Converging patterns in hospital organization: beyond the professional bureaucracy. Health Policy. 2005;74(3):261–281. doi: 10.1016/j.healthpol.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 113.Poulton BC, West MA. Effective multidisciplinary teamwork in primary health care. J Adv Nurs. 1993;18(6):918–925. doi: 10.1046/j.1365-2648.1993.18060918.x. [DOI] [PubMed] [Google Scholar]
- 114.Davies H, Nutley S, Mannion R. Organisational culture and quality of health care. Qual Health Care. 2000;9(2):111–119. doi: 10.1136/qhc.9.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sachweh A, von Kodolitsch Y, Kölbel T, et al. I-SWOT als Instrument zur individuell optimierten Therapie bei thorakoabdominalem Aortenaneurysma [I-SWOT as an instrument to individually optimize therapy of thoracoabdominal aortic aneurysm: effective, norm-compliant, and meeting the needs] Gefäßchirug. 2016 In press. German. [Google Scholar]
- 116.Hoyle D. ISO 9000 Quality Systems Handbook: Using the Standards as a Framework for Business Improvement. Butterworth-Heinemann; 2009. [Google Scholar]
- 117.Bergeson SC, Dean JD. A systems approach to patient-centered care. JAMA. 2006;296(23):2848–2851. doi: 10.1001/jama.296.23.2848. [DOI] [PubMed] [Google Scholar]
- 118.Sheikhzadeh S, Kusch ML, Rybczynski M, et al. A simple clinical model to estimate the probability of Marfan syndrome. QJM. 2012;105(6):527–535. doi: 10.1093/qjmed/hcs008. [DOI] [PubMed] [Google Scholar]
- 119.David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg. 1992;103(4):617–621. [PubMed] [Google Scholar]