Abstract
Introduction
The Göttingen Minipig (GM) is used as large animal model in articular cartilage research. The aim of the study was to introduce osteoarthritis (OA) in the GM by resecting the anterior cruciate ligament (ACLR) according to Pond and Nuki, verified by histological and magnetic resonance imaging (MRI) scoring as well as analysis of gene and protein expression.
Materials and Methods
The eight included skeletally mature female GM were assessed after ACLR in the left and a sham operation in the right knee, which served as control. 26 weeks after surgery the knee joints were scanned using a 3-Tesla high-field MR tomography unit with a 3 T CP Large Flex Coil. Standard proton-density weighted fat saturated sequences in coronal and sagittal direction with a slice thickness of 3 mm were used. The MRI scans were assessed by two radiologists according to a modified WORMS-score, the X-rays of the knee joints by two evaluators. Osteochondral plugs with a diameter of 4mm were taken for histological examination from either the main loading zone or the macroscopic most degenerated parts of the tibia plateau or condyle respectively. The histological sections were blinded and scored by three experts according to Little et al. Gene expression analysis was performed from surrounding cartilage. Expression of adamts4, adamts5, acan, col1A1, col2, il-1ß, mmp1, mmp3, mmp13, vegf was determined by qRT-PCR. Immunohistochemical staining (IH) of Col I and II was performed. IH was scored using a 4 point grading (0—no staining; 3-intense staining).
Results and Discussion
Similar signs of OA were evident both in ACLR and sham operated knee joints with the histological scoring result of the ACLR joints with 6.48 ± 5.67 points and the sham joints with 6.86 ± 5.84 points (p = 0.7953) The MRI scoring yielded 0.34 ± 0.89 points for the ACLR and 0.03 ± 0.17 for the sham knee joints. There was no correlation between the histological and MRI scores (r = 0.10021). The gene expression profiles as well as the immunohistochemical findings showed no significant differences between ACLR and sham knee joints. In conclusion, both knee joints showed histological signs of OA after 26 weeks irrespective of whether the ACL was resected or not. As MRI results did not match the histological findings, MRI was obviously unsuitable to diagnose the OA in GM. The analysis of the expression patterns of the 10 genes could not shed light on the question, whether sham operation also induced cartilage erosion or if the degeneration was spontaneous. The modified Pond-Nuki model may be used with reservation in the adult minipig to induce an isolated osteoarthritis.
Introduction
Most of the in vivo animal models used for the investigation of osteoarthritis (OA) have undergone an intervention in the targeted joint [1,2,3,4]. Changes in the articular cartilage were documented after different periods following the operation as shown in literature after1-14 days [5], 2–6 weeks [6] and 6–52 weeks [7]. Instability should induce changes similar to that in human articular cartilage as described in several publications [8,9,10]. The Pond Nuki [11] model may reflect the degenerative changes in natural OA in dogs and humans [12]. However, the resection of the anterior cruciate ligament (ACL) as starting point of degenerative changes of articular cartilage still remains an injury induced OA [13]. Therefore, development of degenerative changes of articular cartilage is expected in stifle joints after resection of the ACL but not in untreated contralateral joints [6,14]. Development and grading of OA is mainly determined by histological analyses [4,6,14]. In humans, grade and prevalence of OA may be detected by MRI scanning [15,16] and through X-ray examination [17]. Thus, correlation of histological changes and MRI scanning is needed in diagnosis of OA. Emergence of OA is characterized by alterations of gene expression patterns in degenerating cartilage [18,19,20]. That is also true for the animal model of ACLT in the minipig [5].Here the published data address several genes with different behaviour of regulation. Thus, e.g. col II may be upregulated according to one [21] but downregulated according to another trial with similar study design [22].
The pig and notably the Göttingen Minipig (GM) is often used in articular cartilage research [5,17,23,24] and is also a common model for the study of regeneration of focal cartilage defects [7,25,26] but the transection of the ACL has rarely been performed in this species up to now [6]. For the articular cartilage research skeletal mature individuals are required as older pigs may mirror the human condition better, as OA is mostly an affliction of older people whether or not there were previous injuries [27,28].
The aim of the study was to induce OA in adult GM by resection of the anterior cruciate ligament (ACLR), verified by histological and MRI scoring as well as analysis of gene and protein expression.
Material and Methods
Animals and surgery
Eleven skeletally mature female GM (Faculty of Agricultural Sciences, Animal Breeding and Genetics, Georg-August-Universität, Göttingen, Germany) underwent resection of the anterior cruciate ligament in the left knee. The right knee served as control by sham operation. Two minipigs served as pilot animals. According to the ARRIVE guidelines for reporting animal research [29] we have to notify that one animal was replaced because it had to be killed in accordance with the animal welfare officer due to a very unlikely recovery from an infection of the left knee joint one week after surgery. The life time was 26 weeks.
8 animals were therefore included in our study. Their age was on average 24.75 ± 0.66 months, with a median of 25 months, ranging from 23–25 months. The animal body weights (BW) were on average 55.4 kg ± 9.4 kg, a median of 55.2 kg ranging from 44–67 kg. The study was approved by the ethical committee of the Regierungspräsidium Karlsruhe, Abteilung 3—Landwirtschaft, Ländlicher Raum, Veterinär- und Lebensmittelwesen, Karlsruhe, Germany. The approval has the number: 35–9185.81/G-186/09. The animal welfare officer of the IBF (Interfakultäre Biomedizinische Forschungseinrichtung, Heidelberg, Germany) accompanied the study.
The intramuscular premedication was carried out with azaperon (4 mg/kg; Stresnil, Janssen, Beerse, Belgium), ketamin (10 mg/kg; Ketavet 10%, Vet Way, Elvington, England) and midazolam (1 mg/kg; Midazolam-ratiopharm, Ratiopharm, Ulm, Germany), whereas intravenous application of propofol (1–2mg/kg; Propofol-Lipuro, Braun, Melsungen, Germany) initiated the anaesthesia and allowed the endotracheal intubation. The anesthesia was then maintained with isoflurane (1.5-vol%; Forene®, Abbott, Wiesbaden, Germany) under artificial respiration. Perioperative infection prophylaxis was done by intravenously administered cefazolin (2 g/animal; Basocef Trockensubstanz, Deltaselect, Pfullingen, Germany). Surgery was performed under continuous monitoring of vital parameters and depth of narcosis. After skin incision between patella and tuberosity, the subcutaneous tissue was retraced with the patellar ligament as a key structure. The knee joint was then opened medially of the patellar ligament and the patella was luxated if necessary. Afterwards, the depicted anterior cruciate ligament (ACL) was cut and resected with a hook electrode (Aesculap AG, Tuttlingen, Germany) preventing a reconnection of the parts. Afterwards, closing of the wound was done in layers after lavage. Then the wound was covered by a plaster to prevent dirt from entering the wound. A drawer test was performed to confirm instability by the resection of the ACL. The right knee was equally opened, purged and closed, but no cut/resection took place. Analgesia was performed with fentanyl (0.2 mg/animal; Fentanyl-Janssen, Janssen, Neuss, Germany) and carprofen (4 mg/kg; Rimadyl Rind, Pfitzer, Berlin, Germany). All animals were closely monitored for signs of pain, illness and unusual favouring of the hind legs after operation.
Housing and sacrifice of the animals
The animals were allowed to acclimatize before the operation procedure. Water was provided ad libidum. Full weight bearing and free motion was possible before and after surgery. After the operation, the animals were monitored daily. Carprofen (4 mg/kg; Rimadyl Rind, Pfitzer, Berlin, Germany) was applied when needed. 10 days after surgery, the animals were transferred to a licensed farmer and were cared for the remaining life time. 26 weeks after the operation, the animals were first assessed by MRI and x-ray under anaesthesia (see below) and then killed by intravenous application of 20 mL saturated KCl-solution in deep narcosis under electrocardiographic supervision. Afterwards, a positive drawer sign in the left knee confirmed an inoperable ACL, but there was no positive sign in the right knee.
Preparing the animals for MRI
As the pilot trials showed, the animals had to be continuously sedated to prevent movements of the animals which would distort the scanning process. Thus, the MRI assessment was performed under general anesthesia with pentobarbital (Narcoren®, Merial GmbH, Halbergmoos, Germany) given continuously i.v. (10mg/kg per hour). The medication was applied via a perfusor (Terufusion TE-311, Terumo, Eschborn, Germany) under artificial ventilation (Servo 300A, Maquet, Rastatt, Germany). Premedication was performed analogous to the ACL-surgery. Furthermore, a central vein catheter was placed into the jugular vein to provide a safe and large venous access [30]. Additionally, a transurethral catheter was placed [30] and the animals were wrapped in diapers to prevent pollution. The assessments were performed under constant monitoring. Soft towel paper was plugged into their ears to reduce noise exposure.
MRI assessment
On the scheduled day of killing, the MRI-scans were performed consecutively on both knee joints of each minipig. A 3-Tesla high-field magnetic resonance tomography unit (Magnetom Trio, Siemens, Erlangen, Germany) and a 3T CP Large Flex Coil (Siemens, Erlangen, Germany) were used. After a localizing scan, scanning procedures with standard proton-density weighted fat saturated sequence in coronal and sagittal direction, slice thickness 3 mm with 10% gap, adapted from human scanning procedures were applied. The digital scans were then blinded and afterwards assessed by two radiologists who had more than 8 years experience each in musculoskeletal MRI (DD, AU) on an external work station (MacPro, Apple Inc., Cupertin, CA). An FDA approved OsiriX MD Imaging Software was used. The WORMS-score [31] for degenerative signs of the cartilage was adopted. Finally a consensus between the two experts’ assessments was reached.
X-ray assessment
Both knee joints of each minipig, which were still under anaesthesia, were x-rayed in anterior-posterior position with manually extended limb and in lateral position. The focal distance was 1.3 m and the x-ray tube voltage was 66 kV. The exposure time varied as it was automated by the device (DigitalDiagnost, Philips, Hamburg, Germany). The images were then appraised according to the Kellgren and Lawrence [32] scoring system by one senior physician (MS) and one medical student (MK).
Sample collection
After killing the pig, the left and right knee of each minipig were opened and macroscopically examined for signs of change. Afterwards, a punch with 4 mm in diameter was used to excise a sample from the medial and lateral condyle and the medial and lateral tibia plateau surface. Each sample was taken from the most affected area or, if no change was macroscopically visible, from the main loading zone. These samples were then fixated in 4% formaldehyde for histology and immunohistochemistry. Now the cartilage in a diameter of 10 mm in the zone around the punched hole was excised down to the bone with a scalpel and stored in liquid nitrogen for later isolation and processing of the RNA. The used instruments were rendered RNAse free by treatment with hydrogen peroxide [33].
Histology
The formalin-fixed femoral and tibial osteochondral specimens from the joints were washed and decalcified with EDTA (Merck, Darmstadt, Germany) for 3–4 weeks. The samples were then washed to remove EDTA, then automatically (TP1020 Leica, Wetzlar, Germany) dehydrated and afterwards embedded in paraffin with a casting implement (EG1140C Leica, Wetzlar, Germany) for cutting. 5 μm thick slides were sectioned (RM2165 Leica, Wetzlar, Germany) and mounted on Superfrost-Plus glass slides (R. Langenbrick, Emmendingen, Germany). Prior to staining, the slides were deparaffinised with xylene and rehydrated in a graded series of ethanol/water blend. To show overall tissue morphology, the deparaffinised slides were subjected to an adapted hematoxylin/eosin (HE-staining) staining protocol [34] for an unsophisticated assessment of cell and tissue morphology by a light microscope (DMRE Leica, Wetzlar, Germany) with an attached camera (DFC 300 FX Leica, Wetzlar, Germany). We did not aim to perform a statistically based analysis by evaluation of the HE—stained tissue. Furthermore, a safranin-o staining was conducted to evaluate the distribution of proteoglycans in the cartilage and the slides were surveyed with the aforementioned equipment. The slides were blinded, examined and assessed by 3 experts (WR, MW-E, MS) according to the Little-score [35]. Afterwards, a consensus between the 3 experts was reached.
HE-staining
After deparaffinization and rehydration of the slides, they were stained with Mayers hematoxylin for the duration of 10 minutes (Merck, Darmstadt, Germany) and then washed in tap water for 15 minutes. Now they were stained with eosin (Carl Roth, Karlsruhe, Germany) for 3 minutes, followed by dehydration in an ascending alcoholic concentration. The conservation was carried out with Eukitt (O. Kindler GmbH, Freiburg, Germany).
Safranin O-staining
The slides were deparaffinised, rehydrated and stained in Weigerts hematoxylin (Carl Roth, Karlsruhe, Germany) for 8 minutes, washed in tap water for 10 minutes, stained with Fast Green (Merck, Darmstadt, Germany) for 5 minutes and differentiated with 1% acetic acid (Merck, Darmstadt, Germany) for 5 seconds. Afterwards, the specific staining of the proteoglycans was done with Safranin-o (Sigma-Aldrich Chemie GmbH, Munich, Germany). Then the slides were dehydrated in an ascending alcoholic concentration and conserved in eukitt (O.Kindler GmbH, Freiburg, Germany).
Immunohistochemistry
Additionally, the slides were subjected to an immunohistochemistry with COL1A1 (sc59772, Santa Cruz Biotechnology Inc., Heidelberg, Germany) and COL2 (ab3092, abcam, Cambridge, UK) antibodies. After deparaffinization of the slides with xylene and rehydration in a graded series of ethanol/water blend, they were then fixed for 30 min in H2O2 and afterwards subjected to antigen retrieval (heat induced: 10mM sodium citrate, pH 6.0) for 20 minutes. After they had been washed and then blocked with 2% BSA for 80 minutes, the slides were incubated for 70 minutes with the respective antibody (Dilution 1:100 COL1A1, 1:50 COL2) at room temperature and then washed again. The secondary antibody (Vectastain Universal Elite Kit, Vector Laboratories, Burlingame CA, US) in a 1:100 dilution was now applied for 50 minutes and, after additional washing, the ABC-complex (Vectastain Universal Elite Kit, Vector Laboratories, Burlingame CA, US) was applied for another 30 minutes. After this process the slides were stained with 3,3’-diaminobenzidine (Vector Laboratories, Burlingame CA, US) for 4 minutes in the dark and then washed for 5 minutes in tap water. After dehydration in an ascending alcoholic concentration and xylene, the slides were conserved in eukitt (O.Kindler GmbH, Freiburg, Germany). The individual slides were examined using a light microscope (DMRE Leica, Wetzlar, Germany) with an attached camera (DFC 300 FX Leica, Wetzlar, Germany). The slides were assessed according to their staining with 0 (no staining), 1 (little), 2 (moderate) and 3 (distinct) points (GR).
Quantitative PCR
The cartilage samples stored in liquid nitrogen were homogenized (POLYTRON PT 3000, Kinematica AG, Lucerne, Switzerland) in TRIzol® (Life Technologies GmbH, Darmstadt, Germany) and the RNA was isolated according to the manufacturer’s instructions. Afterwards, the RNA-samples were cleaned with a mini-kit column (Mini Kit, Qiagen, Hilden, Germany). The cleaned RNA was then transcribed into cDNA (Sensiscript RT Kit, Qiagen, Hilden, Germany). To obtain a standard curve for each gene, cDNA was submitted to a PCR with the specific primers for each gene: collagen 1A1 (col1A1), collagen 2 (col2), aggrecan (acan), interleukin 1ß (il-1ß), matrix metalloproteinase -1 (mmp1), -3 (mmp3), -13 (mmp13), Vascular endothelial growth factor (vegf), ADAM metallopeptidase with thrombospondin type 1 motif, 4 and 5 (adamts4 and adamts5) and ß-actin (actb); (primer sequences see Table 1). The resulting PCR-product was cleaned with an agarose gel followed by gel extraction (QIAquick Gel Extraction Kit, Qiagen, Hilden). Resulting DNA-concentration was measured and ten fold dilutions were conducted for each gene. Afterwards, a quantitative real time PCR (qRT-PCR) was carried out in duplicate for each dilution on a qPCR-System (Mx3005 P Stratagene, Waldbronn, Germany) with the QuantiTect SYBR-Green PCR Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions and gene specific annealing temperatures. Thus, a standard curve for each gene was created. Each sample RNA was transcribed as mentioned above and submitted to a qRT-PCR in duplicate, with the housekeeping gene ß-actin as reference [36].
Table 1. Sequences of the used primers and their references.
| Name | Sequence (F: forward / R: reverse) | PCR-Product size [base pairs] | Author |
|---|---|---|---|
| Collagen 1A1 | F: CCAACAAGGCCAAGAAGAAG | 64 | Gredes et al. |
| R: ATGGTACCTGAGGCCGTTCT | 2007 [37] | ||
| Collagen 2 | F: CACGGATGGTCCCAAAGG | 102 | Steck et al. |
| R: ATACCAGCAGCTCCCCTCT | 2009 [38] | ||
| Aggrecan | F: TTCCCTGAGGCCGAGAAC | 194 | Zou et al. |
| R: GGGCGGTAATGGAACACAAC | 2008 [39] | ||
| Interleukin 1ß | F: CAGCCATGGCCATAGTACCT | 216 | Tayade et al. |
| R: CCACGATGACAGACACCATC | 2006 [40] | ||
| MMP 1 | F: TCAGTTCGTCCTCACTCCAG | 321 | Le Graverend et al. |
| R: TTGGTCCACCTGTCATCTTC | 2002 [41] | ||
| MMP 3 | F: AATGATCACTTTTGCAGTTCGAGAA | 76 | Zeni et al. |
| R: GGCATGAGCCAAAACTTTTCC | 2007 [42] | ||
| MMP 13 | F: TTGATGATGATGAAACCTGGA | 69 | Gredes et al. |
| R: ACTCATGGGCAGCAACAAG | 2007 [37] | ||
| VEGF | F: GAGACCAGAAACCCCACGAA | 138 | Zhou et al. |
| R: GCACACAGGACGGCTTGAA | 2009 [43] | ||
| ADAMTS-4 | F: CAGGGTCCCATGTGCAACGT | 115 | Huang et al. |
| R: CATCTGCCACCACCAGGGTCT | 2011 [44] | ||
| ADAMTS-5 | F: TTCGACATCAAGCCATGGCAACTG | 197 | Huang et al. |
| R: AAGATTTACCATTAGCCGGGCGG | 2011 [44] | ||
| ß-actin | F: CAAGGAGAAGCTCTGCTACG | 245 | Steck et al. |
| R: AGAGGTCCTTCCTGATGTCC | 2009 [38] |
Statistical Analysis
Statistical analysis was done using the software SAS 9.3 (SAS, Heidelberg, Germany), Excel 2010 (Microsoft, Redmond, WA, USA) and Origin 8.6.0.G (OriginLab Corporation, Northampton, MA, USA). Correlation was performed between the histological and MRI results, also between the histological and gene expression results and finally, between the histological and immunohistochemical findings. The difference between the results representing the ACLR side and the sham side (right) of the knee was tested using the t-test procedure. The level of significance was 0.05. In the box and whisker plots the grey boxes represent 25% to 75% of the values, the whiskers show the minima/ maxima and the small black square represents the mean, whereas the line in the grey box illustrates the median.
Sample size calculation was done for the study regarding changes in the menisci in the stifle joints, which is described elsewhere [45].
Results
From the 11 GM that were operated, two served as pilot animals to establish the MRI procedures in particular and one replaced an animal which had to be killed as it was suffering from an infection of the ACLR knee joint.
At the day of their killing the included animals showed normal gait patterns.
The drawer test was performed for all knee joints immediately after the animals had been killed. Whereas all the ACLR knee joints showed a positive drawer sign with higher laxity, the sham knee joints showed a negative drawer sign with a hard stop.
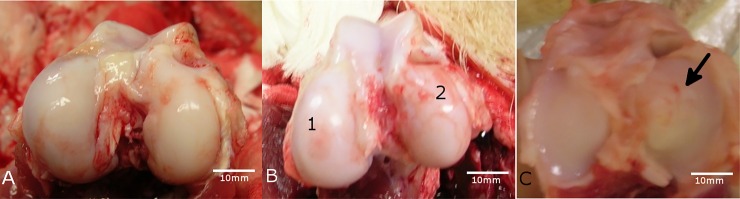
The macroscopical assessment of the tibial and condylar cartilages of the knee joints showed various states of deterioration, from smooth hyaline cartilage layer (no change) up to vanished cartilage with exposition of the subchondral bone (Fig 1A–1C).
Fig 1. Macroscopic overview.
A) The femoral condyles of the ACLR knee joint of animal #8952 showed no visible change/deterioration of the cartilage. B) The femoral condyles of the ACLR knee joint of animal #8955 showed different characteristics in form of a reduced cartilage coating (1) in the lateral condyle and even erosion of the cartilage (2) in the medial condyle. C) The lateral tibia plateau surface of the sham-operated knee joint of animal #8958 showed a slightly rough cartilage surface (arrow). A-C: The scale bars were determined with the corresponding x-ray images using the program RadiAnt DICOM viewer (build 3.4.1.13367, retrieved from http://www.radiantviewer.com; October 12th 2016; Medixant (Poznan, Poland)).
The histological analysis of the cartilage samples exhibited variable degeneration grades throughout the examined sites of the knee joints (Figs 2–6).
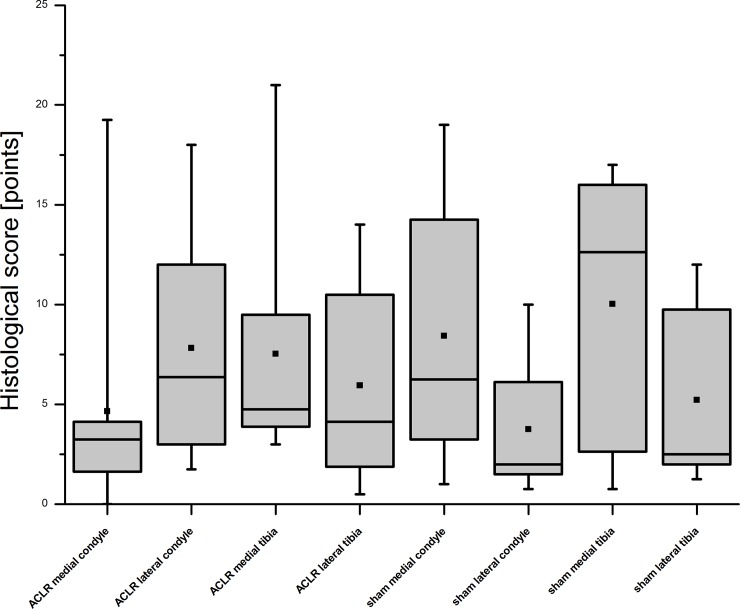
Fig 2. Results of the histological scoring after consensus.
The score for each area of interest–the medial/lateral condyle and the medial/lateral tibiaplateau of the ACLR and sham-operated knee joints- of eight Göttingen Minipigs are shown. The used score [35] ranges from 0 points with no changes up to 25 points. As the results of the scoring showed, the sham knee joints were also affected with no significant differences between the ACLR and sham operated knee joints.
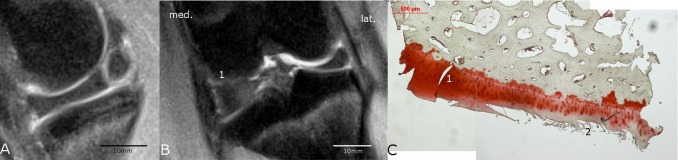
Fig 6.
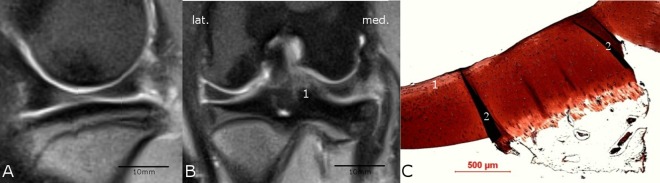
Comparison of MRI (A and B) with histological findings (C). The medial condyle region of the ACLR knee joint of animal #8955 showed in the sagittal MRI-scan (A) through the medial compartment no salience but the coronal MRI-scan (B) showed a signal irregularity (1) in the cartilage layer and was therefore rated as pathologically changed. The scale bars for A and B were determined with the program RadiAnt DICOM viewer (see caption Fig 1). The histological safranin-o staining (C) showed a severe degeneration (Little-score: 19.25 points) with a fissure (1) reaching to the subchondral lamella and severe erosion (2) of the cartilage. Reduced safranin-o staining of the remaining cartilage tissue indicated a reduced GAG content and a severe loss of chondrocytes could be seen. The histological and MRI results did match.
The changes ranged from none with a smooth surface (Fig 3C) or very small surface irregularities of the cartilage (Fig 4C) to fissures in the cartilage surface (Fig 5C) up to erosion of the whole cartilage (Fig 6C). Also the chondrocyte density varied and a cloning of cells was evident. The interterritorial staining was reduced in some of the samples. The outcome of the histological scoring for each area is presented in Fig 2. The mean score of the ACLR medial condyle sample was 4.66 ± 5.69 points, respectively 7.81 ± 5.48 points for the ACLR lateral condyle. The ACLR medial and lateral tibia plateaus were scored as 7.53 ± 5.66 and 5.94 ± 4.85 points. The sham medial and lateral condyle samples showed a score of 8.44 ±6.20 respectively 3.75 ± 3.11 points. And the sham medial and lateral tibia plateaus displayed 10.03 ± 6.47 and 5.22 ± 4.18 points. No significant differences (p = 0.7953) between the ACLR and sham-op knees were found (6.48 ± 5.67 vs. 6.86 ± 5.84 points).
Fig 3.
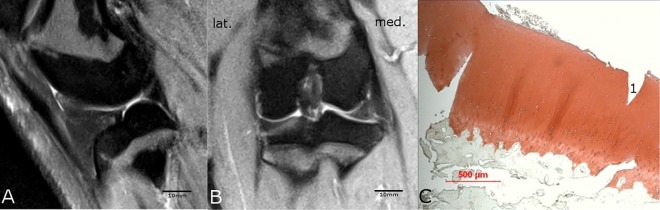
Comparison of MRI (A and B) with histological findings (C). The medial condyle region of the ACLR knee joint of animal #8958 showed no salience neither in the sagittal scan through the medial compartment of the knee (A) nor in the coronal MRI-scan (B). The scale bars for A and B were determined with the program RadiAnt DICOM viewer (see caption Fig 1). The histological safranin-o staining (C) showed a normal cartilage tissue (Little-score: 0 points). The histological and MRI results did match.
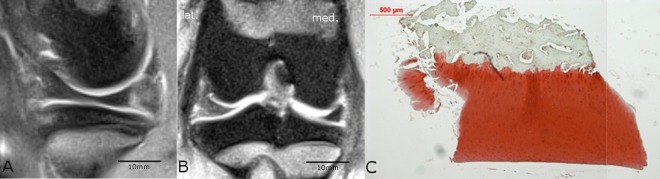
Fig 4.
Comparison of MRI (A and B) with histological findings (C). The medial tibia plateau region of the ACLR knee joint of animal #8952 showed in the sagittal MRI-scan (A) through the medial compartment no salience but in the coronal MRI-scan (B) a signal irregularity (1) of the normally bright appearing cartilage was present and was therefore rated as pathological; no cyst or osteophytes were present. The scale bars for A and B were determined with the program RadiAnt DICOM viewer (see caption Fig 1). C) The histological safranin-o staining showed a mild degeneration (Little-score: 4.0 points) with only a slight reduction in staining (1). The darker formations in the cartilage (2) were interpreted as creases of the slice and were not included in the scoring. Thus, the histological and MRI findings did not match.
Fig 5.
Comparison of MRI (A and B) with histological findings (C). The lateral tibia plateau region of the sham knee joint of animal #8958 showed no salience neither in the sagittal MRI-scan (A) through the lateral compartment of the knee nor in the coronal MRI-scan (B) and was therefore rated as unchanged. The scale bars for A and B were determined with the program RadiAnt DICOM viewer (see caption Fig 1). In contrast to the MRI results, the histological safranin-o staining (C) showed a moderate degeneration (Little-score: 10.5 points) with a fissure (1) in the cartilage and detachment of the topmost layer of the cartilage tissue. The histological finding and the MRI result did not concur.
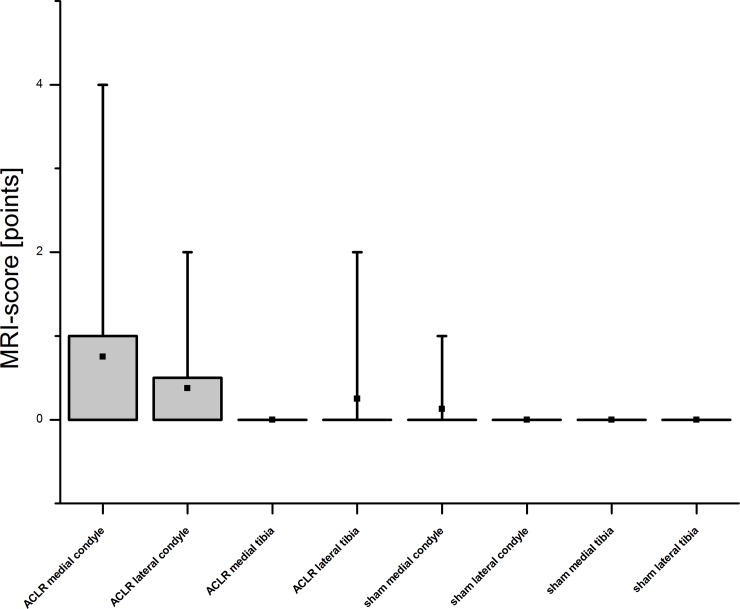
In the MRI examination only few changes of the cartilage were evident (Fig 7) in terms of reduced or even vanished signal strength or other irregularities (Figs 4B and 6B). The successful resection of the ACL was verified in the MRI scans (Fig 8). However, no subchondral edema could be detected and the cartilage layer was thin in the assessed regions. The ACLR medial and lateral condyle showed a MRI-score of 0.75 ± 1.39 and 0.38 ± 0.7 while the ACLR lateral tibia plateau showed a score of 0.25 ± 0.66 and the medial tibia plateau a score of 0. On the sham side, the medial condyle showed a score of 0.13 ± 0.33, whereas the lateral condyle and the medial/lateral tibia plateaus showed a score of 0. No correlation was evident between the histological and MRI scores (r = 0.10021). Also after unblinding the samples, closer examination of the histological findings and MRI scoring showed concurrence in the detected degeneration signs (Figs 3 and 6) in some cases, but in others no concurrence was found (Figs 4 and 5).
Fig 7. MRI scoring.
MRI scans of eight Göttingen Minipigs were scored according to an adapted WORMS-score. The score ranges from 0–22 points. The results showed that in the samples, either from ACLR or sham knee joints, no or only minor changes were visible in the MRI images.
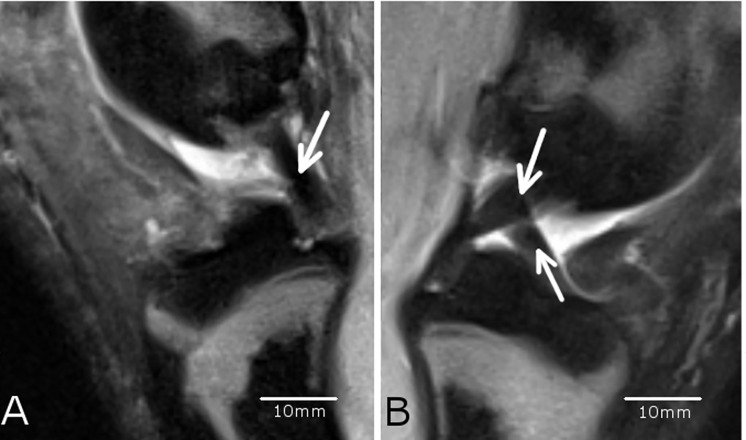
Fig 8. Presence of the ligaments.
The ACLR knee joint A) of animal #4085 showed only the ligamentum cruciatum posterius (arrow) whereas the sham knee joint B) of the same animal showed both cruciate ligaments were present (arrows). This showed that the operation of resecting the ligamentum cruciatum anterius was successful (A). A, B: The scale bars were determined with the program RadiAnt DICOM viewer (see caption Fig 1).
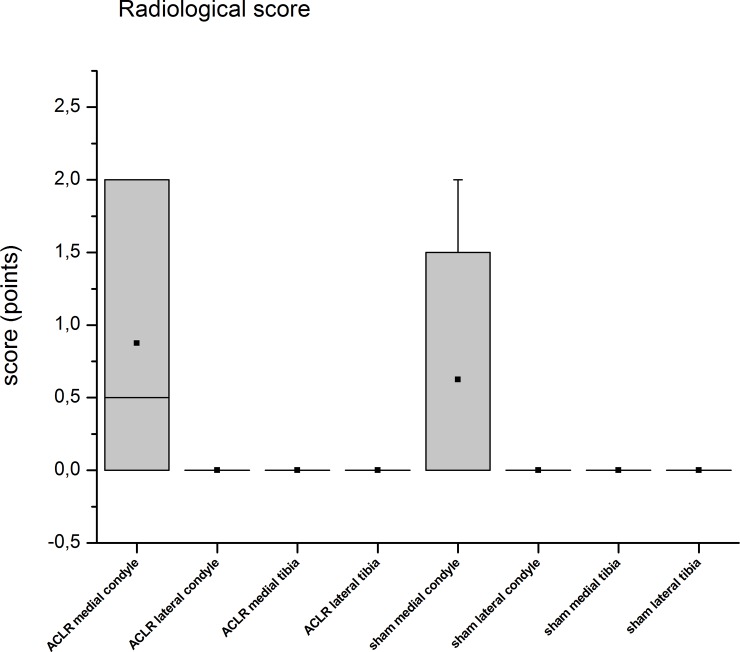
The x-ray assessment revealed signs of degeneration for the medial condyles of either the ACLR or sham knee joints Fig 9. There were no distinct differences between the left and right knee joints. Examination of the narrowing of the joint cavity was not possible, as the legs of the animals had to be extended manually. In some of the ACLR knee joints osteophytes had formed.
Fig 9. Radiological grading.
Radiological grading (from 0 to 4) according to the Kellgren and Lawrence Score [32]. The examined areas of the ACLR and sham sites showed similar findings in terms of degeneration. This also supported our assumption that the sham-operated knee joints may not qualify as a reliable control group. Another aspect of the x-ray analysis was that only the medial condyles of the ACLR and sham sites showed changes of any kind. This would indicate that changes would be initially starting in the medial condyle.
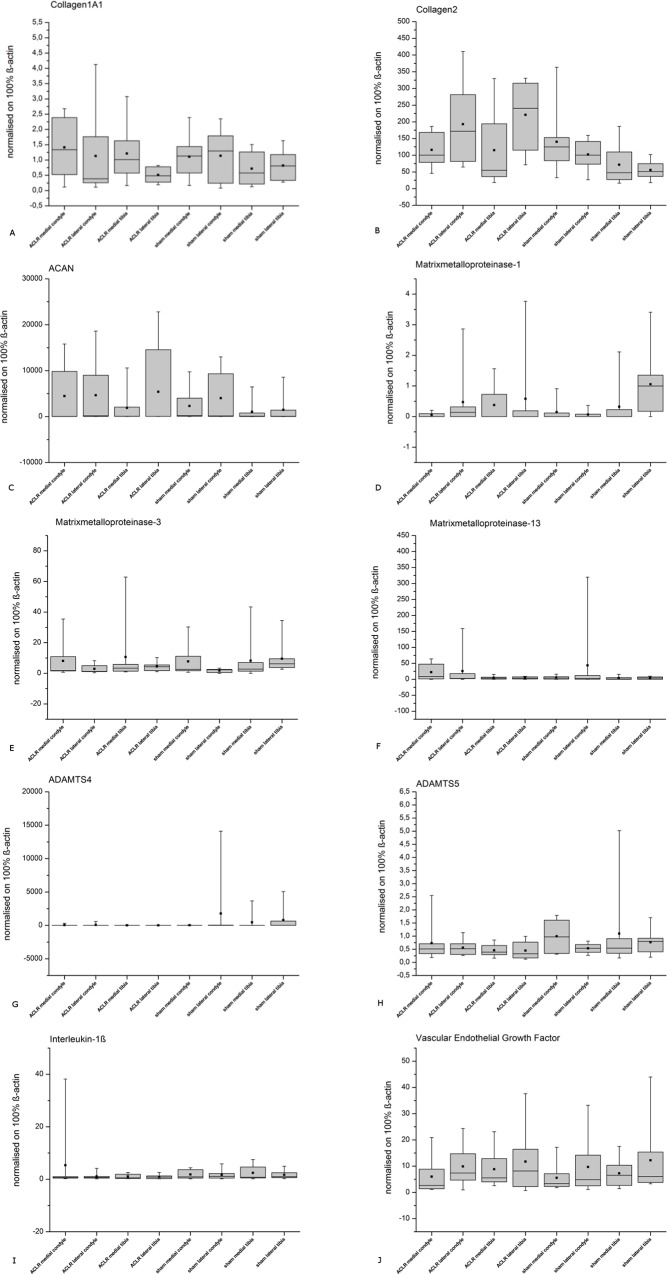
No evident changes in the gene expression profiles were obvious for any of the tested genes, neither in the ACLR nor in the sham group. As the results showed small differences in the mean and high standard deviations, no significant differences were assumed at all (Fig 10). No correlation between the histology and gene expression was seen (r-values Table 2).
Fig 10. Gene Expression.
Normalized (ß-actin) gene expression for the evaluated genes from the examined regions of ACLR and sham knee joints. The results showed no evident differences between the ACLR and the sham operated side for the assessed genes A) col1A1, D) mmp1, E) mmp3, F) mmp13, H) adamts5, I) il-1ß and J) vegf. This would concur with the histological findings, where the sham operated group was as degenerated as the ACLR group and therefore not usable as a serious control group. The gene expressions of B) col2, C) acan and G) adamts4 at first sight appeared to be differently expressed but as standard deviation was high, significant differences were not found. So no significant differences between the ACLR and sham sites were found in terms of gene expression in the end and therefore, no discrimination between an induced (ACLR) and spontaneous (sham) OA could be made.
Table 2. Correlation gene expression vs histology.
Correlation (r-values) of the gene expression findings with the scored histological findings.
| Name | r-value |
|---|---|
| Collagen 1A1 (col1A1) | 0,20395 |
| Collagen 2 (col2) | 0,14545 |
| Aggrecan (acan) | -0,19936 |
| Matrix metalloproteinase 1 (mmp1) | -0,09115 |
| Matrix metalloproteinase 3 (mmp3) | -0,07496 |
| Matrix metalloproteinase 13 (mmp13) | -0,06827 |
| ADAM metallopeptidase with thrombospondin type 1 motif, 4 (adamts4) | -0,17432 |
| ADAM metallopeptidase with thrombospondin type 1 motif, 5 (adamts5) | 0,03295 |
| Interleukin 1ß (il-1ß) | -0,17442 |
| Vascular endothelial growth factor (vegf) | -0,12195 |
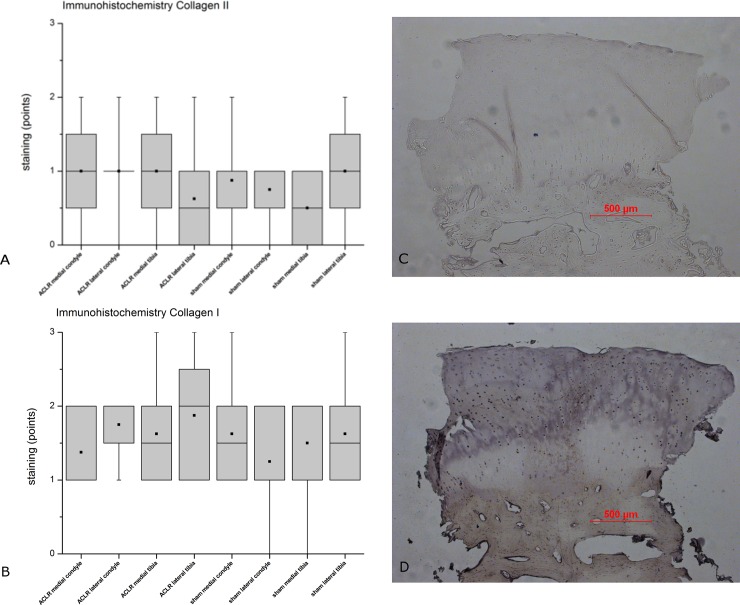
The immunohistochemical analysis of collagens I and II of the samples is shown in Fig 11. Analogous to the histology and gene expression, no differences between the ACLR and sham knee joints for either collagen I or II were found. Neither could a correlation between the histological and immunohistochemical findings be found (r = 0.11607 for collagen I and r = 0.07921 for collagen II).
Fig 11. Immunohistochemical staining.
Results of the immunohistochemical staining of the examined regions of ACLR and sham operated knee joints with A) collagen II and B) collagen I. The score ranges from 0 (no staining) to 3 points. The results indicated no evident differences between the ACLR side and the sham side for neither Col II (A) nor Col I (B). This was in concurrence with our histological results (Fig 2). Sample of an immunhistological staining of collagen II (C) and collagen I (D) from the moderate degenerated (histological score: 9 points) sham operated side of the lateral tibia plateau of animal #8952 with an intense staining for collagen I (3 points) and a weak staining for collagen II (1 point).
Discussion
This study was aiming to induce osteoarthritis (OA) in Göttingen Minipig (GM) through the resection of the anterior cruciate ligament (ACLR) according to Pond and Nuki [11]. The resection of the ACL rather than a transection was chosen to prevent a possible self-healing of the ligament, which was described by Costa-Paz et al. [46]. The findings were then to be verified by histological and MRI scoring as well as an analysis of gene and protein expression. Although the Pond-Nuki model is widely used with dogs [12,47,48], rabbits [1,49], cats [50], lapines [14] or rats [4,51], the model seems to be rarely used with GM, which is a common large animal model in articular cartilage research [5,17,52,53]. The Pond-Nuki- procedure with minipigs was described recently by Wei et al. [6] and the same model was used in Yucatan minipigs [5,54], the latter to study anterior cruciate ligament healing but not degeneration of the articular cartilage. To our knowledge, most animal models for degeneration of the cartilage are models in which a secondary osteoarthritis is induced either by creating an instability in the knee joint by ACLT or ACLT plus menisectomy [1,3,55,56,57], mechanically damaging the cartilage as groove model [3] or damaging the cartilage via chemicals e.g. by monosodiumiodoacetate [58]. Several small animal models for spontaneous OA are known [58,59], a common one is the model with Dunkin Hartley guinea pigs that develop spontaneous OA dependent on age [60]. With large animals, models of spontaneous OA exist as well, e.g. with dogs [61], that highlight the influence of age and breed, and macaques [62]. It was reported that the medial tibial plateau was the most severely affected site [62,63], which was only partially consistent with our findings (Fig 2). Unfortunately, the expression patterns in our study show no indication of the occurrence of spontaneous or induced OA.
The animal models for inducing a secondary osteoarthritis mainly use the histological “Mankin-score” [64] or a modified version [65,66,67,68] to score the degeneration of the cartilage. The original histopathology score was devised by the OARSI histopathology initiative [69] to evaluate OA histopathology. The follow-up initiative (2010) devised species specific consensus scoring systems [35,70,71,72,73,74,75] which are reasonably easy to use and can be readily adopted. There was no specific score available for the GM, thus the OARSI scoring system of Little et al. 2010 [35] for sheep and goats was used for the supposed comparability of the GM including size.
In literature, untreated knees are reported to have mostly a zero or very low mankin-score [55,76,77] but reports of higher scores are also published [78,79]. The results depend also on the area the cartilage was taken from, e.g. the medial or lateral, condyle or the tibia plateau [78,79]. It is reported that treated knee joints often revealed a higher level of degeneration than untreated knee joints [1,4,41,55,66,76,77] but some [78,79] showed similar levels of degeneration.
In the presented study, the analysis of the histopathology showed variations in the level of degeneration in all parts of all knees, as was also shown by Lorenz et al. [12]. The different levels of degeneration in our study were represented by fissures in variable length and quantity, by variable staining, a disparate chondrocyte density, differing cell cloning and tidemark integrity. However, we found no significant differences between the ACLR- and sham-operated joints unlike Frost-Christensen et al. [3]. But Frost-Christensen et al. [3] also resected the medial meniscus and therefore added an additional trauma. The unexpected similarities between ACLR- and sham-operated knee joints may have different explanations. The BW may be a risk factor to develop OA as discussed by [80,81] also for metabolic factors. The GM used in the presented study had a mean BW of 55kg at an age of approx. 24 months. The breeder of the animals (Relliehausen Experimental Farm, Dassel, Germany) reports a BW of 35–40kg for 24 months old GM of as normal [82]. The used animals in the presented study showed a 1.37 fold higher weight than assumed. Thus, the enhanced BW may contribute to the development of the OA in the pigs in both knee joints. Another reason might be the fact that the animals did not move around extensively during their life time in the present study. But as Miyataka et al. [83] showed, even forced running had small effects on cartilage degeneration in mice and Moriyama et al. [84] reported no degenerative changes due to exercise in rats. Cruz et al. [85] showed that a 20min of uninterrupted daily exercise had no effect on the joints of pigs but with additional obstacles it did. Elsaid et al. [86] found that the exercise of joints in rats resulted in an increased cartilage degeneration. Thus it is not clear if exercising may induce or accelerate degenerative changes in articular cartilage. Another problem with exercise in pigs may be that swines are hardly adapted to an exercise protocol or to protect weight-bearing as stated by Chu et al. [87]. Yet another explanation might be that the resection of only the anterior cruciate ligament and the imbalance of kinetics in the knee did not have the expected impact on the induction of osteoarthritis [88] in articular cartilage. However, in our study the sham-operated knee joints also showed signs of degeneration, which is at least in parts contrary to the findings of [89,90,91,92]. But one can assume that the sham site was exposed to higher forces as the contralateral ACLR site was protected. This could have induced degeneration by overstress leading to a secondary degeneration at the sham–operated side. What is more, the sham–operation itself could have induced changes in the joint [92], even though the inner structures were not altered through surgery despite arthrotomy and irrigation [89,90,91]. Thus it seems unlikely, that the sham–operation itself may be the cause of the higher degrees of change in the articular cartilage. Further studies [93,94,95,96,97] were able to show that the cartilage of sham operated animals was not distinguishable from non operated controls in terms of degenerative changes. Neither Calvo et al. [93] nor McDevitt et al. [96] could find significant differences between the non operated controls and the sham operated knees in their animal models with rabbits and dogs respectively. This was also confirmed later for a large animal model with sheep by O’Brien et al. [97]. Therefore the inclusion of a control group of animals without surgical treatment seemed not to be justified when the study protocol was performed also in terms of animal welfare (http://eur-lex.europa.eu, European directives on the protection of animals used for scientific purposes, DIRECTIVE 2010/63/EU, visited July 25th 2016 and [98]).
Yet another risk factor is age. GM reach their skeletal maturity with the closure of the growth plates which takes place between 18 and 22 month of age ((http://minipigs.dk, visited July 25th 2016; cited also by Chu et al. [87]). As growing pigs were shown to possess a higher intrinsic cartilage repair potential [99] they should be skeletally mature. On the other hand they should not be much older to reduce the danger of degenerative changes through to age [100]. These degenerative changes through aging were also addressed in human cartilage [101,102].
A further explanation for the changes in the sham-operated knee joints may be that each operation triggers an inflammatory response of the body which could lead to degeneration of the cartilage [103,104]. In the course of this, proinflammatory cytokines like IL-1ß are known to induce production of MMP´s which in return are degrading components of the articular cartilage [104]. Aigner et al. [18] on the other hand described a down-regulation of the gene expression of many genes in the IL-1 pathway–including IL-1ß—in primary OA cartilage.
We did not find any differences in the gene expression of IL-1ß in the present study between the ACLR and sham-operated group. Thus, we cannot exclude that the sham operation may have induced a process of inflammation leading to the changes.
It is possible that a more or less developed stage of OA was prevalent in the animals when included in the study. Looking at the results of the gene expression of the assessed markers we were not able to detect any differences that could help to differentiate between the outcomes of the sham and the ACLR side.
MRI serves as an important non-invasive tool to detect OA. Especially the early detection of OA has recently been the focus of several publications [105,106,107]. However, MRI morphology often does not correlate with the clinical outcome after cartilage repair surgery. It could also be that specific parameters that correlate best can vary according to the type of procedure performed [108,109].
Histology is the golden standard [106,107,110] for assessing matrix changes associated with the progression of OA. Hence it is important to work on a validation to correlate image findings in MRI with tissue histology, so histology could be an appropriate ex vivo reference for comparison with MRI [107].
Surprisingly, a correlation of the histological scoring with the MRI-scoring by use of the modified WORMS-score showed no concurrence. Kreinest et al. [45] report similar findings, as degenerative changes of the menisci were not detected by MRI also in the same animals as we assessed in the present study.
For the detection of OA with MRI, lots of different sequences are available and used [1,111,112,113]. There is general agreement that cartilage damage evaluation with standard MRI examination remains problematic. However, recently cartilage specific MRI sequences were developed with different degrees in specificity and sensitivity [114,115]. Furthermore, there are significant differences in the detection of OA depending on the severity of the disease. In a study by Bachmann et al. [111], 14 different MRI-sequences were utilized to detect cartilage changes in different OA stages and the results were compared with histology and arthroscopy. Here it was obvious that a severe OA could be detected with SE-sequences up to 94% and mild osteoarthritis could be discovered in 10% of the specimen [111]. In several other studies different MRI sequences were used to detect cartilage degeneration [15,116,117]. It was confirmed that even after MRI examinations with FLASH and DESS sequences low correlation in terms of sensitivity and specificity was found for both sequences in normal to mild Mankin grades [112]. Only moderate to severe changes were diagnosed with higher significance and specificity. The possibility of diagnosing early cartilage changes with high accuracy could not be proven by our used sequences. These results are confirmed by Casula et al. [118], reporting that the loss of cartilage and the quality of remaining tissue in the lesion site is not directly associated with each other, because quantitative MRI parameters (especially T1 relaxation time, T2 relaxation time, and delayed gadolinium-enhanced MRI) were not linearly related to arthroscopic grading in OA.
On the other hand, other studies show that quantitative MRI is able to detect early osteoarthritis changes as classified by the OARSI histological system [106]. Streitparth et al. [119] and Tsai et al. [120] reported a correlation between the histological grading and the MRI results. So it is obvious, that there is a variety of studies which work with different parameters, so that the results cannot be compared. Another aspect is the different intra-articular anatomical geometry between the porcine and the human knee and the difference in size. So there may not be comparability between human and porcine studies. Furthermore, clinical MRI diffusion protocols are seriously affected by various artefacts among them geometrical distortion and susceptibility effects [105]. In our case, it is possible that the histological and corresponding MRI regions may have been marginally different. This is related to a potential mismatch in plane orientation and disproportion in image resolution. In the present study MRI slice thickness of 3 mm has to match a histological osteochondral plug of 4 mm in diameter. However, after unblinding the samples, we compared and tried to allocate the areas of histological changes to the MRI scans and vice versa but did not always find a match in the MRI. Saadat et al. [16] showed that cartilage thickness and surface lesions could be assessed in human cartilage by the MRI sequences but that signal changes of the cartilage were not suited to indicate the amount of cartilage degeneration. Fischbach et al. [121] and Wachsmuth et al. [122] however reported difficulties in detecting cartilage lesions with MRI. This may explain the lack of a match between our histological and MRI findings, because the structure of the cartilage, especially lesions and fissures, is a main “point contributor” to the histological Little score [35]. On the other side, fissures could not sufficiently be detected by the MRI in our study, so its contribution to the MRI score was insignificant. The fact that the articular cartilage of the Minipig knee joints used in our study is thinner [87,123] than the cartilage of human knee joints [123,124] may be another explanation for the difficulties in finding the discovered histological degeneration in the MRI images. Wei et al. [6] showed differences between the control group and the treated group in quantitative MRI analysis by delayed gadolinium enhanced MRI of cartilage with the dGEMRIC technique over a time period of 2 to 6 weeks with a positive correlation of the GAG content to T1, Gd values and negative correlation to T2 values [6]. The blinding of the MRI scans may not avoid bias of the evaluators as the ACL was visibly absent after resection in the scans (Fig 8). Though this was a good validation of a successful surgery, an unbiased assessment of the MRI scans was hardly possible. This may be reflected by the higher scoring of the left condyles, both medially and laterally (Fig 7). However, this problem can hardly be avoided as the ACL as an area of interest is an inner anatomical structure of the stifle joint close to the articular cartilage. The radiological assessment of the knee joints showed no severe signs of degeneration. This may be due to the fact that the x-ray examination was done by manually pulling the limb to get a good view of the joint space. Therefore the examination of the narrowing of the joint cavity as described by [32] was not possible, which is an important factor in the Kellgren and Lawrence score [32].
A literature search (n = 81) for gene expression profiles of osteoarthritic and normal cartilage was performed for the genes which were to be assessed in this study. The identified genes were assessed in terms of their expression level. As the descriptive statistic shows, there are no evident changes in expression patterns between the two groups (Fig 10).
The results of the gene expression profiles in our study differ from the findings of other authors who for example showed an enhanced expression of col2 [18,21,125] or of col1a1 [12,18,21,22,66,125,126,127] in degenerated cartilage. However, col1a1 was upregulated in the menisci of the animals used in the presented study which was identified as degeneration of the meniscal tissue [45]; Thus a change in the assessed articular cartilage should have been detected. For other genes, for example acan, our findings were shared with some authors but differed from the findings of others [12,18,21,41,125,127,128,129]. The lack of difference in the gene expression results between the ACLR- and sham-operated groups in our study could be expected as it is in accordance with our histological findings. Due to that, we cannot discriminate between verum and control group in terms of gene expression as the control group (sham operated) showed similar degenerative changes of articular cartilage to the ACLR-group.
As in our study the sham-operated site also developed OA, we looked at the expression patterns of genes thought to be involved in the OA process that caused the differences between the sham and ACLR joints. Different levels of gene expression could help to identify genes relevant for the development of spontaneous (sham-site) or induced (ACLR-site) OA. But as there were no differences, we could not distinguish between a spontaneous or induced OA through the gene expression profiles.
An obstacle in the present study was the small amount of obtained cartilage tissue for gene expression profiling, which was done in duplicates only; however, several studies used a similar approach [130,131,132].
In our study, distribution of collagen I and II in ACLR and sham knee joints was variable for all included animals and did not correlate in any way with the histological findings. A variability of collagen II distribution in animals was also seen by Bernstein et al. [23]. Another publication by Barley et al. [133] reported an increased staining of collagen II and a decreased staining of collagen I in normal cartilage. This may be due to the fact that in our study the standard deviation was high and the sample number was low and therefore further studies need to be performed. The fact that the results showed high standard deviations may indicate a high individual variability of the animals in terms of degeneration grade. Therefore an increase in the number of animals may provide more valid results. On the other hand the verum (ACLR) and the control (sham) knee joint originate from the identical individual, so that the inter-individual variability of the animals is lessened. Our study design requested a life time of the GM for 26 weeks after the ACLR operation to maximize possible degenerative effects. Other authors used shorter time spans of up to 14 days [5] or up to 6 weeks [6] but with other species, longer life time [11] were used. A longer life span with an unbalanced knee joint is expected to raise the risk of developing OA or worsen an already existing condition in terms of the histological/radiological findings. However, the time of observation over 26 weeks revealed OA on both the ACLR and the sham side.
An increased expression in the RNA analysis is not necessarily associated with an increased protein level [134,135,136]. So the absence of protein detection of most of the examined genes may be a limitation of the presented study. However, most relevant proteins Col II and Col I were examined by IH (Fig 11C and 11D) showing no significant differences between the sham and verum group (Fig 11A and 11B) as was also seen in the gene expression profile (Fig 10B and 10A). One has to consider that further investigation of the proteins (e.g. Western Blotting) is material consuming. The amount of material of articular cartilage in the presented study was limited. According to the protocol (see above) cartilage tissue was available originating from an area with 10 mm in diameter minus the middle region of 4 mm in diameter serving for the histological examination. The aim of this procedure was to gain samples for the histological and the gene expression in immediate vicinity of each other. Therefore it was not possible to extend the range of the harvesting area.
Conclusion
OA was detected by histological findings in both knee joints of Göttingen Minipigs after 26 weeks regardless of the intervention. MRI results did not match the results of scoring the degenerative histological changes. Furthermore, the gene expression analyses of 10 promising genes did not help to answer the question, whether the degenerative changes may be induced or idiopathic. Further studies analyzing the articular cartilage may reveal differences between sham operated knee joints and after ACLR at the protein level. Regarding the findings in the presented study the resection of the ACL does not seem to be an effective method to induce isolated OA in adult minipigs.
Supporting Information
(XLSX)
Acknowledgments
Thanks to Dr. Eric Steck for technical support
Thanks to Elmar Forsch for help especially with anaesthesia
Thanks to Prof. Dr. med. Henrik Michaely, Susanne Lattenkamp, Kerstin Görlitz and Katrin Koziel for the help with the MRI
Thanks to Cornelia Patschull and Ariane Planer for the help with the x-rays
Thanks to Prof. Dr. Henner Simianer, Dr. Christian Gärke and Arne Oppermann for providing the animals
Thanks to Dr. Kristianna Becker, Dr. Susanne Bog, Theo Huber, Kai Lorenz and family Bühler for help with animal care
Thanks to Dr. Joachim Brade for help with the statistical evaluations
Thanks to Dr. Torsten Schattenberg for help with conducting the operations
Thanks to Renate Clüver for her assistance with the English language
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Medical Faculty Mannheim, Heidelberg University, Germany and the Deutsche Forschungsgemeinschaft and Ruprecht-Karls-Universität Heidelberg, Germany within the funding programme Open Access Publishing.
References
- 1.Boulocher C, Chereul E, Langlois JB, Armenean M, Duclos ME, et al. (2007) Non-invasive in vivo quantification of the medial tibial cartilage thickness progression in an osteoarthritis rabbit model with quantitative 3D high resolution micro-MRI. Osteoarthritis Cartilage 15: 1378–1387. 10.1016/j.joca.2007.04.012 [DOI] [PubMed] [Google Scholar]
- 2.Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, et al. (2009) Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum 60: 840–847. 10.1002/art.24304 [DOI] [PubMed] [Google Scholar]
- 3.Frost-Christensen LN, Mastbergen SC, Vianen ME, Hartog A, DeGroot J, et al. (2008) Degeneration, inflammation, regeneration, and pain/disability in dogs following destabilization or articular cartilage grooving of the stifle joint. Osteoarthritis Cartilage 16: 1327–1335. 10.1016/j.joca.2008.03.013 [DOI] [PubMed] [Google Scholar]
- 4.Jay GD, Fleming BC, Watkins BA, McHugh KA, Anderson SC, et al. (2010) Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum 62: 2382–2391. 10.1002/art.27550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haslauer CM, Elsaid KA, Fleming BC, Proffen BL, Johnson VM, et al. (2013) Loss of extracellular matrix from articular cartilage is mediated by the synovium and ligament after anterior cruciate ligament injury. Osteoarthritis Cartilage 21: 1950–1957. 10.1016/j.joca.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei B, Zong M, Yan C, Mao F, Guo Y, et al. (2015) Use of quantitative MRI for the detection of progressive cartilage degeneration in a mini-pig model of osteoarthritis caused by anterior cruciate ligament transection. J Magn Reson Imaging. [DOI] [PubMed] [Google Scholar]
- 7.Gotterbarm T, Breusch SJ, Schneider U, Jung M (2008) The minipig model for experimental chondral and osteochondral defect repair in tissue engineering: retrospective analysis of 180 defects. Lab Anim 42: 71–82. 10.1258/la.2007.06029e [DOI] [PubMed] [Google Scholar]
- 8.Heijink A, Gomoll AH, Madry H, Drobnic M, Filardo G, et al. (2012) Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 20: 423–435. 10.1007/s00167-011-1818-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nebelung W, Wuschech H (2005) Thirty-five years of follow-up of anterior cruciate ligament-deficient knees in high-level athletes. Arthroscopy 21: 696–702. 10.1016/j.arthro.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 10.Sharma L, Lou C, Felson DT, Dunlop DD, Kirwan-Mellis G, et al. (1999) Laxity in healthy and osteoarthritic knees. Arthritis Rheum 42: 861–870. [DOI] [PubMed] [Google Scholar]
- 11.Pond MJ, Nuki G (1973) Experimentally-induced osteoarthritis in the dog. Ann Rheum Dis 32: 387–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenz H, Wenz W, Ivancic M, Steck E, Richter W (2005) Early and stable upregulation of collagen type II, collagen type I and YKL40 expression levels in cartilage during early experimental osteoarthritis occurs independent of joint location and histological grading. Arthritis Res Ther 7: R156–165. 10.1186/ar1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Meer BL, Meuffels DE, van Eijsden WA, Verhaar JA, Bierma-Zeinstra SM, et al. (2015) Which determinants predict tibiofemoral and patellofemoral osteoarthritis after anterior cruciate ligament injury? A systematic review. Br J Sports Med. [DOI] [PubMed] [Google Scholar]
- 14.Makela JT, Rezaeian ZS, Mikkonen S, Madden R, Han SK, et al. (2014) Site-dependent changes in structure and function of lapine articular cartilage 4 weeks after anterior cruciate ligament transection. Osteoarthritis Cartilage 22: 869–878. 10.1016/j.joca.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 15.Rehnitz C, Kupfer J, Streich NA, Burkholder I, Schmitt B, et al. (2014) Comparison of biochemical cartilage imaging techniques at 3 T MRI. Osteoarthritis Cartilage 22: 1732–1742. 10.1016/j.joca.2014.04.020 [DOI] [PubMed] [Google Scholar]
- 16.Saadat E, Jobke B, Chu B, Lu Y, Cheng J, et al. (2008) Diagnostic performance of in vivo 3-T MRI for articular cartilage abnormalities in human osteoarthritic knees using histology as standard of reference. Eur Radiol 18: 2292–2302. 10.1007/s00330-008-0989-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hissnauer TN, Baranowsky A, Pestka JM, Streichert T, Wiegandt K, et al. (2010) Identification of molecular markers for articular cartilage. Osteoarthritis Cartilage 18: 1630–1638. 10.1016/j.joca.2010.10.002 [DOI] [PubMed] [Google Scholar]
- 18.Aigner T, Fundel K, Saas J, Gebhard PM, Haag J, et al. (2006) Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum 54: 3533–3544. 10.1002/art.22174 [DOI] [PubMed] [Google Scholar]
- 19.Aigner T, Zien A, Gehrsitz A, Gebhard PM, McKenna L (2001) Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum 44: 2777–2789. [DOI] [PubMed] [Google Scholar]
- 20.Steck E, Boeuf S, Gabler J, Werth N, Schnatzer P, et al. (2012) Regulation of H19 and its encoded microRNA-675 in osteoarthritis and under anabolic and catabolic in vitro conditions. J Mol Med (Berl) 90: 1185–1195. [DOI] [PubMed] [Google Scholar]
- 21.Fan Z, Bau B, Yang H, Soeder S, Aigner T (2005) Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes, but less responsive to catabolic stimulation with interleukin-1beta. Arthritis Rheum 52: 136–143. 10.1002/art.20725 [DOI] [PubMed] [Google Scholar]
- 22.Yin J, Yang Z, Cao YP, Ge ZG (2011) Characterization of human primary chondrocytes of osteoarthritic cartilage at varying severity. Chin Med J (Engl) 124: 4245–4253. [PubMed] [Google Scholar]
- 23.Bernstein P, Dong M, Graupher S, Corbeil D, Gelinsky M, et al. (2009) Sox9 expression of alginate-encapsulated chondrocytes is stimulated by low cell density. J Biomed Mater Res A 91: 910–918. 10.1002/jbm.a.32308 [DOI] [PubMed] [Google Scholar]
- 24.Kaab MJ, Gwynn IA, Notzli HP (1998) Collagen fibre arrangement in the tibial plateau articular cartilage of man and other mammalian species. J Anat 193 (Pt 1): 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niemietz T, Zass G, Hagmann S, Diederichs S, Gotterbarm T, et al. (2014) Xenogeneic transplantation of articular chondrocytes into full-thickness articular cartilage defects in minipigs: fate of cells and the role of macrophages. Cell Tissue Res 358: 749–761. 10.1007/s00441-014-1982-x [DOI] [PubMed] [Google Scholar]
- 26.Ruehe B, Niehues S, Heberer S, Nelson K (2009) Miniature pigs as an animal model for implant research: bone regeneration in critical-size defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108: 699–706. 10.1016/j.tripleo.2009.06.037 [DOI] [PubMed] [Google Scholar]
- 27.Chaganti RK, Lane NE (2011) Risk factors for incident osteoarthritis of the hip and knee. Curr Rev Musculoskelet Med 4: 99–104. 10.1007/s12178-011-9088-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, et al. (2014) Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis 73: 1659–1664. 10.1136/annrheumdis-2013-203355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2012) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage 20: 256–260. 10.1016/j.joca.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 30.Schwarz ML SR, Mauermann E, Werner A, Steil V, Forsch E, Obertacke U, Becker K LL (2010) Scintigraphic evaluation of bone formation in Göttingen minipigs. Scand J Lab Anim Sci 37: 13–18. [Google Scholar]
- 31.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, et al. (2004) Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12: 177–190. 10.1016/j.joca.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 32.Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16: 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lasch P, Petras T, Ullrich O, Backmann J, Naumann D, et al. (2001) Hydrogen peroxide-induced structural alterations of RNAse A. J Biol Chem 276: 9492–9502. 10.1074/jbc.M008528200 [DOI] [PubMed] [Google Scholar]
- 34.Gray P. (1954) The Microtomist's Formulary and Guide New York: The Blakiston Company, Inc. 820 p. [Google Scholar]
- 35.Little CB, Smith MM, Cake MA, Read RA, Murphy MJ, et al. (2010) The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in sheep and goats. Osteoarthritis Cartilage 18 Suppl 3: S80–92. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson BS, Nam H, Hopkins RG, Morrison RF (2010) Impact of reference gene selection for target gene normalization on experimental outcome using real-time qRT-PCR in adipocytes. PLoS One 5: e15208 10.1371/journal.pone.0015208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gredes T (2007) Untersuchung der Genaktivität im Kiefergelenkknorpel des Schweins (Sus scrofa domesticus) nach Vorverlagerung des Unterkiefers. Dissertation der Ernst-Moritz-Arndt-Universität, Greifswald.
- 38.Steck E, Fischer J, Lorenz H, Gotterbarm T, Jung M, et al. (2009) Mesenchymal stem cell differentiation in an experimental cartilage defect: restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev 18: 969–978. 10.1089/scd.2008.0213 [DOI] [PubMed] [Google Scholar]
- 39.Zou L, Zou X, Chen L, Li H, Mygind T, et al. (2008) Multilineage differentiation of porcine bone marrow stromal cells associated with specific gene expression pattern. J Orthop Res 26: 56–64. 10.1002/jor.20467 [DOI] [PubMed] [Google Scholar]
- 40.Tayade C, Black GP, Fang Y, Croy BA (2006) Differential gene expression in endometrium, endometrial lymphocytes, and trophoblasts during successful and abortive embryo implantation. J Immunol 176: 148–156. [DOI] [PubMed] [Google Scholar]
- 41.Le Graverand MP, Eggerer J, Vignon E, Otterness IG, Barclay L, et al. (2002) Assessment of specific mRNA levels in cartilage regions in a lapine model of osteoarthritis. J Orthop Res 20: 535–544. 10.1016/S0736-0266(01)00126-7 [DOI] [PubMed] [Google Scholar]
- 42.Zeni P, Doepker E, Schulze-Topphoff U, Huewel S, Tenenbaum T, et al. (2007) MMPs contribute to TNF-alpha-induced alteration of the blood-cerebrospinal fluid barrier in vitro. Am J Physiol Cell Physiol 293: C855–864. 10.1152/ajpcell.00470.2006 [DOI] [PubMed] [Google Scholar]
- 43.Zhou QY, Fang MD, Huang TH, Li CC, Yu M, et al. (2009) Detection of differentially expressed genes between Erhualian and Large White placentas on day 75 and 90 of gestation. BMC Genomics 10: 337 10.1186/1471-2164-10-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang CY, Lai KY, Hung LF, Wu WL, Liu FC, et al. (2011) Advanced glycation end products cause collagen II reduction by activating Janus kinase/signal transducer and activator of transcription 3 pathway in porcine chondrocytes. Rheumatology (Oxford) 50: 1379–1389. [DOI] [PubMed] [Google Scholar]
- 45.Kreinest M, Reisig G, Strobel P, Dinter D, Attenberger U, et al. (2016) A Porcine Animal Model for Early Meniscal Degeneration—Analysis of Histology, Gene Expression and Magnetic Resonance Imaging Six Months after Resection of the Anterior Cruciate Ligament. PLoS One 11: e0159331 10.1371/journal.pone.0159331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costa-Paz M, Ayerza MA, Tanoira I, Astoul J, Muscolo DL (2012) Spontaneous healing in complete ACL ruptures: a clinical and MRI study. Clin Orthop Relat Res 470: 979–985. 10.1007/s11999-011-1933-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goranov NV (2007) Serum markers of lipid peroxidation, antioxidant enzymatic defense, and collagen degradation in an experimental (Pond-Nuki) canine model of osteoarthritis. Vet Clin Pathol 36: 192–195. [DOI] [PubMed] [Google Scholar]
- 48.Libicher M, Ivancic M, Hoffmann M, Wenz W (2005) Early changes in experimental osteoarthritis using the Pond-Nuki dog model: technical procedure and initial results of in vivo MR imaging. Eur Radiol 15: 390–394. 10.1007/s00330-004-2486-y [DOI] [PubMed] [Google Scholar]
- 49.Batiste DL, Kirkley A, Laverty S, Thain LM, Spouge AR, et al. (2004) Ex vivo characterization of articular cartilage and bone lesions in a rabbit ACL transection model of osteoarthritis using MRI and micro-CT. Osteoarthritis Cartilage 12: 986–996. 10.1016/j.joca.2004.08.010 [DOI] [PubMed] [Google Scholar]
- 50.Herzog W, Adams ME, Matyas JR, Brooks JG (1993) Hindlimb loading, morphology and biochemistry of articular cartilage in the ACL-deficient cat knee. Osteoarthritis Cartilage 1: 243–251. [DOI] [PubMed] [Google Scholar]
- 51.Nielsen RH, Stoop R, Leeming DJ, Stolina M, Qvist P, et al. (2008) Evaluation of cartilage damage by measuring collagen degradation products in joint extracts in a traumatic model of osteoarthritis. Biomarkers 13: 79–87. 10.1080/13547500701615108 [DOI] [PubMed] [Google Scholar]
- 52.Gavenis K, Heussen N, Hofman M, Andereya S, Schneider U, et al. (2014) Cell-free repair of small cartilage defects in the Goettinger minipig: the effects of BMP-7 continuously released by poly(lactic-co-glycolid acid) microspheres. J Biomater Appl 28: 1008–1015. 10.1177/0885328213491440 [DOI] [PubMed] [Google Scholar]
- 53.Jung M, Kaszap B, Redohl A, Steck E, Breusch S, et al. (2009) Enhanced early tissue regeneration after matrix-assisted autologous mesenchymal stem cell transplantation in full thickness chondral defects in a minipig model. Cell Transplant 18: 923–932. 10.3727/096368909X471297 [DOI] [PubMed] [Google Scholar]
- 54.Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, et al. (2007) Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res 25: 81–91. 10.1002/jor.20282 [DOI] [PubMed] [Google Scholar]
- 55.Altman RD, Dean D (1989) Pain in osteoarthritis. Introduction and overview. Semin Arthritis Rheum 18: 1–3. [DOI] [PubMed] [Google Scholar]
- 56.Hanashi D, Koshino T, Uesugi M, Saito T (2002) Effect of femoral nerve resection on progression of cartilage degeneration induced by anterior cruciate ligament transection in rabbits. J Orthop Sci 7: 672–676. 10.1007/s007760200119 [DOI] [PubMed] [Google Scholar]
- 57.Inoue K, Masuko-Hongo K, Okamoto M, Nishioka K (2005) Induction of vascular endothelial growth factor and matrix metalloproteinase-3 (stromelysin) by interleukin-1 in human articular chondrocytes and synoviocytes. Rheumatol Int 26: 93–98. 10.1007/s00296-004-0513-6 [DOI] [PubMed] [Google Scholar]
- 58.Kuyinu EL, Narayanan G, Nair LS, Laurencin CT (2016) Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res 11: 19 10.1186/s13018-016-0346-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCoy AM (2015) Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet Pathol 52: 803–818. 10.1177/0300985815588611 [DOI] [PubMed] [Google Scholar]
- 60.Jimenez PA, Glasson SS, Trubetskoy OV, Haimes HB (1997) Spontaneous osteoarthritis in Dunkin Hartley guinea pigs: histologic, radiologic, and biochemical changes. Lab Anim Sci 47: 598–601. [PubMed] [Google Scholar]
- 61.Liu W, Burton-Wurster N, Glant TT, Tashman S, Sumner DR, et al. (2003) Spontaneous and experimental osteoarthritis in dog: similarities and differences in proteoglycan levels. J Orthop Res 21: 730–737. 10.1016/S0736-0266(03)00002-0 [DOI] [PubMed] [Google Scholar]
- 62.Carlson CS, Loeser RF, Purser CB, Gardin JF, Jerome CP (1996) Osteoarthritis in cynomolgus macaques. III: Effects of age, gender, and subchondral bone thickness on the severity of disease. J Bone Miner Res 11: 1209–1217. 10.1002/jbmr.5650110904 [DOI] [PubMed] [Google Scholar]
- 63.Huebner JL, Johnson KA, Kraus VB, Terkeltaub RA (2009) Transglutaminase 2 is a marker of chondrocyte hypertrophy and osteoarthritis severity in the Hartley guinea pig model of knee OA. Osteoarthritis Cartilage 17: 1056–1064. 10.1016/j.joca.2009.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mankin HJ, Dorfman H, Lippiello L, Zarins A (1971) Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am 53: 523–537. [PubMed] [Google Scholar]
- 65.Huebner JL, Hanes MA, Beekman B, TeKoppele JM, Kraus VB (2002) A comparative analysis of bone and cartilage metabolism in two strains of guinea-pig with varying degrees of naturally occurring osteoarthritis. Osteoarthritis Cartilage 10: 758–767. [DOI] [PubMed] [Google Scholar]
- 66.Karlsson C, Dehne T, Lindahl A, Brittberg M, Pruss A, et al. (2010) Genome-wide expression profiling reveals new candidate genes associated with osteoarthritis. Osteoarthritis Cartilage 18: 581–592. 10.1016/j.joca.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 67.Lafeber FP, van der Kraan PM, van Roy HL, Vitters EL, Huber-Bruning O, et al. (1992) Local changes in proteoglycan synthesis during culture are different for normal and osteoarthritic cartilage. Am J Pathol 140: 1421–1429. [PMC free article] [PubMed] [Google Scholar]
- 68.Sakakibara Y, Miura T, Iwata H, Kikuchi T, Yamaguchi T, et al. (1994) Effect of high-molecular-weight sodium hyaluronate on immobilized rabbit knee. Clin Orthop 299: 282–292. [PubMed] [Google Scholar]
- 69.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, et al. (2006) Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage 14: 13–29. 10.1016/j.joca.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 70.Cook JL, Kuroki K, Visco D, Pelletier JP, Schulz L, et al. (2010) The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the dog. Osteoarthritis Cartilage 18 Suppl 3: S66–79. [DOI] [PubMed] [Google Scholar]
- 71.Gerwin N, Bendele AM, Glasson S, Carlson CS (2010) The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage 18 Suppl 3: S24–34. [DOI] [PubMed] [Google Scholar]
- 72.Glasson SS, Chambers MG, Van Den Berg WB, Little CB (2010) The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 18 Suppl 3: S17–23. [DOI] [PubMed] [Google Scholar]
- 73.Kraus VB, Huebner JL, DeGroot J, Bendele A (2010) The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthritis Cartilage 18 Suppl 3: S35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laverty S, Girard CA, Williams JM, Hunziker EB, Pritzker KP (2010) The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthritis Cartilage 18 Suppl 3: S53–65. [DOI] [PubMed] [Google Scholar]
- 75.McIlwraith CW, Frisbie DD, Kawcak CE, Fuller CJ, Hurtig M, et al. (2010) The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the horse. Osteoarthritis Cartilage 18 Suppl 3: S93–105. [DOI] [PubMed] [Google Scholar]
- 76.Wei L, Fleming BC, Sun X, Teeple E, Wu W, et al. (2010) Comparison of differential biomarkers of osteoarthritis with and without posttraumatic injury in the Hartley guinea pig model. J Orthop Res 28: 900–906. 10.1002/jor.21093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wenz W, Graf J, Brocai DR, Breusch SJ, Mittnacht M, et al. (1998) [Effectiveness of intra-articular application of hyaluronic acid on early forms of femoropatellar arthrosis—an experimental study in dogs]. Z Orthop Ihre Grenzgeb 136: 298–303. 10.1055/s-2008-1053741 [DOI] [PubMed] [Google Scholar]
- 78.Tiraloche G, Girard C, Chouinard L, Sampalis J, Moquin L, et al. (2005) Effect of oral glucosamine on cartilage degradation in a rabbit model of osteoarthritis. Arthritis Rheum 52: 1118–1128. 10.1002/art.20951 [DOI] [PubMed] [Google Scholar]
- 79.Visco DM, Hill MA, Widmer WR, Johnstone B, Myers SL (1996) Experimental osteoarthritis in dogs: a comparison of the Pond-Nuki and medial arthrotomy methods. Osteoarthritis Cartilage 4: 9–22. [DOI] [PubMed] [Google Scholar]
- 80.Haywood J, Yammani RR (2016) Free fatty acid palmitate activates unfolded protein response pathway and promotes apoptosis in meniscus cells. Osteoarthritis Cartilage 24: 942–945. 10.1016/j.joca.2015.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McNulty AL, Miller MR, O'Connor SK, Guilak F (2011) The effects of adipokines on cartilage and meniscus catabolism. Connect Tissue Res 52: 523–533. 10.3109/03008207.2011.597902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oppermann A (2016) Personal communication: The mean weight of 24month old Göttingen Minipigs
- 83.Miyatake K, Muneta T, Ojima M, Yamada J, Matsukura Y, et al. (2016) Coordinate and synergistic effects of extensive treadmill exercise and ovariectomy on articular cartilage degeneration. BMC Musculoskelet Disord 17: 238 10.1186/s12891-016-1094-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moriyama H, Kanemura N, Brouns I, Pintelon I, Adriaensen D, et al. (2012) Effects of aging and exercise training on the histological and mechanical properties of articular structures in knee joints of male rat. Biogerontology 13: 369–381. 10.1007/s10522-012-9381-8 [DOI] [PubMed] [Google Scholar]
- 85.Cruz R, Ramirez C, Rojas OI, Casas-Mejia O, Kouri JB, et al. (2015) Menisectomized miniature Vietnamese pigs develop articular cartilage pathology resembling osteoarthritis. Pathol Res Pract 211: 829–838. 10.1016/j.prp.2015.07.012 [DOI] [PubMed] [Google Scholar]
- 86.Elsaid KA, Zhang L, Waller K, Tofte J, Teeple E, et al. (2012) The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin's effect in exercised joints following ACL transection. Osteoarthritis Cartilage 20: 940–948. 10.1016/j.joca.2012.04.021 [DOI] [PubMed] [Google Scholar]
- 87.Chu CR, Szczodry M, Bruno S (2010) Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev 16: 105–115. 10.1089/ten.TEB.2009.0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fleming BC, Hulstyn MJ, Oksendahl HL, Fadale PD (2005) Ligament Injury, Reconstruction and Osteoarthritis. Curr Opin Orthop 16: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Appleton CT, Pitelka V, Henry J, Beier F (2007) Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum 56: 1854–1868. 10.1002/art.22711 [DOI] [PubMed] [Google Scholar]
- 90.Stoop R, Buma P, van der Kraan PM, Hollander AP, Billinghurst RC, et al. (2001) Type II collagen degradation in articular cartilage fibrillation after anterior cruciate ligament transection in rats. Osteoarthritis Cartilage 9: 308–315. 10.1053/joca.2000.0390 [DOI] [PubMed] [Google Scholar]
- 91.Vilensky JA, O'Connor BL, Brandt KD, Dunn EA, Rogers PI (1994) Serial kinematic analysis of the trunk and limb joints after anterior cruciate ligament transection: Temporal, spatial, and angular changes in a canine model of osteoarthritis. J Electromyogr Kinesiol 4: 181–192. 10.1016/1050-6411(94)90019-1 [DOI] [PubMed] [Google Scholar]
- 92.Jackson DW, McDevitt CA, Simon TM, Arnoczky SP, Atwell EA, et al. (1992) Meniscal transplantation using fresh and cryopreserved allografts. An experimental study in goats. Am J Sports Med 20: 644–656. [DOI] [PubMed] [Google Scholar]
- 93.Calvo E, Palacios I, Delgado E, Sanchez-Pernaute O, Largo R, et al. (2004) Histopathological correlation of cartilage swelling detected by magnetic resonance imaging in early experimental osteoarthritis. Osteoarthritis Cartilage 12: 878–886. 10.1016/j.joca.2004.07.007 [DOI] [PubMed] [Google Scholar]
- 94.Eltawil NM, De Bari C, Achan P, Pitzalis C, Dell'accio F (2009) A novel in vivo murine model of cartilage regeneration. Age and strain-dependent outcome after joint surface injury. Osteoarthritis Cartilage 17: 695–704. 10.1016/j.joca.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hulmes DJ, Marsden ME, Strachan RK, Harvey RE, McInnes N, et al. (2004) Intra-articular hyaluronate in experimental rabbit osteoarthritis can prevent changes in cartilage proteoglycan content. Osteoarthritis Cartilage 12: 232–238. 10.1016/j.joca.2003.11.007 [DOI] [PubMed] [Google Scholar]
- 96.McDevitt C, Gilbertson E, Muir H (1977) An experimental model of osteoarthritis; early morphological and biochemical changes. J Bone Joint Surg Br 59: 24–35. [DOI] [PubMed] [Google Scholar]
- 97.O'Brien EJ, Beveridge JE, Huebner KD, Heard BJ, Tapper JE, et al. (2013) Osteoarthritis develops in the operated joint of an ovine model following ACL reconstruction with immediate anatomic reattachment of the native ACL. J Orthop Res 31: 35–43. 10.1002/jor.22187 [DOI] [PubMed] [Google Scholar]
- 98.Curzer HJ, Perry G, Wallace MC, Perry D (2016) The Three Rs of Animal Research: What they Mean for the Institutional Animal Care and Use Committee and Why. Sci Eng Ethics 22: 549–565. 10.1007/s11948-015-9659-8 [DOI] [PubMed] [Google Scholar]
- 99.Vasara AI, Hyttinen MM, Pulliainen O, Lammi MJ, Jurvelin JS, et al. (2006) Immature porcine knee cartilage lesions show good healing with or without autologous chondrocyte transplantation. Osteoarthritis Cartilage 14: 1066–1074. 10.1016/j.joca.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 100.Rosenthal AK, Ryan LM (1994) Ageing increases growth factor-induced inorganic pyrophosphate elaboration by articular cartilage. Mech Ageing Dev 75: 35–44. [DOI] [PubMed] [Google Scholar]
- 101.Aigner T, Rose J, Martin J, Buckwalter J (2004) Aging theories of primary osteoarthritis: from epidemiology to molecular biology. Rejuvenation Res 7: 134–145. 10.1089/1549168041552964 [DOI] [PubMed] [Google Scholar]
- 102.Verzijl N, DeGroot J, Ben ZC, Brau-Benjamin O, Maroudas A, et al. (2002) Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum 46: 114–123. [DOI] [PubMed] [Google Scholar]
- 103.Berenbaum F (2013) Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 21: 16–21. 10.1016/j.joca.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 104.Zayed N, Afif H, Chabane N, Mfuna-Endam L, Benderdour M, et al. (2008) Inhibition of interleukin-1beta-induced matrix metalloproteinases 1 and 13 production in human osteoarthritic chondrocytes by prostaglandin D2. Arthritis Rheum 58: 3530–3540. 10.1002/art.23958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen B, Roeder E, Vuissoz PA, Gillet P, Felblinger J, et al. (2013) Respective interest of T2 mapping and diffusion tensor imaging in assessing porcine knee cartilage with MR at 3 Teslas. Biomed Mater Eng 23: 263–272. 10.3233/BME-130750 [DOI] [PubMed] [Google Scholar]
- 106.Lukas VA, Fishbein KW, Lin PC, Schar M, Schneider E, et al. (2015) Classification of histologically scored human knee osteochondral plugs by quantitative analysis of magnetic resonance images at 3T. J Orthop Res 33: 640–650. 10.1002/jor.22810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palmer AJ, Brown CP, McNally EG, Price AJ, Tracey I, et al. (2013) Non-invasive imaging of cartilage in early osteoarthritis. Bone Joint J 95-B: 738–746. 10.1302/0301-620X.95B6.31414 [DOI] [PubMed] [Google Scholar]
- 108.Blackman AJ, Smith MV, Flanigan DC, Matava MJ, Wright RW, et al. (2013) Correlation between magnetic resonance imaging and clinical outcomes after cartilage repair surgery in the knee: a systematic review and meta-analysis. Am J Sports Med 41: 1426–1434. 10.1177/0363546513485931 [DOI] [PubMed] [Google Scholar]
- 109.Salzmann GM, Erdle B, Porichis S, Uhl M, Ghanem N, et al. (2014) Long-term T2 and Qualitative MRI Morphology After First-Generation Knee Autologous Chondrocyte Implantation: Cartilage Ultrastructure Is Not Correlated to Clinical or Qualitative MRI Outcome. Am J Sports Med 42: 1832–1840. 10.1177/0363546514536682 [DOI] [PubMed] [Google Scholar]
- 110.Orth P, Peifer C, Goebel L, Cucchiarini M, Madry H (2015) Comprehensive analysis of translational osteochondral repair: Focus on the histological assessment. Prog Histochem Cytochem 50: 19–36. 10.1016/j.proghi.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 111.Bachmann G, Heinrichs C, Jurgensen I, Rominger M, Scheiter A, et al. (1997) [Comparison of different MRT techniques in the diagnosis of degenerative cartilage diseases. In vitro study of 50 joint specimens of the knee at T1.5]. Rofo 166: 429–436. 10.1055/s-2007-1015453 [DOI] [PubMed] [Google Scholar]
- 112.Bittersohl B, Mamisch TC, Welsch GH, Stratmann J, Forst R, et al. (2009) Experimental model to evaluate in vivo and in vitro cartilage MR imaging by means of histological analyses. Eur J Radiol 70: 561–569. 10.1016/j.ejrad.2008.02.031 [DOI] [PubMed] [Google Scholar]
- 113.Eckstein F, Burstein D, Link TM (2006) Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed 19: 822–854. 10.1002/nbm.1063 [DOI] [PubMed] [Google Scholar]
- 114.Evangelopoulos DS, Huesler M, Ahmad SS, Aghayev E, Neukamp M, et al. (2015) Mapping tibiofemoral gonarthrosis: an MRI analysis of non-traumatic knee cartilage defects. Br J Radiol 88: 20140542 10.1259/bjr.20140542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.von Engelhardt LV, Kraft CN, Pennekamp PH, Schild HH, Schmitz A, et al. (2007) The evaluation of articular cartilage lesions of the knee with a 3-Tesla magnet. Arthroscopy 23: 496–502. 10.1016/j.arthro.2006.12.027 [DOI] [PubMed] [Google Scholar]
- 116.Bittersohl B, Hosalkar HS, Miese FR, Schibensky J, Konig DP, et al. (2015) Zonal T2* and T1Gd assessment of knee joint cartilage in various histological grades of cartilage degeneration: an observational in vitro study. BMJ Open 5: e006895 10.1136/bmjopen-2014-006895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, et al. (2006) Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis 65: 433–441. 10.1136/ard.2005.039370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Casula V, Hirvasniemi J, Lehenkari P, Ojala R, Haapea M, et al. (2014) Association between quantitative MRI and ICRS arthroscopic grading of articular cartilage. Knee Surg Sports Traumatol Arthrosc. [DOI] [PubMed] [Google Scholar]
- 119.Streitparth F, Schottle P, Schlichting K, Schell H, Fischbach F, et al. (2009) Osteochondral defect repair after implantation of biodegradable scaffolds: indirect magnetic resonance arthrography and histopathologic correlation. Acta Radiol 50: 765–774. 10.1080/02841850902980272 [DOI] [PubMed] [Google Scholar]
- 120.Tsai PH, Lee HS, Siow TY, Chang YC, Chou MC, et al. (2013) Sequential change in T2* values of cartilage, meniscus, and subchondral bone marrow in a rat model of knee osteoarthritis. PLoS One 8: e76658 10.1371/journal.pone.0076658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fischbach F, Bruhn H, Unterhauser F, Ricke J, Wieners G, et al. (2005) Magnetic resonance imaging of hyaline cartilage defects at 1.5T and 3.0T: comparison of medium T2-weighted fast spin echo, T1-weighted two-dimensional and three-dimensional gradient echo pulse sequences. Acta Radiol 46: 67–73. [DOI] [PubMed] [Google Scholar]
- 122.Wachsmuth L, Keiffer R, Juretschke HP, Raiss RX, Kimmig N, et al. (2003) In vivo contrast-enhanced micro MR-imaging of experimental osteoarthritis in the rabbit knee joint at 7.1T1. Osteoarthritis Cartilage 11: 891–902. [DOI] [PubMed] [Google Scholar]
- 123.Ahern BJ, Parvizi J, Boston R, Schaer TP (2009) Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthritis Cartilage 17: 705–713. 10.1016/j.joca.2008.11.008 [DOI] [PubMed] [Google Scholar]
- 124.Bowers ME, Trinh N, Tung GA, Crisco JJ, Kimia BB, et al. (2008) Quantitative MR imaging using "LiveWire" to measure tibiofemoral articular cartilage thickness. Osteoarthritis Cartilage 16: 1167–1173. 10.1016/j.joca.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fukui N, Miyamoto Y, Nakajima M, Ikeda Y, Hikita A, et al. (2008) Zonal gene expression of chondrocytes in osteoarthritic cartilage. Arthritis Rheum 58: 3843–3853. 10.1002/art.24036 [DOI] [PubMed] [Google Scholar]
- 126.Attia E, Brown H, Henshaw R, George S, Hannafin JA (2010) Patterns of gene expression in a rabbit partial anterior cruciate ligament transection model: the potential role of mechanical forces. Am J Sports Med 38: 348–356. 10.1177/0363546509348052 [DOI] [PubMed] [Google Scholar]
- 127.Stoker AM, Cook JL, Kuroki K, Fox DB (2006) Site-specific analysis of gene expression in early osteoarthritis using the Pond-Nuki model in dogs. J Orthop Surg Res 1: 8 10.1186/1749-799X-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Matyas JR, Adams ME, Huang D, Sandell LJ (1995) Discoordinate gene expression of aggrecan and type II collagen in experimental osteoarthritis. Arthritis Rheum 38: 420–425. [DOI] [PubMed] [Google Scholar]
- 129.Jefferies D, Farquharson C, Thomson J, Smith W, Seawright E, et al. (2002) Differences in metabolic parameters and gene expression related to osteochondrosis/osteoarthrosis in pigs fed 25-hydroxyvitamin D3. Vet Res 33: 383–396. 10.1051/vetres:2002024 [DOI] [PubMed] [Google Scholar]
- 130.Foldager CB, Munir S, Ulrik-Vinther M, Soballe K, Bunger C, et al. (2009) Validation of suitable house keeping genes for hypoxia-cultured human chondrocytes. BMC Mol Biol 10: 94 10.1186/1471-2199-10-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grcevic D, Jajic Z, Kovacic N, Lukic IK, Velagic V, et al. (2010) Peripheral blood expression profiles of bone morphogenetic proteins, tumor necrosis factor-superfamily molecules, and transcription factor Runx2 could be used as markers of the form of arthritis, disease activity, and therapeutic responsiveness. J Rheumatol 37: 246–256. 10.3899/jrheum.090167 [DOI] [PubMed] [Google Scholar]
- 132.Jacquet R, Hillyer J, Landis WJ (2005) Analysis of connective tissues by laser capture microdissection and reverse transcriptase-polymerase chain reaction. Anal Biochem 337: 22–34. 10.1016/j.ab.2004.09.033 [DOI] [PubMed] [Google Scholar]
- 133.Barley RD, Adesida AB, Bagnall KM, Jomha NM (2010) Immunohistochemical characterization of reparative tissue present in human osteoarthritic tissue. Virchows Arch 456: 561–569. 10.1007/s00428-010-0890-z [DOI] [PubMed] [Google Scholar]
- 134.Greenbaum D, Colangelo C, Williams K, Gerstein M (2003) Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 4: 117 10.1186/gb-2003-4-9-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guo Y, Xiao P, Lei S, Deng F, Xiao GG, et al. (2008) How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim Biophys Sin (Shanghai) 40: 426–436. [DOI] [PubMed] [Google Scholar]
- 136.Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13: 227–232. 10.1038/nrg3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.