Abstract
Purpose
Pain relief after exercise, exercise-induced hypoalgesia (EIH), is established across the lifespan. Conditioned pain modulation (CPM: pain inhibits pain) may be a mechanism for EIH.
Methods
In 55 adolescents, pressure pain thresholds were measured before and after exercise (deltoid, quadriceps, and nail bed) and during CPM at the nail bed and deltoid test stimulus sites. The relation between EIH and CPM was explored.
Results
EIH occurred at deltoid and quadriceps; CPM occurred at nail bed and deltoid. CPM and EIH correlated at deltoid; adolescents with greater CPM experienced greater pain relief after exercise. At this site, CPM predicted 5.4% of EIH. Arm lean mass did not add a significant effect. Peak exercise pain did not influence EIH. Adolescents with none, minimal, moderate, or severe peak exercise pain experienced similar EIH.
Conclusions
A potential relation exists between CPM and EIH in adolescents. Pediatric physical therapists should consider the CPM response when prescribing exercise as a pain management tool.
Keywords: diffuse noxious inhibitory control, body mass index, exercise induced hypoalgesia, aerobic exercise
Introduction
A decrease in pain following exercise is exercise-induced hypoalgesia (EIH), which is well established in adults1–3 and more recently in adolescents across the weight status.4 EIH is an example of endogenous pain modulation and produces systemic effects; pain relief occurs throughout the body.2,3 In young healthy adults, aerobic exercise of higher intensity produces greater EIH than lower intensity exercise,5 and isometric contractions that are reported as painful produce greater EIH than non-painful contractions.6
Conditioned pain modulation (CPM) is the concept that ‘pain inhibits pain’ and is a measure of central endogenous pain modulation.7 In the clinic, any physical therapy intervention that is reported as unpleasant (i.e. exercise, thermal modalities, and electrical stimulation) could work through the mechanism of CPM. With CPM in the research setting, a noxious stimulus (conditioning stimulus) decreases pain perception of a subsequent noxious stimulus (test stimulus).8 A greater conditioning stimulus produces greater CPM.8 Young healthy adults and adolescents demonstrate a consistent robust CPM response that tends to decline with increasing age.9–14 Taken together, these results suggest exercise that is painful may activate descending inhibitory pathways resulting in subsequent pain relief (i.e., EIH). In young and older adults, CPM predicts EIH.3,11,15 While not causal, this suggests that CPM may produce an additive EIH effect when exercise is reported as painful.
Previously we have shown that EIH and CPM exist in adolescents and are individually correlated with lean mass regardless of weight status.4,13 The purpose of this study is to determine if a relation exists between CPM and EIH in these adolescents. We hypothesize that the CPM and EIH response in adolescents are related and potentially linked through lean mass.
Methods
Subjects
Sixty-two adolescents (15.1 ± 1.8 years; 29 male and 33 female) were recruited from a Milwaukee, Wisconsin. These participants were enrolled as part of a larger research study investigating the association between inflammatory markers, physical fitness, and pain in adolescents of varying weight status.
Adolescents participated in three experimental sessions. The first session included measurement of weight status and experimental pain, (pressure pain threshold [PPT]) to familiarize the adolescents to the pressure algometer (Algomed).4 After the first session, adolescents participated in either the EIH or CPM session in a counterbalanced manner. The EIH session involved measurement of PPTs three times at 3 sites, left nail bed, left deltoid muscle, and right quadriceps muscle, before and after a maximal aerobic treadmill test (VO2Max Bruce Protocol)]4 The magnitude of EIH is the increase in PPTs following exercise.4 The adolescents rated their exercise pain using a Numerical Rating Scale (NRS) 0–10 with the anchors: 0 as “no pain” and 10 as “worst pain” during each stage of the treadmill test and upon completion. The CPM session involved body composition testing (Dual-energy X-ray Absorptiometry [DXA] scan) and the CPM protocol.12,13 For the CPM protocol, PPTs were measured at the nail bed and deltoid muscle with the right foot in a control condition (room temperature cool water bath) followed by a noxious condition (ice water bath); the time between the neutral and ice water conditions was twenty minutes.12,13 The ice water was the conditioning stimulus and PPTs were the test stimulus. Two trials at each site, (nail bed and deltoid) were completed for CPM to limit the exposure to the ice water condition. The absolute difference in PPTs between the noxious and control conditions is the magnitude of CPM.11,13
Statistical Analysis
The two PPTs were averaged for the CPM testing at each site (CPMNail and CPMDelt); the three PPTs were averaged for the EIH testing at each site (EIHNail, EIHDelt, and EIHQuad). In addition, the EIH and CPM were averaged across all sites (EIHAll & CPMAll). For each EIH and CPM session, repeated measures ANOVAs (trial [pre and post-exercise] or [cool water and ice water]) were done with site as a factor within the analysis. Post-hoc Pearson correlations were computed for EIH at the deltoid muscle (EIHDelt), quadriceps muscle (EIHQuad), and average EIH across muscles (EIHDeltQuad) with CPM at the nail bed (CPMNail), deltoid muscle (CPMDelt), and average CPM at the two sites (CPMNailDelt). From significant Pearson correlations, a regression analysis was completed with EIHDelt as the dependent variable and CPMDelt entered as Step 1. Because lean mass has been shown to influence EIH and CPM,4,13 lean mass of the left arm taken from the DXA results was included in Step 2. Data were analyzed using Statistical Package for the Social Sciences (SPSS, version 23, IBM, Chicago, IL) for statistics.
To determine if pain during exercise contributed to the EIH response, adolescents were classified into groups based on the peak pain ratings (NRS 0–10) during the treadmill test: no pain (0/10 NRS), minimal pain (1–3/10 NRS), moderate pain (4–6/10 NRS), and severe pain (7–10/10 NRS).16,17 Repeated measures ANOVA (trial [pre and post-exercise] x site [deltoid and quadriceps muscles]) was done with peak exercise pain groups (no pain, minimal pain, moderate pain, and severe pain)16,17 as a between subject factor. An alpha level of p<0.05 was used for all analyses.
Results
Fifty-five adolescents completed the EIH and CPM protocols (Table 1). Detailed EIH and CPM results are reported elsewhere4,13 but in summary, fit and unfit adolescents reported an increase in PPTs following exercise (EIH) and were unchanged with quiet rest.4 For CPM, PPTs increased in the ice water compared with the neutral water for adolescents in a similar manner across weight status (normal versus overweight/obese) and sex.13
Table 1.
Participant Characteristics
| n=55 | |
|---|---|
| Sex (male) | 26 |
| Age (years) | 15.2 ± 1.8 |
| BMI z score | 0.98 ± 0.93 |
| Lean mass -- Whole Body (kg) | 46.8 ± 10.9 |
| Lean mass -- Left Arm (kg) | 2.7 ± 0.9 |
| Peak Pain During Maximal Exercise (NRS 0–10) | 4.3 ± 2.9 |
| CPMNailDelt (kPa) | 84.6 ± 85.2 |
| CPMNail (kPa) | 77.4 ± 100.1 |
| CPMDelt (kPa) | 91.8 ± 123.4 |
| EIHAll (kPa) | 29.5 ± 87.4 |
| EIHNail (kPa) | 0.8 ± 74.9 |
| EIHDelt (kPa) | 27.9 ± 69.5 |
| EIHQuad (kPa) | 56.1 ± 101.4 |
| EIHDeltQuad (kPa) | 42.0 ± 64.3 |
Data are represented as mean ± SD
Abbreviations:
BMI, body mass index
CPM, conditioned pain modulation
Delt, deltoid muscle
EIH, exercise induced hypoalgesia
kg, kilogram
kPa, kilopascals
Nail, nail bed
NRS, Numerical Rating Scale
Quad, quadriceps muscle
Within the exercise session alone, EIH is site specific (trial x site: p<0.001) with significant increases in pain thresholds at the deltoid (p=0.004) and quadriceps (p<0.001) but no significance at nail bed (p>0.05). Within the CPM session alone, CPM is similar across sites (trial x site: p=0.47) with significant increase in pain threshold at the nail bed (p<0.001) and deltoid (p<0.001) while the foot is submerged in ice water compared with neutral water.
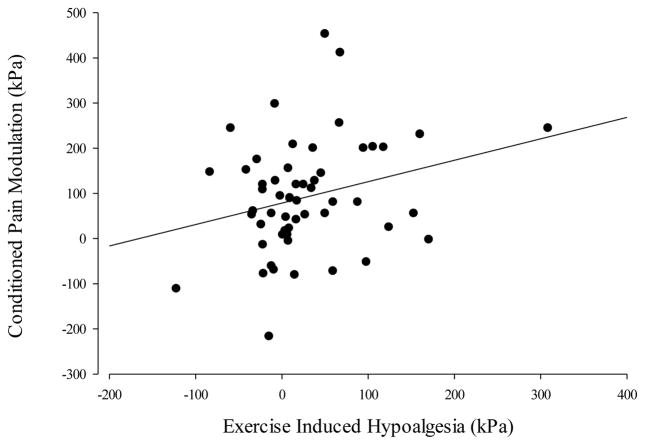
CPMDelt was positively correlated with EIHDelt (r=0.27, p=0.05) in that adolescents who experienced greater conditioned pain modulation at the deltoid muscle also experienced greater pain relief after exercise at the deltoid muscle. (Table 2, Figure 1). CPMDelt predicted 5.4% of the variance in the EIHDelt response (F=4.074, p=0.049) and lean mass did not add a significant effect (F=2.09, p=0.13).
Table 2.
Relation Between CPM and EIH at the Deltoid and Nail bed Sites
| CPMNailDelt | CPMNail | CPMDelt | |
|---|---|---|---|
|
|
|||
| EIHDeltQuad | r = 0.20, p = 0.15 | r = 0.07, p = 0.61 | r = 0.21, p = 0.12 |
|
| |||
| EIHDelt | r = 0.23, p = 0.09 | r = 0.06, p = 0.67 | r = 0.27, p = 0.05 * |
|
| |||
| EIHQuad | r = 0.09, p = 0.50 | r = 0.05, p = 0.72 | r = 0.09, p = 0.52 |
Abbreviations:
CPM, conditioned pain modulation
Delt, deltoid
EIH, exercise induced hypoalgesia
Nail, nail bed
Quad, quadriceps
Figure 1. Relation of Exercise Induced Hypoalgesia and Conditioned Pain Modulation.
Exercise induced hypoalgesia experienced at the deltoid muscle is positively correlated with conditioned pain modulation at the deltoid muscle (r=0.27, p=0.05).
Peak Pain with Maximal Aerobic Exercise
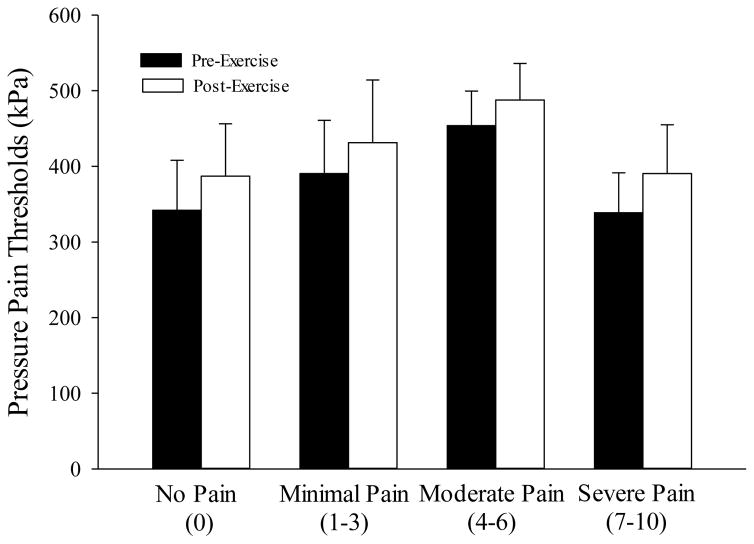
EIH was similar between the pain groups (trial x site x peak pain: p>0.05); peak pain reported during maximal aerobic exercise did not influence EIH (Figure 2).
Figure 2. Exercise Induced Hypoalgesia by Peak Exercise Pain Groups.
Pre and post exercise pressure pain thresholds (EIHDeltQuad) shown by peak exercise pain groupings (no pain, n=9; minimal pain, n=14; moderate pain, n=18; severe pain, n=14). EIH was similar between the peak exercise pain groups (trial x peak pain: p>0.05).
Discussion
This is the first study to support that CPM predicts EIH in adolescents following maximal treadmill running. We have previously shown this predictive relation in adults following maximal isometric contractions held to task failure.11 In young and older adults, CPM uniquely predicted 8.8% of the variance in the EIH.11 Taken together, there is a predictive relation between CPM and EIH that occurs from adolescence through older adulthood and following exhaustive aerobic and isometric exercise. Others have shown a positive relation between CPM and EIH in adults following 15 minutes of cycling at moderate/high intensity.15 Conversely, there was no association between CPM and EIH following low and high intensity cycling or isometric contractions.3 Thus, there are equivocal results in the relation between EIH and CPM, which may be related to exercise dose with more consistent results when the exercise is completed to exhaustion.
The mixed results may also be related to the measurement site. Our study demonstrated that the CPM and EIH relation was site specific. There was a positive association between the CPM response at the deltoid muscle and the EIH response at the deltoid muscle only. When comparing CPM and EIH averages across testing sites or between dissimilar sites (e.g., comparing quadriceps muscle with deltoid muscle), there were no associations. Similarly, Lemley et al. reported that CPM was predictive of EIH when the index finger was the measurement site for both protocols.11 One potential explanation is that the sensitivity to change for both CPM and EIH is similar when using the same site for both protocols. Similar to our protocol, Vaegter et al. used multiple sites (leg and arm) in the measurement of EIH and CPM3,15 with mixed results; although the CPM and EIH response by site was not measured but rather the average CPM and EIH across sites. Our results in which the systemic responses of CPM and EIH were measured at the same measurement site (deltoid) show a significant small effect.
No studies to our knowledge have investigated the role of body composition in the relation between EIH and CPM. Independent of exercise, Price et al. has shown no difference in CPM efficiency between obese and normal weight adults using a test site with little excess subcutaneous fat (forehead).18 Previously, we have shown in adolescents that lean mass was related to both the CPM and EIH responses; lean mass of the arm uniquely predicted 10% of the CPM magnitude and lean mass of the body was correlated with the EIH magnitude.4,13 Despite previous research showing that lean mass was related to both CPM and EIH independently, lean mass does not appear to account for the relation between CPM and EIH.
Since exercise is often painful, peak pain during exercise was measured as a potential conditioning stimulus. The average peak pain reported during maximal aerobic exercise by the adolescents was moderately painful with a wide-range of peak pain reported. Furthermore, peak pain reported during exercise did not influence the EIH response in our adolescent population. Thus, despite the relation between CPM and EIH at the deltoid muscle, our results show that exercise pain does not influence the EIH response; adolescents who experience none, minimal, moderate, or severe peak pain during exercise experience similar EIH. When physical therapists use exercise for pain relief in an adolescent population, it is not necessary for the exercise to be painful in order for pain relief to occur.
Considering the small relation between EIH and CPM and the similar EIH across the peak pain exercise groups, our results suggest that there are likely multiple mechanisms that are responsible for EIH.11,19 For example, we have previously shown the magnitude of EIH is related to sedentary behavior across weight status.4 Future pediatric research is necessary to evaluate other multi-factorial mechanisms such as psychosocial factors as well as specific pain conditions in a variety of ages. From a clinical perspective, assessment of CPM in pediatric populations by physical therapists has the potential to assist with clinical decision making about the use of exercise as a pain management tool in adolescents. With the current focus on decreasing pain medications, alternate pain relief options, such as exercise and endogenous pain modulation, are necessary for adolescents experiencing pain.
Acknowledgments
Grant, Scholarship, & Fellowship Support:
Foundation for Physical Therapy -- Promotion of Doctoral Studies (PODS) I Scholarship (SS)
Foundation for Physical Therapy – Promotion of Doctoral Studies (PODS) II Scholarship (SS)
American Association of University Women – Dissertation Fellowship (SS)
Marquette University President’s Council -- Raynor Fellowship (SS)
National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 8UL1TR000055 (SS & MHB)
Footnotes
Conflicts of Interest Statement:
The authors declare no conflict of interest.
References
- 1.Koltyn KF. Exercise-induced hypoalgesia and intensity of exercise. Sports Med. 2002;32(8):477–487. doi: 10.2165/00007256-200232080-00001. [DOI] [PubMed] [Google Scholar]
- 2.Kosek E, Lundberg L. Segmental and plurisegmental modulation of pressure pain thresholds during static muscle contractions in healthy individuals. Eur J Pain. 2003;7(3):251–258. doi: 10.1016/S1090-3801(02)00124-6. [DOI] [PubMed] [Google Scholar]
- 3.Vaegter HB, Handberg G, Graven-Nielsen T. Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. Pain. 2014;155(1):158–167. doi: 10.1016/j.pain.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Stolzman S, Danduran M, Hunter SK, Bement MH. Pain response after maximal aerobic exercise in adolescents across weight status. Med Sci Sports Exerc. 2015;47(11):2431–2440. doi: 10.1249/MSS.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naugle KM, Fillingim RB, Riley JL., 3rd A meta-analytic review of the hypoalgesic effects of exercise. J Pain. 2012;13(12):1139–1150. doi: 10.1016/j.jpain.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoeger Bement MK, Dicapo J, Rasiarmos R, Hunter SK. Dose response of isometric contractions on pain perception in healthy adults. Med Sci Sports Exerc. 2008;40(11):1880–1889. doi: 10.1249/MSS.0b013e31817eeecc. [DOI] [PubMed] [Google Scholar]
- 7.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23(5):611–615. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]
- 8.van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain. 2010;11(5):408–419. doi: 10.1016/j.jpain.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Edwards RR, Ness TJ, Weigent DA, Fillingim RB. Individual differences in diffuse noxious inhibitory controls (DNIC): Association with clinical variables. Pain. 2003;106(3):427–437. doi: 10.1016/j.pain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Lariviere M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain. 2007;23(6):506–510. doi: 10.1097/AJP.0b013e31806a23e8. [DOI] [PubMed] [Google Scholar]
- 11.Lemley KJ, Hunter SK, Bement MK. Conditioned pain modulation predicts exercise-induced hypoalgesia in healthy adults. Med Sci Sports Exerc. 2015;47(1):176–184. doi: 10.1249/MSS.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 12.Stolzman S, Lemley K, Hoffmeister K, Coate M, Drendel A, Hoeger Bement M. Conditioned pain modulation and exercise-induced hypoalgesia in adolescents. Pediatric physical therapy: the official publication of the Section on Pediatrics of the American Physical Therapy Association. 2014;26(1):154-154–155. [Google Scholar]
- 13.Stolzman S, Hoeger Bement M. Lean mass predicts conditioned pain modulation in adolescents across weight status. Eur J Pain. 2016 doi: 10.1002/ejp.821. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Tsao JC, Seidman LC, Evans S, Lung KC, Zeltzer LK, Naliboff BD. Conditioned pain modulation in children and adolescents: Effects of sex and age. J Pain. 2013;14(6):558-558–567. doi: 10.1016/j.jpain.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaegter HB, Handberg G, Jorgensen MN, Kinly A, Graven-Nielsen T. Aerobic exercise and cold pressor test induce hypoalgesia in active and inactive men and women. Pain Med. 2015;16(5):923–933. doi: 10.1111/pme.12641. [DOI] [PubMed] [Google Scholar]
- 16.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? grading pain severity by its interference with function. Pain. 1995;61(2):277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 17.Turner JA, Franklin G, Heagerty PJ, et al. The association between pain and disability. Pain. 2004;112(3):307–314. doi: 10.1016/j.pain.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Price RC, Asenjo JF, Christou NV, Backman SB, Schweinhardt P. The role of excess subcutaneous fat in pain and sensory sensitivity in obesity. Eur J Pain. 2013;17(9):1316–1326. doi: 10.1002/j.1532-2149.2013.00315.x. [DOI] [PubMed] [Google Scholar]
- 19.Ellingson LD, Koltyn KF, Kim JS, Cook DB. Does exercise induce hypoalgesia through conditioned pain modulation? Psychophysiology. 2014;51(3):267–276. doi: 10.1111/psyp.12168. [DOI] [PubMed] [Google Scholar]