Abstract
Aim: The vascular endothelium plays a key role in the pathophysiology of atherosclerosis. Flow-mediated dilation (FMD) is a novel way of assessing endothelial function. Cilostazol is a unique antiplatelet drug that also has the potential to improve endothelial function. The objective of this present study was to investigate the effects of cilosatzol on endothelial function as assessed by FMD.
Methods: Fifty-one patients with coronary artery disease (CAD) were assigned to one of two groups: the Cilostazol(+) group (with cilostazol) and Cilostazol(−) group (without cilostazol). In addition to conventional dual antiplatelet therapy with aspirin and clopidogrel/ticlopidine, the Cilostazol(+) group (n = 27) was also given cilostazol (100 mg/day). The Cilostazol(−) group (n = 24) did not receive cilostazol. FMD was assessed at enrollment and after 6–9 months.
Results: The FMD of both the Cilostazol(+) and Cilostazol(−) groups remained similar at 5.2 (interquartile range: 3.8–8.5) to 5.4 (interquartile range: 4.2–6.7) (P = 0.29) and 5.0 (interquartile range: 3.6–6.4) to 4.9 (interquartile range: 4.0–7.0) (P = 0.38), respectively. However, the diameters of the baseline and maximal brachial arteries tended to increase in the Cilostazol(−) group (baseline: 4.2 ± 0.7 to 4.4 ± 0.7, P = 0.18; maximal: 4.5 ± 0.7 to 4.6 ± 0.7 P = 0.22), whereas that of the Cilostazol(−) group tended to decrease (baseline: 4.1 ± 0.6 to 3.9 ± 0.5, P = 0.10; maximal: 4.3 ± 0.7 to 4.1 ± 0.5, P = 0.05). The rates of change in the baseline diameter (Cilostazol(+): 3.7 ± 9.8% vs. Cilostazol(−): −3.8 ± 12.2%, P = 0.03) and maximal diameter (Cilostazol(+): +3.1 ± 8.9% vs. Cilostazol(−): −4.4 ± 12.0%, P = 0.02) were significantly different.
Conclusion: Although cilostazol didn't affect the FMD, there was a significant difference in the rates of change in baseline and maximal brachial artery diameter. This may have a beneficial effect in patients with cardiovascular disease.
Keywords: Cilostazol, Endothelial Function, Flow-mediated dilation, Coronary artery disease
See editorial vol. 23: 1147–1149
Introduction
Cardiovascular disease (CVD) is the number one cause of death throughout the world1). The World Health Organization (WHO) reported that an estimated 17.5 million people died from CVD in 2012, which represented 31% of global deaths. Among these deaths, an estimated 7.4 million people died due to coronary artery disease (CAD), while 6.7 million died due to stroke. In contrast, in 2000, an estimated 6 million died due to CAD, while 5.7 million died due to stroke. Accordingly, the number of deaths caused by CVDs has increased over the past decade. It is therefore essential to elucidate the endothelial functions that play a key role in the pathophysiology of atherosclerosis.
The endothelium is not only a passive barrier but also an active transducer, as it participates in a variety of paracrine factors that act locally in the blood vessel walls and lumen2). Under healthy conditions, the endothelium produces a wide range of factors that regulate vascular tone, adhesion of circulating blood cells to the vessel wall, thrombus formation, smooth muscle cell proliferation, and vessel wall inflammation. However, under the existence of risk factors such as hypercholesterolemia, hypertension, smoking, and diabetes mellitus, the phenotype of the endothelium may be altered such that it accelerates inflammation, thrombosis, vasoconstriction, and the formation of atherosclerotic lesions3). This maladaptive endothelial phenotype appears in early atherosclerosis and is associated with the classical cardiovascular risk factors.
The flow-mediated dilation (FMD) test has emerged as a major non-invasive tool for assessing endothelial function in clinical settings4–6). FMD acts as a marker of Nitric Oxide (NO)-mediated endothelium-dependent vasodilator function. Impaired FMD is an indicator of endothelial dysfunction and is reported to be a predictor of future CVD7, 8). The FMD value has also been found to be a predictor of changes in the levels of carotid plaque, independent of the classical cardiovascular risk factors9). Because endothelial dysfunction is a systemic process, an impaired FMD measurement in the forearm indicates the presence of systemic endothelial dysfunction. Thus, impaired FMD is an early marker of atherosclerosis. Notably, FMD has been shown to be strongly correlated with coronary endothelial function10).
Cilostazol is a unique antiplatelet drug that selectively and reversibly targets phosphodiesterase III (PDE-III)11). PDE-III is usually present in the platelets, endothelial cells, and smooth muscle cells, where it increases cyclic adenosine monophosphate concentrations, resulting in vasodilation. It also increases sensitivity to endogenous vasodilators, such as prostaglandins, and inhibits both the primary and secondary platelet aggregation induced by collagen, adenosine diphosphate (ADP), arachidonic acid, and epinephrine. Cilostazol is also reported to reduce angiographic restenosis after percutaneous transluminal angioplasty with provisional nitinol stenting for femoropopliteal lesions12). Additionally, the clinical outcomes of patients with acute myocardial infarction who are treated with cilostazol, in addition to conventional dual antiplatelet therapy, are favorable in comparison to those who are treated with conventional dual antiplatelet therapy13).
Aim
Although FMD has been well assessed and cilostazol has been widely used in patients with atherosclerosis, the effect of cilostazol on endothelial function as assessed by FMD has not been elucidated in patients with coronary artery disease. The present study was therefore performed to investigate the effect of cilostazol on endothelial function as assessed by FMD in patients with coronary artery disease.
Methods
Study Subjects
This study was a prospective, open-label, randomized trial. Patients were eligible if they had angina requiring percutaneous coronary intervention (PCI) within two months prior to enrollment. The exclusion criteria included heart failure (New York Heart Association functional class II—IV), left ventricular ejection fraction < 40%, end-stage renal failure requiring hemodialysis, atrial fibrillation, active systemic inflammatory disease, collagen disease, active hepatic disease, malignancy, the usage of anticoagulation therapy, and a history of acute myocardial infarction within the last 3 months. A total of 51 eligible patients were prospectively recruited from September 2010 to March 2012 at Showa University Fujigaokoa Hospital. The patients were randomly assigned to either the Cilostazol(+) group (with cilostazol) or the Cilostazol(−) group (without cilostazol). On top of conventional dual antiplatelet therapy (DAPT) with aspirin (100 mg/day) and clopidogrel (75 mg/day)/ticlopidine (200 mg/day), the patients in the Cilostazol(+) group (n = 27) were given cilostazol (100 mg/day). The patients in the Cilostazol(−) group (n = 24) were not given cilostazol. The patients' endothelial function and blood biochemistry were assessed at enrollment and at 6–9 months after the start of the study. In all patients, coronary risk factors were controlled according to Japanese circulation guidelines14, 15). Clinical follow-up visits were scheduled every 1–3 months. At the time of enrollment and 6 to 9 months after enrollment, all of the patients underwent endothelial function and blood biochemistry assessments. Routine angiographic follow-up was not mandatory. All adverse clinical events were assessed.
This study was approved by the Institutional Ethics Review Committee. Written informed consent was obtained from each patient before participation. This study was registered under the UMIN protocol registration system (ID UMIN 000004065).
The Assessment of Endothelial Function
All subjects were instructed to fast after dinner on the day before the assessment and to refrain from smoking and ingesting any alcohol or caffeine during the study period. All assessments were conducted from 9 to 10:30 AM. All subjects rested for at least 15 min in a seated position in a quiet, dark, air-conditioned room before each FMD assessment. A longitudinal image of the brachial artery was recorded at baseline using an ultrasound with a 10M-Hz linear array transducer probe (UNEX, Nagoya, Japan). A forearm-cuff was then inflated for 5 min at 50 mmHg above the SBP (systolic blood pressure) just before the FMD assessment. After deflation of the cuff, the diastolic diameter of the brachial artery was semi-automatically recorded continuously for 2 min. The FMD was then estimated as the percent change in vessel diameter from baseline to maximum dilatation during reactive hyperemia. Because FMD is highly dependent on the baseline diameter of the vessel, we also compared the baseline and maximal diameters in each group. Experienced technicians were blinded to the clinical data of the study participants. Intra- and inter-observer correlation coefficients for FMD were considerably high (0.99 and 0.92, respectively).
The Assessment of Angiography
Lesion characteristics were assessed according to AHA classification. Drug eluting stent (DES) usage, stent diameter and length, the number of stents, and the number of stents per vessel were described. Target lesion revascularization (TLR) and target vessel revascularization (TVR) were assessed.
Blood Biochemistry
Blood samples were collected from all patients after overnight fasting at baseline and at 6–9 months. High-sensitive C-reactive protein (CRP) tests were conducted by SRL, Inc (Tokyo, Japan). Health Sciences Research Institute, Inc. (Yokohama, Japan) assessed the levels of brain natriuretic peptide (BNP). All other biochemical analyses were performed inhouse.
Statistical Analyses
All statistical analyses were performed using the JMP software program (JMP, Version 11; SAS Institute Inc., NC, USA). The sample size was calculated by a power analysis using preliminary data with the following assumptions: a Type I error of 0.05 (2-tailed), 80% power, a mean increase in FMD of 10% in the Cilostazol(+) group, a mean increase in FMD of 0% in the Cilostazol(−) group, and a standard deviation of 10% in each group. It was determined that a minimum of 17 patients (total of 34 patients) would be required to obtain 80% power in detecting a difference in the %change of FMD from enrollment to 6–9 months.
The patients' age, laboratory data, baseline brachial artery diameter, and maximal brachial artery diameter were expressed as the mean ± SD. The stent diameter, stent length, stent number, stent number per lesion, and FMD were expressed as the median (interquartile range). Comparisons between continuous variables were analyzed using either the t-test or Wilcoxon's rank sum test, as appropriate. The categorical variables were analyzed using the chi-square test. The enrollment and follow-up data were compared using a paired t-test or Wilcoxon's singed rank test, as appropriate. P values of < 0.05 were considered to be statistically significant.
Results
Patient disposition is summarized in Fig. 1. Fifty-one patients were randomly assigned to the Cilostazol(+) (n = 27) and Cilostazol(−) groups (n = 24). All of the patients survived for the duration of the study period. In the Cilostazol(+) group, cilostazol treatment was stopped in 2 patients due to palpitation and 1 patient due to minor bleeding; we could not follow 2 patients due to poor compliance. In the Cilosatazol(−) group, we could not follow 2 patients due to poor compliance. There was no single major bleeding episode. Accordingly, the final analysis included 22 patients in the Cilostazol(+) group and 22 patients in the Cilostazol(−) group.
Fig. 1.
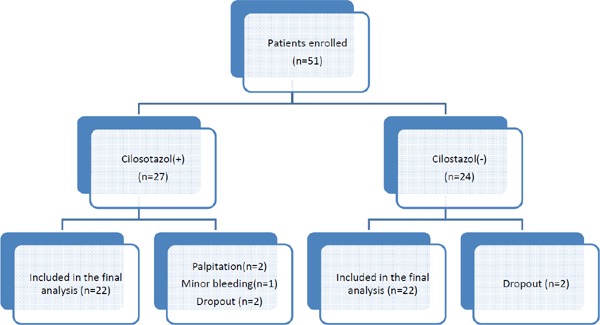
Patient disposition flow chart
Table 1 shows the baseline clinical characteristics of the study patients. There were no significant differences in terms of age, gender, hypertension, hyperlipidemia, diabetes, chronic kidney disease, current smoking status, cerebrovascular disease, peripheral artery disease, previous myocardial infarction or unstable angina pectoris. There were no statistically significant differences between the two groups with regard to the use of major medications, including angiotensin converting enzyme inhibitor (ACE-I)/angiotensin II receptor blockers (ARBs), β-blockers, calcium channel blockers (CCB), statins and nitrate/nicorandil.
Table 1. The baseline clinical characteristics of patients.
| Overall (n = 44) | Cilostazol (+) (n = 22) | Cilostazol (−) (n = 22) | P value | |
|---|---|---|---|---|
| Age, years | 67 ± 8 | 67 ± 7 | 66 ± 10 | 0.81 |
| Male, n (%) | 34 (77.3%) | 16 (72.7%) | 18 (81.8%) | 0.47 |
| Hypertension, n (%) | 32 (72.7%) | 18 (81.8%) | 14 (63.6%) | 0.18 |
| Hyperlipidemia, n (%) | 38 (86.4%) | 19 (86.4%) | 19 (86.4%) | 1.00 |
| Diabetes, n (%) | 19 (43.2%) | 10 (45.5%) | 9 (40.9%) | 0.76 |
| CKD, n (%) | 5 (11.4%) | 1 (4.6%) | 4 (18.1%) | 0.15 |
| Current Smoker, n (%) | 13 (29.6%) | 5 (22.7%) | 8 (36.3%) | 0.32 |
| CVD, n (%) | 2 (4.6%) | 0 | 2 (9.1%) | 0.15 |
| PAD, n (%) | 1 (2.3%) | 0 | 1 (4.6%) | 0.31 |
| Previous MI | 10 (22.7%) | 5 (22.7%) | 5 (22.7%) | 1.00 |
| UAP, n (%) | 16 (38.4%) | 6 (37.5%) | 10 (45.5%) | 0.21 |
| Medication, n (%) | ||||
| ACE-I/ARB | 26 (59.1%) | 14 (63.6%) | 12 (54.6%) | 0.54 |
| β-blokers | 13 (29.6%) | 4 (18.2%) | 9 (40.9%) | 0.10 |
| CCB | 19 (43.2%) | 11 (50.0%) | 8 (36.4%) | 0.36 |
| Statin | 40 (90.9%) | 19 (86.4%) | 21 (95.5%) | 0.29 |
| Nitrate/Nicorandil | 7 (15.9%) | 5 (22.7%) | 2 (9.1%) | 0.22 |
The data are shown as the mean ± SD or n (%). CKD, chronic kidney disease; CVD, cerebrovascular disease; PAD, peripheral artery disease; Previous MI, previous myocardial infarction; EF, ejection fraction; UAP, unstable angina pectoris; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium-channel blocker.
The procedural characteristics are shown in Table 2. We did not observe any significant differences in the number of treated lesions, type B2 or C lesions, target vessel location, indications of de novo lesions, indications of in-stent restenosis, prevalence of previous PCI, prevalence of previous CABG or drug eluting stent (DES) usage. Regarding stent usage, the diameter, length, number of stents, and number of stents per lesion were similar. At 6–9 months, target vessel and target lesion revascularization was comparable between the two groups.
Table 2. The characteristics of the procedures.
| Overall (n = 44) | Cilostazol (+) (n = 22) | Cilostazol (−) (n = 22) | P value | |
|---|---|---|---|---|
| Number of treated lesions, n (%) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.87 |
| Target Vessel, n (%) | ||||
| LMT | 2 (4.6%) | 1 (4.6%) | 1 (4.6%) | 1.00 |
| RCA | 18 (40.9%) | 10 (45.5%) | 8 (36.4%) | 0.54 |
| LAD | 32 (72.7%) | 16 (72.7%) | 16 (72.7%) | 1.00 |
| LCX | 14 (31.8%) | 6 (27.3%) | 8 (36.4%) | 0.52 |
| De novo, n (%) | 41 (93.2%) | 22 (100.0%) | 19 (86.4%) | 0.07 |
| ISR, n (%) | 8 (18.2%) | 4 (18.2%) | 4 (18.2%) | 1.00 |
| Type B2 or C, n (%) | 12 (27.3%) | 6 (27.3%) | 6 (27.3%) | 1.00 |
| Previous PCI, n (%) | 11 (25%) | 7 (31.8%) | 4 (18.2%) | 0.30 |
| Previous CABG, n (%) | 1 (2.3%) | 1 (4.6%) | 0 (0%) | 0.31 |
| DES usage, n (%) | 35 (79.6%) | 19 (86.4%) | 16 (72.8%) | 0.26 |
| Stent diameter, mm | 3.0 (3.0–3.5) | 3.0 (2.75–3.5) | 3.5 (3–3.5) | 0.48 |
| Stent length, mm | 18 (15–23) | 18.0 (15.0–23.0) | 18.0 (14.0–23.0) | 0.78 |
| Stent number, n | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.5 (1.0–2.3) | 0.66 |
| Stent number per vessel, n | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.62 |
| TVR (6–9 months), n (%) | 9 (20.5%) | 5 (22.7%) | 4 (18.2%) | 0.71 |
| TLR (6–9 months), n (%) | 7 (15.9%) | 4 (18.2%) | 3 (13.6%) | 0.68 |
Data are n (%) or median and (interquartile range). LMT, left main trunk; RCA, right coronary artery; LAD, left anterior descending; LCX, left circumflex; ISR, in-stent restenosis; Previous PCI, previous percutaneous coronary intervention; Previous CABG, previous coronary artery bypass graft; DES, drug-eluting stent; TLR, target lesion revascularization; TVR target vessel revascularization.
Table 3 shows the laboratory data at enrollment and at 6–9 months in each group. Upon enrollment, all laboratory data were comparable between the two groups. There were no significant differences in serum creatinine, LDL cholesterol, triglyceride, or glycohemoglobin levels over time in either group. In both groups, the level of HDL cholesterol increased over time; a significant difference was only observed in the Cilostazol(−) group. The LDL/HDL ratio significantly decreased over time in both groups. BNP decreased significantly in the Cilostazol(+) group. BNP also decreased in the Cilostazol(−) group, but these data were not statistically significant. High-sensitive CRP levels were assessed in a limited number of patients (Cilostazol(+) group, n = 19; Cilostazol(−) group, n = 15). In these patients, high-sensitive CRP levels decreased from 0.29 ± 0.74 mg/dl to 0.12 ± 0.15 mg/dl in the Cilostazol(+) group, but these data were not significant (P = 0.21), while there was a non-significant increase from 0.19 ± 0.31 mg/dl to 0.32 ± 0.73 mg/dl in the Cilostazol(−) group (P = 0.64).
Table 3. Laboratory data of patients at enrollment and at 6–9 Months.
| Cilostazol (+) (n = 22) |
Cilostazol (−) (n = 22) |
|||||
|---|---|---|---|---|---|---|
| Enrollment | At 6–9 months | P-value | Enrollment | At 6–9 months | P-value | |
| Cre, mg/dl | 0.81 ± 0.14 | 0.82 ± 0.17 | 0.33 | 0.88 ± 0.24 | 0.87 ± 0.27 | 0.37 |
| LDL cholesterol, mg/dl | 110 ± 27 | 103 ± 24 | 0.30 | 102 ± 35 | 90 ± 28 | 0.16 |
| HDL cholesterol, mg/dl | 54 ± 18 | 58 ± 20 | 0.07 | 50 ± 10 | 53 ± 11 | 0.01 |
| LDL/HDL ratio | 2.2 ± 1.1 | 2.0 ± 0.9 | 0.01 | 2.1 ± 0.8 | 1.8 ± 0.8 | 0.02 |
| TG, mg/dl | 134 ± 52 | 130 ± 50 | 0.56 | 142 ± 93 | 156 ± 82 | 0.53 |
| Hb A1c, % | 6.4 ± 0.8 | 6.6 ± 1.1 | 0.31 | 6.7 ± 1.3 | 6.6 ± 1.2 | 0.87 |
| BNP, pg/ml | 63 ± 121 | 24 ± 19 | 0.01 | 69 ± 96 | 62 ± 90 | 0.68 |
The data are shown as the mean ± SD. Cre, serum creatine; TG, triglyceride; Hb A1c, hemoglobin A1c; BNP, brain natrium peptide.
Table 4 shows the results of FMD, baseline brachial artery diameter, maximal brachial artery diameter, systolic blood pressure, diastolic blood pressure, and heart rate at each time point. The FMD of both the Cilostazol(+) and Cilostazol(−) groups remained similar at 5.2 (interquartile range:3.8–8.5) to 5.4 (interquartile range:4.2–6.7) (P = 0.29) and 4.9 (interquartile range:4.0–7.0) to 5.0 (interquartile range: 3.6–6.4) (P = 0.38), respectively. The percent change in FMD was −6.9% (interquartile range:−23.5–16.6%) in the Clisotazol(+) group and +7.0% (interquartile range:−21.8–49.6%) in the Cilostazol(−) group and was not statistically significant (P = 0.34). Although the FMD did not change significantly in either group, the baseline brachial artery diameter and maximal brachial artery diameter increased in the Cilostazol(−) group (baseline: 4.2 ± 0.7 to 4.4 ± 0.7, P = 0.18; maximal: 4.5 ± 0.7 to 4.6 ± 0.7, P = 0.22) and decreased in the Cilostazol(+) group (baseline: 4.1 ± 0.6 to 3.9 ± 0.5, P = 0.10; maximal: 4.3 ± 0.7 to 4.1 ± 0.5, P = 0.05). There were statistically significant differences in the percent change (Fig. 2) in the baseline diameter (Cilostazol(+): +3.7 ± 9.8% vs. Cilostazol(−): −3.8 ± 12.2%) and in the maximal diameter (Cilostazol(−): −3.1 ± 8.9% vs. Cilostazol(−): −4.4 ± 12.0%). The trends in blood pressure and heart rate were similar in both groups.
Table 4. FMD (flow-mediated dilation), baseline diameter of brachial artery, maximal diameter of brachial artery, SBP (systolic blood pressure), DBP (diastolic blood pressure), and HR (heart rate) at enrollment and 6.9 months thereafter.
| Cilostazol (+) (n = 22) |
Cilostazol (−) (n = 22) |
|||||
|---|---|---|---|---|---|---|
| Enrollment | At 6–9 months | P-value | Enrollment | At 6–9 months | P-value | |
| FMD, % | 5.2 (3.8–8.5) | 5.4 (4.2–6.7) | 0.29 | 5.0 (3.6–6.4) | 4.9 (4.0–7.0) | 0.38 |
| baseline diameter, mm | 4.2 ± 0.7 | 4.4 ± 0.7 | 0.18 | 4.1 ± 0.6 | 3.9 ± 0.5 | 0.10 |
| maximal diameter, mm | 4.5 ± 0.7 | 4.6 ± 0.7 | 0.22 | 4.3 ± 0.7 | 4.1 ± 0.5 | 0.05 |
| SBP, mmHg | 125 ± 16 | 131 ± 16 | 0.15 | 125 ± 17 | 130 ± 19 | 0.28 |
| DBP, mmHg | 69 ± 7 | 73 ± 11 | 0.21 | 69 ± 13 | 70 ± 10 | 0.74 |
| HR, n/min | 67 ± 11 | 65 ± 12 | 0.64 | 70 ± 10 | 67 ± 8 | 0.20 |
Fig. 2.
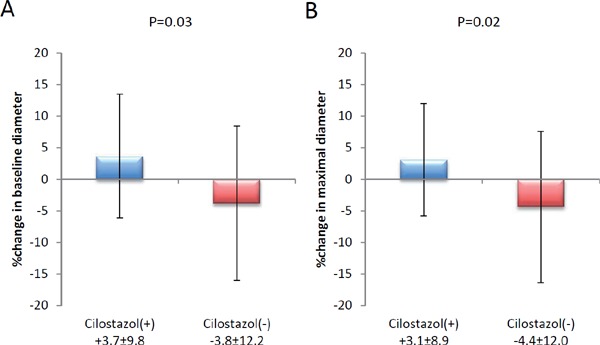
The percent change in baseline and maximal brachial artery diameter
Discussion
To the best of our knowledge, this is the first study to investigate the effect of cilostazol on endothelial function as assessed by FMD in patients with coronary artery disease. We did not find cilostazol to affect FMD. However, there was a greater rate of change in the baseline and maximal brachial artery diameters in the Cilostazol(+) group between enrollment and follow-up in comparison to the Cilostazol(−) group. This dilation of brachial artery diameter is consistent with its well-described vasodilator effect and may be of potential benefit to patients with cardiovascular disease, especially those with peripheral artery disease.
There are several previous reports (Supplemental Table 1) that have studied the effects of cilostazol on endothelial function as assessed by FMD; however, these reports did not involve patients with coronary artery disease and the durations of treatment were not as long as our study16–20). Three reports16–18) investigating younger smokers found that 2 weeks of cilostazol treatment resulted in FMD improvement. These studies16–18) indicated that younger smokers are more likely to respond to cilostazol treatment. One report19) examined patients with silent cerebral lacunar infarction and hypercholesterolemia who received a combination of probucol and cilostazol for four weeks, and found that treatment resulted in FMD improvement. Although these cases showed similar clinical characteristics, the FMD values at enrollment were much lower than those of our study and the patient's LDL cholesterol levels at enrollment were much higher (134 ± 27 mg/dl in the Probucol + Cilostazol group, 136 ± 30 mg/dl in the aspirin group) than in our study (108 ± 28 mg/dl in the Cilostazol(+) group, 102 ± 32 mg/dl in the Cilostazol(−) group). Thus, several factors existed that could be improved by more aggressive drug therapy. In one report20) Raynaud's syndrome patients who received cilostazol for 6 weeks showed no FMD improvement. However, similar to our study, the patients showed a significant increase in brachial artery diameter.
The addition of cilostazol, on top of conventional dual antiplatelet therapy, has been reported to be beneficial for patients with coronary artery disease who require coronary stents; however, these reports mainly measure improvement by the reduction of instent restenosis21, 22). On the other hand, there are reports that show that the addition of cilostazol on top of conventional dual antiplatelet therapy did not result in better clinical outcomes in patients with coronary artery disease who required coronary stents in comparison to conventional dual antiplatelet therapy23, 24). Although the endpoints of these studies differed from our own, the patient demographics were quite similar. Thus, with the knowledge of these conflicting data, that cilostazol did not improve the FMD values of the patients in our study, was not entirely unexpected.
Unlike patients with coronary artery disease, patients with peripheral artery disease are more likely to benefit from cilostazol. Cilostazol has been reported to reduce not only the risk of restenosis but also the risk of amputation in patients with cardiovascular disease25). The vasodilatory effect of cilostazol that we observed in our study can be beneficial to such patients. However, in spite of these effects, there is not enough evidence to support that the administration of cilostazol can reduce mortality.
Cilostazol is contraindicated for patients with severe heart failure since other PDE inhibitors such as milrinone26) and vesnarinon27), which are used as inotropic agents for patients with severe heart failure, have been shown to be associated with a worse clinical outcome. However, in the present study, there was a significant decrease in BNP levels in the Cilostazol(+) group. This result indicates the possible utility of cilostazol in the treatment of patients with coronary artery disease but without heart failure.
Study Limitations
There are several limitations associated with this study. First, this was not a double-blind study and there was no placebo, although the endothelial functions were assessed in a blinded manner. Second, FMD is highly dependent on the baseline brachial diameter28); a large baseline vessel diameter is correlated with a low FMD value. As a result, the vasodilation effect of cilostazol likely affected FMD readings. Third, the study was performed at a single center with a small study population and we did not administer the maximum dose of cilostazol. However, in the present study, the addition of cilostazol did not result in any improvement in FMD. The percent change in FMD was somewhat worse in the Cilostazol(+) group but did not reach statistical significance. We are therefore of the opinion that it is very difficult to show that the addition of cilostazol results in an improvement in FMD, even if we increase the number of cases.
Conclusion
Although cilostazol did not affect FMD in patients with coronary artery disease, the increased baseline and maximal brachial artery diameter and the significant difference in the rate of change, suggests that cilostazol may be beneficial for patients with cardiovascular disease.
Acknowledgments
We thank Fuyuki Asano, Yuki Honda, Tomomi Futagawa, Misako Mori, Mamoru Hatakeyama and the entire cardiology staff and also the physiological laboratory staff for their valuable support.
Disclosures
The author has received honorarium from Terumo R&D, Goodman CO., LTD and Abbott Vascular Japan.
Supplemental Table 1. Comparisons with previous publications.
| Study demographic |
FMD data in cilostazol group |
|||||||
|---|---|---|---|---|---|---|---|---|
| Author | Case characteristics | Case (n) | Age | Duration | Therapy | Enrollment | Follow-up | P-value |
| Mori et al. | Coronary artery disease | 22 | 67 ± 7 | 6–9 months | Cilostazol (100 mg/day) and conventional dual antiplatelet theraphy | 5.2 (3.8–8.5) | 5.4 (4.2–6.7) | 0.29 |
| 22 | 66 ± 10 | Conventional dual antiplatelet theraphy | 5.0 (3.6–6.4) | 4.9 (4.0–7.0) | 0.38 | |||
| Oida et al. | Smoker | 20 | 38 ± 5.2 | 2 weeks | Cilostazol (150 mg/day) | 4.2 ± 1.2 | 7.8 ± 3.5 | 0.003 |
| Kim et al. | Smoker | 20 | 29.1 ± 1.9 | 2 weeks | Cilostazol (200 mg/day) | 8.0 ± 2.1 | 12.2 ± 5.1 | 0.003 |
| Non-smoker | 10 | 28 ± 1.1 | 12.0 ± 4.5 | 16.1 ± 3.7 | 0.041 | |||
| Jeong et al. | Smoker | 20 (cross over with 1 week interval) | 29 ± 2 | 2 weeks | Cilostazol (200 mg/day) | 7.7 ± 1.9 | 8.8 ± 2.0 | 0.016 |
| Sarpogrelate (200 mg/day) | 7.4 ± 1.9 | 8.8 ± 1.9 | 0.021 | |||||
| Takae et al. | Silent cerebral Lacunar infarcts and hypercholesterolemia | 17 | 72 ± 7 | 4 weeks | Cilostazol (200 mg/day) and Probucol (500 mg/day) | 2.69 ± 1.51 | 3.53 ± 1.69 | < 0.05 |
| 17 | 72 ± 8 | Aspirin (100 mg/day) | 2.63 ± 1.48 | 2.92 ± 1.39 | NS | |||
| Rajagopalan et al. | Raynaud's syndrome (primary) | 19 | 47 ± 12 | 6 weeks | Cilostazol (200 mg/day) | 4.06 (2.5–6.1) | −0.77 (−2.4–3.5) | NS |
| Placebo | Not available | |||||||
| Raynaud's syndrome (secondary) | 20 | 41 ± 13 | Cilostazol (200 mg/day) | 2.23 (0.05–6.3) | 2.95 (1.7–7.4) | NS | ||
| Placebo | Not available | |||||||
FMD = flow-mediated dilation.
References
- 1). WHO. The top 10 causes of death: Fact sheet N°310. World Heal. Organ. 2013. http://www.who.int/mediacentre/factsheets/fs310/en/
- 2). Vita JA., Keaney JF. Endothelial function: a barometer for cardiovascular risk? Circulation 2002; 106: 640-642 [DOI] [PubMed] [Google Scholar]
- 3). Levine GN, Keaney JF, Vita JA. Cholesterol reduction in cardiovascular disease. Clinical benefits and possible mechanisms. N Engl J Med 1995; 332: 512-521 [DOI] [PubMed] [Google Scholar]
- 4). Stoner L, Sabatier MJ. Use of Ultrasound for Non-Invasive Assessment of Flow-Mediated Dilation. J Atheroscler Thromb 2012; 19: 407-421 [DOI] [PubMed] [Google Scholar]
- 5). Poredos P, Jezovnik MK. Testing endothelial function and its clinical relevance. J Atheroscler Thromb 2013; 20: 1-8 [DOI] [PubMed] [Google Scholar]
- 6). Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992; 340: 1111-1115 [DOI] [PubMed] [Google Scholar]
- 7). Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001; 104: 2673-2678 [DOI] [PubMed] [Google Scholar]
- 8). Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26: 631-640 [DOI] [PubMed] [Google Scholar]
- 9). Lind L. Flow-Mediated Vasodilation was Found to be an Independent Predictor of Changes in the Carotid Plaque Status During a 5-Year Follow-Up. -A Prospective Investigation of the Vasculature in the Uppsala Seniors (PIVUS) Study. J Atheroscler Thromb 2014; 21: 161-168 [DOI] [PubMed] [Google Scholar]
- 10). Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager Ma., Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 1995; 26: 1235-1241 [DOI] [PubMed] [Google Scholar]
- 11). Goto S. Cilostazol: Potential mechanism of action for antithrombotic effects accompanied by a low rate of bleeding. Atheroscler Suppl 2005; 6: 3-11 [DOI] [PubMed] [Google Scholar]
- 12). Iida O, Yokoi H, Soga Y, Inoue N, Suzuki K, Yokoi Y, Kawasaki D, Zen K, Urasawa K, Shintani Y, Miyamoto A, Hirano K, Miyashita Y, Tsuchiya T, Shinozaki N, Nakamura M, Isshiki T, Hamasaki T, Nanto S. Cilostazol reduces angiographic restenosis after endovascular therapy for femoropopliteal lesions in the Sufficient Treatment of Peripheral Intervention by Cilostazol study. Circulation 2013; 127: 2307-2315 [DOI] [PubMed] [Google Scholar]
- 13). Chen K-Y, Rha S-W, Li Y-J, Poddar KL, Jin Z, Minami Y, Wang L, Kim EJ, Park CG, Seo HS, Oh DJ, Jeong MH, Ahn YK, Hong TJ, Kim YJ, Hur SH, Seong IW, Chae JK, Cho MC, Bae JH, Choi DH, Jang YS, Chae IH, Kim CJ, Yoon JH, Chung WS, Seung KB, Park SJ. Triple versus dual antiplatelet therapy in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation 2009; 119: 3207-3214 [DOI] [PubMed] [Google Scholar]
- 14). Guidelines for elective percutaneous coronary intervention in patients with stable coronary artery disease (JCS 2011) published in 2012--digest version. Circ J 2013; 77: 1590-1607 [DOI] [PubMed] [Google Scholar]
- 15). Guidelines for Secondary Prevention of Myocardial Infarction (JCS 2011). Circ J 2013; 77: 231-248 [DOI] [PubMed] [Google Scholar]
- 16). Oida K, Ebata K, Kanehara H, Suzuki J, Miyamori I. Effect of cilostazol on impaired vasodilatory response of the brachial artery to ischemia in smokers. J Atheroscler Thromb 2003; 10: 93-98 [DOI] [PubMed] [Google Scholar]
- 17). Kim KS, Park HS, Jung IS, Park J-H, Ahn KT, Jin S-A, Park YK, Kim JH, Lee J-H, Choi SW, Jeong J-O, Seong I-W. Endothelial dysfunction in the smokers can be improved with oral cilostazol treatment. J Cardiovasc Ultrasound 2011; 19: 21-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Jeong IS, Park J-H, Jin SA, Kim JH, Lee J-H, Choi SW, Jeong J-O, Seong I-W. Oral sarpogrelate can improve endothelial dysfunction as effectively as oral cilostazol, with fewer headaches, in active young male smokers. Heart Vessels 2013; 28: 578-582 [DOI] [PubMed] [Google Scholar]
- 19). Takase B, Nagata M, Hattori H, Tanaka Y, Ishihara M. Combined therapeutic effect of probucol and cilostazol on endothelial function in patients with silent cerebral lacunar infarcts and hypercholesterolemia: a preliminary study. Med Princ Pract 2014; 23: 59-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Rajagopalan S, Pfenninger D, Somers E, Kehrer C, Chakrabarti A, Mukherjee D, Brook R, Kaplan MJ. Effects of cilostazol in patients with Raynaud's syndrome. Am J Cardiol 2003; 92: 1310-1315 [DOI] [PubMed] [Google Scholar]
- 21). Lee SW, Chun KJ, Park SW, Kim HS, Kim YH, Yun SC, Kim WJ, Lee JY, Park DW, Lee CW, Hong MK, Rhee KS, Chae JK, Ko JK, Park JH, Lee JH, Choi SW, Jeong JO, Seong IW, Jon S, Cho YH, Lee NH, Kim JH, Park SJ. Comparison of Triple Antiplatelet Therapy and Dual Antiplatelet Therapy in Patients at High Risk of Restenosis After Drug-Eluting Stent Implantation (from the DECLARE-DIABETES and -LONG Trials). Am J Cardiol Elsevier Inc.; 2010; 105: 168-173 [DOI] [PubMed] [Google Scholar]
- 22). Lee SW, Park SW, Kim YH, Yun SC, Park DW, Lee CW, Hong MK, Kim HS, Ko JK, Park JH, Lee JH, Choi SW, Seong IW, Cho YH, Lee NH, Kim JH, Chun KJ, Park SJ. Drug-Eluting Stenting Followed by Cilostazol Treatment Reduces Late Restenosis in Patients With Diabetes Mellitus. The DECLARE-DIABETES Trial (A Randomized Comparison of Triple Antiplatelet Therapy With Dual Antiplatelet Therapy After Drug-Eluting Stent I. J Am Coll Cardiol 2008; 51: 1181-1187 [DOI] [PubMed] [Google Scholar]
- 23). Suh JW, Lee SP, Park KW, Lee HY, Kang HJ, Koo BK, Cho YS, Youn TJ, Chae IH, Choi DJ, Rha SW, Bae JH, Kwon TG, Bae JW, Cho MC, Kim HS. Multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease: Results of the CILON-T (influence of cilostazol-based triple antiplatelet therapy on is. J Am Coll Cardiol Elsevier Inc.; 2011; 57: 280-289 [DOI] [PubMed] [Google Scholar]
- 24). Youn YJ, Lee JW, Ahn SG, Lee SH, Choi H, Yu CW, Hong YJ, Kwon HM, Hong MK, Jang Y, Yoon J. Multicenter randomized trial of 3-month cilostazol use in addition to dual antiplatelet therapy after biolimus-eluting stent implantation for long or multivessel coronary artery disease. Am Heart J Mosby, Inc.; 2014; 167: 241-248.e1 [DOI] [PubMed] [Google Scholar]
- 25). Warner CJ, Greaves SW, Larson RJ, Stone DH, Powell RJ, Walsh DB, Goodney PP. Cilostazol is associated with improved outcomes after peripheral endovascular interventions. J Vasc Surg Society for Vascular Surgery; 2014; 59: 1607-1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med 1991; 325: 1468-1475 [DOI] [PubMed] [Google Scholar]
- 27). Cohn JN, Goldstein SO, Greenberg BH, Lorell BH, Bourge RC, Jaski BE, Gottlieb SO, McGrew F, DeMets DL, White BG. A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure Vesnarinone Trial Investigators. N Engl J Med 1998; 339: 1810-1816 [DOI] [PubMed] [Google Scholar]
- 28). Atkinson G, Batterham AM. The clinical relevance of the percentage flow-mediated dilation index. Curr Hypertens Rep 2015; 17: 4. [DOI] [PubMed] [Google Scholar]


