Abstract
Chemotherapy-induced peripheral neuropathy (CIPN), characterized by pain and numbness in hands and feet, is a common side effect of cancer treatment. In most patients, symptoms of CIPN subside after treatment completion. However, in a substantial subgroup, CIPN persists long into survivorship. Impairment in pain resolution pathways may explain persistent CIPN. We investigated the contribution of T cells and endogenous interleukin (IL)-10 to resolution of CIPN. Paclitaxel-induced mechanical allodynia was prolonged in T-cell-deficient (Rag1−/−) mice compared with wild-type (WT) mice. There were no differences between WT and Rag1−/− mice in severity of paclitaxel-induced mechanical allodynia. Adoptive transfer of either CD3+ or CD8+, but not CD4+, T cells to Rag1−/− mice normalized resolution of CIPN. Paclitaxel treatment increased the number of T cells in lumbar dorsal root ganglia (DRG), where CD8+ T cells were the major subset. Inhibition of endogenous IL-10 signaling by intrathecal injection of anti-IL-10 to WT mice or Rag1−/− mice reconstituted with CD8+ T cells delayed recovery from paclitaxel-induced mechanical allodynia. Recovery was also delayed in IL-10 knock-out mice. Conversely, administration of exogenous IL-10 attenuated paclitaxel-induced allodynia. In vitro, IL-10 suppressed abnormal paclitaxel-induced spontaneous discharges in DRG neurons. Paclitaxel increased DRG IL-10 receptor expression and this effect requires CD8+ T cells. In conclusion, we identified a novel mechanism for resolution of CIPN that requires CD8+ T cells and endogenous IL-10. We propose that CD8+ T cells increase DRG IL-10 receptor expression and that IL-10 suppresses the abnormal paclitaxel-induced spontaneous discharges by DRG neurons to promote recovery from CIPN.
SIGNIFICANCE STATEMENT Chemotherapy-induced peripheral neuropathy persists after completion of cancer treatment in a significant subset of patients, whereas others recover. Persistent neuropathy after completion of cancer treatment severely affects quality of life. We propose that understanding how neuropathy resolves will identify novel avenues for treatment. We identified a novel and critical role for CD8+ T cells and for endogenous IL-10 in recovery from paclitaxel-induced neuropathy in mice. Enhancing the capacity of CD8+ T cells to promote resolution or increasing IL-10 signaling are promising targets for novel interventions. Clinically, peripheral blood CD8+ T-cell function and/or the capacity of individuals to produce IL-10 may represent biomarkers of risk for developing persistent peripheral neuropathy after completion of cancer treatment.
Keywords: CD8+ T cells, chemotherapy-induced peripheral neuropathy, IL-10
Introduction
Nearly 70% of cancer patients report symptoms of peripheral neuropathy during chemotherapeutic treatment (Postma et al., 1995; Polomano and Bennett, 2001; Quasthoff and Hartung, 2002; Dougherty et al., 2004; Cata et al., 2006; Kannarkat et al., 2007; Windebank and Grisold, 2008; Pachman et al., 2012; Seretny et al., 2014; Vichaya et al., 2015). This chemotherapy-induced peripheral neuropathy (CIPN) is a common cause of dose reduction or termination of chemotherapy, leading to suboptimal cancer therapy. Notably, symptoms of CIPN do not always resolve upon treatment completion and continue long into survivorship in ∼30% of affected cancer patients, thereby significantly reducing their quality of life (Miltenburg and Boogerd, 2014; Seretny et al., 2014).
The chemotherapeutic agent paclitaxel is used frequently to treat ovarian, breast, and lung cancer (Rowinsky et al., 1990; Rowinsky et al., 1993; Choy et al., 1994; Gianni et al., 1995). Mechanisms of paclitaxel-induced neuropathy remain unknown, although there is growing evidence that paclitaxel administration targets peripheral sensory neurons, leading to damage of neuronal mitochondria (Varbiro et al., 2001; Flatters and Bennett, 2006; Peters et al., 2007; Krukowski et al., 2015). In addition, there is evidence that the production of chemokine monocyte chemoattractant protein-1 in dorsal root ganglia (DRG) attracts proinflammatory monocytes to the DRG, causing endogenous increases in proinflammatory cytokines (Ledeboer et al., 2007; Boyette-Davis et al., 2011; Zhang et al., 2013; Zhang et al., 2016). These proinflammatory cytokines can (further) sensitize peripheral sensory neurons, leading to abnormal spontaneous discharges, hyperexcitability, and allodynia (Wieseler-Frank et al., 2005; Binshtok et al., 2008). Moreover, inhibition of monocyte infiltration into DRG and suppression of proinflammatory cytokine production prevents the development of paclitaxel-induced mechanical allodynia in rodent models (Liu et al., 2010; Zhang et al., 2013; Huang et al., 2014). Both paclitaxel-induced neuronal mitochondrial damage and endogenous production of proinflammatory cytokines are hypothesized to underlie the development of CIPN (Wieseler-Frank et al., 2005; Flatters and Bennett, 2006).
Although proinflammatory cytokine production by macrophages plays a role in CIPN, little is known about the role of the adaptive immune system in CIPN. There is evidence that CD4+ T cells, which can produce anti-inflammatory cytokines such as interleukin (IL)-4, IL-10, and transforming growth factor (TGF)-β, can reduce pain in models of surgical nerve injury (Serpe et al., 1999; Serpe et al., 2003; Austin et al., 2012; Lees et al., 2015; Walsh et al., 2015). Neuropathic pain induced by chronic constriction injury of the sciatic nerve increases the number of CD4+ T regulatory (Treg) cells in DRG (Lees et al., 2015). Peripheral depletion of Tregs from mice worsened mechanical allodynia in this model of chronic neuropathic pain. Furthermore, increasing the number of CD4+ Tregs attenuated mechanical allodynia in response to sciatic nerve injury (Austin et al., 2012; Lees et al., 2015). Others have shown that exogenous administration of anti-inflammatory cytokines such as IL-10 and TGF-β suppresses allodynia in surgery (Chacur et al., 2004; Milligan et al., 2005; Shen et al., 2013) and in a model of chemotherapy-induced neuropathic pain (Ledeboer et al., 2007). In addition, we showed that inhibition of endogenous IL-10 signaling by intrathecal administration of IL-10 antibody prolonged thermal hyperalgesia in a model of transient inflammatory pain (Willemen et al., 2010).
A possible contribution of T cells or endogenous production of anti-inflammatory cytokines to CIPN has not yet been identified. We investigated the contribution of specific T-cell subsets and of endogenous IL-10 signaling to the development and resolution of paclitaxel-induced mechanical allodynia. We identify CD8+ T cells as the critical cell subset that, together with endogenous IL-10, are required for resolution of CIPN.
Materials and Methods
Animals.
Adult (8–12 weeks of age) male C57BL/6J mice, as well as Rag1−/−, IL-10−/−, and IL-4−/− mice in a C57BL/6J background, were obtained from The Jackson Laboratory. Five-week-old male Sprague Dawley rats (Harlan Laboratories) were used for DRG electrophysiology experiments. Rodents were housed at The University of Texas M.D. Anderson Cancer Center Animal Facility, the Texas A&M Health Science Center Program for Animal Resources, or at the Utrecht University Central Animal Facility in the Netherlands. Rodents were housed on a regular 12 h light/dark cycle and had ad libitum access to food and water. All procedures were consistent with the National Institutes of Health's Guidelines for the Care and Use of Laboratory Animals and the Ethical Issues of the International Association for the Study of Pain (Zimmermann, 1983) and were approved by the institutional animal care and use committees of the respective institutions.
Chemotherapeutic treatment.
Paclitaxel (6 mg/ml) in 50% El Kolipher (Sigma-Aldrich) and 50% ethanol (Sigma-Aldrich) were diluted in sterile saline and administered at a dose of 2 mg/kg intraperitoneally on day 0 and day 2.
T-cell isolation and adoptive transfer.
Spleens were collected from CO2-asphyxiated mice. Single-cell suspensions were obtained by passing spleens through a 70 μm mesh filter, after which cells were washed twice with PBS plus 0.1% bovine serum albumin. T-cell subpopulations were purified via the Miltenyi Biotec Pan CD3+, CD4+, or CD8+ T-cell negative selection kits according to the manufacturer's instructions. One day before the first paclitaxel administration, T-cell populations (CD3+: 8 million per mouse; CD4+ or CD8+: 3 million per mouse) or PBS were injected intravenously into the tail vein in a volume of 100 μl.
Von Frey test for mechanical allodynia.
Mechanical allodynia as a readout for CIPN was measured as the hindpaw withdrawal response to von Frey hair stimulation by an investigator blinded to genotype and treatment using the up-and-down method, as we described previously (Wang et al., 2011; Mao-Ying, 2014; Krukowski et al., 2015). Mice were placed in a Plexiglas enclosure (10 × 10 × 13 cm3) with a mesh floor for 30 min before testing. Subsequently, a series of von Frey hairs (0.02, 0.07, 0.16, 0.4, 0.6, 1.0, and 1.4 g; Stoelting) were applied perpendicular to the midplantar surface of hindpaw. A trial began with the application of the 0.16 g hair. A positive response was defined as a clear paw withdrawal or shaking. Whenever a positive response occurred, the next lower hair was applied, and whenever a negative response occurred, the next higher hair was applied. The testing consisted of five stimuli after the first change in response occurred and the pattern of response was converted to a 50% von Frey threshold using the method described previously (Chaplan et al., 1994).
Immunohistochemical analysis.
Mice were killed by CO2 asphyxiation and lumbar DRG (L4–L6) were removed on day 7 or day 21 after paclitaxel treatment. DRG were fixed in 4% paraformaldehyde, sucrose protected, frozen in optimal cutting temperature compound plus 30% sucrose (2:1), and sliced into 6 μm sections. The sections were incubated for 2 h at room temperature in 0.1 m PBS, 0.1% saponin containing 5% normal donkey serum, and 2% bovine serum albumin. Subsequently, sections were incubated with primary antibody for 24 h at 4°C, followed by incubation with the secondary antibody for 24 h at 4°C. For T-cell quantification, DRG sections of equivalent size (L4–L6) were stained with anti-CD3 monoclonal antibody (rat, 1:100; BD Biosciences), followed by Alexa Fluor-488 donkey anti-rat (1:500; Invitrogen). No staining was observed in slides stained with secondary antibody alone. The number of T cells per slice was counted in five randomly selected DRG slices of equivalent size per mouse using a Leica DM4 SPE confocal microscope with a 40 × objective.
Flow cytometric analysis.
For flow cytometry, lumbar DRG (L4–L6) were collected on day 7 after the start of paclitaxel or vehicle treatment. DRG from three mice per group were pooled. Single-cell suspensions were prepared using both loose-fitting and tight-fitting pestles. Leukocyte cells were enriched on a Percoll gradient (30/70%) by centrifuging for 30 min at 500 × g. Leukocytes were stained for 30 min on ice using anti-CD4 (eFluor 450-conjugated; eBioscience), anti-CD8 (PE-conjugated; eBioscience), and anti-CD45 (APC-Cy7-conjugated; BD Biosciences).
To assess circulating cell populations, we obtained peripheral blood by cardiac puncture into a heparinized collection tube. Blood (100 μl) was aliquoted into flow cytometry staining tubes and stained with surface antibodies for 30 min at room temperature. Surface antibodies included anti-CD45 (APC-conjugated; BD Biosciences), CD3 (FITC-conjugated; BD Biosciences), CD8 (PE-conjugated; BD Biosciences), and CD4 (PerCp-Cy5.5-conjugated; BD Biosciences). Leukocyte subpopulations were identified as follows: CD4+ T-cell subsets were CD45+, CD4+, and CD8− and CD8+ T-cell subsets were CD45+, CD8+, and CD4−. After surface antibody staining, red blood cells were lysed (BD Biosciences). Cells were analyzed on a Fortessa or C6 Accuri flow cytometer (BD Biosciences).
mRNA expression analysis.
Lumbar DRG (L4–L6) or lumbar spinal cords (L4–L6) were rapidly removed from CO2-asphyxiated mice and snap frozen. Total RNA was extracted using TRIzol/chloroform (Invitrogen) and converted into cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems). TaqMan probe Il10 (IL-10 exon 3–5, NM_010548.2), Il10ra (IL-10 receptor 1 (IL-10R1) exon 1–2, NM_008348.2), Cd3e (CD3 exon 5–6, NM_007648.4), and Cd8a (CD8 exon 1–2, NM_001081110.2) were purchased from Integrated DNA Technologies and quantitative amplification was performed using the Maxima Probe Master Mix (Thermo Scientific). All samples were analyzed in triplicate using an annealing temperature of 60°C and normalized by Gapdh expression (GAPDH exon 2–3, NM_008084).
Intrathecal injections.
All intrathecal injections (5 μl) were performed under isoflurane anesthesia as described previously (Eijkelkamp et al., 2010). Earlier studies have shown that proteins injected intrathecally enter both DRG and spinal cord (Abram et al., 2006; Jacques et al., 2012; Homs et al., 2014; Laumet et al., 2015). Goat anti-IL-10 IgG (Sigma-Aldrich), mouse anti-TGF-β (R&D Systems), or normal goat IgG (IgG), Sigma-Aldrich) as a control (10 μg/mouse) were administered intrathecally on days 6–10. The IL-4/IL-10 synerkine is a fusion protein of human IL-4 and human IL-10 connected via a linker sequence (Eijkelkamp et al., 2016) and was administered intrathecally (1 μg/mouse) on days 5–8.
Spontaneous discharge of cultured DRG neurons.
Spontaneous discharge of rat DRG neurons was performed as described previously (Li et al., 2015). Briefly, rats were treated with paclitaxel (2 mg/kg, i.p.) every other day for a total of 4 d. One day after treatment completion, rats were deeply anesthetized and the DRG (L4–L5) were extracted and placed in a culture dish containing 1 ml of trypsin (0.125 mg/ml; Hyclone) and 1 ml of type IA collagenase (2 mg/ml) in Dulbecco's modified Eagle medium (DMEM; Sigma-Aldrich). Chemical dissociation of tissue was performed by shaking and heating the chamber. Cells were washed and mechanically dispersed with a polished Pasteur pipette, plated on poly-l-ornithine-coated glass sheets, and held in culture dishes with DMEM until use (Li et al., 2014). The cells were used for patch-clamp recordings within 6 h after plating. Whole-cell patch-clamp recordings were performed as described previously (Li et al., 2014). The cells were transferred to a recording chamber placed on an inverted microscope (Eclipse Ti; Nikon) and perfused with oxygenated artificial CSF (2 ml/min) at room temperature. Only neurons with a stable resting membrane potential of at least −40 mV and evoked spikes that overshot 0 mV were used. Series resistance was compensated to 70%.
Statistical analysis.
Data are expressed as mean ± SEM. Statistical analysis was performed using repeated-measures ANOVA (one or two way) or repeated-measures t test followed by Bonferroni analysis. Statistical significance was set at p < 0.05.
Results
Contribution of T cells to paclitaxel-induced mechanical allodynia
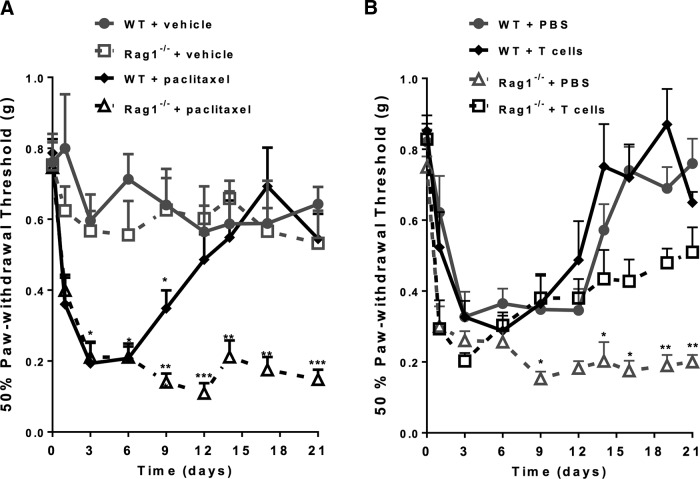
To investigate the role of T cells in paclitaxel-induced mechanical allodynia, we used Rag1−/− mice that are deficient in mature T and B cells and compared them with wild-type (WT) C57BL/6 mice. Mice were treated with paclitaxel (2 mg/kg, i.p., on days 0 and 2) or vehicle, followed by measurement of mechanical allodynia using von Frey hairs. No differences were detected in baseline sensitivity to mechanical stimuli when comparing WT and Rag1−/− mice. After paclitaxel treatment, WT and Rag1−/− mice displayed identical onset and maximal severity of mechanical allodynia (Fig. 1A). However, the resolution of mechanical allodynia was severely delayed in Rag1−/− mice: WT mice treated with paclitaxel had completely recovered by day 12, whereas mechanical allodynia persisted for at least 21 d in Rag1−/− mice (Fig. 1A).
Figure 1.
Contribution of T cells to paclitaxel-induced mechanical allodynia. A, Paclitaxel (2 mg/kg, i.p.) was administered to WT (C57BL/6) and Rag1−/− mice on day 0 and day 2. Mechanical allodynia was measured using von Frey hairs and the 50% paw withdrawal threshold was calculated using the up-and-down method. WT + vehicle (circles, gray line); Rag1−/− + vehicle (open squares, gray dashed line); WT + paclitaxel (diamonds black line); and Rag1−/− + paclitaxel (open triangles, black dashed line). Two-way repeated-measures ANOVA revealed a main effect of time (p < 0.01), a group effect (p < 0.01), a genotype effect (p < 0.01) and a 3-way interaction among group, genotype, and time (p < 0.01). Bonferroni post hoc analysis showed differences between groups (WT + vehicle vs WT + paclitaxel or Rag1−/− + vehicle vs Rag1−/− + paclitaxel) at various time points; n = 8–10/group. **p < 0.01, ***p < 0.001. B, WT and Rag1−/− mice received PBS or CD3+ T cells intravenously 1 d before paclitaxel treatment as in A. Shown are: WT + PBS (circles, gray line); WT + T cells (diamonds, black line); Rag1−/− + PBS (open triangles, gray dashed line); and Rag1−/− + T cells (open squares, black dashed line). Two-way repeated-measures ANOVA revealed a main effect of time (p < 0.01), a group effect (p < 0.01), a treatment effect (T cell vs PBS, p < 0.01), and a 3-way interaction among group, treatment, and time (p < 0.01). Bonferroni post hoc analysis showed differences between groups (WT+ PBS vs WT + PBS or Rag1−/− + PBS vs Rag1−/− + T cells) at various time points; n = 6–8/group. *p < 0.05, **p < 0.01.
To determine the contribution of T cells to recovery from CIPN, we adoptively transferred CD3+ T cells to Rag1−/− mice. CD3+ T cells, isolated from spleens of C57BL/6 mice, were injected intravenously into the tail veins of Rag1−/− and WT mice 1 d before paclitaxel treatment. The results in Figure 1B show that Rag1−/− mice reconstituted with CD3+ T cells recovered from paclitaxel-induced mechanical allodynia at rates comparable to WT mice. T-cell transfer did not affect the recovery of CIPN in WT mice and did not affect the onset or maximal level of paclitaxel-induced mechanical allodynia in WT or Rag1−/− mice. These data indicate that T cells are crucial for recovery from CIPN.
T-cell localization after paclitaxel treatment
Next, we investigated whether the T cells that were transferred to Rag1−/− mice migrated to lumbar DRG that contain the cell bodies of the primary sensory neurons innervating the hindpaws. Adoptively transferred CD3+ T cells migrated to the DRG of paclitaxel-treated Rag1−/− mice. The number of CD3+ T cells in the DRG of Rag1−/− mice after T-cell transfer was similar to that observed in WT mice on day 21 after paclitaxel treatment (Table 1). As expected, no CD3+ T cells were present in the DRG of Rag1−/− mice that did not receive T cells.
Table 1.
Homing of T cells to DRG after adoptive transfer
| Mouse strain | Intravenous treatment | CD3+ T cells |
|---|---|---|
| C57BL/6 | Saline | 11.7 ± 2.5 |
| C57BL/6 | T cells | 8.5 ± 1.8 |
| Rag1−/− | Saline | 0.0 ± 0.0 |
| Rag1−/− | T cells | 13.1 ± 2.5 |
Rag1−/− mice received CD3+ T cells on day −1 and were treated with paclitaxel on days 0 and 2. DRG were collected on day 21. DRG were stained with anti-CD3 antibodies and T-cell numbers/DRG slice of 5 randomly selected slices were quantified; n = 3 mice/group. Two-way ANOVA measured no differences in the number of C57BL/6 + T cells or Rag1−/− + T cells in the DRG.
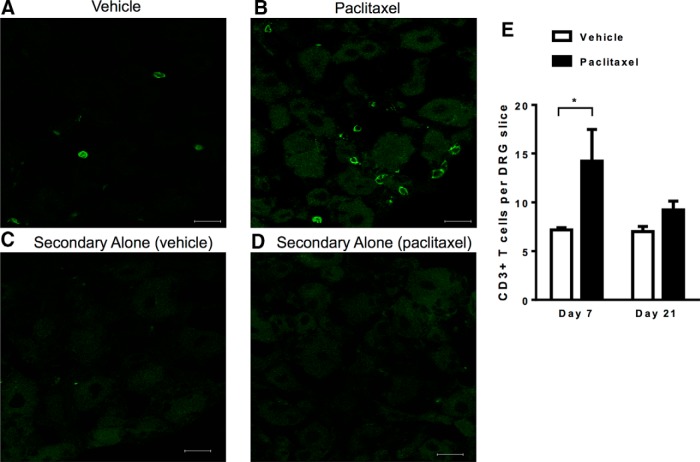
Recovery from paclitaxel-induced mechanical allodynia started from 7 d after paclitaxel treatment (Fig. 1). At this time point, there was a significant increase in the number of CD3+ T cells in the lumbar DRG isolated from paclitaxel-treated mice compared with those isolated from vehicle-treated WT mice (Fig. 2). Flow cytometric analysis revealed that the majority of T cells in the DRG were CD8+ T cells and that paclitaxel treatment did not alter this subset distribution (Table 2). The total number of circulating peripheral blood T cells on day 7 was similar between vehicle-treated and paclitaxel-treated mice (Table 3). Increased T-cell numbers in the DRG had normalized by day 21 (Fig. 2E). No T cells were detected in the spinal cord dorsal horn by immunohistochemical analysis in paclitaxel-treated or vehicle-treated mice. Quantitative RT-PCR analysis identified CD3 and CD8 mRNA in the spinal cords of WT mice, no changes were detected in response to paclitaxel treatment (Table 4). These findings are consistent with a previous report showing no T-cell infiltration into the spinal cord in a model of oxaliplatin-induced peripheral neuropathy (Janes et al., 2015).
Figure 2.
T-cell localization after paclitaxel treatment. WT mice were treated with paclitaxel or vehicle as in Figure 1A and DRG were collected on day 7. A–D, Representative examples of immunofluorescence analysis of DRG stained with CD3 antibody. Shown are: vehicle treated (A), paclitaxel treated (B), vehicle stained with secondary antibody alone (C), and paclitaxel stained with secondary antibody alone (D). E, Quantification of T-cell numbers/DRG slice. Five slices per mouse were quantified; n = 3–4 mice/group. *p < 0.05, two-way ANOVA followed by Bonferroni post test.. Scale bar, 20 μm.
Table 2.
Investigation of T-cell subpopulations in DRG
| Treatment | CD4+ T cells | CD8+ T cells |
|---|---|---|
| Vehicle | 39.1 ± 10.5 | 60.8 ± 10.5 |
| Paclitaxel | 35.9 ± 3.1 | 64.0 ± 3.1 |
C57BL/6J mice were treated with paclitaxel and DRG were collected on day 7. T-cell subpopulations in DRG were investigated by flow cytometry using CD45, CD3, CD8, and CD4 antibodies. Data represent the percentage of positive cells within the total population of CD3+ T cells. Samples were pooled from 3 mice/group; n = 2. For the CD8+ and CD4+ subpopulations, the percentage of total T cells was quantified.
Table 3.
Peripheral blood T-cell numbers on day 7
| Treatment | CD3+ T cells | CD4+ T cells | CD8+ T cells |
|---|---|---|---|
| Vehicle | 28.2 ± 0.7 | 13.6 ± 0.6 | 8.675 ± 0.1 |
| Paclitaxel | 30.9 ± 1.3 | 14.4 ± 0.9 | 9.55 ± 0.6 |
C57BL/6J mice were treated with paclitaxel and blood was collected on day 7. T-cell subpopulations were assessed by flow cytometry using CD45, CD3, CD8, and CD4 antibodies. Data represent the percentage of each subset within the CD45+ leukocyte population. Student's t test did not reveal differences in CD3+, CD4+, or CD8+ percentage between vehicle-treated and paclitaxel-treated mice.
Table 4.
T-cell levels in the spinal cord on day 7
| Treatment | Mouse strain | CD3 | CD8 |
|---|---|---|---|
| Vehicle | C57BL/6 | 1.0 ± 0.09 | 1.0 ± 0.21 |
| Paclitaxel | C57BL/6 | 1.104 ± 0.15 | 1.64 ± 0.35 |
CD3 and CD8 mRNA expression levels in lumbar spinal cord were examined on day 7 after paclitaxel treatment. Student's t test did not reveal significant differences between groups; n = 7/group.
Identification of the T-cell subset responsible for recovery from paclitaxel-induced mechanical allodynia
To investigate which subset of T cells is required for promoting recovery of mechanical allodynia, we transferred either CD4+ T cells or CD8+ T cells to Rag1−/− mice 1 d before paclitaxel administration. Rag1−/− mice receiving CD8+ T cells recovered from paclitaxel-induced mechanical allodynia with kinetics similar to recovery in WT mice (Fig. 3). In contrast, mechanical allodynia persisted in Rag1−/− mice receiving CD4+ T cells (Fig. 3). The number of circulating CD4+ and CD8+ T cells on days 19–21 was comparable between reconstituted Rag1−/− and WT mice, indicating that transfer of both subsets was successful (Table 5).
Figure 3.
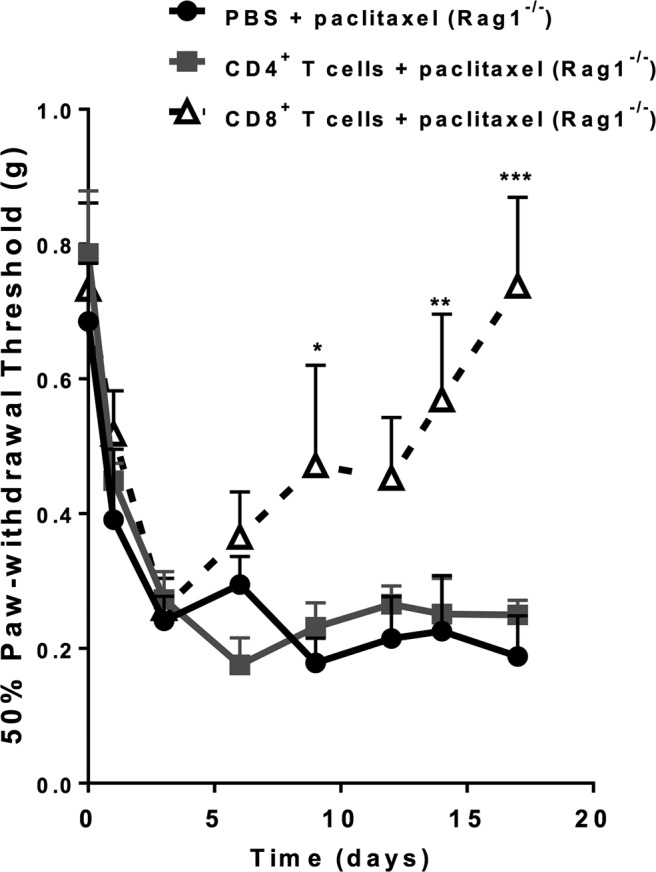
Identification of the T-cell subset responsible for recovery from paclitaxel-induced mechanical allodynia. Paclitaxel (2 mg/kg, i.p.) was administered to Rag1−/− mice on day 0 and day 2. CD8+ or CD4+ T cells were adoptively transferred to Rag1−/− mice 1 d before the first paclitaxel treatment. Shown are: PBS + paclitaxel (circles, black line); CD4+ T cells + paclitaxel (squares, gray line); and CD8+ T cells (open triangles, dashed black line). Mechanical allodynia was measured as in Figure 1. Two-way repeated-measures ANOVA showed a main effect of time (p < 0.0001), group (p < 0.03), and a group-by-time interaction (p < 0.001). Bonferroni post hoc analysis for CD8+ T cells versus saline: *p < 0.05, **p < 0.01, ***p < 0.001; n = 4–6/group. No significant differences were observed between mice receiving CD4+ T cells or saline.
Table 5.
Confirmation of adoptive T-cell transfer
| Treatment | Mouse strain | Intravenous treatment | CD4+ T cells | CD8+ T cells |
|---|---|---|---|---|
| Vehicle | C57BL/6 | N/A | 15.3 ± 0.9 | 10.8 ± 0.5 |
| Paclitaxel | C57BL/6 | N/A | 15.7 ± 1.1 | 10.4 ± 0.1 |
| Paclitaxel | Rag1−/− | PBS | 0.3 ± 0.0 | 0.4 ± 0.1 |
| Paclitaxel | Rag1−/− | CD8+ T cells | 0.7 ± 0.0 | 9.3 ± 0.7 |
| Paclitaxel | Rag1−/− | CD4+ T cells | 15.3 ± 1.6 | 1.7 ± 0.3 |
Effective adoptive transfers were confirmed by investigating T-cell blood percentages at experiment termination (days 19–21). Blood was collected by cardiac puncture and flow cytometry staining was done with CD45, CD8, and CD4 antibodies. The percentage of CD45+ leukocytes was determined for the CD4+ and CD8+ T-cell populations. One-way ANOVA did not reveal differences in CD4+ or CD8+ percentages among vehicle-treated C57BL/6 mice, paclitaxel-treated mice, or Rag1−/− mice that received either CD4+ or CD8+ T cells.
N/A, Not applicable.
Impact of endogenous IL-10 on recovery from paclitaxel-induced mechanical allodynia
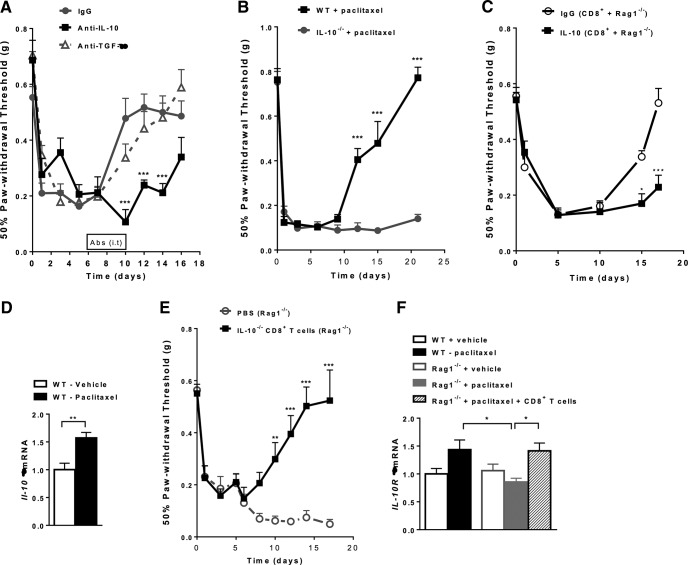
CD8+ T cells exert their regulatory effects in part through the production of anti-inflammatory cytokines such as IL-10 and TGF-β (Abel et al., 2006; Dai et al., 2010; Saraiva and O'Garra, 2010; Gabryšová et al., 2014; Kashi et al., 2014). We used neutralizing antibodies specific for IL-10 or TGF-β to examine the contribution of these anti-inflammatory cytokines to recovery from paclitaxel-induced mechanical allodynia in WT mice. IL-10- and TGF-β-blocking antibodies or an isotype control antibody were administered intrathecally to target both DRG and spinal cord (Abram et al., 2006; Jacques et al., 2012; Homs et al., 2014; Laumet et al., 2015). Mice were injected with the antibodies daily from days 6–10 after paclitaxel administration and mechanical allodynia was measured. Neutralizing antibodies against IL-10 delayed recovery from paclitaxel-induced mechanical allodynia significantly (Fig. 4A). In contrast, intrathecal administration of a TGF-β blocking antibody did not change the recovery times compared with a control antibody. To further evaluate the importance of IL-10 in recovery from CIPN, IL-10−/− mice were treated with paclitaxel and mechanical allodynia was followed over time. The results in Figure 4B show that mechanical allodynia was prolonged in IL-10−/− mice until at least day 21. Next, we analyzed the contribution of endogenous IL-10 signaling in CD8+ T-cell-mediated resolution of CIPN. Rag1−/− mice were reconstituted with CD8+ T cells and treated with paclitaxel, followed by daily intrathecal administration of IL-10-blocking antibody or IgG control antibody (day 6–10). The intrathecal administration of IL-10 antibody prevented resolution of CIPN in the Rag1−/− mice reconstituted with CD8+ T cells, whereas reconstituted mice treated with control IgG recovered by day 17 (Fig. 4C). These data demonstrate that CD8-mediated resolution of CIPN is dependent on endogenous IL-10 signaling.
Figure 4.
Impact of endogenous IL-10 on recovery from paclitaxel-induced mechanical allodynia. A, Paclitaxel (2 mg/kg, i.p.) was administered to WT mice on day 0 and day 2. Anti-IL10, anti-TGF-β, or control antibodies (10 μg/mouse/d) were administered intrathecally from days 6–10, and mechanical allodynia was followed over time. Shown are: IgG (circles, gray line); anti-IL-10 (squares, black line); and anti-TGF-β (open triangles, gray dashed line). Two-way repeated-measures ANOVA showed a main effect of time (p < 0. 001), a group effect (p < 0.01), and a group-by-time interaction (p < 0.001). Bonferroni post hoc analysis of anti-IL10 versus isotype-control antibody: ***p < 0.001; n = 8–10/group. B, Paclitaxel (2 mg/kg, i.p.) was administered to IL-10−/− or WT mice on day 0 and day 2. Shown are: IL-10−/− mice (gray circles), WT mice (black squares). Two-way repeated-measures ANOVA showed a main effect of time (p < 0.001), a group effect (p < 0.001), and a group-by-time interaction (p < 0.001). Bonferroni post hoc analysis: ***p < 0.001; n = 6–7/group. C, Paclitaxel (2 mg/kg, i.p.) was administered to Rag1−/− mice on day 0 and day 2. CD8+ cells isolated from WT mice were adoptively transferred to Rag1−/− mice 1 d before the first paclitaxel treatment. Anti-IL10 or control IgG antibodies (10 μg/mouse/d) were administered intrathecally from days 6–10 and mechanical allodynia was followed over time. Shown are: IgG CD8+ Rag1−/− (open circles) and IL-10 CD8+ Rag1−/− (black squares). Two-way repeated-measures ANOVA showed a main effect of time (p < 0.001), a group effect (p < 0.001), and a group-by-time interaction (p < 0.001). Bonferroni post hoc analysis: *p < 0.05, ***p < 0.001; n = 8/group. D, Paclitaxel (2 mg/kg, i.p.) was administered to WT mice on day 0 and day 2. IL-10 mRNA expression levels in lumbar spinal cord were examined on day 7 after paclitaxel treatment. Student's t test measured differences between vehicle-treated (open bars) and paclitaxel-treated (black bars) mice. **p < 0.01; n = 8/group. E, Paclitaxel (2 mg/kg, i.p.) was administered to Rag1−/− mice on day 0 and day 2. CD8+ cells isolated from IL-10−/− mice were adoptively transferred to Rag1−/− mice 1 d before the first paclitaxel treatment and mechanical allodynia was assessed. Shown are: PBS (open circles, gray dashed line) and IL-10−/− + CD8+ T cells (squares, black line). One-way repeated-measures ANOVA showed a main effect of time (p < 0.001), a group effect (p < 0.001), and a group-by-time interaction (p < 0.001). Bonferroni post hoc analysis: **p < 0.01; ***p < 0.001; n = 8/group. F, IL-10 receptor mRNA levels were assessed in lumbar DRG of WT mice, Rag1−/− mice, and Rag1−/− mice reconstituted with CD8+ T cells at day 7 after paclitaxel treatment. Shown are: WT + vehicle (open black bars); WT + paclitaxel (black bars); Rag1−/− + vehicle (open gray bars); Rag1−/− + paclitaxel (gray bars); and Rag1−/− + paclitaxel + CD8+ T cells (patterned bars). One-way ANOVA revealed significant differences between groups. *p < 0.05; n = 6–10/group.
Next, we investigated whether paclitaxel increases IL-10 mRNA and/or IL-10R1 expression in lumbar DRG and/or spinal cord. Paclitaxel increased IL-10 mRNA in the spinal cord (Fig. 4D), but not in DRG (data not shown). It is possible that CD8+ T cells infiltrating the DRG produce low levels of IL-10 that we did not detect using quantitative RT-PCR. To address this possibility, we transferred CD8+ T cells from IL-10−/− mice to Rag1−/− mice. However, CD8+ T cells from IL-10−/− mice were fully capable of promoting resolution of paclitaxel-induced allodynia in Rag1−/− mice (Fig. 4E). These data demonstrate that the CD8+ T-cell population is necessary for the resolution of CIPN, but the CD8+T cell is not the source of the IL-10 required for this resolution.
Interestingly, paclitaxel increased the expression of mRNA encoding IL-10R1 in the DRG of WT mice, but not in the DRG of Rag1−/− mice. Paclitaxel did induce an increase in IL-10R1 mRNA in DRG of Rag1−/− mice reconstituted with CD8+ T cells (Fig. 4F).
Effect of IL-10 on paclitaxel-induced mechanical allodynia and spontaneous activity of DRG neurons
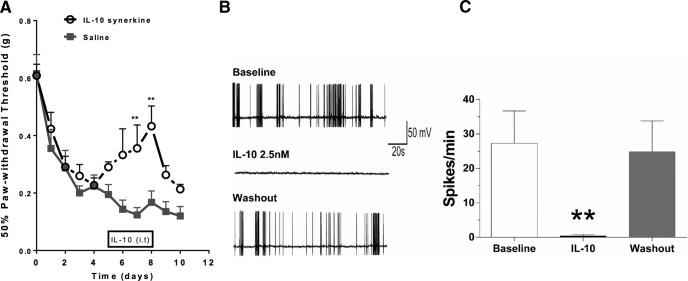
Knowing that IL-10 is necessary for resolution of mechanical allodynia, we investigated whether administration of exogenous IL-10 suppresses CIPN in T-cell-deficient mice. Rag1−/− mice were treated intrathecally with IL-10 synerkine or PBS from days 5 to 8 after paclitaxel treatment. The results shown in Figure 5A demonstrate that IL-10 relieves CIPN transiently. Next, we analyzed the effect of IL-10 on paclitaxel-induced spontaneous discharges in DRG neurons (Li et al., 2015). Consistent with earlier studies, DRG neurons displayed abnormal spontaneous discharges after in vivo exposure to paclitaxel. The addition of IL-10 inhibited these spontaneous discharges transiently, indicating that IL-10 suppresses paclitaxel-induced spontaneous discharges without affecting neuronal survival (Fig. 5B,C).
Figure 5.
Effect of IL-10 on paclitaxel-induced mechanical allodynia and spontaneous discharges of DRG neurons. A, IL-10 synerkine (1 μg/mouse) was administered intrathecally from days 5–8 after the start of paclitaxel treatment and mechanical allodynia was monitored. Shown are: IL-10 synerkine (open circles, black dashed line) and saline (squares, gray line). Two-way repeated-measures ANOVA revealed a main effect of time (p < 0.001), a group effect (p < 0.01), and a group-by-time interaction (p < 0.05). Bonferroni post hoc test: **p < 0.01; n = 10/group. B, Lumbar DRG neurons from paclitaxel-treated rats were cultured in vitro. Spontaneous activity was recorded at baseline, after the addition of IL-10, and after washout. C, Bar graphs summarize results obtained in seven cells from four paclitaxel-treated animals.
Discussion
We propose the novel concept that understanding the endogenous mechanisms that regulate resolution of CIPN will allow for the identification of therapeutic targets to promote recovery in patients with persistent CIPN. Here, we demonstrate for the first time that CD8+ T cells are essential for recovery from paclitaxel-induced mechanical allodynia. In addition, we identify a thus far unappreciated key role for endogenous IL-10 in recovery from CIPN by showing that genetic deletion or antibody-mediated inhibition of IL-10 prolonged paclitaxel-induced mechanical allodynia. The CD8+ T-cell-mediated resolution of CIPN is dependent on endogenous IL-10 signaling, but CD8+ T cells are not the source of the IL-10 needed for resolution. Notably, CD8+ T cells are required to increase the expression of IL-10R1 in the DRG of paclitaxel-treated mice. Consistent with a role for IL-10 in the resolution of CIPN, intrathecal administration of exogenous IL-10 reduced paclitaxel-induced mechanical allodynia in T-cell-deficient Rag1−/− mice. Finally, we show that in vitro IL-10 suppressed the abnormal spontaneous discharges of DRG neurons exposed to paclitaxel in vivo. These findings identify a novel and critical role for CD8+ T cells and IL-10 in recovery from paclitaxel-induced mechanical allodynia and are initial steps toward identifying the mechanisms responsible for recovery from CIPN.
Our finding that T cells—in particular, CD8+ T cells—are critical for recovery from CIPN is novel and unexpected. Consistent with a role in resolution, the number of CD8+ T cells in lumbar DRG was increased at day 7 after paclitaxel, when the WT mice started to recover from mechanical allodynia. At this point, it is unclear whether the resident T cells that were present in the DRG of naive mice proliferated during recovery from paclitaxel treatment or if paclitaxel induces infiltration of additional T cells into the DRG.
Our present data demonstrate that transfer of CD8+ T cells was sufficient to normalize resolution of CIPN in Rag1−/− mice, whereas CD4+ T cells had no effect. There are conflicting data on the role of CD4+ T cells in other neuropathy models. In nerve damage- and inflammation-induced mechanical allodynia, depletion of CD4+ Tregs leads to worsened pain. There is evidence that CD4+ Tregs infiltrate into the DRG in these models and expansion of CD4+ Tregs relieves pain transiently (Austin et al., 2012; Lees et al., 2015). Others have observed that CD4+ cell infiltration into the spinal cord contributes to persistent pain in response to nerve ligation (Cao and DeLeo, 2008; Costigan et al., 2009). In our model of CIPN, Rag1−/− mice that did not have T cells had a normal onset and severity of pain and transfer of CD4+ T cells was not sufficient to normalize resolution in Rag1−/− mice, whereas transfer of CD8 T cells induced recovery. These findings indicate that CD4+ T cells are neither sufficient nor required for resolution of CIPN. Similarly, we do not have evidence that B cells, which are also lacking in Rag1−/− mice, are required for onset, severity, or resolution of CIPN. Nevertheless, it is possible that expansion of selective subsets of CD4+ T-cell subpopulations (e.g., CD4+ Tregs or Th2 cells) or B cells are capable of modulating CIPN.
Recent studies have identified sexual dimorphisms in the contribution of T cells and microglia to inflammatory pain induced by complete Freund's adjuvant and to neuropathic pain induced by nerve injury (Sorge et al., 2011, 2015). Consistent with our present data, Sorge et al. (2015) showed that there was no difference between male and female T-cell-deficient mice and WT mice in the onset or severity of inflammatory and neuropathic pain. These observations support our conclusion that T cells are not required for the development of pain. The same study identified a sex difference in the contribution of the adaptive immune system, presumably T cells, to neuropathic and inflammatory pain under conditions in which microglial inhibitors were applied. Under these conditions, it became clear that microglia activity is required for pain in males. In contrast, females do not need to engage microglial activity to develop chronic pain, but only depend on microglia activity in the absence of T cells. It should be noted that there is little to no evidence for microglia activation in models of CIPN even though most of these studies were performed in males (Zhang et al., 2012; Di Cesare Mannelli et al., 2013; Janes et al., 2014; Robinson et al., 2014; Vichaya et al., 2015). Importantly, resolution of pain was not investigated in the study by Sorge et al. (2015). However, we have preliminary evidence that resolution of cisplatin-induced neuropathy is prolonged in female T-cell-deficient Rag2−/− mice and that transfer of CD8+ T cells normalizes resolution (data not shown). It remains to be determined whether there are sex differences in the contribution of T cells to resolution of inflammatory and neuropathic pain.
We show that both CD8+ T cells and endogenous IL-10 are required for the resolution of CIPN. Genetic deletion of IL-10 delayed recovery from paclitaxel-induced mechanical allodynia markedly and these mice do have CD8+ T cells. In addition, blockade of spinal cord and DRG IL-10 signaling by intrathecal administration of anti-IL-10 delayed recovery from paclitaxel-induced mechanical allodynia markedly in both WT mice and in Rag1−/− mice reconstituted with CD8+ T cells. These findings support a model in which CD8+ T cells promote resolution of CIPN via an IL-10-mediated pathway. Consistent with a key role for IL-10 in the resolution of pain, daily intrathecal injections of IL-10 synerkine to Rag1−/− mice resulted in transient reduction of paclitaxel-induced mechanical allodynia. It is possible that increasing either the dose or the duration of treatment with IL-10 synerkine would lead to full recovery of paclitaxel-treated mice. Indeed, previous studies indicate that IL-10 suppresses pain transiently in response to nerve ligation or exposure to paclitaxel (Chacur et al., 2004; Milligan et al., 2005; Ledeboer et al., 2007; Shen et al., 2013). Prolonged increases in IL-10 induced by intrathecal administration of an IL-10-encoding construct leads to persistent reduction of pain (Ledeboer et al., 2007).
We propose that at least part of the beneficial effect of IL-10 is mediated via direct effects of IL-10 on IL-10 receptors that are expressed by DRG neurons. In support of this hypothesis, we show that in vitro IL-10 suppresses the abnormal discharges of DRG neurons that are induced by in vivo exposure to paclitaxel. Moreover, it has been shown that in vitro culture of normal DRG neurons with IL-10 reverses voltage-gated sodium currents, thereby reducing neuronal excitability (Chen et al., 2011; Shen et al., 2013). The question arises whether the in vivo paclitaxel-induced increase in spinal cord IL-10 promotes resolution of CIPN via an effect on IL-10 receptors in DRG and/or spinal cord. One possibility would be that IL-10 produced in the spinal cord binds to IL-10 receptors on projections of DRG neurons in lamina I and II of the spinal cord to suppress spontaneous discharges. Although speculative, spinal cord IL-10 may also diffuse to the DRG to have suppressive effects on spontaneous activity generated in DRG somata and/or have effects on spinal cord glia and/or infiltrating macrophages, leading to reduced pain (Zhu et al., 2016). Suppression of spontaneous activity in DRG neurons would be expected to reduce spontaneous pain that the animals with CIPN may experience. However, DRG neurons that develop spontaneous activity are also hyperexcitable to afferent inputs (Zhang and Dougherty, 2014). Therefore, suppression of spontaneous activity likely also reduces mechanical allodynia, as shown here in response to IL-10 treatment in vivo.
In conclusion, the work presented here is the first to identify a novel key regulatory role for CD8+ T cells and endogenous IL-10 production in recovery from paclitaxel-induced mechanical allodynia. We have also demonstrated that CD8+ T cells are required to upregulate IL-10 receptors in the DRG during resolution of CIPN and that, in vitro, IL-10 suppresses the paclitaxel-induced spontaneous discharges of DRG neurons directly. Further studies should elucidate the mechanisms via which CD8+ T cells increase IL-10 receptors and how IL-10 signaling suppresses the abnormal spontaneous activity of DRG neurons. Increased understanding of the role of CD8+ T cells and IL-10 signaling in the resolution of CIPN could lead to novel interventions to prevent and treat this persistent side effect of cancer treatment.
Footnotes
This work was supported by a STAR award from the University of Texas System and the National Institutes of Health (Grants R21 CA183736, RO1 NS073939, and RO1 NS074999 and National Cancer Institute Support Grant P30 CA016672 to MD Anderson Cancer Center). We thank Xiao Jiao Huo for technical assistance and Christine Steen-Louws for producing the IL-4/IL-10 synerkine fusion protein.
C.E.H. has a patent for the synerkine used in this study. The remaining authors declare no competing financial interests.
References
- Abel M, Sène D, Pol S, Bourlière M, Poynard T, Charlotte F, Cacoub P, Caillat-Zucman S. Intrahepatic virus-specific IL-10-producing CD8 T cells prevent liver damage during chronic hepatitis C virus infection. Hepatology. 2006;44:1607–1616. doi: 10.1002/hep.21438. [DOI] [PubMed] [Google Scholar]
- Abram SE, Yi J, Fuchs A, Hogan QH. Permeability of injured and intact peripheral nerves and dorsal root ganglia. Anesthesiology. 2006;105:146–153. doi: 10.1097/00000542-200607000-00024. [DOI] [PubMed] [Google Scholar]
- Austin PJ, Kim CF, Perera CJ, Moalem-Taylor G. Regulatory T cells attenuate neuropathic pain following peripheral nerve injury and experimental autoimmune neuritis. Pain. 2012;153:1916–1931. doi: 10.1016/j.pain.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152:308–313. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, DeLeo JA. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol. 2008;38:448–458. doi: 10.1002/eji.200737485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cata JP, Weng HR, Lee BN, Reuben JM, Dougherty PM. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol. 2006;72:151–169. [PubMed] [Google Scholar]
- Chacur M, Gutierrez JM, Milligan ED, Wieseler-Frank J, Britto LR, Maier SF, Watkins LR, Cury Y. Snake venom components enhance pain upon subcutaneous injection: an initial examination of spinal cord mediators. Pain. 2004;111(1–2):65–76. doi: 10.1016/j.pain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen X, Pang RP, Shen KF, Zimmermann M, Xin WJ, Li YY, Liu XG. TNF-alpha enhances the currents of voltage gated sodium channels in uninjured dorsal root ganglion neurons following motor nerve injury. Exp Neurol. 2011;227:279–286. doi: 10.1016/j.expneurol.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Choy H, Akerley W, Safran H, Clark J, Rege V, Papa A, Glantz M, Puthawala Y, Soderberg C, Leone L. Phase I trial of outpatient weekly paclitaxel and concurrent radiation therapy for advanced non-small-cell lung cancer. J Clin Oncol. 1994;12:2682–2686. doi: 10.1200/JCO.1994.12.12.2682. [DOI] [PubMed] [Google Scholar]
- Costigan M, Moss A, Latremoliere A, Johnston C, Verma-Gandhu M, Herbert TA, Barrett L, Brenner GJ, Vardeh D, Woolf CJ, Fitzgerald M. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci. 2009;29:14415–14422. doi: 10.1523/JNEUROSCI.4569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Wan N, Zhang S, Moore Y, Wan F, Dai Z. Cutting edge: programmed death-1 defines CD8+CD122+ T cells as regulatory versus memory T cells. J Immunol. 2010;185:803–807. doi: 10.4049/jimmunol.1000661. [DOI] [PubMed] [Google Scholar]
- Di Cesare Mannelli L, Pacini A, Bonaccini L, Zanardelli M, Mello T, Ghelardini C. Morphologic features and glial activation in rat oxaliplatin-dependent neuropathic pain. J Pain. 2013;14:1585–1600. doi: 10.1016/j.jpain.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Eijkelkamp N, Heijnen CJ, Willemen HL, Deumens R, Joosten EA, Kleibeuker W, den Hartog IJ, van Velthoven CT, Nijboer C, Nassar MA, Dorn GW, 2nd, Wood JN, Kavelaars A. GRK2: a novel cell-specific regulator of severity and duration of inflammatory pain. J Neurosci. 2010;30:2138–2149. doi: 10.1523/JNEUROSCI.5752-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp N, Steen-Louws C, Hartgring SA, Willemen HL, Prado J, Lafeber FP, Heijnen CJ, Hack CE, van Roon JA, Kavelaars A. IL4–10 fusion protein is a novel drug to treat persistent inflammatory pain. J Neurosci. 2016;36:7353–7363. doi: 10.1523/JNEUROSCI.0092-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122:245–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabryšová L, Howes A, Saraiva M, O'Garra A. The regulation of IL-10 expression. Curr Top Microbiol Immunol. 2014;380:157–190. doi: 10.1007/978-3-662-43492-5_8. [DOI] [PubMed] [Google Scholar]
- Gianni L, Munzone E, Capri G, Villani F, Spreafico C, Tarenzi E, Fulfaro F, Caraceni A, Martini C, Laffranchi A. Paclitaxel in metastatic breast cancer: a trial of two doses by a 3-hour infusion in patients with disease recurrence after prior therapy with anthracyclines. J Natl Cancer Inst. 1995;87:1169–1175. doi: 10.1093/jnci/87.15.1169. [DOI] [PubMed] [Google Scholar]
- Homs J, Pagès G, Ariza L, Casas C, Chillón M, Navarro X, Bosch A. Intrathecal administration of IGF-I by AAVrh10 improves sensory and motor deficits in a mouse model of diabetic neuropathy. Mol Ther Methods Clin Dev. 2014;1:7. doi: 10.1038/mtm.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZZ, Li D, Liu CC, Cui Y, Zhu HQ, Zhang WW, Li YY, Xin WJ. CX3CL1-mediated macrophage activation contributed to paclitaxel-induced DRG neuronal apoptosis and painful peripheral neuropathy. Brain Behav Immun. 2014;40:155–165. doi: 10.1016/j.bbi.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Jacques SJ, Ahmed Z, Forbes A, Douglas MR, Vigenswara V, Berry M, Logan A. AAV8(gfp) preferentially targets large diameter dorsal root ganglion neurones after both intra-dorsal root ganglion and intrathecal injection. Mol Cell Neurosci. 2012;49:464–474. doi: 10.1016/j.mcn.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Janes K, Esposito E, Doyle T, Cuzzocrea S, Tosh DK, Jacobson KA, Salvemini D. A3 adenosine receptor agonist prevents the development of paclitaxel-induced neuropathic pain by modulating spinal glial-restricted redox-dependent signaling pathways. Pain. 2014;155:2560–2567. doi: 10.1016/j.pain.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes K, Wahlman C, Little JW, Doyle T, Tosh DK, Jacobson KA, Salvemini D. Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav Immun. 2015;44:91–99. doi: 10.1016/j.bbi.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannarkat G, Lasher EE, Schiff D. Neurologic complications of chemotherapy agents. Curr Opin Neurol. 2007;20:719–725. doi: 10.1097/WCO.0b013e3282f1a06e. [DOI] [PubMed] [Google Scholar]
- Kashi VP, Ortega SB, Karandikar NJ. Neuroantigen-specific autoregulatory CD8+ T cells inhibit autoimmune demyelination through modulation of dendritic cell function. PLoS One. 2014;9:e105763. doi: 10.1371/journal.pone.0105763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski K, Nijboer CH, Huo X, Kavelaars A, Heijnen CJ. Prevention of chemotherapy-induced peripheral neuropathy by the small-molecule inhibitor pifithrin-mu. Pain. 2015;156:2184–2192. doi: 10.1097/j.pain.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumet G, Garriga J, Chen SR, Zhang Y, Li DP, Smith TM, Dong Y, Jelinek J, Cesaroni M, Issa JP, Pan HL. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci. 2015;18:1746–1755. doi: 10.1038/nn.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, Martin D, Maier SF, Johnson KW, Leinwand LA, Chavez RA, Watkins LR. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21:686–698. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees JG, Duffy SS, Perera CJ, Moalem-Taylor G. Depletion of Foxp3+ regulatory T cells increases severity of mechanical allodynia and significantly alters systemic cytokine levels following peripheral nerve injury. Cytokine. 2015;71:207–214. doi: 10.1016/j.cyto.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Toll-like receptor 4 signaling contributes to paclitaxel-induced peripheral neuropathy. J Pain. 2014;15:712–725. doi: 10.1016/j.jpain.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Adamek P, Zhang H, Tatsui CE, Rhines LD, Mrozkova P, Li Q, Kosturakis AK, Cassidy RM, Harrison DS, Cata JP, Sapire K, Zhang H, Kennamer-Chapman RM, Jawad AB, Ghetti A, Yan J, Palecek J, Dougherty PM. The cancer chemotherapeutic paclitaxel increases human and rodent sensory neuron responses to TRPV1 by activation of TLR4. J Neurosci. 2015;35:13487–13500. doi: 10.1523/JNEUROSCI.1956-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Lu N, Cui Y, Yang T, Zhao ZQ, Xin WJ, Liu XG. Prevention of paclitaxel-induced allodynia by minocycline: Effect on loss of peripheral nerve fibers and infiltration of macrophages in rats. Mol Pain. 2010;6:76. doi: 10.1186/1744-8069-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O'Connor K, Martin D, Forsayeth JR, Maier SF, Johnson K, Chavez RA, Leinwand LA, Watkins LR. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci. 2005;21:2136–2148. doi: 10.1111/j.1460-9568.2005.04057.x. [DOI] [PubMed] [Google Scholar]
- Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev. 2014;40:872–882. doi: 10.1016/j.ctrv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30:3687–3696. doi: 10.1200/JCO.2012.41.7238. [DOI] [PubMed] [Google Scholar]
- Peters CM, Jimenez-Andrade JM, Kuskowski MA, Ghilardi JR, Mantyh PW. An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain Res. 2007;1168:46–59. doi: 10.1016/j.brainres.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polomano RC, Bennett GJ. Chemotherapy-evoked painful peripheral neuropathy. Pain Med. 2001;2:8–14. doi: 10.1046/j.1526-4637.2001.002001008.x. [DOI] [PubMed] [Google Scholar]
- Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6:489–494. doi: 10.1093/oxfordjournals.annonc.a059220. [DOI] [PubMed] [Google Scholar]
- Mao-Ying QL, Kavelaars A, Krukowski K, Huo XJ, Zhou W, Price TJ, Cleeland C, Heijnen CJ. The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. PLoS One. 2014;9:e100701. doi: 10.1371/journal.pone.0100701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/PL00007853. [DOI] [PubMed] [Google Scholar]
- Robinson CR, Zhang H, Dougherty PM. Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience. 2014;274:308–317. doi: 10.1016/j.neuroscience.2014.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst. 1990;82:1247–1259. doi: 10.1093/jnci/82.15.1247. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK, Noe DA, Ettinger DS, Christian MC, Lubejko BG, Fishman EK, Sartorius SE, Boyd MR, Donehower RC. Phase I and pharmacological study of the pulmonary cytotoxin 4-ipomeanol on a single dose schedule in lung cancer patients: hepatotoxicity is dose limiting in humans. Cancer Res. 1993;53:1794–1801. [PubMed] [Google Scholar]
- Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155:2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Coers S, Sanders VM, Jones KJ. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:393–402. doi: 10.1016/S0889-1591(03)00028-X. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KF, Zhu HQ, Wei XH, Wang J, Li YY, Pang RP, Liu XG. Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Exp Neurol. 2013;247:466–475. doi: 10.1016/j.expneurol.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31:15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varbiro G, Veres B, Gallyas F, Jr, Sumegi B. Direct effect of Taxol on free radical formation and mitochondrial permeability transition. Free Radic Biol Med. 2001;31:548–558. doi: 10.1016/S0891-5849(01)00616-5. [DOI] [PubMed] [Google Scholar]
- Vichaya EG, Chiu GS, Krukowski K, Lacourt TE, Kavelaars A, Dantzer R, Heijnen CJ, Walker AK. Mechanisms of chemotherapy-induced behavioral toxicities. Front Neurosci. 2015;9:131. doi: 10.3389/fnins.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JT, et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J Clin Invest. 2015;125:2547. doi: 10.1172/JCI82458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Heijnen CJ, Eijkelkamp N, Garza Carbajal A, Schedlowski M, Kelley KW, Dantzer R, Kavelaars A. GRK2 in sensory neurons regulates epinephrine-induced signalling and duration of mechanical hyperalgesia. Pain. 2011;152:1649–1658. doi: 10.1016/j.pain.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Wieseler-Frank J, Maier SF, Watkins LR. Immune-to-brain communication dynamically modulates pain: physiological and pathological consequences. Brain Behav Immun. 2005;19:104–111. doi: 10.1016/j.bbi.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Willemen HL, Eijkelkamp N, Wang H, Dantzer R, Dorn GW, 2nd, Kelley KW, Heijnen CJ, Kavelaars A. Microglial/macrophage GRK2 determines duration of peripheral IL-1beta-induced hyperalgesia: contribution of spinal cord CX3CR1, p38 and IL-1 signaling. Pain. 2010;150:550–560. doi: 10.1016/j.pain.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Dougherty PM. Enhanced excitability of primary sensory neurons and altered gene expression of neuronal ion channels in dorsal root ganglion in paclitaxel-induced peripheral neuropathy. Anesthesiology. 2014;120:1463–1475. doi: 10.1097/ALN.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li Y, de Carvalho-Barbosa M, Kavelaars A, Heijnen CJ, Albrecht PJ, Dougherty PM. Dorsal root ganglion infiltration by macrophages contributes to paclitaxel chemotherapy induced peripheral neuropathy. J Pain. 2016;17:775–786. doi: 10.1016/j.jpain.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yoon SY, Zhang H, Dougherty PM. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J Pain. 2012;13:293–303. doi: 10.1016/j.jpain.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Boyette-Davis JA, Kosturakis AK, Li Y, Yoon SY, Walters ET, Dougherty PM. Induction of monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR2 in primary sensory neurons contributes to paclitaxel-induced peripheral neuropathy. J Pain. 2013;14:1031–1044. doi: 10.1016/j.jpain.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Chen X, Liu Z, Peng YP, Qiu YH. Interleukin-10 protection against lipopolysaccharide-induced neuro-inflammation and neurotoxicity in ventral mesencephalic cultures. Int J Mol Sci. 2016;17:E25. doi: 10.3390/ijms17010025. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]