Abstract
Background
Migraine is a disabling neurological disorder often complicated by gastrointestinal conditions such as gastric stasis. The association between migraine and gastric stasis has received very little attention in the literature, but the existing evidence suggests that they may share a common etiology.
Results
Patients with migraine and those with gastric stasis exhibit abnormal autonomic nervous system function. Furthermore, empirical studies demonstrate that migraineurs experience significant delays in gastric emptying, both during and outside of attacks, when compared to non-migrainous controls.
Conclusion
More research is needed to establish the relationship between gastric stasis and migraine burden and to determine the impact of gastric stasis on migraine treatment.
Keywords: Autonomic diseases, migraine, gastrointestinal, gastric stasis
Introduction
Migraine is a common, incapacitating headache disorder characterized by severe headache attacks that last from four to 72 hours (1). Approximately 18% of women and 6% of men worldwide suffer from migraines (2). In general, migraines are underdiagnosed and undertreated (3), and emerging data continue to shed light on the disabling nature of this disorder and its associated comorbidities. Migraines are often accompanied by exacerbating gastrointestinal symptoms such as nausea and vomiting (1). One gastrointestinal condition that often accompanies migraine is gastric stasis (GS) (4).
Gastric stasis, also called gastroparesis (5), is defined as delayed emptying of the stomach in the absence of mechanical obstruction, and its clinical manifestations include nausea, vomiting, bloating, and weight loss (6). An estimated 4% of the adult population suffers from GS (7). Gastric stasis disproportionately affects women (82%), and the average age of onset is 33.7 years old (8). Based on a study of 146 patients with GS, the most common etiologies of GS were: idiopathic origins (36%), diabetes (29%), post-gastric surgery (especially when the vagal nerve is damaged) (13%), Parkinson’s disease (7.5%), collagen vascular disorders (4.8%), and intestinal pseudo-obstruction (4.1%) (8).
In a survey completed by 500 migraine patients in 1966, 96% of patients experienced gastrointestinal symptoms (4). More recently, Lipton and colleagues reported that 73% of migraine patients experience nausea and 29% experience vomiting (9). In a meta-analysis of women with migraine, nausea and vomiting were reported by 61.6% of those with migraine with aura and 66.0% of migraineurs without aura (10). There are several potential causes of migraine-associated nausea and vomiting. These symptoms may arise because of increased activation of the sympathetic nervous system (11) (or they may be adverse effects of the narcotics (e.g. opioids) used to treat headaches (12). Alter, migraine-associated nausea and vomiting may be associated with comorbid GS in these patients (6). However, in both of these studies, it was not clear whether patients’ symptoms could be specifically attributed to GS.
It is hypothesized that both migraine and GS arise because of autonomic nervous system (ANS) dysfunction (13–16). Gastric stasis during migraine may be attributed to increased sympathetic nervous system activity, decreased parasympathetic nervous system activity, or both (4). Gastric stasis and vomiting during migraine may complicate treatment by causing a delay or inconsistent absorption of analgesics (17). Very few studies of GS in migraineurs have been performed, and little is known about the physiological link between these conditions. This review provides key insights on GS in migraineurs and sheds light on the need for more research on this topic.
ANS dysfunction in migraine and pathophysiology of GS
Autonomic dysfunction in migraine
Autonomic symptoms including nausea, vomiting, diarrhea, cutaneous vasoconstriction, vasodilation, piloerection, and diaphoresis are common during migraine (18). A study by Shechter and colleagues showed that compared with controls and nondisabled migraineurs, resting diastolic blood pressure was higher (p < 0.10) in disabled migraineurs (69.75 mm vs. 71.58 mm vs. 73.21 mm, respectively). Additionally, pulse rate (RR interval) variation was significantly lower (p < 0.001) in disabled migraine subjects compared with nondisabled migraineurs and controls (1.19 vs. 1.26 vs. 1.26, respectively) (18). Autonomic dysregulation has also been studied using thermography. Dalla Volta et al. demonstrated that migraineurs experience vasomotor fluctuations during and between migraine attacks (19).
Several brain regions have been implicated in the pathophysiology of migraine (Figure 1), and ANS derangement during migraine can be attributed to dysfunction of these brain regions (20–22). For example, the midbrain periaqueductal gray matter (PAG), a region of densely layered neurons, regulates the autonomic response to antinociceptive and autonomic signals (23,24). The PAG is a major nodal point of modulation of craniovascular nociception and, through its role in the descending pain modulation system, contributes to migraine pathophysiology (25). Investigators assessed iron homeostasis in the PAG of 34 patients with either episodic migraine or chronic daily headache (defined as those who experience migraine without aura on ≥15 days per month due to medication overuse, and who previously suffered episodic migraine without aura) or in control subjects. High-resolution magnetic resonance imaging (MRI) suggested that iron homeostasis in the PAG is impaired in the episodic migraine and chronic daily headache groups. Furthermore, iron deposition in the PAG appeared to be related to the frequency of headaches – the more frequent and longer the duration of migraine, the more likely the deposition of iron (20). A more recent study confirms the relationship between migraine attack frequency and PAG function (26). High attack frequency is associated with increased PAG functional connectivity to some brain areas (e.g. supramarginal gyrus, ventral prefrontal cortex, anterior insula, nucleus cuneiformis, hypothalamus) and is associated with reduced connectivity to others (e.g. dorsomedial prefrontal cortex, postcentral gyrus, precentral gyrus, anterior cingulate, amygdala, angular gyrus, medial thalamus). This correlation is significantly (p = 0.05) associated with migraineurs compared to controls. The severity of migraine attacks also influences PAG activation. Cao et al. reported that activation of the PAG occurs during a severe migraine attack but not during a mild or moderate attack (27).
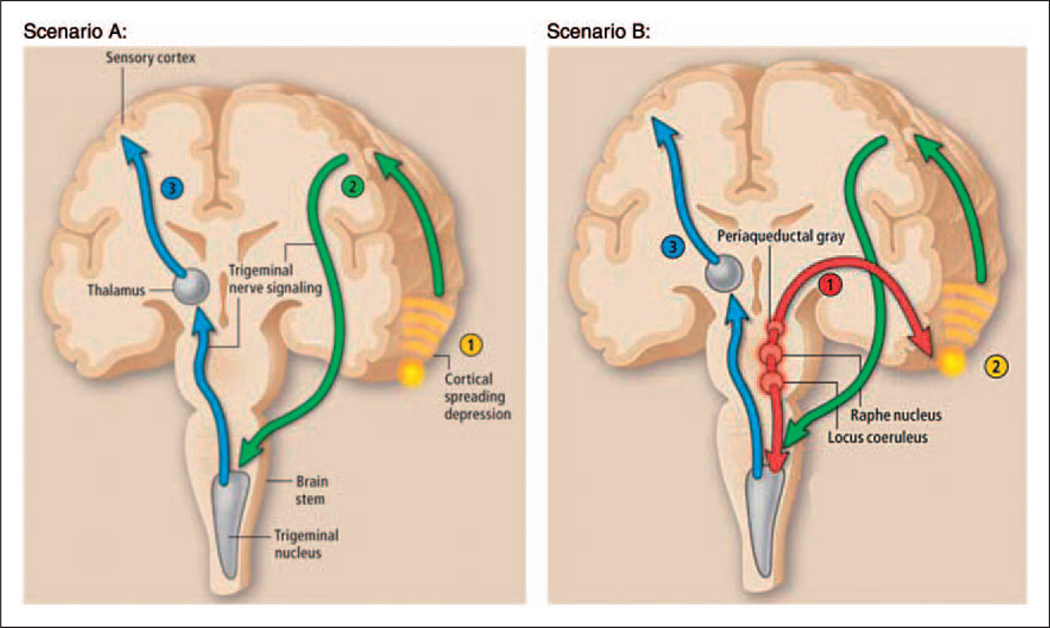
Figure 1.
Neuronal control of headache pain
Scenario A: (1) Hyperexcitable neurons trigger cortical spreading depression. Cortical spreading depression stimulates the release of neurotransmitters and ions. (2) These neurotransmitters and ions relay pain signals to the trigeminal nucleus in the brain stem, which (3) sends a pain signal to the thalamus, ultimately resulting in the sensation of pain.
Scenario B: (1) The raphe nucleus, the locus coeruleus, and the periaqueductal gray function abnormally. (2) Dysfunction of the brain stem leads to spreading depression in the cortex or subcortex, and through signals transmitted by neurotransmitters and ions, subsequently activates the trigeminal system. (3) Alternatively, brain stem dysfunction can directly activate pain pathways. Reprinted with permission.22
Whereas perturbation of neuronal processes in the brain can lead to migraines (20,26–29), direct stimulation of various regions of the brain can alleviate migrainous symptoms (30,31). Stimulation of the sphenopalatine ganglion, a nucleus for autonomic fibers, relieves headache pain associated with refractory migraine (30). Additionally, preliminary results from a small case series suggest that stimulating the vagus nerve, a preganglionic neuron belonging to the parasympathetic division of the ANS (32), improves drug-refractory chronic migraine (31).
Pathophysiology of GS
Normal gastrointestinal motility is mediated through the function of smooth muscle cells, interstitial cells of Cajal, enteric nerves, and the vagus nerve (Figure 2) (6,33). Diseases that affect the enteric nervous system often negatively impact the ability of the stomach to empty, usually resulting in pathological delay in gastric emptying. A prolonged delay in gastric emptying leads to GS (6): the failure of the stomach to perform its mechanical functions of storage and evacuation (34). Deleterious health consequences associated with untreated GS include esophagitis, Mallory-Weiss tear from chronic vomiting, malnutrition, volume depletion with acute renal failure, electrolyte disturbances, and bezoar formation (7).
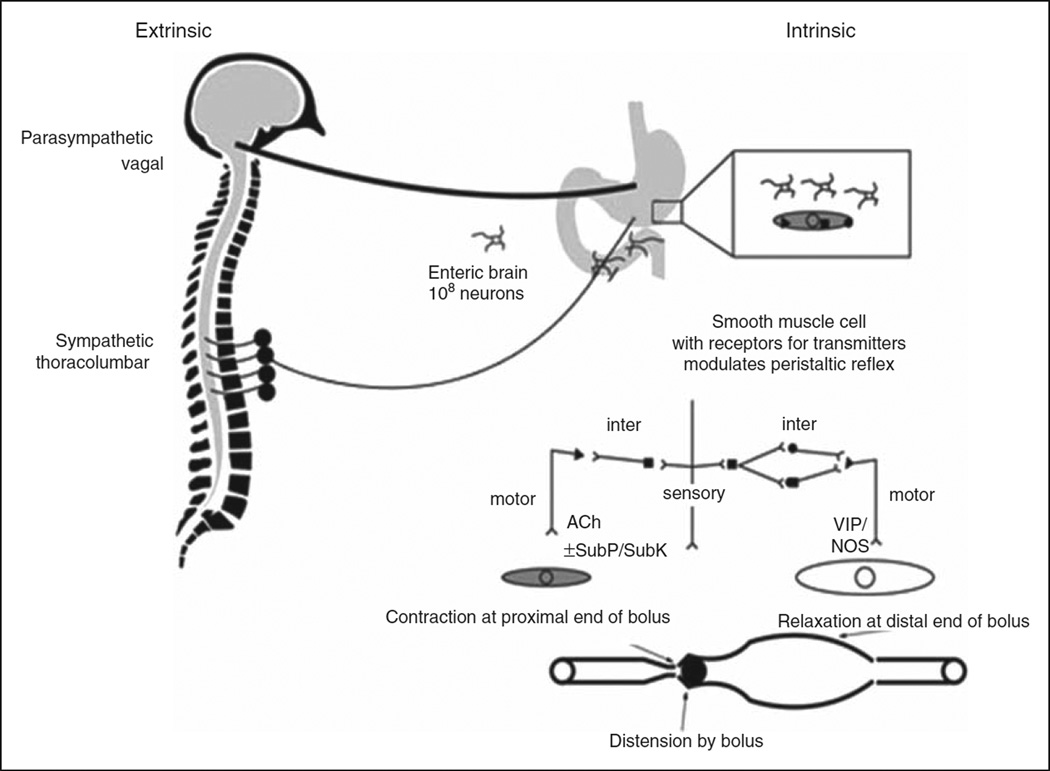
Figure 2.
Control of gastrointestinal function by the brain. Sympathetic and parasympathetic innervation of gut modulates gastrointestinal function. Neurotransmitters released from the enteric neurons control peristalsis. The major neurotransmitters involved are acetylcholine (ACh), substance P (SubP), vasoactive intestinal polypeptide (VIP) and nitric oxide. NOS: nitric oxide synthase; SubK: substance K. Adapted with permission.33
Sensory information from the gastrointestinal tract is transmitted via the afferent vagus, and efferent output to the gastrointestinal tract is regulated in response to changing homeostatic conditions. Demyelinating lesions in the brain stem, presumably affecting the vagal nerve nuclei, may give rise to GS (13). In a case series, two female patients presented with intractable nausea, vomiting, cerebellar deficits, dysphagia, and paresthesias. MRI of their brains revealed an area of demyelination in the medullary region. Both of these patients had an abnormal gastric emptying test. Treatment of the brain lesions with corticosteroids led to simultaneous improvement in gastric emptying and reduction in size of the brain lesions, suggesting a common etiology (13).
There are several ways to measure the rate of gastric emptying, but gastric scintigraphy is the standard for clinical measurement of gastric motility (35). Gastric scintigraphy involves radiolabeling of a solid or liquid component of a meal. Scintigraphy, a method whereby external detectors capture emitted internal radiation, is used to measure gastric counts. The counts correlate directly with the volume of the meal remaining. Gastric scintigraphy is considered the gold standard in the field, as it is an unbiased method not subject to any assumptions about the geometric shape of the stomach. One caveat to this method is that it is not highly sensitive (35). Another method employed in the studies described herein is epigastric impedance. Epigastric impedance involves applying alternating current (AC) currents to the epigastrium to detect changes in gastric contents. A major benefit of the epigastric impedance method is that it is noninvasive (36).
Clinical implications of GS in migraine and migraine treatment
The rate of gastric emptying impacts the rate and efficiency of absorption of drugs with high intestinal permeability. Variations in the gastric emptying rate influence the therapeutic outcome of orally administered drugs (37). Early experimental evidence suggests that migraine patients absorb oral formulations of headache medications less efficiently than non-migrainous controls (38–40). In 1974, Volans measured the rate and efficiency of absorption of effervescent aspirin during a migraine attack and during the headache- free period: 19 out of 42 migraine patients experienced a delay in aspirin absorption during a migraine attack, but this impairment was not observed during a headache-free period (40). Similar results were obtained in a study evaluating the rate and efficiency of absorption of tolfenamic acid in migraine patients during the migraine phase and the migraine-free phase. Absorption was significantly delayed during the migraine phase compared to the migraine-free phase (tmax (h), 2.86 vs. 1.69; p < 0.05). Additionally, absorption, as measured by area under the curve (AUC)(0–2 h), was significantly lower during the migraine period (2.00) versus the migraine-free period (4.07) (38). Tokola and Neuvonen also investigated the effect of migraine on the absorption of oral paracetamol in nine patients during the migraine phase and the migraine-free phase. The peak serum concentration (µmol 1−1) of paracetamol was lower during the migraine phase (109) than it was during the migraine-free period (14). The AUC(0–2h), AUC(0–4h), and AUC(0–6h) were significantly decreased during migraine attacks (17% vs. 14% vs. 12%, respectively) (39). These results suggest that migraineurs experience abnormalities in gastrointestinal function during a migraine attack, but not during the headache-free phase. However, more recent studies challenge this notion (41–43), and these studies will be discussed in a later section.
Triptans are effective and safe for acute migraine treatment. Following oral administration of triptans to persons with migraine, the plasma concentrations vary from patient to patient. This variation may be due to differences in rates of stomach emptying in migraineurs and is likely to become more pronounced during migraine attacks when many migraineurs experience GS (44). Thomsen and colleagues assessed the rate of zolmitriptan absorption in 20 patients during a migraine attack and in 18 of these patients during the migraine-free period. Zolmitriptan was less rapidly absorbed during an attack than it was during the migraine-free period. At four hours post-dose, the maximum plasma concentration of zolmitriptan (Cmax) and AUC0–4 ranged from 0 to 27.9 ng/ml and 0 to 60.8 ng/ml.h, respectively, during a migraine attack. During the migraine-free period, Cmax and AUC0–4 ranged from 3.5 to 26.3 ng/ml and 9.4 to 79.5 ng/ml.h, respectively (45). Experimental evidence has shown that the efficiency of triptans in treating migraines is increased when combined with gastrokinetic drugs. For example, when rizatriptan was combined with trimebutine, 30 of 64 migraine attacks (46.8%) resolved completely one hour post-dose, compared with eight attacks (12.5%) when treated with rizatriptan alone (p < 0.01). The combination was more effective than rizatriptan alone at two hours (73.4% vs. 31.2%; p < 0.001) as well as four hours post-dose (79.7% vs. 31.2%; p < 0.001) (46). Together, these studies suggest that despite their common usage among persons with migraine, there are barriers to the efficacy of oral triptans.
Delayed or inconsistent absorption of conventional orally administered headache medications during migraine may be caused by GS (17). Metoclopramide is Food and Drug Administration (FDA)-approved for the short-term treatment of GS in the United States (47). It is also effective for the treatment of acute migraine. It is believed to stimulate gastrointestinal motility by sensitizing tissues to the actions of acetylcholine or by 5-HT receptor agonism (48). However, there are major side effects associated with metoclopramide, including extrapyramidal reactions, acute dystonic reactions, akathisia, drug-induced Parkinsonism, tardive dyskinesia, and hyperprolactinemia. In fact, the FDA has placed a black-box warning on metoclopramide because of the risk of related side effects (47). As an alternative, many non-oral formulations of migraine medications, including injectables, and transdermal patches, are available that bypass the gastrointestinal tract (49–52).
Evaluating the link between migraine and GS
Few empirical studies have explored the relationship between GS and migraine. The results of these studies are described in this section and summarized in Table 1 (41,42,53). Boyle and colleagues demonstrated a correlation between migraine severity and delayed gastric emptying as measured by the epigastric impedance method. The severity of patients’ headaches was subjectively assessed based on a four-point scale: none, mild, moderate and severe. When pain was mild, the time to half emptying (t50%) was significantly (p < 0.01) delayed by less than 30 minutes, compared to a normal gastric emptying time during the headache-free period. However, gastric half-emptying time was delayed in excess of 30 minutes (p < 0.01) compared to a normal half-emptying time during the headache-free phase, when headache pain was moderate or severe (53).
Table 1.
Summary of gastric emptying studies in migraineurs.
| Time to half emptying | ||
|---|---|---|
| Method of detection | Headache group | t1/2 (min) |
| Epigastric impedance (53) | • Mild attack | < 30 |
| • Moderate or severe attack | > 30 | |
| • Control | 9 ± 5 | |
| Gastric scintigraphy (42) | • Interictal | 188.8 |
| • Ictal | 149.9 | |
| • Controls | 111.8 | |
| Gastric scintigraphy (41) | • Interictal | 243 |
| • Spontaneous migraine | 124 | |
| • Induced migraine | 182 | |
| • Control | 112 | |
Recent studies show that GS occurs during spontaneous migraine attacks, visually induced migraine attacks, and during the headache-free, interictal period (41,42). Gastric scintigraphy was used to measure the gastric emptying rate of 10 migraine patients and 10 age- and sex-matched controls. In migraineurs, the time to half emptying after an induced migraine attack was delayed ictally (78%) and interictally (80%). Compared to non-migrainous controls, the time to half emptying during the interictal period was significantly longer in migraineurs (188.8 min vs. 111.8 min; p < 0.05) (42). These results suggest that migraineurs suffer from GS both during and outside of an induced attack, contrary to previous beliefs that GS occurred only during an attack (4,38,39). In a subsequent study, Aurora and colleagues confirmed that the time to half emptying was delayed after a spontaneous migraine attack as well (41).
Cyclic vomiting syndrome (CVS) is an incapacitating, functional disorder that is characterized by recurrent episodes of nausea, vomiting, and in some cases, abdominal pain, separated by periods of relative wellness. CVS is thought to be a part of the migraine spectrum (54). CVS is less well understood in adults than it is in children (55), but studies have shed light on CVS in adults and have confirmed an association between CVS and migraine in this patient population (55,56). For example, Fleisher and colleagues retrospectively analyzed the clinical characteristics of 41 adult patients with CVS. Of the 40 patients who provided sufficient data to be included in the analysis, 28 (70%) experienced migraine during or between episodes of CVS (55). In a separate retrospective analysis of 92 adult patients with CVS, Hejazi et al. determined that 27 patients (29%) had a personal history of migraine (56). Cyclic vomiting syndrome is associated with ANS dysfunction and possibly results from GS (16,57). In fact, gastric electrical stimulation (GES), a therapy that has been used to treat GS (58), has been shown to improve recalcitrant CVS (59). Christensen and colleagues analyzed the association between CVS and migraine in patients with GS (60). Sixty-seven patients with diabetic GS were divided into two groups based on the absence or presence of cyclic vomiting symptoms. There was a significant (p = 0.02) between-group difference in the prevalence of migraine headaches in the group with cyclic vomiting symptoms compared to those without such symptoms (47.4% vs. 20.7%) (60). Taken together, the studies discussed in this section demonstrate a link between migraine, GS, and ANS dysfunction. However, more work is needed to enhance our understanding of this phenomenon.
Conclusions
Little is known about the physiological link between GS and migraine. Recent evidence has confirmed that migraineurs experience GS (41,42). ANS dysfunction appears to lie at the heart of migraine and GS, which are often comorbid conditions, but the directionality of this association remains unclear (15,16,20,28,61). Migraineurs experienced delays in gastric emptying during and outside of an attack (41,42), suggesting that abnormal gastric motility is not limited to an acute event but is instead a clinical feature of migraine. Indirect evidence suggests that GS may complicate treatment of migraine (38–40). Consequently, transdermal, injectable, and other non-oral formulations of migraine medications that bypass the gastrointestinal tract have been developed for the treatment of migraine, especially in patients with comorbid GS. More research is needed to establish the relationship between GS and migraine burden, including determining the impact of GS on migraine-associated disability, health-related quality of life, and healthcare resource use. It would also be of interest to elucidate which specific aberrations in brain function correlate with GS in migraineurs. Finally, additional research could reveal that it may be of value for clinicians to assess whether migraine patients have GS and consider this condition in their treatment strategy.
Clinical implications.
The association between migraine and gastric stasis has received little attention in the literature, but existing evidence suggests that they may share a common etiology; this review provides insights on gastric stasis in migraineurs.
Autonomic nervous system dysfunction appears to lie at the heart of migraine and gastric stasis, which are often comorbid conditions, but the directionality of this association remains unclear.
Migraineurs experience delays in gastric emptying during and outside of an attack, suggesting that abnormal gastric motility is not limited to an acute event but is instead a clinical feature of migraine.
Indirect evidence suggests that gastric stasis may complicate treatment of migraine.
Acknowledgments
The authors would like to acknowledge Lashon Pringle, PhD, of Imprint Publication Science, New York, NY, for her contributions to the paper. Imprint was funded by Allergan Inc.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Dr. Aurora serves on the editorial board of Headache; is listed as author on a patent re: Gastric stasis and improvement with onabotulinumtoxinA (Allergan Inc.); has received research support from Advanced Bionics, Alexza Pharmaceuticals, Astra-Zeneca, Allergan Inc., Boston Scientific, CAPNIA, GlaxoSmithKline, MAP Pharmaceuticals Inc., Merck and Co, Ortho-McNeil/Janssen, Neuralieve Inc., NuPath Inc., Takeda Pharmaceutical Company Limited, Winston Pharmaceuticals Inc., and the National Institutes of Health (NIH); has served as a consultant for Astra Zeneca, Valeant Pharmaceuticals International, NMT Medical Inc., Ortho-McNeil/Janssen, Pfizer Inc., Medtronic Inc., Merck and Co., GlaxoSmithKline, Allergan Inc., Neuralieve Inc., NuPath Inc.; and has received honoraria from Merck and Co., GlaxoSmithKline, Allergan Inc., Nautilius, NuPath Inc., and Zogenix Inc.
Dr. Papapetropoulos was a full-time employee of Allergan Inc., at the time of writing, and held stock options at Allergan Inc.
Dr. Kori is a full-time employee of MAP Pharmaceuticals and holds stock and stock options at MAP Pharmaceuticals.
Dr. Abell is a consultant, investigator, and licensor for Medtronic Inc., and is a consultant for Rhythm Pharmaceuticals. He is also funded by NIH Grant U01DK074007-05 and NIH Grant U01DK074007-04-S1 ARRA.
Footnotes
Conflicts of interest
Dr. Kedar reports no disclosures.
References
- 1.The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, Lipton RB, Celentano DD, et al. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64–69. [PubMed] [Google Scholar]
- 3.Buse DC, Rupnow MF, Lipton RB. Assessing and managing all aspects of migraine: Migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Mayo Clin Proc. 2009;84:422–435. doi: 10.1016/S0025-6196(11)60561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volans GN. Migraine and drug absorption. Clin Pharmacokinet. 1978;3:313–318. doi: 10.2165/00003088-197803040-00004. [DOI] [PubMed] [Google Scholar]
- 5.Parrish CR. Gastrointestinal issues in persons with diabetes. In: Powers MA, editor. Handbook of diabetes medical nutrition therapy. Gaithersburg, MD: Aspen Publishers; 1996. pp. 618–637. [Google Scholar]
- 6.Masaoka T, Tack J. Gastroparesis: Current concepts and management. Gut Liver. 2009;3:166–173. doi: 10.5009/gnl.2009.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waseem S, Moshiree B, Draganov PV. Gastroparesis: Current diagnostic challenges and management considerations. World J Gastroenterol. 2009;15:25–37. doi: 10.3748/wjg.15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–2404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 9.Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: Data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 10.Schurks M, Buring JE, Kurth T. Migraine, migraine features, and cardiovascular disease. Headache. 2010;50:1031–1040. doi: 10.1111/j.1526-4610.2009.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spierings EL. Symptomatology and pathogenesis of migraine. J Pediatr Gastroenterol Nutr. 1995;21(Suppl 1):S37–S41. doi: 10.1097/00005176-199501001-00011. [DOI] [PubMed] [Google Scholar]
- 12.Porreca F, Ossipov MH. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: Mechanisms, implications, and management options. Pain Med. 2009;10:654–662. doi: 10.1111/j.1526-4637.2009.00583.x. [DOI] [PubMed] [Google Scholar]
- 13.Reddymasu SC, Bonino J, McCallum RW. Gastroparesis secondary to a demyelinating disease: A case series. BMC Gastroenterol. 2007;7:3. doi: 10.1186/1471-230X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peroutka SJ. Migraine: A chronic sympathetic nervous system disorder. Headache. 2004;44:53–64. doi: 10.1111/j.1526-4610.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- 15.Peroutka SJ. Re: A sympathetic view of “2003 Wolff Award: Possible parasympathetic contributions to peripheral and central sensitization during migraine”. Headache. 2004;44:731–732. doi: 10.1111/j.1526-4610.2004.04134_5.x. [DOI] [PubMed] [Google Scholar]
- 16.Chelimsky TC, Chelimsky GG. Autonomic abnormalities in cyclic vomiting syndrome. J Pediatr Gastroenterol Nutr. 2007;44:326–330. doi: 10.1097/MPG.0b013e31802bddb7. [DOI] [PubMed] [Google Scholar]
- 17.Rapoport AM, Freitag F, Pearlman SH. Innovative delivery systems for migraine: The clinical utility of a transdermal patch for the acute treatment of migraine. CNS Drugs. 2010;24:929–940. doi: 10.2165/11317540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Shechter A, Stewart WF, Silberstein SD, et al. Migraine and autonomic nervous system function: A population-based, case-control study. Neurology. 2002;58:422–427. doi: 10.1212/wnl.58.3.422. [DOI] [PubMed] [Google Scholar]
- 19.Dalla Volta G, Anzola GP, DiMonda V. The disappearance of the “cold patch“ in recovered migraine patients: Thermographic findings. Headache. 1991;31:305–309. doi: 10.1111/j.1526-4610.1991.hed3105305.x. [DOI] [PubMed] [Google Scholar]
- 20.Welch KM, Nagesh V, Aurora SK, et al. Periaqueductal gray matter dysfunction in migraine: Cause or the burden of illness? Headache. 2001;41:629–637. doi: 10.1046/j.1526-4610.2001.041007629.x. [DOI] [PubMed] [Google Scholar]
- 21.Kruit MC, Launer LJ, Ferrari MD, et al. Infarcts in the posterior circulation territory in migraine. The population- based MRI CAMERA study. Brain. 2005;128:2068–2077. doi: 10.1093/brain/awh542. [DOI] [PubMed] [Google Scholar]
- 22.Dodick DW, Gargus JJ. Why migraines strike. Sci Am. 2008;299:56–63. doi: 10.1038/scientificamerican0808-56. [DOI] [PubMed] [Google Scholar]
- 23.Bandler R, Carrive P. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 1988;439:95–106. doi: 10.1016/0006-8993(88)91465-5. [DOI] [PubMed] [Google Scholar]
- 24.Smith GS, Savery D, Marden C, et al. Distribution of messenger RNAs encoding enkephalin, substance P, somatostatin, galanin, vasoactive intestinal polypeptide, neuropeptide Y, and calcitonin gene-related peptide in the midbrain periaqueductal grey in the rat. J Comp Neuro. 1994;350:23–40. doi: 10.1002/cne.903500103. [DOI] [PubMed] [Google Scholar]
- 25.Moskowitz MA. The trigeminovascular system. In: Olesen J, Tfelt-Hansen P, Welch KMA, editors. The Headaches. New York: Raven Press, Ltd; 1993. pp. 97–104. [Google Scholar]
- 26.Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol. 2011;70:838–845. doi: 10.1002/ana.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Y, Aurora SK, Nagesh V, et al. Functional MRI-BOLD of brainstem structures during visually triggered migraine. Neurology. 2002;59:72–78. doi: 10.1212/wnl.59.1.72. [DOI] [PubMed] [Google Scholar]
- 28.Kruit MC, van Buchem MA, Launer LJ, et al. Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: The population-based MRI CAMERA study. Cephalalgia. 2010;30:129–136. doi: 10.1111/j.1468-2982.2009.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welch KM, Cao Y, Aurora S, et al. MRI of the occipital cortex, red nucleus, and substantia nigra during visual aura of migraine. Neurology. 1998;51:1465–1469. doi: 10.1212/wnl.51.5.1465. [DOI] [PubMed] [Google Scholar]
- 30.Tepper SJ, Rezai A, Narouze S, et al. Acute treatment of intractable migraine with sphenopalatine ganglion electrical stimulation. Headache. 2009;49:983–989. doi: 10.1111/j.1526-4610.2009.01451.x. [DOI] [PubMed] [Google Scholar]
- 31.Cecchini AP, Mea E, Tullo V, et al. Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: Preliminary data. Neurol Sci. 2009;30(Suppl 1):S101–S104. doi: 10.1007/s10072-009-0073-3. [DOI] [PubMed] [Google Scholar]
- 32.McCorry LK. Physiology of the autonomic nervous system. Am J Pharm Educ. 2007;71:78. doi: 10.5688/aj710478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellow JE, Delvaux M, Azpiroz F, et al. Principles of applied neurogastroenterology: Physiology/motility-sensation. Gut. 1999;45(Suppl 2):II17–II24. doi: 10.1136/gut.45.2008.ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramkumar D, Schulze KS. Gastroduodenal motility. Curr Opin Gastroenterol. 2003;19:540–545. doi: 10.1097/00001574-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: A joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103:753–763. doi: 10.1111/j.1572-0241.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 36.McClelland GR, Sutton JA. Epigastric impedance: A non-invasive method for the assessment of gastric emptying and motility. Gut. 1985;26:607–614. doi: 10.1136/gut.26.6.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nyholm D, Lennernäs H. Irregular gastrointestinal drug absorption in Parkinson’s disease. Expert Opin Drug Metab Toxicol. 2008;4:193–203. doi: 10.1517/17425255.4.2.193. [DOI] [PubMed] [Google Scholar]
- 38.Tokola RA, Neuvonen PJ. Effects of migraine attack and metoclopramide on the absorption of tolfenamic acid. Br J Clin Pharmacol. 1984;17:67–75. doi: 10.1111/j.1365-2125.1984.tb05001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tokola RA, Neuvonen PJ. Effect of migraine attacks on paracetamol absorption. Br J Clin Pharmacol. 1984;18:867–871. doi: 10.1111/j.1365-2125.1984.tb02557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volans GN. Absorption of effervescent aspirin during migraine. Br Med J. 1974;4:265–268. doi: 10.1136/bmj.4.5939.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aurora S, Kori S, Barrodale P, et al. Gastric stasis occurs in spontaneous, visually induced, and interictal migraine. Headache. 2007;47:1443–1446. doi: 10.1111/j.1526-4610.2007.00922.x. [DOI] [PubMed] [Google Scholar]
- 42.Aurora SK, Kori SH, Barrodale P, et al. Gastric stasis in migraine: More than just a paroxysmal abnormality during a migraine attack. Headache. 2006;46:57–63. doi: 10.1111/j.1526-4610.2006.00311.x. [DOI] [PubMed] [Google Scholar]
- 43.Cheng LK, O’Grady G, Du P, et al. Gastrointestinal system. Wiley Interdiscip Rev Syst Biol Med. 2010;2:65–79. doi: 10.1002/wsbm.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrari A, Tiraferri I, Neri L, et al. Why pharmacokinetic differences among oral triptans have little clinical importance: A comment. J Headache Pain. 2011;12:5–12. doi: 10.1007/s10194-010-0258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomsen LL, Dixon R, Lassen LH, et al. 311C90 (zolmitriptan), a novel centrally and peripheral acting oral 5-hydroxytryptamine-1D agonist: A comparison of its absorption during a migraine attack and in a migraine-free period. Cephalalgia. 1996;16:270–275. doi: 10.1046/j.1468-2982.1996.1604270.x. [DOI] [PubMed] [Google Scholar]
- 46.Krymchantowski AV, Filho PF, Bigal ME. Rizatriptan vs. rizatriptan plus trimebutine for the acute treatment of migraine: A double-blind, randomized, crossover, placebo-controlled study. Cephalalgia. 2006;26:871–874. doi: 10.1111/j.1468-2982.2006.01136.x. [DOI] [PubMed] [Google Scholar]
- 47.Lee A, Kuo B. Metoclopramide in the treatment of diabetic gastroparesis. Expert Rev Endocrinol Metab. 2010;5:653–662. doi: 10.1586/eem.10.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marmura MJ. Use of dopamine antagonists in treatment of migraine. Curr Treat Options Neurol. 2012;14:27–35. doi: 10.1007/s11940-011-0150-9. [DOI] [PubMed] [Google Scholar]
- 49.Phillips WJ, Tollefson B, Johnson A, et al. Relief of acute pain in chronic idiopathic gastroparesis with intravenous phentolamine. Ann Pharmacother. 2006;40:2032–2036. doi: 10.1345/aph.1H255. [DOI] [PubMed] [Google Scholar]
- 50.Fuseau E, Petricoul O, Moore KH, et al. Clinical pharmacokinetics of intranasal sumatriptan. Clin Pharmacokinet. 2002;41:801–811. doi: 10.2165/00003088-200241110-00002. [DOI] [PubMed] [Google Scholar]
- 51.Fox AW. Subcutaneous sumatriptan pharmacokinetics: Delimiting the monoamine oxidase inhibitor effect. Headache. 2010;50:249–255. doi: 10.1111/j.1526-4610.2009.01568.x. [DOI] [PubMed] [Google Scholar]
- 52.Dahlöf CG. Non-oral formulations of triptans and their use in acute migraine. Curr Pain Headache Rep. 2005;9:206–212. doi: 10.1007/s11916-005-0064-x. [DOI] [PubMed] [Google Scholar]
- 53.Boyle R, Behan PO, Sutton JA. A correlation between severity of migraine and delayed gastric emptying measured by an epigastric impedance method. Br J Clin Pharmacol. 1990;30:405–409. doi: 10.1111/j.1365-2125.1990.tb03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pareek N, Fleisher DR, Abell T. Cyclic vomiting syndrome: What a gastroenterologist needs to know. Am J Gastroenterol. 2007;102:2832–2840. doi: 10.1111/j.1572-0241.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 55.Fleisher DR, Gornowicz B, Adams K, et al. Cyclic vomiting syndrome in 41 adults: The illness, the patients, and problems of management. BMC Med. 2005;3:20. doi: 10.1186/1741-7015-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hejazi RA, Lavenbarg TH, McCallum RW. Spectrum of gastric emptying patterns in adult patients with cyclic vomiting syndrome. Neurogastroenterol Motil. 2010;22:1298–1302. e338. doi: 10.1111/j.1365-2982.2010.01584.x. [DOI] [PubMed] [Google Scholar]
- 57.Ladabaum U, Hasler WL. Novel approaches to the treatment of nausea and vomiting. Dig Di. 1999;17:125–132. doi: 10.1159/000016917. [DOI] [PubMed] [Google Scholar]
- 58.Soffer E, Abell T, Lin Z, et al. Review article: Gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681–694. doi: 10.1111/j.1365-2036.2009.04082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grover I, Shivangi K, Kedar A, et al. Gastric stimulation is an option for patients with refractory cyclical vomiting syndrome: One year follow up; Presented at: Digestive Disease Week (DDW) 2011; Chicago, IL. 7–11 May 2011; poster Tu1388. [Google Scholar]
- 60.Christensen CJ, Johnson WD, Abell TL. Patients with cyclic vomiting pattern and diabetic gastropathy have more migraines, abnormal electrogastrograms, and gastric emptying. Scand J Gastroenterol. 2008;43:1076–1081. doi: 10.1080/00365520802085411. [DOI] [PubMed] [Google Scholar]
- 61.Peroutka SJ. 2008: The year in review. Headache. 2009;49:796–802. doi: 10.1111/j.1526-4610.2009.01422.x. [DOI] [PubMed] [Google Scholar]