Abstract
Background
Psychosis is hypothesized to occur on a spectrum between psychotic disorders and healthy individuals. In the middle of the spectrum are individuals who endorse psychotic-like experiences (PLEs) that may not impact daily functioning or cause distress. Individuals with PLEs show alterations in both cognitive ability and functional connectivity of several brain networks, but the relationship between PLEs, cognition, and functional networks remains poorly understood.
Methods
We analyzed resting-state fMRI data, a range of neuropsychological tasks, and questions from the Achenbach Adult Self Report (ASR) in 468 individuals from the Human Connectome Project. We aimed to determine whether global efficiency of specific functional brain networks supporting higher-order cognition (the fronto-parietal network (FPN), cingulo-opercular network (CON), and default mode network (DMN)) was associated with PLEs and cognitive ability in a non-psychiatric sample.
Results
21.6% of individuals in our sample endorsed at least one PLE. PLEs were significantly negatively associated with higher-order cognitive ability, CON global efficiency, and DMN global efficiency, but not crystallized knowledge. Higher-order cognition was significantly positively associated with CON and DMN global efficiency. Interestingly, the association between PLEs and cognitive ability was partially mediated by CON global efficiency and, in a subset of individuals who tested negative for drugs (N=405), the participation coefficient of the right anterior insula (a hub within the CON).
Conclusions
These findings suggest that CON integrity may represent a shared mechanism that confers risk for psychotic experiences and the cognitive deficits observed across the psychosis spectrum.
Keywords: psychotic-like experiences, resting-state networks, imaging, psychosis, cognition, graph theory
Introduction
Psychotic experiences, such as delusions and hallucinations, occur primarily in the context of serious mental health disorders. However research suggests that approximately 8–20% of clinically healthy individuals endorse occasional psychotic-like experiences (PLEs) that may or may not impact functioning (1, 2). As such, psychotic experiences occur on a continuum, with a subset of the general population experiencing PLEs in the absence of a diagnosable disorder, while another subset (e.g., individuals with schizophrenia) experience frequent and debilitating psychosis in conjunction with other psychiatric symptoms (3).
Under this continuum model, the psychosis phenotype expressed in the general population should show relationships with risk factors and neurobiological abnormalities associated with clinical psychosis, but with a lesser degree of severity (3). Research to date has provided some evidence in support of these predictions, revealing associations between PLEs and sub-clinical negative (4) and affective symptoms (5, 6). Interestingly, several studies have also shown associations between PLEs in the general population and cognitive ability (7, 8), an area of functioning that is robustly impaired in psychotic disorders (9). In fact, cognitive deficits are often present in individuals who later develop schizophrenia (10), and youths on the psychosis spectrum have been shown to have lower predicted age based on their cognitive functioning (11), suggesting that mechanisms supporting cognitive ability also affect mechanisms that give rise to the psychosis phenotype.
Recently, research exploring the neurobiological underpinnings of both PLEs and cognitive ability has focused on the role of brain networks in their development and maintenance (12–15). Brain networks derived from resting-state fMRI represent reliable and robust patterns of functional connectivity (16), and these networks appear to support different domains of information processing (17). Research has revealed that three networks supporting higher-order cognitive ability – the cingulo-opercular network (CON), fronto-parietal network (FPN), and default mode network (DMN) – have abnormal functional connectivity in psychotic disorders (18–20), which has been associated with both impairments in cognitive ability (21, 22) and psychotic symptoms (23, 24). Data from psychosis-spectrum youths has also revealed significantly altered functional connectivity of both the CON and DMN during rest, which was associated with cognitive ability (15), suggesting that functionally relevant connectivity abnormalities can be observed across the psychosis spectrum.
Given the complex literature on functional connectivity in psychotic disorders, which often reveals both increased and decreased connectivity between network nodes, graph analysis of a network’s global efficiency allows for a parsimonious measure of a network’s functional integration (25). Global efficiency also appears to be a predictive measure, as global efficiency of the whole brain graph has been shown to be decreased in individuals with subclinical psychotic experiences (26), as well as in schizophrenia (27), and correlates with positive symptoms (28) and IQ (29). Although some studies have shown increased whole brain global efficiency in the context of psychosis (30, 31), previous work from our group looking at specific functional networks (CON and FPN) – taking into account the connectivity of only the nodes within that network -- have revealed associations between reduced global efficiency of these specific networks and decreased cognitive ability (32). Therefore, based on these studies, as well as consistently observed abnormalities in DMN connectivity in psychotic disorders (e.g. (33)), we predict that inefficient functioning of the FPN, CON, as well as the DMN, may contribute to the phenotypes of both psychosis and impaired cognition.
In the current study we tested the a priori hypothesis that reduced functional network efficiency of the CON, FPN, and DMN would be associated with both increased PLEs and reduced cognitive ability in a large cohort of relatively healthy young adults. This hypothesis was based on previous literature showing that greater PLEs (2, 7) and lower network efficiency (32) are associated with lower cognitive ability – associations we hoped to replicate and extend to the general population, in order to test the psychosis continuum model. Additionally, we hypothesized that efficiency of these networks would mediate the relationship between psychosis and cognitive ability, suggesting a shared mechanism between these two phenotypes.
Methods
Participants
Participants were recruited for the Human Connectome Project (HCP), a large-scale study focused on collecting high-quality neuroimaging data and extensive individual phenotyping in adult twins and their non-twin siblings (34). 468 individuals from the “S500” release (Table 1) were included in the current study after signing informed consent documents approved by the Washington University Internal Review Board. Potential participants were excluded if they or their sibling had a severe neurodevelopment disorder, a documented and treated neuropsychiatric disorder, neurological disorder, or other physical disorder that could influence brain function (34, 35).
Table 1.
Subject demographics
| Demographics | All Subjects (N=468) | Subjects who did NOT endorse any PLEs (N=367) | Subjects who endorsed at least one PLE (N=101) | Difference Between Those Who Did/Did Not Endorse PLEs |
|---|---|---|---|---|
| Age mean (SD), years | 29.17 (3.51) | 29.44 (3.49) | 28.21 (3.42) | t(466)=3.15, p=.002 |
| Gender (%female/%male) | 58.3/41.7 | 61.6/38.4 | 46.5/53.5 | χ2 = 7.38, p=.007 |
| Race (%white/%African American/%other) | 74.1/20.7/5.2 | 79.3/16.3/4.4 | 55.4/36.6/8.0 | χ2 = 34.26, p<.001 |
| Subject Education mean (SD), years | 14.85 (1.85) | 15.02 (1.79) | 14.20 (1.94) | t(465)=4.04, p<.001 |
| Annual Household Income (%<$10K/%10K-39,999/%40K-74,999/%75K-100K, %>100K/%missing data) | 7.1/32/29.5/15.8/15.0/0.6 | 4.9/30.2/30.5/16.3/17.4/0.5 | 14.9/38.6/25.7/13.9/5.9/1.0 | χ2 = 25.30, p=.001 |
Demographic variables for all subjects; demographics for only those subjects who did not endorse any PLEs (i.e. summed score on the four questions of interest from the Achenbach Adult Self Report Scale (ASR) = 0), and demographics for only those subjects who scored > 0 on the four questions of interest from the ARS, indicating endorsement of at least one PLE. Statistical analyses revealed differences in the demographic data between those who endorsed no PLEs on the ASR, and those who endorsed at least one PLE (summed score > 0). Individuals who endorsed at least one PLE were significantly younger, more likely to be male, more likely to be African-American, had lower subject education, and lower annual household income on average.
Procedure
Participants completed testing over the course of two days at the Washington University School of Medicine. Each day included a drug screen, two 15-minute resting-state scans (as well as other scans not discussed in these analyses), and extensive behavioral assessments.
Cognitive Testing
Cognitive testing involved paradigms from the NIH Toolbox (www.nihtoolbox.org) and the University of Pennsylvania Computerized Neuropsychological Testing Battery (36, 37). Measures of cognition analyzed in the current study included the Picture Sequencing Task (episodic memory), Wisconsin Card Sort (executive function/cognitive flexibility), Flanker Task (executive function/inhibition), Penn Matrices Test (fluid intelligence), Penn Word Memory Test (episodic memory), and List Sorting Task (working memory). Given that the CON, FPN, and DMN are believed to support all of these cognitive processes, and given that our interest in the current study was to measure “overall” cognitive functioning as supported by these networks, scores from these six tasks were included in a principal components analysis (PCA), and the first component was used as a measure of higher-order cognitive ability (35% of the total variance). Crystallized knowledge was measured as the average z-scores from two measures of language and reading comprehension: Picture Vocabulary and Oral Reading Recognition. NIH Toolbox scores were age and sex normed.
Psychotic-Like Experiences
Participants completed the Achenbach Adult Self Report (ASR) for ages 18–59 (38). Within the ASR, four questions were identified as measuring experiences similar to the positive symptoms of psychosis. These questions included: “I hear sounds or voices that other people think aren’t there”, “I see things that other people think aren’t there”, “I do things that other people think are strange”, and “I have thoughts that other people would think are strange”. Each question was scored on a scale of 0 (Not True), 1 (Somewhat or Sometimes True), or 2 (Very True or Often True). Scores for these four psychosis questions were summed to yield a PLEs score. Although the ASR is not commonly used for the measurement of PLEs, as these items are somewhat limited, we believe they provide a useful assessment of PLEs in the general population, given that the HCP did not include more complex measurements of clinical symptomatology.
Imaging Procedure
HCP imaging procedures have been detailed extensively in the literature (39, 40). All subjects were run on a customized Siemens Connectom 3T scanner with a 32-channel head coil and completed T1-weighted and T2-weighted structural scans (0.7mm isotropic), as well as four 15-minute resting-state BOLD scans, with their eyes open and fixated on a crosshair. Resting-state scans were acquired using a T2*-weighted multi-band (MB=8) EPI sequence with 72 axial slices per volume, 2x2x2mm voxels, FOV=208mm, TE=33.1ms, TR=720ms, and flip angle=52 degrees.
Preprocessing Pipelines
FMRI data from the HCP was run through minimal preprocessing pipelines (40) (see supplement for more details). Subsequently, CIFTI-format resting-state data (gray matter surface vertices plus subcortical gray matter voxels, collectively termed “grayordinates”) underwent additional functional connectivity preprocessing. Six rigid body motion parameters, their first derivatives, and their squares were used as regressors to correct for motion, and additional denoising was performed using FMRIB’s ICA-based X-noiseifier (FIX)(41),(42). Mean grayordinate timecourse was then regressed from the timeseries of each grayordinate, followed by temporal band-pass filtering (0.009–0.08Hz). All four resting-state runs were concatenated following preprocessing, yielding 60 minutes (4800 frames) of resting-state data. Movement estimates (average root mean square across frames in a run) were not associated with PLEs (r = −.02, p = .67), DMN efficiency (r=.02, p=.68), FPN efficiency (r=−.02, p=.68), or participation coefficients (all r’s <|−.09|, all p’s ≥.05), but were associated with cognition (r = −.15, p=.001) and CON efficiency (r=−.19, p<.001). However, all analyses reported below held in pattern and effect sizes when covarying for motion estimates.
Graph Analysis
R to z transformed correlations were calculated between each cortical parcel in the Gordon parcellation scheme (43, 44), which includes 333 parcels assigned to 12 different functional brain networks. The Gordon parcellation was used because it has been shown to have greater parcel homogeneity and greater differentiation relative to a null model than seven other alternative parcellation schemes. We also used the same network assignments as the Gordon parcellation. Graph analysis was performed using functional connectivity data from parcels that comprised CON, FPN, and DMN (44). Global efficiency was the main metric of interest, as networks with higher global efficiency are thought to be more functionally integrated, with higher potential for information transfer between nodes within that network (25). Additionally, work from our group revealed that higher global efficiency of the CON and FPN is associated with better cognitive ability in both health and schizophrenia (32).
Graph analysis was performed on weighted, undirected graphs using the following steps: 1) thresholding of each individual subject’s whole brain graph from 5%–10% edge density in 1% increments (all other connections reassigned as zeros), 2) isolation of parcels from the CON (n=40), FPN (n=24), and DMN (n=41) from thresholded whole brain graphs, 3) calculation of global efficiency for a priori networks using algorithms from the Brain Connectivity Toolbox (BCT) (25) at each edge density, 4) average global efficiency values from all six edge densities, yielding a single global efficiency value for each network. Follow-up analyses also included the participation coefficient of four a priori parcels (bilateral anterior insula, bilateral anterior cingulate cortex). Participation coefficient is a measure of a node’s connectedness with other networks (more information on graph metrics provided in Supplement). Participation coefficient values for our a priori nodes were averaged across the 5%–10% thresholds and included in subsequent analyses.
Data Analysis
Associations between cognition, psychosis, and network metrics were calculated using bivariate correlations, with follow-up partial correlations including potential confounding factors (gender, depression, anxiety, and head motion) using SPSS v20. Given our strong a priori hypotheses, one-tailed significance tests were performed for all correlations, and only a priori networks (CON, FPN, and DMN) were assessed. Spearman’s correlations were also performed to assess robustness of results in non-parametric tests (Supplement), and analysis of data without global mean confound regression was performed and is included in the Supplement. Bonferroni methods were used to correct for multiple comparisons of correlations with our network metrics (global efficiency: .05/3=.017; participation coefficient: .05/4=.0125) for both psychosis and cognition. Mediation analyses were performed using the PROCESS macro in SPSS (45), with bias-corrected 95% confidence intervals using 1000 bootstrap samples. The analogous correlation analyses were also performed after approximately adjusting for family structure in a Mixed Generalized Linear Model, with “family ID” as the highest level and “subject” as the second level (46), in order to assess whether significant findings were due to increased correlations between genetically related subjects. Finally, analyses were performed excluding individuals who tested positive for drugs on the day of the testing (N=63), as well as when excluding a single subject with a psychosis score of six (all other scores were <6). Analyses controlling for these potential confounds, as well as variables such as age, sex, race, education, THC and tobacco use, are provided in the Supplement.
Results
PLEs and Cognitive Ability
21.6% of individuals reported at least one PLE. Of those 101 subjects, 7 subjects endorsed hearing sounds or voices other people think aren’t there (7 = sometimes true), 5 endorsed seeing things other people think aren’t there (4 = sometimes true; 1 = often true), 82 endorsed doing things that other people think are strange (68 = sometimes true; 14 = often true), and 67 endorsed having thoughts that other people would think are strange (56 = sometimes true; 11 = often true).
PLEs were significantly negatively associated with cognitive ability (r = −.15, p<.001), indicating that individuals with more self-reports of PLEs had worse higher-order cognition (Figure 1a). Interestingly, these individuals were significantly more likely to be male and African-American, had significantly lower personal education, significantly lower household income, and were one year younger on average that those who did not endorse any PLEs (Table 1), which is in line with the demographic differences reported between psychosis and non-psychosis spectrum youth from the Philadelphia Neurodevelopmental Cohort (47). Additionally, individuals who endorsed at least one PLE had significantly lower cognitive ability than individuals who endorsed no PLEs (t(466)=3.15, p=.002) (Figure 1b). Interestingly, PLEs were not significantly associated with crystallized knowledge (r =−.07, p=.08), there were no differences in crystallized knowledge between those who did and did not endorse PLEs (t(466)=.82, p=.42), and Meng’s z-test for comparing the strength of correlations (48) revealed that PLES were significantly more strongly associated with higher-order cognition than crystallized knowledge (z=−2.01, p=.02).
Figure 1.
Psychotic-like experiences (PLEs) were significantly negatively associated with a) higher-order cognition. The relationship remained when removing the subject with a score of six (r=−.16, p<.001). b) Higher-order cognitive ability was significantly reduced in individuals who endorsed at least one PLE (summed score on four questions of interest from ASR were >0) (t(466)=3.15, p=.002), compared to those who endorsed no PLEs, however there was no difference between groups in crystallized knowledge (t(466)=.82, p=.42). PLEs were also significantly negatively associated with c) cingulo-opercular network (CON) global efficiency. The relationship remained significant when removing the subject with a score of six (r=−.10, p=.02). PLEs were measured using four questions from the Achenbach Adult Self Report Scale (“I hear sounds or voices that other people think aren’t there”, “I see things that other people think aren’t there”, “I do things that other people think are strange”, and “I have thoughts that other people would think are strange”). Higher-order cognition was measured as the first factor from a principal components factor analysis including the: Picture Sequencing Task (episodic memory), Wisconsin Card Sort (executive function/cognitive flexibility), Flanker Task (executive function/inhibition), Penn Matrices Test (fluid intelligence), Penn Word Memory Test (episodic memory), and List Sorting Task (working memory).
PLEs and Functional Network Efficiency
PLEs were significantly negatively associated with the global efficiency of the CON (r =−.12, p=.004) (Figure 1c) and the DMN (r =−.11, p=.009), indicating that individuals with more PLEs had lower global efficiency of the CON and DMN. Global efficiency of the FPN was not significantly related to PLEs (r =−.01, p=.38).
Cognition and Functional Network Efficiency
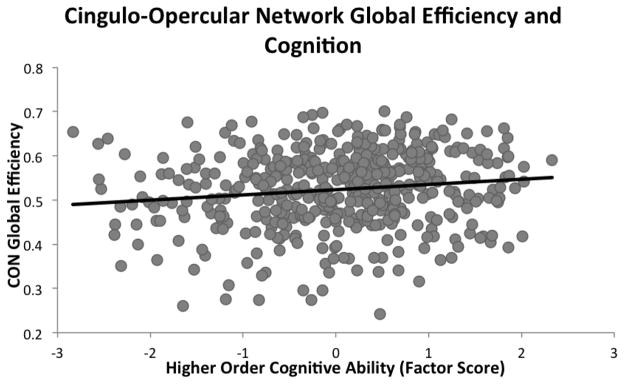
Higher-order cognitive ability was positively associated with global efficiency of the CON (r=.14, p=.001), indicating that individuals with better cognitive ability had higher CON global efficiency (Figure 2). Cognitive ability was not, however, associated with global efficiency of the FPN (r=−.03, p=.26) or the DMN (−.03, p=.26). Crystallized knowledge was not associated with global efficiency of any of the three networks (all r’s<.05, all p’s>.12).
Figure 2.
Global efficiency of the cingulo-opercular network (CON) significantly positively predicted higher-order cognitive ability. Global efficiency measures a network’s functional integration and potential for information transfer, suggesting that more functionally efficient CON supports better cognitive ability.
Mediation Analysis
Mediation analysis revealed that CON global efficiency partially mediated the relationship between PLEs and cognitive ability (indirect effect (path ab) bias corrected 95% CI[−.0417, −.003]). This mediation was partial, as PLEs continued to significantly predict cognitive ability with CON global efficiency in the model (t(2,465)= −3.08, p=.002) (Figure 3). In addition, CON global efficiency remained a significant partial mediator when head motion was included as a covariate in the model (path ab indirect effect 95% CI [−.035, −.001]). Global efficiency of the DMN did not significantly mediate the relationship between PLEs and cognition (indirect effect 95% CI[−.002, .026]). These findings suggest that the observed association between increased PLEs and worse cognitive ability is in part accounted for by lower global efficiency of the CON.
Figure 3.
Cingulo-opercular network (CON) global efficiency partially mediated the association between psychotic-like experiences (PLEs) and cognition. The 95% bias corrected confidence interval for indirect effect (path ab), which measures the reduction of the effect between PLEs and cognition ability when CON global efficiency is included in the model, is [−0.0417, −0.003], indicating a significant reduction, and therefore a significant mediation. The mediation is partial, since the association between PLEs and cognition remains significantly greater than zero (path c’) with CON global efficiency in the model.
Cognition, PLEs, and Participation
We performed follow-up analyses to assess whether connectedness of specific CON hub nodes was also related to PLEs and cognition. Right anterior insula (rAI) participation was significantly associated with cognitive ability (r=.12, p=.006), but not PLEs (r=−.06, p=.09). rAI participation did not significantly mediate the association between PLEs and cognitive ability in all subjects. However, when subjects who tested positive for drugs were removed, rAI participation was more strongly correlated with PLEs (r=−.10, p=.04) and was a significant partial mediator of PLEs and cognition (indirect effect 95% CI[−.0397, −.001]). This partial mediation was marginally significant when controlling for motion (indirect effect 95% CI[−.0381, 0.0001]). No other a priori hub nodes significantly correlated with cognition (all r’s<.08, all p’s>.04) or PLEs (all r’s<|−.07|, all p’s>.06) after correcting for multiple comparisons.
In order to assess whether rAI participation and CON global efficiency were independent predictors of cognition and PLEs, we included both variables as mediators in the same model, which included all subjects. We found that CON global efficiency continued to be a significant partial mediator of the relationship between PLEs and cognition (path ab indirect effect 95% CI [−0.037, −0.001]), but rAI participation was not a significant mediator in this model (95%CI [−0.024, 0.001]). These findings suggest that the CON’s partial mediation of the relationship between PLEs and cognitive ability is not due to the connectedness of the rAI with other resting-state networks, and may instead be more dependent on the functional efficiency within the CON itself.
Discussion
The current study reveals a significant association between PLEs and higher-order cognition in the general population, showing that healthy individuals who self-report more PLEs have lower higher-order cognitive ability, but not lower crystallized knowledge. Further, we demonstrate that the relationship between PLEs and cognition is partially mediated by the global efficiency of the CON, suggesting that functional integration of the CON is associated with both cognitive ability and the experience of PLEs. Interestingly, this mediation was also present for the participation coefficient of the rAI, although the strength of the mediation was somewhat less robust. We observed a significant partial mediation when drug users were removed, but this association was trending when motion was included as a covariate. Therefore, these findings suggest that connectedness of the rAI with other networks may also be an important underlying factor in the experience of PLEs and cognitive performance, but is likely a small piece of a much larger puzzle.
Although effect sizes for the relationships observed in the current study were small (r’s <.20), the magnitude is not unexpected, given that these relationships are being observed in individuals who are not diagnosed with a psychotic disorder, and have not sought more than 12 months of psychiatric treatment for any disorder, suggesting limited impairment due to lifetime psychiatric symptoms. The fact that these findings are both in line with the existing literature and can be detected in the general population provides further support for the relationship between psychosis, cognitive ability, and functional network efficiency across the psychosis spectrum. That said, these small effect sizes may also be the consequence, at least in part, of using a relatively crude measure of PLEs, only four questions from the Achenbach Adult Self-Report Scale (ASR). Using the ASR as our only measure of PLEs most likely reduced the variability of reported experiences in our sample, and therefore our ability to detect meaningful associations. Despite this limitation, these findings are the first to identify a common mechanism (i.e., the functional integrity of the CON) that is associated with both PLEs and cognitive ability in a non-psychiatric population. The small effect sizes observed in our sample do, however, suggest that other mechanisms may also influence both cognitive function and the experience of PLEs.
The CON is a functional brain network comprised of two major hub nodes: the dorsal anterior cingulate cortex and the anterior insula. This network is strongly coupled with the FPN and DMN, and nodes within the CON (primarily the anterior insula) have been shown to dynamically mediate activity between the FPN and DMN to gate salient information for further cognitive processing (49); a mediation that has been shown to be degraded in patients with schizophrenia (50). Meta-analysis of task-based fMRI data has revealed that nodes within the CON have increased activation during many different cognitive tasks, implicating this network in supporting a range of higher-order cognitive processes (51, 52). Further, strength of the rAI’s modulation of FPN and DMN activity during rest correlates with cognitive performance in patients with schizophrenia (53), suggesting that disruption of the rAI contributes to cognitive impairments observed in schizophrenia. In addition, global efficiency of the CON and the participation coefficient of the rAI have been found to correlate with cognition in both schizophrenia patients and healthy controls (32), suggesting that more robust functional integration of this network supports better cognitive ability.
The CON is also thought to play a crucial role in the generation and maintenance of psychotic symptoms (54, 55). Kapur and colleagues have suggested that individuals with psychotic disorders inappropriately assign salience to environmental stimuli, as modulated by activity in the CON (56). They posit that individuals then work to explain why a stimulus is salient, leading to the development and maintenance of delusional thought and hallucinatory experiences (57, 58). Providing support for this theory, neuroimaging research has shown that activity of the rAI increases when individuals with schizophrenia experience auditory hallucinations (59), and that stronger functional connectivity of the anterior cingulate cortex correlates with psychotic symptom severity in individuals at risk for developing schizophrenia (60). Furthermore, severity of delusions and hallucinations was negatively correlated with BOLD activation of the right insula and the anterior cingulate in a group of unmedicated first episode psychosis patients (61), suggesting that disruption of these nodes contributes to the experience of psychosis. Finally, individuals with schizotypal personality disorder, which is a disorder characterized by a relatively pervasive pattern of PLEs, have been shown to have significantly reduced gray matter volume of the insula and dorsolateral prefrontal cortex, and left insula volume had reduced functional connectivity with the putamen (62), further implicating the insula in PLEs across the psychosis spectrum.
Given the role of the CON in supporting higher-order cognitive ability, as well as its hypothesized involvement in the development and maintenance of psychotic symptoms, the current findings provide evidence that these relationships occur on a continuum that can be observed in healthy young adults. Previous studies have shown a link between PLEs in the general population and cognitive deficits, with one study revealing that worse verbal fluency skills relating to higher levels of PLEs (7), while data from the Philadelphia Neurodevelopmental Cohort revealed that psychosis-spectrum youths have a lower predicted age based on their cognitive ability (11). Additionally, healthy college students reporting PLEs have reduced functional connectivity of both the DMN and the CON with subcortical regions (e.g. thalamus, basal ganglia), and lower connectivity between the cerebellar network and the rAI was associated with higher positive symptom scores (12). Of note, neither this study in healthy individuals nor a previous study from our group with schizophrenia patients revealed robust differences in global efficiency between those with and without psychosis, limiting the strength of these findings in supporting the psychosis continuum model. The current study, however, is the first to demonstrate that efficiency of that CON mediates the association between PLEs and cognitive ability, identifying a potential mechanism underlying the psychosis continuum.
One important question that remains, however, is why the PLEs experienced by otherwise healthy individuals remain subclinical and do not appear to greatly impact functioning. Longitudinal research has shown that, of healthy individuals reporting PLEs at baseline, 8% reported persistent subclinical PLEs, 84% reported that their PLEs remitted, and 8% converted to a psychotic disorder (63). Although the cause for conversion to a psychotic disorder remains unknown, research has shown that transitory PLEs can become more persistent in the context of environmental risk factors, such as trauma (64), urbanicity (64, 65), and cannabis use (66), all of which have been previously identified as risk factors for the development of a psychotic disorders (67–69). Furthermore, there is a well-established genetic risk for psychotic symptoms (70), as evidenced by familial clustering of positive symptoms such as magical thinking and perceptual aberration (71, 72). Therefore, the psychosis-proneness continuum model suggests that a broadly distributed psychosis risk exists in the general population that, in some environments, leads individuals to experience only transitory PLEs, while in other environments leads to the expression of a more persistent psychosis phenotype that causes clinical distress (3). Given the forthcoming genotyping of these HCP subjects, future studies can analyze the contribution of genetic risk to the current mediation model and further explore this hypothesized psychosis continuum.
Conclusions
The current study reveals significant associations between psychotic-like experiences, cognitive ability, and the functional integrity of the cingulo-opercular network in individuals from the general population. Our findings reveal that the global efficiency of the CON, and possibly the participation coefficient of the right anterior insula, a hub within the CON, are partial mediators in the association between PLEs and cognitive ability, revealing that lower CON functional integration and lower rAI participation are associated with experiencing more PLEs and exhibiting worse higher-order cognitive ability. The identification of the CON as a potential mediator of PLEs and cognitive ability informs future research in both patients with psychotic disorders and healthy individuals, and helps to elucidate the neural underpinnings of complex psychotic disorders such as schizophrenia.
Supplementary Material
Acknowledgments
Data was collected as part of the Human Connectome Project (1U54MH091657-01).
Footnotes
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scott J, Chant D, Andrews G, McGrath J. Psychotic-like experiences in the general community: the correlates of CIDI psychosis screen items in an Australian sample. Psychological medicine. 2006;36:231–238. doi: 10.1017/S0033291705006392. [DOI] [PubMed] [Google Scholar]
- 2.Barnett JH, McDougall F, Xu MK, Croudace TJ, Richards M, Jones PB. Childhood cognitive function and adult psychopathology: associations with psychotic and non-psychotic symptoms in the general population. The British journal of psychiatry : the journal of mental science. 2012;201:124–130. doi: 10.1192/bjp.bp.111.102053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychological medicine. 2009;39:179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 4.van Os J, Hanssen M, Bijl RV, Ravelli A. Strauss (1969) revisited: a psychosis continuum in the general population? Schizophrenia research. 2000;45:11–20. doi: 10.1016/s0920-9964(99)00224-8. [DOI] [PubMed] [Google Scholar]
- 5.Krabbendam L, Myin-Germeys I, De Graaf R, Vollebergh W, Nolen WA, Iedema J, et al. Dimensions of depression, mania and psychosis in the general population. Psychological medicine. 2004;34:1177–1186. doi: 10.1017/s0033291703001892. [DOI] [PubMed] [Google Scholar]
- 6.Stefanis NC, Hanssen M, Smirnis NK, Avramopoulos DA, Evdokimidis IK, Stefanis CN, et al. Evidence that three dimensions of psychosis have a distribution in the general population. Psychological medicine. 2002;32:347–358. doi: 10.1017/s0033291701005141. [DOI] [PubMed] [Google Scholar]
- 7.Krabbendam L, Myin-Germeys I, Hanssen M, van Os J. Familial covariation of the subclinical psychosis phenotype and verbal fluency in the general population. Schizophrenia research. 2005;74:37–41. doi: 10.1016/j.schres.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Simons CJ, Jacobs N, Jolles J, van Os J, Krabbendam L. Subclinical psychotic experiences and cognitive functioning as a bivariate phenotype for genetic studies in the general population. Schizophrenia research. 2007;92:24–31. doi: 10.1016/j.schres.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychological bulletin. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 10.Khandaker GM, Barnett JH, White IR, Jones PB. A quantitative meta-analysis of population-based studies of premorbid intelligence and schizophrenia. Schizophrenia research. 2011;132:220–227. doi: 10.1016/j.schres.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gur RC, Calkins ME, Satterthwaite TD, Ruparel K, Bilker WB, Moore TM, et al. Neurocognitive growth charting in psychosis spectrum youths. JAMA psychiatry. 2014;71:366–374. doi: 10.1001/jamapsychiatry.2013.4190. [DOI] [PubMed] [Google Scholar]
- 12.Orr JM, Turner JA, Mittal VA. Widespread brain dysconnectivity associated with psychotic-like experiences in the general population. NeuroImage Clinical. 2014;4:343–351. doi: 10.1016/j.nicl.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shim G, Oh JS, Jung WH, Jang JH, Choi CH, Kim E, et al. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behavioral and brain functions : BBF. 2010;6:58. doi: 10.1186/1744-9081-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole MW, Repovs G, Anticevic A. The Frontoparietal Control System: A Central Role in Mental Health. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2014 doi: 10.1177/1073858414525995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satterthwaite TD, Vandekar SN, Wolf DH, Bassett DS, Ruparel K, Shehzad Z, et al. Connectome-wide network analysis of youth with Psychosis-Spectrum symptoms. Molecular psychiatry. 2015;20:1508–1515. doi: 10.1038/mp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA psychiatry. 2014;71:109–118. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Littow H, Huossa V, Karjalainen S, Jaaskelainen E, Haapea M, Miettunen J, et al. Aberrant Functional Connectivity in the Default Mode and Central Executive Networks in Subjects with Schizophrenia - A Whole-Brain Resting-State ICA Study. Frontiers in psychiatry. 2015;6:26. doi: 10.3389/fpsyt.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orliac F, Naveau M, Joliot M, Delcroix N, Razafimandimby A, Brazo P, et al. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophrenia research. 2013;148:74–80. doi: 10.1016/j.schres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biological psychiatry. 2011;69:967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unschuld PG, Buchholz AS, Varvaris M, van Zijl PC, Ross CA, Pekar JJ, et al. Prefrontal brain network connectivity indicates degree of both schizophrenia risk and cognitive dysfunction. Schizophrenia bulletin. 2014;40:653–664. doi: 10.1093/schbul/sbt077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manoliu A, Riedl V, Zherdin A, Muhlau M, Schwerthoffer D, Scherr M, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophrenia bulletin. 2014;40:428–437. doi: 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophrenia research. 2010;117:21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Drakesmith M, Caeyenberghs K, Dutt A, Zammit S, Evans CJ, Reichenberg A, et al. Schizophrenia-like topological changes in the structural connectome of individuals with subclinical psychotic experiences. Human brain mapping. 2015;36:2629–2643. doi: 10.1002/hbm.22796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su TW, Hsu TW, Lin YC, Lin CP. Schizophrenia symptoms and brain network efficiency: A resting-state fMRI study. Psychiatry research. 2015;234:208–218. doi: 10.1016/j.pscychresns.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Su TP, Zhou Y, Chou KH, Chen IY, Jiang T, et al. Anatomical insights into disrupted small-world networks in schizophrenia. NeuroImage. 2012;59:1085–1093. doi: 10.1016/j.neuroimage.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 29.van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, et al. Functional connectivity and brain networks in schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo CY, Su TW, Huang CC, Hung CC, Chen WL, Lan TH, et al. Randomization and resilience of brain functional networks as systems-level endophenotypes of schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:9123–9128. doi: 10.1073/pnas.1502052112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheffield JM, Repovs G, Harms MP, Carter CS, Gold JM, MacDonald AW, III, et al. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82–93. doi: 10.1016/j.neuropsychologia.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khadka S, Meda SA, Stevens MC, Glahn DC, Calhoun VD, Sweeney JA, et al. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biological psychiatry. 2013;74:458–466. doi: 10.1016/j.biopsych.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K. The WU-Minn Human Connectome Project: an overview. NeuroImage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TE, Bucholz R, et al. The Human Connectome Project: a data acquisition perspective. NeuroImage. 2012;62:2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, et al. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;25:766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 37.Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, et al. Function in the human connectome: task-fMRI and individual differences in behavior. NeuroImage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Achenbach TM. The Achenbach System of Empirically Based Assessment (ASEBA): Development, Findings, Theory and Applications. University of Vermont Research Center for Children, Youth and Families; Burlington, VT: 2009. [Google Scholar]
- 39.Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, et al. Resting-state fMRI in the Human Connectome Project. NeuroImage. 2013;80:144–168. doi: 10.1016/j.neuroimage.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, et al. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. NeuroImage. 2014;95:232–247. doi: 10.1016/j.neuroimage.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. NeuroImage. 2014;90:449–468. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb Cortex. 2016;26:288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior research methods, instruments, & computers : a journal of the Psychonomic Society, Inc. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 46.Ganjgahi H, Winkler AM, Glahn DC, Blangero J, Kochunov P, Nichols TE. Fast and powerful heritability inference for family-based neuroimaging studies. NeuroImage. 2015;115:256–268. doi: 10.1016/j.neuroimage.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calkins ME, Moore TM, Merikangas KR, Burstein M, Satterthwaite TD, Bilker WB, et al. The psychosis spectrum in a young U.S. community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13:296–305. doi: 10.1002/wps.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng XL, Rosenthal R, Rubin DB. Comparing Correlated Correlation-Coefficients. Psychological bulletin. 1992;111:172–175. [Google Scholar]
- 49.Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. NeuroImage. 2014;99:180–190. doi: 10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 50.Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79:814–828. doi: 10.1016/j.neuron.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in cognitive sciences. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moran LV, Tagamets MA, Sampath H, O’Donnell A, Stein EA, Kochunov P, et al. Disruption of anterior insula modulation of large-scale brain networks in schizophrenia. Biological psychiatry. 2013;74:467–474. doi: 10.1016/j.biopsych.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. Journal of psychiatry & neuroscience : JPN. 2012;37:17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Reality distortion is related to the structure of the salience network in schizophrenia. Psychological medicine. 2011;41:1701–1708. doi: 10.1017/S0033291710002205. [DOI] [PubMed] [Google Scholar]
- 56.Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis--linking biology, pharmacology and phenomenology of psychosis. Schizophrenia research. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. The American journal of psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 58.Freeman D, Garety PA, Kuipers E, Fowler D, Bebbington PE. A cognitive model of persecutory delusions. The British journal of clinical psychology/the British Psychological Society. 2002;41:331–347. doi: 10.1348/014466502760387461. [DOI] [PubMed] [Google Scholar]
- 59.Sommer IE, Diederen KM, Blom JD, Willems A, Kushan L, Slotema K, et al. Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain : a journal of neurology. 2008;131:3169–3177. doi: 10.1093/brain/awn251. [DOI] [PubMed] [Google Scholar]
- 60.Jacobson S, Kelleher I, Harley M, Murtagh A, Clarke M, Blanchard M, et al. Structural and functional brain correlates of subclinical psychotic symptoms in 11–13 year old schoolchildren. NeuroImage. 2010;49:1875–1885. doi: 10.1016/j.neuroimage.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 61.Smieskova R, Roiser JP, Chaddock CA, Schmidt A, Harrisberger F, Bendfeldt K, et al. Modulation of motivational salience processing during the early stages of psychosis. Schizophrenia research. 2015;166:17–23. doi: 10.1016/j.schres.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Yan C, Yin DZ, Fan MX, Cheung EF, Pantelis C, et al. Neurobiological changes of schizotypy: evidence from both volume-based morphometric analysis and resting-state functional connectivity. Schizophrenia bulletin. 2015;41(Suppl 2):S444–454. doi: 10.1093/schbul/sbu178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanssen M, Bak M, Bijl R, Vollebergh W, van Os J. The incidence and outcome of subclinical psychotic experiences in the general population. The British journal of clinical psychology/the British Psychological Society. 2005;44:181–191. doi: 10.1348/014466505X29611. [DOI] [PubMed] [Google Scholar]
- 64.Spauwen J, Krabbendam L, Lieb R, Wittchen HU, van Os J. Evidence that the outcome of developmental expression of psychosis is worse for adolescents growing up in an urban environment. Psychological medicine. 2006;36:407–415. doi: 10.1017/S0033291705006902. [DOI] [PubMed] [Google Scholar]
- 65.Spauwen J, Krabbendam L, Lieb R, Wittchen HU, van Os J. Does urbanicity shift the population expression of psychosis? Journal of psychiatric research. 2004;38:613–618. doi: 10.1016/j.jpsychires.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophrenia bulletin. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- 67.Pedersen CB, Mortensen PB. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Archives of general psychiatry. 2001;58:1039–1046. doi: 10.1001/archpsyc.58.11.1039. [DOI] [PubMed] [Google Scholar]
- 68.Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophrenia bulletin. 2012;38:661–671. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 70.Cardno AG, Marshall EJ, Coid B, Macdonald AM, Ribchester TR, Davies NJ, et al. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Archives of general psychiatry. 1999;56:162–168. doi: 10.1001/archpsyc.56.2.162. [DOI] [PubMed] [Google Scholar]
- 71.Linney YM, Murray RM, Peters ER, MacDonald AM, Rijsdijk F, Sham PC. A quantitative genetic analysis of schizotypal personality traits. Psychological medicine. 2003;33:803–816. doi: 10.1017/s0033291703007906. [DOI] [PubMed] [Google Scholar]
- 72.MacDonald AW, 3rd, Pogue-Geile MF, Debski TT, Manuck S. Genetic and environmental influences on schizotypy: a community-based twin study. Schizophrenia bulletin. 2001;27:47–58. doi: 10.1093/oxfordjournals.schbul.a006859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.