Abstract
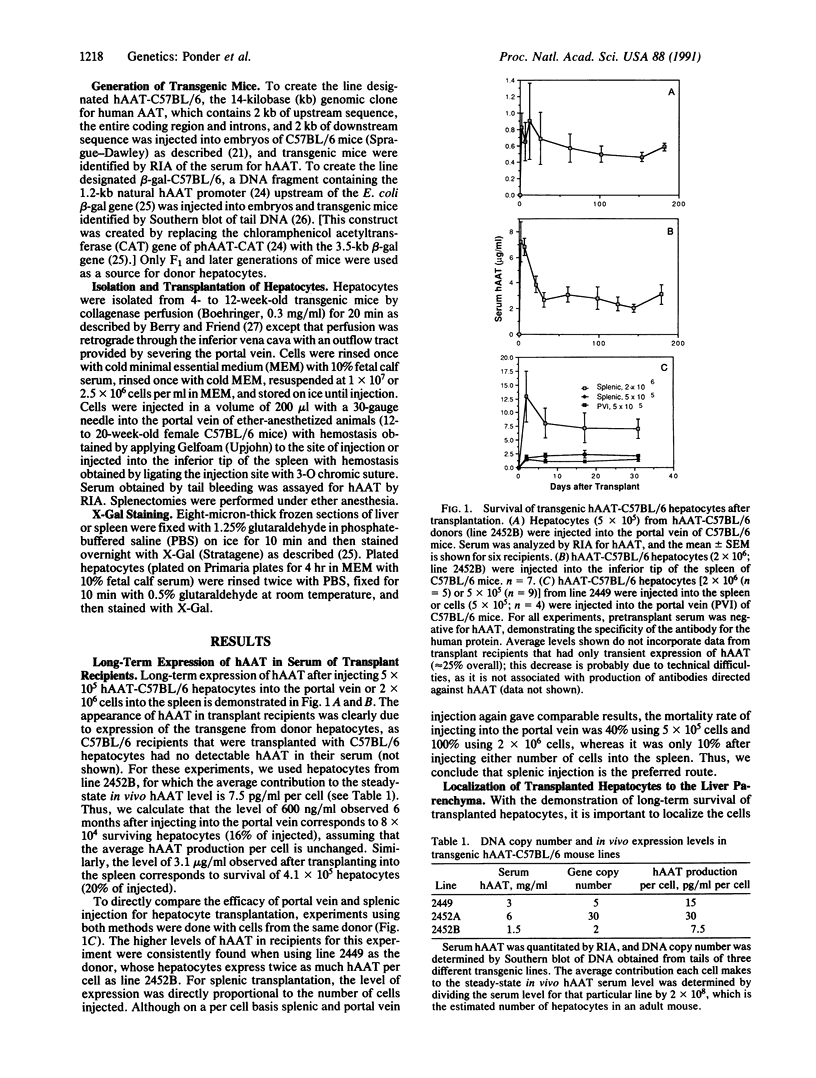
One approach to gene therapy for hepatic diseases is to remove hepatocytes from an affected individual, genetically alter them in vitro, and reimplant them into a receptive locus. Although returning hepatocytes to the liver itself would be advantageous, the feasibility of this approach has never been evaluated due to the inability to distinguish donor from host hepatocytes. To unambiguously identify transplanted hepatocytes after transplantation, and to better quantitate their number and degree of liver function, two transgenic mouse lines were generated in a C57BL/6 background. The first expresses the Escherichia coli beta-galactosidase gene from the relatively liver-specific human alpha 1-antitrypsin (hAAT) promoter and allows transgenic hepatocytes to be readily identified after 5-bromo-4-chloro-3-indolyl beta-D-galactoside staining; the second produces the hAAT protein under control of the same promoter, which enables hepatocyte survival and maintenance of liver function to be quantitated by measuring the serum levels of hAAT. Hepatocytes isolated from transgenic donors were transplanted into nontransgenic C57BL/6 recipients by intrasplenic injection. Surprisingly, a large fraction of these cells were identified within the liver parenchyma but not the spleen at 2 months after transplantation. The high levels of serum hAAT detected in transplant recipients were stable for greater than 6 months, suggesting that established cells will survive indefinitely. These results have important implications for liver organogenesis and hepatic gene therapy.
Full text
PDF
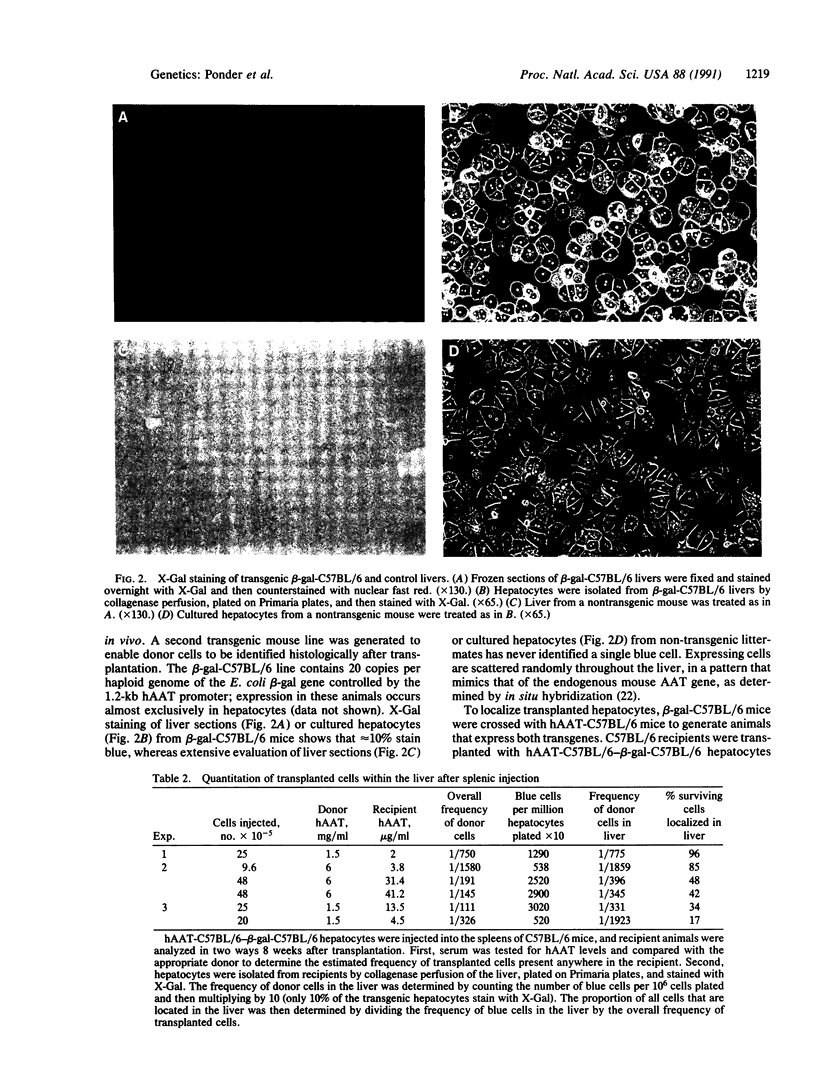

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. D., Thompson J. A., DiPietro J. M., Montgomery K. T., Reid L. M., Anderson W. F. Gene expression in implanted rat hepatocytes following retroviral-mediated gene transfer. Somat Cell Mol Genet. 1989 May;15(3):215–227. doi: 10.1007/BF01534872. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumgardner G. L., Fasola C., Sutherland D. E. Prospects for hepatocyte transplantation. Hepatology. 1988 Sep-Oct;8(5):1158–1161. doi: 10.1002/hep.1840080533. [DOI] [PubMed] [Google Scholar]
- Cuervas-Mons V., Cienfuegos J. A., Maganto P., Rodriguez V., Eroles G., Pinedo I., Santamaria L., Ramos J., Ortiz J. L., Castillo-Olivares J. L. Long-term evaluation of isolated syngeneic hepatocytes transplanted into the normal rat spleen by TC-99M-HIDA scintigraphy. Transplantation. 1985 Jan;39(1):87–90. [PubMed] [Google Scholar]
- Demetriou A. A., Levenson S. M., Novikoff P. M., Novikoff A. B., Chowdhury N. R., Whiting J., Reisner A., Chowdhury J. R. Survival, organization, and function of microcarrier-attached hepatocytes transplanted in rats. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7475–7479. doi: 10.1073/pnas.83.19.7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerhol M. K., Cox D. W. The Pi polymorphism: genetic, biochemical, and clinical aspects of human alpha 1-antitrypsin. Adv Hum Genet. 1981;11:1-62, 371-2. [PubMed] [Google Scholar]
- Fuller B. J. Transplantation of isolated hepatocytes. A review of current ideas. J Hepatol. 1988 Dec;7(3):368–376. doi: 10.1016/s0168-8278(88)80010-2. [DOI] [PubMed] [Google Scholar]
- Groth C. G., Arborgh B., Björkén C., Sundberg B., Lundgren G. Correction of hyperbilirubinemia in the glucuronyltransferase-deficient rat by intraportal hepatocyte transplantation. Transplant Proc. 1977 Mar;9(1):313–316. [PubMed] [Google Scholar]
- Gupta S., Chowdhury N. R., Jagtiani R., Gustin K., Aragona E., Shafritz D. A., Chowdhury J. R., Burk R. D. A novel system for transplantation of isolated hepatocytes utilizing HBsAg-producing transgenic donor cells. Transplantation. 1990 Sep;50(3):472–475. [PubMed] [Google Scholar]
- Henne-Bruns D., Ambrass F. O., Schmiegelow P., Höhne M., Paul D., Kremer B. Intrasplenic hepatocyte transplantation: evaluation of DNA synthesis and proliferation in auxiliary transplanted cells. Res Exp Med (Berl) 1989;189(4):295–302. doi: 10.1007/BF01852262. [DOI] [PubMed] [Google Scholar]
- Hillan K. J., Burt A. D., George W. D., MacSween R. N., Griffiths M. R., Bradley J. A. Intrasplenic hepatocyte transplantation in rats with experimental liver injury: morphological and morphometric studies. J Pathol. 1989 Sep;159(1):67–73. doi: 10.1002/path.1711590114. [DOI] [PubMed] [Google Scholar]
- Jaffe V., Darby H., Selden C., Hodgson H. J. The growth of transplanted liver cells within the pancreas. Transplantation. 1988 Feb;45(2):497–498. doi: 10.1097/00007890-198802000-00053. [DOI] [PubMed] [Google Scholar]
- Jirtle R. L., Biles C., Michalopoulos G. Morphologic and histochemical analysis of hepatocytes transplanted into syngeneic hosts. Am J Pathol. 1980 Oct;101(1):115–126. [PMC free article] [PubMed] [Google Scholar]
- Kelly J. H., Darlington G. J. Modulation of the liver specific phenotype in the human hepatoblastoma line Hep G2. In Vitro Cell Dev Biol. 1989 Feb;25(2):217–222. doi: 10.1007/BF02626182. [DOI] [PubMed] [Google Scholar]
- Koopman P., Povey S., Lovell-Badge R. H. Widespread expression of human alpha 1-antitrypsin in transgenic mice revealed by in situ hybridization. Genes Dev. 1989 Jan;3(1):16–25. doi: 10.1101/gad.3.1.16. [DOI] [PubMed] [Google Scholar]
- Ledley F. D., Darlington G. J., Hahn T., Woo S. L. Retroviral gene transfer into primary hepatocytes: implications for genetic therapy of liver-specific functions. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5335–5339. doi: 10.1073/pnas.84.15.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor G. R., Mogg A. E., Burke J. F., Caskey C. T. Histochemical staining of clonal mammalian cell lines expressing E. coli beta galactosidase indicates heterogeneous expression of the bacterial gene. Somat Cell Mol Genet. 1987 May;13(3):253–265. doi: 10.1007/BF01535207. [DOI] [PubMed] [Google Scholar]
- Makowka L., Lee G., Cobourn C. S., Farber E., Falk J. A., Falk R. E. Allogeneic hepatocyte transplantation in the rat spleen under cyclosporine immunosuppression. Transplantation. 1986 Nov;42(5):537–541. doi: 10.1097/00007890-198611000-00020. [DOI] [PubMed] [Google Scholar]
- Matas A. J., Sutherland D. E., Steffes M. W., Mauer S. M., Sowe A., Simmons R. L., Najarian J. S. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976 May 28;192(4242):892–894. doi: 10.1126/science.818706. [DOI] [PubMed] [Google Scholar]
- Mito M., Ebata H., Kusano M., Onishi T., Saito T., Sakamoto S. Morphology and function of isolated hepatocytes transplanted into rat spleen. Transplantation. 1979 Dec;28(6):499–505. doi: 10.1097/00007890-197912000-00013. [DOI] [PubMed] [Google Scholar]
- Nordlinger B., Bouma M. E., Wang S. R., Ballet F., Verthier N., Huguet C., Infante R. High-yield preparation of porcine hepatocytes for long survival after transplantation in the spleen. Eur Surg Res. 1985;17(6):377–382. doi: 10.1159/000128494. [DOI] [PubMed] [Google Scholar]
- Nordlinger B., Wang S. R., Bouma M. E., Verthier N., Hillan K., Delelo R., Infante R. Can hepatocytes proliferate when transplanted into the spleen? Demonstration by autohistoradiography in the rat. Eur Surg Res. 1987;19(6):381–387. doi: 10.1159/000128726. [DOI] [PubMed] [Google Scholar]
- Ricordi C., Flye M. W., Lacy P. E. Renal subcapsular transplantation of clusters of hepatocytes in conjunction with pancreatic islets. Transplantation. 1988 Jun;45(6):1148–1151. doi: 10.1097/00007890-198806000-00034. [DOI] [PubMed] [Google Scholar]
- Shen R. F., Clift S. M., DeMayo J. L., Sifers R. N., Finegold M. J., Woo S. L. Tissue-specific regulation of human alpha 1-antitrypsin gene expression in transgenic mice. DNA. 1989 Mar;8(2):101–108. doi: 10.1089/dna.1.1989.8.101. [DOI] [PubMed] [Google Scholar]
- Sifers R. N., Carlson J. A., Clift S. M., DeMayo F. J., Bullock D. W., Woo S. L. Tissue specific expression of the human alpha-1-antitrypsin gene in transgenic mice. Nucleic Acids Res. 1987 Feb 25;15(4):1459–1475. doi: 10.1093/nar/15.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D. E., Matas A. J., Steffes M. W., Simmons R. L., Najarian J. S. Transplantation of liver cells in an animal model of congenital enzyme deficiency disease: the Gunn rat. Transplant Proc. 1977 Mar;9(1):317–319. [PubMed] [Google Scholar]
- Vroemen J. P., Blanckaert N., Buurman W. A., Heirwegh K. P., Kootstra G. Treatment of enzyme deficiency by hepatocyte transplantation in rats. J Surg Res. 1985 Sep;39(3):267–275. doi: 10.1016/0022-4804(85)90152-0. [DOI] [PubMed] [Google Scholar]
- Vroemen J. P., Buurman W. A., Heirwegh K. P., van der Linden C. J., Kootstra G. Hepatocyte transplantation for enzyme deficiency disease in congenic rats. Transplantation. 1986 Aug;42(2):130–135. doi: 10.1097/00007890-198608000-00005. [DOI] [PubMed] [Google Scholar]
- Vroemen J. P., Buurman W. A., Schutte B., Maessen J. G., van der Linden C. J., Kootstra G. The cytokinetic behavior of donor hepatocytes after syngenic hepatocyte transplantation into the spleen. Transplantation. 1988 Mar;45(3):600–607. doi: 10.1097/00007890-198803000-00019. [DOI] [PubMed] [Google Scholar]
- Vroemen J. P., Buurman W. A., van der Linden C. J., Visser R., Heirwegh K. P., Kootstra G. Transplantation of isolated hepatocytes into the pancreas. Eur Surg Res. 1988;20(1):1–11. doi: 10.1159/000128734. [DOI] [PubMed] [Google Scholar]
- Vroemen J. P., van der Linden C. J., Buurman W. A., Coenegracht J., Heirwegh K. P., Kootstra G. In vivo dynamic 99mTc-HIDA scintigraphy after hepatocyte transplantation: a new method for the monitoring of graft function. Eur Surg Res. 1987;19(3):140–150. doi: 10.1159/000128693. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Jefferson D. M., Chowdhury J. R., Novikoff P. M., Johnston D. E., Mulligan R. C. Retrovirus-mediated transduction of adult hepatocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3014–3018. doi: 10.1073/pnas.85.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. A., Yee J. K., Skelly H. F., Moores J. C., Respess J. G., Friedmann T., Leffert H. Expression of retrovirally transduced genes in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1987 May;84(10):3344–3348. doi: 10.1073/pnas.84.10.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R. J., Fuller B. J., Attenburrow V. D., Nutt L. H., Hobbs K. E. Functional assessment of hepatocytes after transplantation into rat spleen.. Transplantation. 1982 Feb;33(2):123–126. doi: 10.1097/00007890-198202000-00004. [DOI] [PubMed] [Google Scholar]