Abstract
Aim: Patients with severe aortic stenosis (AS) may have bleeding episodes due to the loss of high-molecular-weight (HMW) von Willebrand factor multimers (VWFMs). The absence of HMW-VWFMs and bleeding tendency are usually corrected after aortic valve replacement (AVR). To investigate the process of VWFM recovery and symptoms in patients with severe AS, we analyzed changes in VWF antigen (VWF:Ag), ADAMTS13 activity (ADAMTS13:AC), and platelet thrombus formation under high shear stress conditions.
Methods: Nine patients with severe AS undergoing AVR were analyzed.
Results: Evident deficiency of HMW-VWFMs was observed in six patients before surgery, which was rapidly restored within 8 days after AVR. Median levels of VWF:Ag before surgery, on postoperative days (PODs) 1, 8, 15, and 22, and one year after AVR were 78.1%, 130%, 224%, 155%, 134%, and 142%, respectively. In contrast, ADAMTS13:AC was 50.5%, 35.5%, 25.5%, 25.1%, 30.3%, and 84.6%, respectively. Preoperative thrombus formation but not surface coverage was significantly lower than that on POD 22, which was considered as normal level in each patient. Compared with preoperative levels, thrombus volume was significantly lower on POD 1, but rapidly increased by POD 8.
Conclusion: Bleeding tendency and loss of HMW-VWFMs observed in patients with severe AS before surgery was rapidly corrected after AVR. Instead, patients were in a VWF-predominant state between POD 8 and 22.
Keywords: Aortic valve stenosis, Acquired von Willebrand syndrome, Thrombus formation, ADAMTS13
See editorial vol. 23: 1141–1143
Introduction
In 1958, Heyde described patients with calcific aortic valve stenosis (AS) who had massive gastrointestinal bleeding with no identifiable cause1). Twenty-eight years later, gastrointestinal bleeding observed in patients with AS was attributed to submucosal angiodysplasia2). Subsequently, King et al. reported an association between unexplained gastrointestinal bleeding and AS that resolved after aortic valve replacement (AVR)3). This unique bleeding syndrome, called Heyde's syndrome, is identified as acquired type 2A von Willebrand syndrome (AVWS). It is characterized by the loss of high-molecular-weight (HMW) von Willebrand factor multimers (VWFMs)4–6). This type of AVWS was also found in patients treated with ventricular assist devices7) and extracorporeal membrane oxygenation therapy8).
VWF is a multimeric glycoprotein that binds platelets at the site of vascular injury and promotes perplatelet–platelet interactions9). HMW-VWFMs are particularly important for hemostasis under high shear stress conditions. In addition, high shear stress induces structural changes in VWF and exposes the peptide bond between 1605 and 1606 of the A2 domain in VWF, the cleavage site of ADAMTS13 (a disintegrin-like and metalloproteinase with thrombospondin type 1 motifs 13)10). Consequently, plasma VWF contributes to two types of emergencies, bleeding and thrombosis11). A recent study showed that lower plasma levels of ADAMTS13 are associated with arterial thrombosis12). Therefore, the ratio of VWF to ADAMTS13 is sometimes used to evaluate the risk of thrombosis13).
In patients with AS, a significant negative correlation was observed in vivo between higher shear stress and loss of HMW-VWFMs5). Further, the absence of HMW-VWFMs and bleeding tendency are usually corrected by valve replacement5). These results suggest that high shear stress arising from passage through a narrowed aortic valve enhances proteolysis of VWF by ADAMTS13 and induces the loss of HMW-VWFMs. However, the process by which platelet thrombus formation is restored and HMW-VWFM recovery occurs after AVR in patients with severe AS has not been fully elucidated.
We investigated plasma levels of VWF antigen (VWF:Ag) with VWFM analysis, plasma levels of ADAMTS13 activity (ADAMTS13:AC), and platelet thrombus formation using a flow chamber system in patients with severe AS before and after valve replacement.
Materials and Methods
Patients
Nine patients who underwent AVR for isolated severe AS between January 2012 and December 2013 at Nara Medical University Hospital were included in this study. Exclusion criteria included acute infection, autoimmune disorder, malignancy, hemodialysis, and more than mild aortic valve regurgitation. Patients undergoing procedures such as coronary artery bypass grafting, mitral or tricuspid valve surgery, or aortic surgery in combination with AVR were also excluded.
AS severity was assessed according to the European Association of Echocardiography/American Society of Echocardiography guidelines14). Briefly, severe AS was defined as an aortic valve area (AVA) > 1.0/cm2 or a mean pressure gradient greater than 40 mmHg by Doppler echocardiography. None of the patients had a history of bleeding or received valve replacements with mechanical or bioprosthetic valves followed by administration of warfarin, anti-platelet agents, or both for prevention of thrombosis. Blood samples were collected from these patients before AVR and on postoperative days (PODs) 1, 8, 15, and 22 and one year after valve replacement. Plasma levels of VWF:Ag and ADAMTS13:AC, VWFMs, and mural thrombus formation were measured using a flow chamber system. The ethics committee of Nara Medical University approved the study and written informed consent was obtained from all patients.
Echocardiographic Evaluation
Using an iE33 xMATRIX Echocardiography System (Philips Healthcare, Andover, MA, USA) or a Vivid E9 Ultrasound System (GE Healthcare, PA, USA), investigators assessed the preoperative hemodynamic performance of the aortic valve with transthoracic echocardiography. The mean and peak transvalvular pressure gradients were calculated with the modified Bernoulli equation, and the effective orifice area (EOA) was calculated using the continuity equation. Postoperative echocardiography was performed one month and one year after AVR. A size mismatch between the patient and the prosthesis was defined as an indexed EOA of < 0.85 cm2 per square meter of body surface area (BSA).
VWF and ADAMTS13
Plasma levels of VWF:Ag were measured by sandwich enzyme-linked immunosorbent assay (ELISA) using rabbit anti-human VWF polyclonal antiserum (DAKO, Glostrup, Denmark)15). The value obtained from normal individuals (n = 20) using this assay was 102 ± 33%. Plasma ADAMTS13:AC was determined using a commercially available chromogenic act-ELISA kit (Kainos Laboratories, Tokyo, Japan)16). The value obtained for normal individuals (n = 55) using act-ELISA was 99 ± 22%. The value of 100% was defined as the level of VWF:Ag and ADAMTS13:AC in pooled normal human plasma (NP). VWFM analysis was performed according to the method by Ruggeri and Zimmerman17) with modifications described by Warren et al.18). The experimental conditions, including western blotting with luminographic detection, were as previously described by Budde et al.19). Multimers were classified as HMW-VWFMs if there were > 10 bands in the VWFM analysis20). HMW bands that were not detected in NP were defined as unusually large (UL) VWFMs.
Platelet Thrombus Formation
Platelet thrombus formation was evaluated by thrombus generation under high shear stress conditions in a parallel plate flow chamber system21, 22). Briefly, whole blood anticoagulated with argatroban was incubated with the fluorescent dye DiOC6 (1 µM), and samples containing DiOC6-labeled platelets were perfused over a type I collagen-coated glass surface under a high shear rate (1500 s−1) for 7 min. DiOC6 fluorescence corresponding to the platelets was examined at an excitation wavelength of 488 nm with a barrier filter at 500 nm. The area covered by adherent platelets (surface coverage) and the volume of each thrombus at 7 min after perfusion were evaluated using confocal laser scanning microscopy (CSLM; FV300; Olympus, Tokyo, Japan). Each flow chamber experiment was performed three times at each time point, and five areas (211 × 317 mm each) randomly selected from each perfusion trial performed with whole blood from a patient with AS were evaluated.
Statistical Analysis
All continuous valuables were expressed as medians (ranges). The Mann -Whitney U test was used to determine significant differences (P < 0.05) between groups. The Wilcoxon signed-rank test was used to compare values from different time points in each group. Discrete variables were compared using Fisher's exact test. Correlations between variables were assessed using Spearman's rank correlation test. Statistical analysis was performed using EZR on the R commander23).
Results
Patient Characteristics
Characteristics of the nine patients are shown in Table 1. Patient age was relatively high (median, 74 years). As shown in Table 2, routine laboratory values were almost normal. Note that severe anemia with hemoglobin < 10 g/dL was not observed in these patients. As there was no apparent gastrointestinal bleeding, Heyde's syndrome could not be diagnosed. However, the aortic valve area was < 1.0/cm2 in all patients, consistent with the criteria for severe AS.
Table 1. Patient characteristics (n = 9).
| median (range) | |
|---|---|
| Age (years) | 74 (69–78) |
| Sex (female/male) | 5/4 |
| BSA (m2) | 1.6 (1.3–1.8) |
| Underlying diseases | patient number |
| Hypertension | 8 |
| Diabetes mellitus | 3 |
| Dyslipidemia | 6 |
| Medication | |
| ACE-I/ARB | 6 |
| CCB | 4 |
| β-blocker | 3 |
| Statin | 6 |
BSA: body surface area
ACE-I: angiotensin-converting enzyme inhibitor
ARB: angiotensin receptor blocker
CCB: calcium channel blocker
Table 2. Laboratory data before aoritic valve replacement (n = 9).
| Median (range) | |
|---|---|
| Hematological examination | |
| Red blood cell count (1010/L) | 397 (325–428) |
| Hematocrit (%) | 38 (31–41) |
| Hemoglobin (g/dL) | 12.7 (10.4–14) |
| Platelet counts (109/L) | 168 (101–206) |
| PT-INR | 1.0 (1.0–1.1) |
| APTT (second) | 28 (26–35) |
| Fibrinogen (mg/dL) | 290 (192–525) |
| Bleeding Time (minute) | 2 (2–3.5) |
| Blood chemistry test | |
| Total bilirubin (mg/dL) | 0.8 (0.5–1.6) |
| Lactase dehydrogenase (LDH, U/L) | 216 (150–364) |
| Blood urea nitrogen (BUN, mg/dL) | 20 (15–32) |
| Creatinine (CRE, mg/dL) | 0.9 (0.5–1.2) |
| Albumin (Alb, g/dL) | 4.3 (3.6–4.8) |
| Aspartate transaminase (AST, U/L) | 21 (15–71) |
| Alanine transaminase (ALT, U/L) | 23 (7–97) |
| Echocardiographic data | |
| Aortic valve area (cm2) | 0.6 (0.3–0.8) |
PT-INR: prothrobin time- international normalized ratio
APTT: activated partial thromboplastin time
Surgical Treatment
One patient received a mechanical valve (On-X Prosthetic Aortic Heart Valve, On-X Life Technologies, Austin, TX, USA), and the other eight patients received bioprosthetic valves (Perimount, Carpentier-Edwards, Irvine, CA, USA). The median blood loss during surgery and the first 24 postoperative hours was 998 mL (range, 170 – 2430 mL) and 744 mL (range, 380 – 1605 mL), respectively. No patient underwent mediastinal re-exploration for bleeding after surgery. All patients had an uneventful postoperative course. As shown in Table 3, the median peak aortic transvalvular pressure gradient was 98 mmHg before surgery, which improved dramatically except in Patient 9, who had a patient–prosthesis mismatch. In six patients, the mean preoperative transvalvular pressure gradient was over 40 mmHg, which is one of the diagnostic criteria for severe AS. The remaining three patients (Patients 5, 8, and 9) had a mean preoperative transvalvular gradient of ≤ 40 mmHg. These values quickly decreased to > 20 mmHg, except in Patient 9, who had a patient–prosthesis mismatch. During the first year of follow-up after surgery, all patients, including the one with the mechanical prosthesis requiring anticoagulation, were asymptomatic. The indexed EOA for Patient 9 at one year was 0.5 cm2 per square meter of BSA.
Table 3. Changes of transthoracic echocardiographic parameters.
| Patient No | ABO blood type | Peak transvalvular gradient (mmHg) |
Mean transvalvular gradient (mmHg) |
||||
|---|---|---|---|---|---|---|---|
| Pre-operation | One month after operation | One year after operation | Pre-operation | One month after operation | One year after operation | ||
| 1 | A | 98 | 11 | 12 | 54 | 5 | 6 |
| 2 | O | 100 | 15 | 26 | 54 | 7 | 11 |
| 3 | AB | 119 | 35 | 26 | 69 | 19 | 13 |
| 4 | A | 132 | 25 | 24 | 72 | 12 | 11 |
| 5 | A | 78 | 18 | 32 | 40 | 8 | 18 |
| 6 | AB | 117 | 19 | 19 | 64 | 9 | 9 |
| 7 | A | 79 | 21 | 20 | 46 | 10 | 9 |
| 8 | A | 54 | 22 | 25 | 32 | 12 | 12 |
| 9 | B | 62 | 51 | 52 | 40 | 25 | 26 |
| Median | 98 | 21 | 25 | 54 | 10 | 11 | |
| Maximum | 132 | 51 | 52 | 72 | 25 | 26 | |
| Minimum | 54 | 11 | 12 | 32 | 5 | 6 | |
Changes in Platelet Count and Plasma Levels of VWF:Ag and ADAMTS13:AC
As shown in Fig. 1, the median platelet count dramatically decreased from 168 × 109/µL before surgery to 72 × 109/µL on POD 1 and gradually improved after POD 8. The median plasma level of VWF:Ag before surgery was 78.1%, which was lower than that in normal individuals. Plasma levels of VWF:Ag on POD 1, 8, 15, and 22 and one year after AVR were 130%, 224%, 155%, 134%, and 142%, respectively, which were significantly higher than preoperative levels. In contrast, median plasma ADAMTS13:AC before surgery was 50.5%, which was also relatively low (Fig. 1). Plasma ADAMTS13:AC on POD 1, 8, 15, and 22 and one year after surgery was 35.5%, 25.5%, 25.1%, 30.3%, and 84.6%, respectively. Plasma levels of ADAMTS13:AC gradually decreased until POD 15, whereas they increased to within the normal range (median, 84.6%) one year after AVR, which was significantly higher than values on POD 1, 8, 15, and 22. The ratios of VWF:Ag to ADAMTS13:AC before surgery, on POD 1, 8, 15, and 22, and one year after surgery were 1.6, 4.5, 8.1, 6.1, 4.1, and 1.64, respectively. On POD 8 and 15, the ratio was significantly higher than that before surgery and one year after AVR. Therefore, patients were assumed to be in a VWF-predominant state between POD 8 and 22, instead of having bleeding tendency before AVR.
Fig. 1.
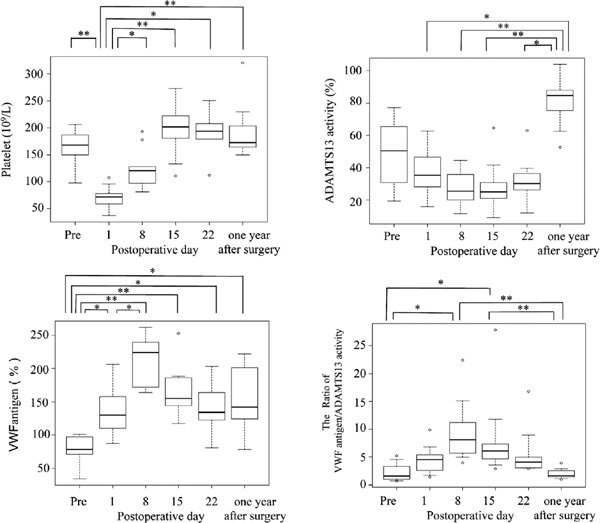
Sequential changes in platelet count, plasma levels of von Willebrand factor (VWF) antigen, and ADAMTS13:AC before and after valve replacement
Median platelet count dramatically decreased from before surgery to postoperative day (POD) 1, and gradually increased after POD 8. Median plasma levels of VWF:Ag before surgery were relatively low compared with those in normal individuals. Levels on POD 1, 8, 15, and 22 and one year after surgery were significantly higher than preoperative levels (P > 0.05). Plasma levels of ADAMTS13:AC before surgery were relatively low, and levels on POD 1, 8, 15, and 22 were clearly lower than those one year after aortic valve replacement. The ratios of VWF:Ag to ADAMTS13:AC dramatically increased between POD 8 and 22. *P < 0.05, **P < 0.01
VWFM Analysis
As shown in Fig. 2, we analyzed VWFMs in plasma samples before surgery, on POD 1, 8, 15, and 22, and one year after surgery. Of nine patients with severe AS, six had an evident deficiency of HMW-VWFMs before surgery. The remaining three patients (Patients 5, 8, and 9) had slightly decreased levels of HMW-VWFMs before surgery. These three patients had relatively mild findings of echocardiographic parameters (Table 3). In all nine patients, levels of HMW-VWFMs increased after surgery, and UL-VWFMs were detectable by POD 8 or 15. These results indicate that patients with severe AS had bleeding tendency before surgery, but the risk of thrombosis increased dramatically after surgery on POD 8 or 15. We found an evident deficiency of HMW-VWFMs in the patient with prosthesis size mismatch one year after AVR (Patient 9). The remaining eight patients did not have HMW-VWFM deficiency one year after surgery.
Fig. 2.
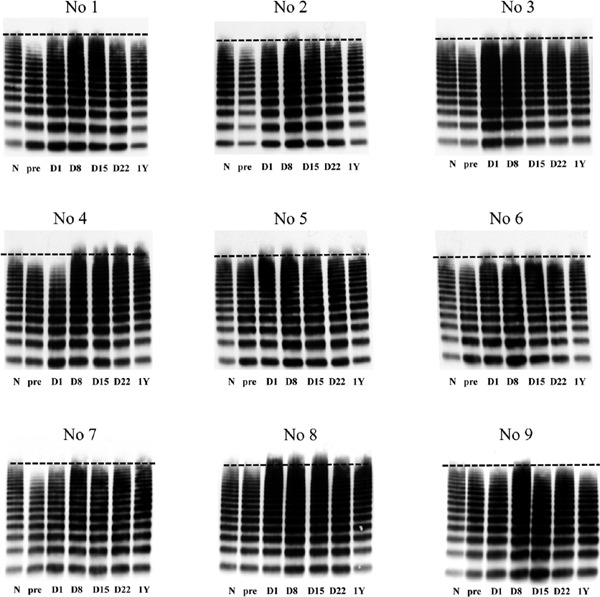
von Willebrand factor multimer (VWFM) analysis
We performed VWFM analysis using plasma collected before surgery (pre), on postoperative days (D) 1, 8, 15, and 22, and one year (1Y) after aortic valve replacement. N indicates normal plasma. Of nine patients with severe aortic stenosis, six had evident deficiency of HMW-VWFMs before surgery. The remaining three (Patients 5, 8, and 9) had slightly decreased levels of HMW-VWFMs before surgery. In all patients, UL-VWFMs were detectable on D 8 or 15.
Thrombosis Formation Under High Shear Stress Conditions
We analyzed thrombosis formation under a high shear rate (1500 s−1) at five time points: before surgery and on POD 1, 8, 15, and 22 using a flow chamber system. Three-dimensional images of mural thrombus generation on a collagen surface in Patient 4 are shown in Fig. 3 as a representative case. Thrombus formation dramatically decreased on POD 1, compared with that before surgery, due to a decrease in platelet count associated with the effects of surgery, such as the use of an extracorporeal circulation device during surgery or dilution of blood by large transfused volumes. However, thrombus formation recovered by POD 8.
Fig. 3.
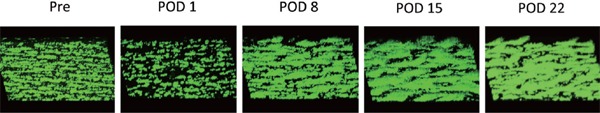
Three-dimensional images of mural thrombus generation on a collagen surface
Representative images from Patient 4 are shown. Less thrombus formation occurred on postoperative day (POD) 1 compared with that prior to surgery. However, the ability to form thrombi was quickly recovered by POD 8 and increased up to POD 22 after aortic valve replacement.
A quantitative analysis of surface coverage and thrombus volume at 7 min after perfusion is shown in Fig. 4. Surface coverage on POD 1 was significantly lower compared with that before surgery (P > 0.05). Compared with POD 1, surface coverage was significantly higher (P > 0.05) on POD 8, 15, and 22. However, preoperative values were the same as those on POD 22. With regard to platelet thrombus formation, thrombus volume was significantly lower on POD 1 and higher on POD 8, 15, and 22 compared with that before AVR (P < 0.05, Fig. 4). These results indicate that surface coverage before surgery was not significantly lower, but thrombus volume before surgery was significantly lower than that on POD 22, which is considered normal in each patient.
Fig. 4.
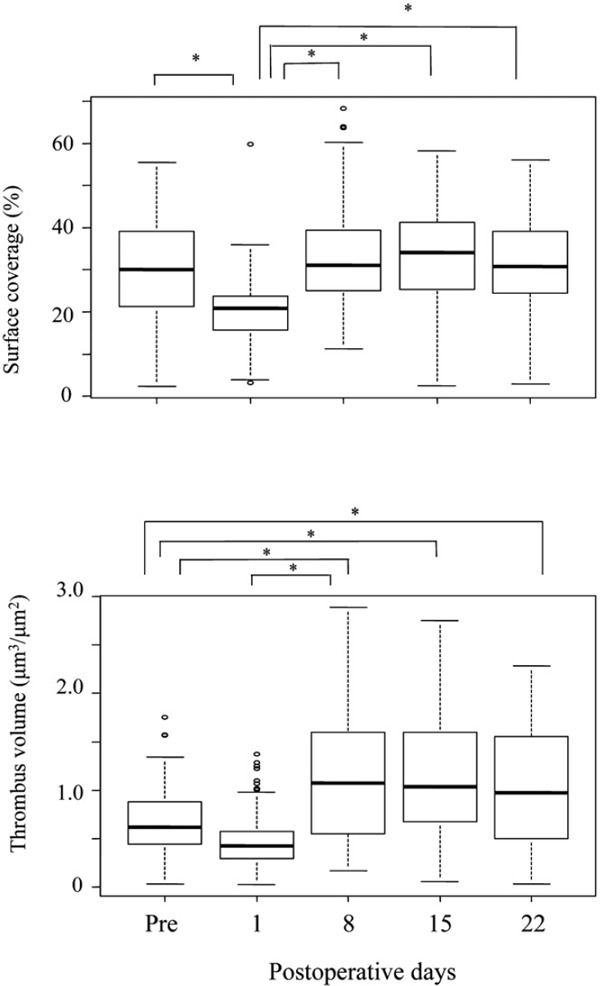
Changes in percentage of surface coverage and thrombus volume before and after valve replacement
A quantitative analysis of surface coverage and volume of thrombus formation at 7 min after perfusion is shown. Surface coverage was significantly lower on postoperative day (POD) 1 compared with that prior to surgery, but increased significantly on POD 8, 15, and 22. However, preoperative values were similar to those on POD 22, which were considered the normal level for each patient. Compared with preoperative values, thrombus volume was significantly lower on POD 1 and higher on POD 8, 15, and 22. Preoperative thrombus volume but not surface coverage was significantly lower compared with POD 22 reference levels. *P < 0.05
Discussion
Recent studies have reported that patients with severe AS have impaired platelet function under high shear stress conditions24, 25). These hemostatic abnormalities are completely corrected by AVR24, 25). However, the correction process has not been fully elucidated. Therefore, we performed serial assessments of mural thrombus formation along with an analysis of VWFMs and plasma levels of VWF:Ag and ADAMTS13:AC before and after AVR in patients with isolated severe AS.
We found that thrombus volume, one indicator of platelet aggregation ex vivo, was reduced in patients with severe AS before AVR. In contrast, the platelet adhesion area was not significantly lower before surgery. Mural thrombus formation at the site of vascular injury contributes to the arrest of bleeding. During the initial stages of thrombus formation, platelet adhesion to denuded vessel walls is initiated by VWF binding to platelet glycoprotein (GP) Ib/IX/V complexes under high shear stress conditions26). This is followed by platelet activation and aggregation, which require an association between GPIIb/IIIa and VWF27, 28). In patients with AS, gastrointestinal bleeding events have been rarely reported, despite a high frequency of HMW-VWFM loss7). After AVR, we found that both surface coverage and thrombus volume were remarkably reduced on POD 1 as a result of the artificial effects of AVR. Low platelet count, a consequence of the use of an extracorporeal circulation device during surgery, and dilution of blood by large transfused volumes during surgery likely contributed to reductions in platelet count. After POD 8, the surface coverage area was almost the same as that before surgery. However, thrombus volume before surgery was significantly lower than baseline values after POD 22. These results suggest that it is platelet aggregation that is mostly impaired in patients with severe AS. Panzer et al.24) also reported that levels of HMW-VWFMs were diminished, and that the loss of HMW-VWFMs affected platelet aggregation more than platelet adhesion in patients with AS, using the cone and plate analyzer Impact-R. They speculated that low-to-intermediate molecular weight VWFMs might be sufficient for platelet adhesion, but HMW-VWFMs are required for platelet aggregation.
Deficiencies in HMW-VWFMs were observed in six of nine patients with AS before surgery. The remaining three patients did not have any evident lack of HMW-VWFMs. Both peak and mean transvalvular gradients of these three patients were the lowest despite an AVA of < 1.0/cm2, which meets the criteria for severe AS. These findings are consistent with the results reported by Vincentelli et al.5) The percentage of HMWM-VWF is inversely correlated with mean transvalvular gradient in patients with severe AS. In six patients, HMW-VWFM deficiency was completely resolved after POD 8. Moreover, all nine patients had UL-VWFMs on POD 8 or 15. Some patients continued to have UL-VWFMs from POD 15 to one year after AVR. One patient (Patient 9) did not have HMW-VWFMs one year after surgery due to a patient–prosthesis mismatch.
Plasma levels of VWF:Ag gradually increased until POD 8 and then gradually decreased. VWF:Ag levels were within the normal range one year after AVR. In contrast, plasma levels of ADAMTS13:AC were relatively low before surgery and gradually decreased until POD 15. At one year after AVR, plasma levels of ADAMTS13:AC increased to the normal range. These results indicate that ADAMTS13:AC was low due to the consumption in cleaving VWF during the preoperative and perioperative periods. To evaluate levels of both VWF and ADAMTS13 together, the ratio of VWF to ADAMTS13 has been used in systemic inflammation29) and liver disease13). We previously reported that a ratio of VWF:Ag to ADAMTS13:AC above 5 might be a risk factor for thromboembolic events in patients after hematopoietic stem cell transplantation30). In this study, the ratio of VWF:Ag to ADAMTS13:AC before surgery was 1.56. It dramatically increased to 9.5 on POD 8 and decreased to 5.4 on POD 22. Finally, it decreased to 1.64 at one year after AVR. Patients were in a VWF-predominant state between POD 8 and 22, and we may pay attention to the thrombosis rather than bleeding even in the early stage after surgery.
There are some limitations to this study. First, the number of patients analyzed in this study was relatively small in a single hospital. Therefore, potential bias might exist and the impact of this study could be limited. However, it is very difficult to perform the VWFM analyses and thrombosis formation in a larger scale of patients because VWFM analysis is a time-consuming examination and platelet thrombus formation should be performed within 3 h of collecting blood. Second, blood samples from patients were collected every 7 days in the perioperative period. Since the factors analyzed in this study such as VWF and ADAMTS13 seemed to change over a short period of time, we might miss the correct sequence of change. Finally, VWF activity was not analyzed in this study. The adhesive activity of VWF depends on its molecular size. Therefore, we could speculate the VWF activity using the result of VWFM analysis.
In conclusion, we found less platelet thrombus formation in patients with severe AS before surgery, but the ability to form platelet thrombi, along with levels of HMW-VWFMs, were rapidly restored by POD 8. We should pay attention to thrombotic events rather than bleeding between the second and third weeks after AVR in patients with severe AS.
Funding Sources
This study was supported in part by research grants from the Ministry of Health, Labour and Welfare of Japan; the Ministry of Education, Culture, Sports, Science and Technology of Japan; and the Takeda Science Foundation.
Conflicts of Interest
MM is on a clinical advisory board for Baxalta.
References
- 1). Heyde EC: Gastrointestinal bleeding in aortic stenosis. N Engl J Med, 1958; 259: 196 [Google Scholar]
- 2). Greenstein RJ, McElhinney AJ, Reuben D, Greenstein AJ: Colonic vascular ectasias and aortic stenosis: coincidence or causal relationship? Am J Surg, 1986; 151: 347-351 [DOI] [PubMed] [Google Scholar]
- 3). King RM, Pluth JR, Giuliani ER: The association of unexplained gastrointestinal bleeding with calcific aortic stenosis. Ann Thorac Surg, 1987; 44: 514-516 [DOI] [PubMed] [Google Scholar]
- 4). Warkentin TE, Moore JC, Morgan DG: Aortic stenosis and bleeding gastrointestinal angiodysplasia: is acquired von Willebrand's disease the link? Lancet, 1992; 340: 35-37 [DOI] [PubMed] [Google Scholar]
- 5). Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F, Bauters A, Decoene C, Goudemand J, Prat A, Jude B: Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med, 2003; 349: 343-349 [DOI] [PubMed] [Google Scholar]
- 6). Tamura T, Horiuchi H, Imai M, Tada T, Shiomi H, Kuroda M, Nishimura S, Takahashi Y, Yoshikawa Y, Tsujimura A, Amano M, Hayama Y, Imamura S, Onishi N, Tamaki Y, Enomoto S, Miyake M, Kondo H, Kaitani K, Izumi C, Kimura T, Nakagawa Y: Unexpectedly High Prevalence of Acquired von Willebrand Syndrome in Patients with Severe Aortic Stenosis as Evaluated with a Novel Large Multimer Index. J Atheroscler Thromb, 2015; 22: 1115-1123 [DOI] [PubMed] [Google Scholar]
- 7). Sucker C, Feindt P, Scharf RE: Aortic stenosis, von Willebrand factor, and bleeding. N Engl J Med, 2003; 349: 1773-1774; author reply 1773–1774 [DOI] [PubMed] [Google Scholar]
- 8). Kalbhenn J, Schmidt R, Nakamura L, Schelling J, Rosenfelder S, Zieger B: Early diagnosis of acquired von Willebrand Syndrome (AVWS) is elementary for clinical practice in patients treated with ECMO therapy. J Atheroscler Thromb, 2015; 22: 265-271 [DOI] [PubMed] [Google Scholar]
- 9). Shiozaki S, Takagi S, Goto S: Prediction of Molecular Interaction between Platelet Glycoprotein Ibalpha and von Willebrand Factor using Molecular Dynamics Simulations. J Atheroscler Thromb, 2015: [DOI] [PubMed] [Google Scholar]
- 10). Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K: Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem, 2001; 276: 41059-41063 [DOI] [PubMed] [Google Scholar]
- 11). Sadler JE: von Willebrand factor: two sides of a coin. J Thromb Haemost, 2005; 3: 1702-1709 [DOI] [PubMed] [Google Scholar]
- 12). Bongers TN, de Bruijne EL, Dippel DW, de Jong AJ, Deckers JW, Poldermans D, de Maat MP, Leebeek FW: Lower levels of ADAMTS13 are associated with cardiovascular disease in young patients. Atherosclerosis, 2009; 207: 250-254 [DOI] [PubMed] [Google Scholar]
- 13). Hugenholtz GC, Adelmeijer J, Meijers JC, Porte RJ, Stravitz RT, Lisman T: An unbalance between von Willebrand factor and ADAMTS13 in acute liver failure: implications for hemostasis and clinical outcome. Hepatology, 2013; 58: 752-761 [DOI] [PubMed] [Google Scholar]
- 14). Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M. American Society of E and European Association of E: Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr, 2009; 22: 1-23; quiz 101–102 [DOI] [PubMed] [Google Scholar]
- 15). Matsumoto M, Kawaguchi S, Ishizashi H, Yagi H, Iida J, Sakaki T, Fujimura Y: Platelets treated with ticlopidine are less reactive to unusually large von Willebrand factor multimers than are those treated with aspirin under high shear stress. Pathophysiology of haemostasis and thrombosis, 2005; 34: 35-40 [DOI] [PubMed] [Google Scholar]
- 16). Kato S, Matsumoto M, Matsuyama T, Isonishi A, Hiura H, Fujimura Y: Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion, 2006; 46: 1444-1452 [DOI] [PubMed] [Google Scholar]
- 17). Ruggeri ZM, Zimmerman TS: Variant von Willebrand's disease: characterization of two subtypes by analysis of multimeric composition of factor VIII/von Willebrand factor in plasma and platelets. The Journal of clinical investigation, 1980; 65: 1318-1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Warren CM, Krzesinski PR, Greaser ML: Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis, 2003; 24: 1695-1702 [DOI] [PubMed] [Google Scholar]
- 19). Budde U, Schneppenheim R, Plendl H, Dent J, Ruggeri ZM, Zimmerman TS: Luminographic detection of von Willebrand factor multimers in agarose gels and on nitrocellulose membranes. Thromb Haemost, 1990; 63: 312-315 [PubMed] [Google Scholar]
- 20). Budde U, Drewke E, Mainusch K, Schneppenheim R: Laboratory diagnosis of congenital von Willebrand disease. Semin Thromb Hemost, 2002; 28: 173-190 [DOI] [PubMed] [Google Scholar]
- 21). Tsuji S, Sugimoto M, Miyata S, Kuwahara M, Kinoshita S, Yoshioka A: Real-time analysis of mural thrombus formation in various platelet aggregation disorders: distinct shear-dependent roles of platelet receptors and adhesive proteins under flow. Blood, 1999; 94: 968-975 [PubMed] [Google Scholar]
- 22). Shida Y, Nishio K, Sugimoto M, Mizuno T, Hamada M, Kato S, Matsumoto M, Okuchi K, Fujimura Y, Yoshioka A: Functional imaging of shear-dependent activity of ADAMTS13 in regulating mural thrombus growth under whole blood flow conditions. Blood, 2008; 111: 1295-1298 [DOI] [PubMed] [Google Scholar]
- 23). Kanda Y: Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant, 2013; 48: 452-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Panzer S, Badr Eslam R, Schneller A, Kaider A, Koren D, Eichelberger B, Rosenhek R, Budde U, Lang IM: Loss of high-molecular-weight von Willebrand factor multimers mainly affects platelet aggregation in patients with aortic stenosis. Thromb Haemost, 2010; 103: 408-414 [DOI] [PubMed] [Google Scholar]
- 25). Takahashi N, Tanabe K, Yoshitomi H, Adachi T, Ito S, Sugamori T, Endo A, Ishibashi Y, Oda T: Impairment of platelet retention rate in patients with severe aortic valve stenosis. J Cardiol, 2013; 62: 171-175 [DOI] [PubMed] [Google Scholar]
- 26). Tobimatsu H, Nishibuchi Y, Sudo R, Goto S, Tanishita K: Adhesive Forces between A1 Domain of von Willebrand Factor and N-terminus Domain of Glycoprotein Ibalpha Measured by Atomic Force Microscopy. J Atheroscler Thromb, 2015; 22: 1091-1099 [DOI] [PubMed] [Google Scholar]
- 27). Matsui H, Sugimoto M, Mizuno T, Tsuji S, Miyata S, Matsuda M, Yoshioka A: Distinct and concerted functions of von Willebrand factor and fibrinogen in mural thrombus growth under high shear flow. Blood, 2002; 100: 3604-3610 [DOI] [PubMed] [Google Scholar]
- 28). Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ: Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood, 2006; 108: 1903-1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Claus RA, Bockmeyer CL, Sossdorf M, Losche W: The balance between von-Willebrand factor and its cleaving protease ADAMTS13: biomarker in systemic inflammation and development of organ failure? Curr Mol Med, 2010; 10: 236-248 [DOI] [PubMed] [Google Scholar]
- 30). Matsumoto M, Kawa K, Uemura M, Kato S, Ishizashi H, Isonishi A, Yagi H, Park YD, Takeshima Y, Kosaka Y, Hara H, Kai S, Kanamaru A, Fukuhara S, Hino M, Sako M, Hiraoka A, Ogawa H, Hara J, Fujimura Y: Prophylactic fresh frozen plasma may prevent development of hepatic VOD after stem cell transplantation via ADAMTS13-mediated restoration of von Willebrand factor plasma levels. Bone Marrow Transplant, 2007; 40: 251-259 [DOI] [PubMed] [Google Scholar]


