Abstract
Aim: Advanced glycation end products (AGE) are considered to be among the critical pathogenic factors involved in the progression of diabetic complications. Skin autofluorescence (AF), a noninvasive measurement of AGE accumulation, has been recognized as a useful and convenient marker for diabetic vascular diseases in Caucasians. This study aimed to evaluate the association of tissue AGE, assessed using skin AF, with coronary artery calcification in Japanese subjects with type 2 diabetes.
Methods: In total, 122 Japanese subjects with type 2 diabetes enrolled in this cross-sectional study underwent multi-slice computed tomography for total coronary artery calcium scores (CACS) estimation and examination with a skin AF reader.
Results: Skin AF positively correlated with age, sex, diabetes duration, pulse wave velocity, systolic blood pressure, serum creatinine, and CACS. In addition, skin AF results negatively correlated with BMI, eGFR, and serum C-peptide concentration. According to multivariate analysis, age and systolic blood pressure showed strong positive correlation and eGFR showed negative correlation with skin AF values. Multiple linear regression analyses revealed a significant positive correlation between skin AF values and logCACS, independent of age, sex, diabetes duration, HbA1c, BMI, IMT, and blood pressure. However, skin AF showed no association with serum levels of AGE, such as Nε-(carboxymethyl) lysine and 3-deoxyglucosone.
Conclusion: Skin AF results positively correlated with CACS in Japanese subjects with type 2 diabetes. This result indicates that AGE plays a role in the pathogenesis of diabetic macrovascular disease. Measurement of skin AF values may be useful for assessing the severity of diabetic complications in Japanese subjects.
Keywords: Advanced glycation end products, Coronary artery calcium scores, Type 2 diabetes, Surrogate marker
Introduction
Atherosclerotic diseases are the leading cause of death in individuals with type 2 diabetes1). In a Japanese prospective population study, patients with type 2 diabetes had approximately 2.0 to 3.5 times higher risk of cardiovascular disease (CVD), including ischemic stroke and coronary heart disease, than nondiabetic subjects2). Thus, detection and treatment of subclinical atherosclerosis in the diabetic population may prevent CVD events and substantially reduce the risk of cardiovascular death.
The coronary artery calcification score (CACS) measured by multi-detector computed tomography (MDCT) is reportedly a better predictor of CVD than traditional noninvasive surrogate markers, such as carotid intima-media thickness (IMT)3). Therefore, CACS is well-accepted and recommended in asymptomatic individuals with an intermediate risk, as assessed according to the Framingham Risk Score, in Western countries4). CACS measurement has also been regarded as being clinically useful for evaluating coronary atherosclerosis in Japan5) where the incidence of coronary heart disease is much lower than in Western countries. In a previous study, we showed CACS to be positively associated with biological markers of oxidative stress6) and to predict the morbidity of CVD in Japanese subjects with type 2 diabetes7).
The risks of diabetic vascular complications are not fully represented by the currently established risk factors, such as HbA1c. Advanced glycation end products (AGE) are the irreversible products of nonenzymatic glycation, resulting from long-term hyperglycemia8). A large number of studies clarified that AGE mainly contributes to the development and progression of vascular complications in diabetes9). Tissue AGE accumulation exerts deleterious effects. One of the mechanisms underlying these effects involves changing the three-dimensional structure of proteins. Another involves the receptor for AGE-mediated activation of oxidative stress and inflammation pathways10). There is accumulating evidence of the relationships between serum AGE levels and vascular disease, but current serum AGE concentrations are not consistently related to diabetic complications according to several studies11). AGE accumulation, as assessed by skin biopsy specimens, reportedly shows a positive association with the presence of vascular disease12). These results raise the possibility that the pathogenic effects of AGE on vascular disease are exerted via tissue accumulation over many years13). A noninvasive technique for evaluating tissue accumulation of several types of fluorescent AGE by measuring skin autofluorescence (AF) was developed14). On the basis of a number of studies conducted mainly in Western countries, skin AF has now been recognized as a predictor of vascular complications in subjects with chronic kidney disease (CKD)15, 16). In addition, the relationships of skin AF with diabetic micro- and macroangiopathy have been intensively examined in subjects with both type 1 and type 2 diabetes17–19). However, the effectiveness of the measurement instrument, the AGE Reader, in diabetic patients has not been sufficiently evaluated in non-Caucasian, including Japanese, populations20, 21). Therefore, we designed this cross-sectional study to clarify the validity of skin AF for predicting the severity of atherosclerosis assessed using baPWV (brachial ankle pulse wave velocity), carotid IMT, and CACS in Japanese subjects with type 2 diabetes who were free of renal dysfunction.
Subject and Methods
Study Subjects
The subjects were 122 type 2 diabetes patients who visited Iwate Medical University Hospital during the period from April 2013 to December 2014. These patients ranged in age from 20 to 80 years. Patients with skin reflectance (R%) below 6% were excluded because of the limitation of the instrument in measuring skin AF accurately in non-Caucasians with relatively dark skin22, 23). Patients were excluded if they had renal dysfunction [estimated glomerular filtration rate (eGFR) below 30 mL min−1 1.73 m−2], any malignancy, an infectious disorder, or a past history of stroke or coronary artery disease. Written informed consent was obtained from all study participants. This study was approved by the Institutional Review Board of Iwate Medical University (Approval number: H25-25).
Measurement of Skin AF
Skin AF was assessed by an autofluorescence reader (AGE reader; Diagnoptics, Groningen, The Netherlands) as previously described14). AF measurement was defined as the average light intensity of the excitation spectrum between 420 nm and 600 nm, divided by the average light intensity of the emission spectrum between 300 nm and 420 nm and multiplied by one hundred and expressed in arbitrary units (AU). Skin AF was measured on the volar surface of the lower arm, approximately 10 – 15 cm below the elbow fold, with the patient in a seated position. The coefficient of variation for intraindividual measurements repeated over a few days was 5.82% (n = 5).
Measurement of Coronary Artery Calcification
A VCT 240 slice MDCT (Aquilion ONE, Toshiba Medical, Tokyo, Japan) was used to obtain plain multislice CT scans. The calcium score analyses of coronary arteries were performed with a 0.5 mm collimation width, a gantry rotation speed of 0.4 s/rotation, 120 kV, and 300 mA using prospective ECG-gated axial scanning. Calcium plaque was defined as reaching a threshold of 130 HU and covering an area of at least 0.51 mm2. The total CACS were determined on a workstation (ZIO Station, ZIO Soft, Inc., Tokyo, Japan) using a software program for coronary artery calcification according to the Agatston method24). The subjects were divided into three groups according to their CACS; the CAC 0, CAC 1–399, and CAC > 400 groups.
Measurements of ABI, baPWV, and Carotid Artery IMT
ABI (ankle brachial pressure index) and baPWV were measured using an automatic waveform analyzer (BP-203RPE; Colin Co., Komaki, Japan), as described previously25). IMT of the carotid arteries was measured using ultrasound diagnostic equipment (LOGIQ 500, GE Yokogawa Medical Systems Corp., Hino, Tokyo, Japan) with an electrical linear transducer (mid-frequency of 7.5 MHz). The common carotid artery, carotid bulb, and portions of the internal and external carotid arteries on both sides were scanned with the subject in the supine position6). We defined the max IMT as the thickest portion detected in the scanned regions. The scans were performed by a trained sonographer.
Biochemical Measurements
Laboratory values were measured employing routine techniques on blood and urine samples obtained after a 12-h overnight fast. Plasma levels of Nε-(carboxymethyl) lysine, 3-deoxyglucosone, and malondialdehyde low density lipoprotein (MDA-LDL) as well as urinary levels of 8-hydroxy-2′-deoxyguanosine (OHdG) and 8-isoprostane were measured by SRL, Inc. (Tokyo, Japan).
Statistical Analysis
Quantitative data are presented as means ± standard deviation (SD). Variables were compared using Spearman's rank-order correlation analysis. We performed multivariate regression analysis using the force entry method to analyze variables independently related to skin AF. A multiple linear regression analysis adjusted for age, gender, body mass index (BMI), diabetes duration, history of smoking, systolic blood pressure, eGFR, skin AF, HbA1c, and max IMT was performed to evaluate parameters independently showing significant correlations with CACS. CACS plus one value were logarithmically converted. Linear regression analysis was performed with the step-down procedure to examine the grade of CACS and skin AF. Differences among the three groups were calculated employing the Kruskal -Wallis test for continuous variables and the Chi-square test for categorical variables. Receiver operating characteristics (ROC) curve analyses were drawn, and the areas under the curve (AUC) were then calculated. The level of significance was set at p < 0.05. SPSS version 21 (SPSS Japan Inc., Tokyo, Japan) was used for all analyses.
Results
The clinical characteristics of our 122 subjects are shown in Table 1. Mean age was 61.0 years, mean diabetes duration was 10.7 years, and 59.0% of the subjects were men. Nearly half (49%) of the subjects were currently smoking or had a smoking history. The mean skin AF value was 2.42 (AU), compatible with that in a previous report on East Asian subjects26).
Table 1. Baseline characteristics of the study participants.
| n = 122 | |
|---|---|
| Gender (male/female) | 72/50 |
| Age (years) | 61.0 ± 13.0 |
| BMI (kg/m2) | 26.4 ± 5.1 |
| Diabetes duration (years) | 10.7 ± 9.3 |
| Hypertension, n (%) | 73 (60) |
| Dislipidemia, n (%) | 89 (73) |
| SBP (mmHg) | 131.7 ± 17.7 |
| DBP (mmHg) | 75.1 ± 12.0 |
| History of smoking (%) | 60 (49) |
| Total cholesterol (mg/dL) | 187.4 ± 34.5 |
| Triglyceride (mg/dL) | 138.3 ± 70.2 |
| HDL cholesterol (mg/dL) | 52.2 ± 16.3 |
| LDL cholesterol (mg/dL) | 110.0 ± 30.0 |
| eGFR (ml/min per 1.73 m2) | 77.4 ± 23.6 |
| Fasting blood glucose (mg/dL) | 153.6 ± 51.1 |
| HbA1c (%) | 8.57 ± 2.33 |
| Urinary 8-isoprostane (pg/mgCr) | 265.2 ± 264.2 |
| Urinary 8-OHdG (ng/mgCr) | 11.4 ± 7.9 |
| MDA-LDL (U/dl) | 133.0 ± 42.3 |
| Nε-(carboxymethyl) lysine (µg/ml) | 3.49 ± 0.96 |
| 3-deoxyglucosone (ng/ml) | 24.37 ± 14.2 |
| max IMT (mm) | 1.64 ± 0.70 |
| mean baPWV (cm/s) | 1569.4 ± 311.4 |
| mean ABI | 1.13 ± 0.96 |
| Coronary artery calcification score, (AU) | 197.03 ± 412.78 |
| Skin autofluorescence (AU) | 2.42 ± 0.417 |
| Metformin, n (%) | 52 (43) |
| DPP-4 inhibitors, n (%) | 53 (44) |
| Statins, n (%) | 50 (41) |
| RAS inhibitors, n (%) | 61 (50) |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobinA1c; OHdG, hydroxydeoxyguanosine; MDA-LDL, malondialdehyde modified low density lipoprotein; IMT, intima-media thickness; baPWV, brachial ankle pulse wave velocity; ABI, ankle brachial index; DPP, dipeptidyl peptidase; RAS, renin-angiotensin system
The skin AF value positively correlated with age (r = 0.375, p < 0.001), diabetes duration (r = 0.338, p < 0.001), systolic blood pressure (r = 0.233, p = 0.01), serum creatinine levels (r = 0.206, p = 0.023), baPWV (r = 0.335, p < 0.001), and logCACS (r = 0.344, p < 0.001) (Table 2). In addition, AF showed negative correlations with BMI (r = −0.203, p = 0.025), eGFR (r = −0.321, p < 0.001), and plasma C-peptide level (r = −0.23, p = 0.011). Interestingly, the plasma levels of AGE, Nε-(carboxymethyl) lysine and an AGE-precursor, 3-doxyglucosone, showed no significant associations with skin AF. In addition, surrogate markers of oxidative stress, such as 8-OHdG, 8-isoprostane, and MDA-LDL, did not correlate with skin AF. Next, we performed multiple linear regression analyses to identify variables independently related to skin AF (Table 3). According to multivariate analysis, age and systolic blood pressure were strongly positively related to skin AF, whereas eGFR showed a negative correlation with skin AF values. Intriguingly, logCACS was identified as a variable independently associated with skin AF, whereas neither max IMT nor baPWV showed a significant association with AF. Certain classes of oral medications, such as metformin, dipeptidyl peptidase (DPP)-4 inhibitors, statins, and renin-angiotensin system (RAS) inhibitors, apparently have minor effects on skin AF values.
Table 2. Correlations of skin AF with clinical parameters.
| Variable | Correlation coefficient | P value |
|---|---|---|
| Age, years | 0.375 | < 0.001 |
| Sex | −0.07 | 0.936 |
| History of smoking | 0.039 | 0.67 |
| Body mass index, kg/m2 | −0.203 | 0.025 |
| Diabetes duration, years | 0.338 | < 0.001 |
| Systolic blood pressure, mmHg | 0.233 | 0.01 |
| Diastolic blood pressure, mmHg | 0.073 | 0.429 |
| HbA1c, % | −0.038 | 0.677 |
| Fasting blood glucose, mg/dl | −0.013 | 0.886 |
| eGFR (ml/min per 1.73 m2) | −0.321 | < 0.001 |
| Serum creatinine, mg/dl | 0.206 | 0.023 |
| LDL-C, mg/dl | 0.031 | 0.733 |
| HDL-C, mg/dl | −0.076 | 0.403 |
| TG, mg/dl | −0.096 | 0.294 |
| Urinary 8-isoprostane, pg/mgCr | −0.121 | 0.193 |
| Urinary 8-OHdG, ng/mgCr | −0.081 | 0.374 |
| MDA-LDL-C, U/dl | −0.01 | 0.915 |
| max IMT, mm | 0.159 | 0.08 |
| mean baPWV, cm/s | 0.335 | < 0.001 |
| mean ABI | −0.66 | 0.47 |
| log CACS, AU | 0.344 | < 0.001 |
| C-peptide, ng/ml | −0.23 | 0.011 |
| N ε-(carboxymethyl) lysine, µ g/ml | 0.086 | 0.377 |
| 3-deoxyglucosone, ng/ml | −0.147 | 0.13 |
| High-sensitivity C-reactive protein, mg/l | −0.07 | 0.446 |
Spearman rank correlation coefficient
Table 3. Determinants of skin AF in multivariate regression analysis.
| Factors | β | P value |
|---|---|---|
| Age | 0.269 | 0.041 |
| Sex | 0.06 | n.s. |
| History of smoking | 0.034 | n.s. |
| BMI | −0.140 | n.s. |
| Systolic blood pressure | 0.218 | 0.017 |
| HbA1c | 0.077 | n.s. |
| Fasting blood glucose | 0.160 | n.s. |
| eGFR | −0.256 | 0.007 |
| max IMT | −0.135 | n.s. |
| baPWV | −0.030 | n.s. |
| logCACS | 0.222 | 0.04 |
| Metformin | −0.137 | n.s. |
| DPP-4 inhibitors | −0.037 | n.s. |
| Statins | −0.149 | n.s. |
| RAS inhibitors | 0.082 | n.s. |
β is the standard coefficient; the multiple coefficient of determina-tion (R2) = 0.308
In our Japanese subjects with type 2 diabetes, CACS showed a strong association with tissue accumulation of AGE, as evaluated employing a skin AGE analyzer. Thus, we next performed further examinations to detect factors influencing CACS. When we stratified patients by tertile of skin AF, a significant increase in logCACS was observed across these tertiles (Fig. 1). This result confirmed the strong association of skin AF with coronary atherosclerosis. Next, we stratified the patients into groups according to the degree of CACS, i.e., CACS = 0 (n = 43), CACS ≥ 1 to 399 (n = 59), and CACS ≥ 400 (n = 20), and performed linear regression analysis. We observed significant increasing trends for age (p < 0.001), diabetes duration (p = 0.01), max IMT (p < 0.001), baPWV (p < 0.001), and skin AF (p = 0.008). In contrast, fasting plasma glucose (p = 0.014) and eGFR (p = 0.001) showed decreasing trends (Table 4). Multiple linear regression analysis, adjusted for age, gender, BMI, diabetes duration, HbA1c, history of smoking, systolic blood pressure, eGFR, skin AF, and max IMT, revealed age (β = 0.366, p < 0.01), max IMT (β = 0.351, p < 0.01) and skin AF (β = 0.169, p = 0.026) to be the only parameters showing independent statistically significant associations with CACS (Table 5). In contrast, a similar multiple linear regression analysis revealed skin AF to not be independently associated with baPWV. Finally, to determine the significance of measuring skin AF as a predictor of subclinical atherosclerosis in Japanese population, the AUC was assessed using ROC analysis of surrogate markers of atherosclerosis. The AUC for skin AF to discriminate CACS > 100, which was reportedly recognized as a cut-off value for predicting cardiovascular morbidity in Japanese people27), was 0.698 (95% CI, 0.602–0795, p < 0.001), a value much higher than those obtained for PWV and max IMT (Fig. 2).
Fig. 1.
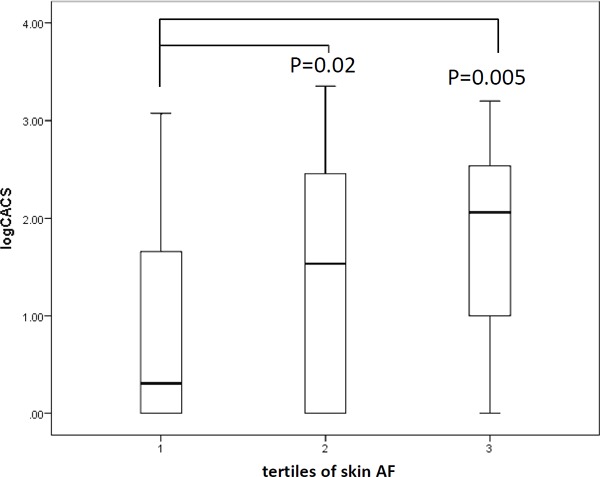
Box plot of logarithmically converted CACS according to stratification of the subjects by tertiles of skin AF.
Table 4. Characteristics of Patients According to Coronary Artery Calcium Scores.
| CACS 0 (n = 43) | CACS 1–399 (n = 59) | CACS ≧400 (n = 20) | P for Trend | |
|---|---|---|---|---|
| Age, years | 51.9 ± 13.7 | 64.6 ± 8.8 | 69.1 ± 8.9 | P < 0.001* |
| Male sex, n (%) | 26 (60) | 33 (56) | 13 (65) | P = 0.8 |
| History of smoking, n (%) | 21 (49) | 31 (53) | 8 (40) | P = 0.6 |
| Body mass index, kg/m2 | 27.4 ± 4.8 | 26.0 ± 5.5 | 25.7 ± 3.8 | P = 0.2 |
| Diabetes duration, years | 8.1 ± 7.3 | 11.0 ± 10.2 | 15.2 ± 9.0 | P = 0.01* |
| Skin AF, AU | 2.27 ± 0.40 | 2.48 ± 0.43 | 2.57 ± 0.32 | P = 0.008* |
| Systolic blood pressure, mmHg | 131.0 ± 17.7 | 132.0 ± 18.8 | 132.3 ± 15.1 | P = 0.9 |
| Diastolic blood pressure, mmHg | 75.4 ± 10.3 | 76.1 ± 13.4 | 71.3 ± 11.1 | P = 0.4 |
| HbA1c, % | 9.66 ± 2.44 | 7.85 ± 2.10 | 8.33 ± 2.02 | P < 0.001* |
| Fasting blood glucose, mg/dl | 172.8 ± 60.4 | 145.0 ± 41.0 | 137.7 ± 45.6 | P = 0.014* |
| eGFR (ml/min per 1.73 m2) | 85.1 ± 20.7 | 76.2 ± 25.3 | 64.4 ± 17.8 | P = 0.001* |
| LDL cholesterol, mg/dl | 107.2 ± 26.5 | 113.3 ± 33.0 | 106.0 ± 27.7 | P = 0.6 |
| HDL cholesterol, mg/dl | 49.4 ± 11.8 | 53.8 ± 16.3 | 53.3 ± 23.3 | P = 0.6 |
| TG, mg/dl | 143.6 ± 67.5 | 141.8 ± 66.7 | 117.0 ± 84.3 | P = 0.4 |
| Urinary 8-isoprostane, pg/mgCr | 260.8 ± 140 | 248 ± 207 | 321 ± 507 | P = 0.2 |
| Urinary 8-OHdG, ng/mgCr | 11.3 ± 7.9 | 11.2 ± 7.4 | 12.3 ± 9.2 | P = 1.0 |
| MDA-LDL-C, U/dl | 125.5 ± 31.9 | 139.6 ± 45.2 | 130.0 ± 51.4 | P = 0.3 |
| High-sensitivity C-reactive protein, mg/l | 0.57 ± 2.44 | 0.19 ± 0.33 | 0.064 ± 0.046 | P = 0.1 |
| N ε-(carboxymethyl) lysine, µ g/ml | 3.55 ± 0.99 | 3.42 ± 1.03 | 3.72 ± 1.08 | P = 0.4 |
| 3-deoxyglucosone, ng/ml | 26.6 ± 18.1 | 23.9 ± 13.2 | 23.6 ± 14.2 | P = 0.9 |
| max IMT, mm | 1.32 ± 0.6 | 1.66 ± 0.61 | 2.3 ± 0.70 | P < 0.001* |
| mean baPWV | 1425 ± 235 | 1579 ± 284 | 1851 ± 344 | P < 0.001* |
Values are means ± SD.
# p < 0.05 (Kruskal Wallis test and the Chi-Square test)
Table 5. Determinants of logCACS in multiple regression analysis.
| Factors | β | P value |
|---|---|---|
| Age | 0.366 | < 0.01 |
| max IMT | 0.351 | < 0.01 |
| skin AF | 0.169 | 0.026 |
β is the standard coefficient; the multiple coefficient of determination (R2)= 0.439
Fig. 2.
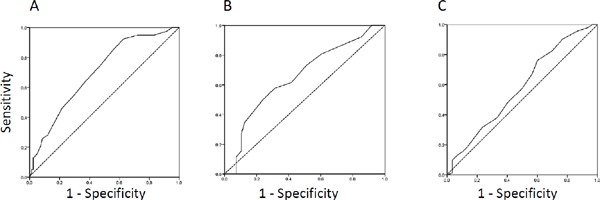
ROC curve analysis of AF for predicting subclinical atherosclerosis.
A. CACS > 100, area under the curve (AUC) = 0.698 (95% CI, 0.602–0795, p < 0.001). B. PWV > 1800 cm/s, AUC = 0.655 (95% CI, 0.538–0773, p < 0.05). C. max IMT > 1.1 mm, AUC = 0.582 (95% CI, 0.460–0704, n.s.).
Discussion
To our knowledge, this study is the first to demonstrate a close relationship between coronary artery calcification and skin AF, reflecting tissue accumulation of AGE in Japanese subjects with type 2 diabetes. Because the incidence of CVD in the Japanese population has been rising in recent decades, a rapid, cost-effective, and simple method of evaluating atherosclerosis in routine practice is urgently needed. Our present observations support the advantages of measuring skin AF, a useful marker for detecting subclinical atherosclerosis.
For the 10 years since the initial report introducing the AGE Reader14), evidence has been mounting that skin AF may predict vascular complications in Caucasians28). Because AGE result from hyperglycemia, a number of studies have been conducted to assess the relationship between skin AF and diabetic microvascular complications, including nephropathy and neuropathy19). Moreover, the associations with surrogate markers of atherosclerosis29), as well as cardiovascular mortality17), have been documented in Caucasian populations. The relationship between coronary artery calcification and skin AF was also examined in Caucasian subjects with type 1 diabetes18) and nondiabetes30). Several studies of Asian populations have identified associations of skin AF with markers of atherosclerosis, including coronary artery calcification in CKD subjects31). Furthermore, the associations of skin AF with the presence of CVD32) and cardiovascular mortality33) were also examined mainly in patients with CKD. Therefore, the present results obtained in Japanese subjects with type 2 diabetes may facilitate understanding the utility of measuring skin AF for predicting atherosclerosis development in Japanese populations.
Because AGE are eliminated by the kidneys, we excluded subjects with renal failure from this study, as decreased clearance would result in tissue AGE accumulation34). Patients with renal dysfunction are regarded as being extremely sensitive to tissue AGE accumulation. In fact, the associations of skin AF with various complications have been most extensively investigated in subjects with CKD16). Although it is still possible that the relationship between skin AF and CACS observed in this study is partially attributable to confounding by renal dysfunction, the statistical significance of this relationship was independent of eGFR in our diabetic subjects.
Skin AF was measured noninvasively, quickly, and conveniently employing a desktop instrument. In addition, the inter-observer variability of skin AF values was relatively small14) as compared to other physiological test values requiring more complex techniques. Furthermore, CACS has been established as a reliable marker for detecting subclinical atherosclerosis and predicting CVD events. In the United States and European countries, CACS measurement is recommended in asymptomatic subjects with intermediate risk (10 – 20% CVD risk over 10 years) for assessing whether preventive therapy is needed4). However, MDCT examinations can be inconvenient and rather expensive for routine practice in subjects with type 2 diabetes, whereas radiation exposure with MDCT, up to 1.2 millisieverts, is not considered to pose a significant risk. The most important aspect of this study is that several surrogate markers, such as carotid IMT, PWV, and CACS, widely used in daily practice, were comparatively assessed to demonstrate the significance of skin AF for the evaluation of atherosclerosis in a Japanese population. Previously, the relationships between skin AF and CACS were only reported in a European population with a small number of diabetic subjects30), American subjects with type 1 diabetes18) and Chinese subjects with CKD31). Furthermore, the relationships between skin AF and IMT were assessed in European nondiabetic subjects29) and Japanese CKD subjects35). The association of skin AF with PWV has not yet been reported. Interestingly, Dekker et al. reported that skin AF correlated with carotid IMT but not with CACS in a study population with a small number of diabetic subjects30). As shown in Fig. 2, our results revealed skin AF to show a stronger association with CACS than either PWV or IMT, suggesting that prolonged hyperglycemia-induced AGE accumulation may be closely associated with the pathogenesis of aortic calcification. Therefore, skin AF measurement holds great promise as a screening tool for diabetic vascular complications.
Accumulating evidence obtained with the AGE reader has raised unexpected issues regarding the pathological roles of tissue AGE aggregation in various disorders, such as rheumatoid arthritis36), foot ulceration37), schizophrenia38), and cognitive dysfunction39). Because the reasons for tissue AGE accumulation being related to these conditions are unclear, further research aimed at both elucidating the underlying mechanism and developing strategies for reducing AGE accumulation is required. Although statins and RAS inhibitors40) reportedly reduce circulating levels of AGE, currently administered medications, such as statins, anti-diabetic, and anti-hypertensive agents, did not alter the skin AF values in our small-group investigation. Clinical trials of interventions with agents that decrease AGE accumulation, i.e., AGE breakers or inhibitors of AGE formation, are expected in the near future. We anticipate that skin AF measurement will ultimately come into widespread use for investigating the roles of AGE in various diseases.
In our subjects, the serum levels of nonfluorescent AGE, such as Nε-(carboxymethyl) lysine and 3-deoxyglucosone, showed no associations with CACS. Furthermore, biological markers of oxidative stress, such as plasma MDA-LDL and urinary 8-OHdG and 8-isoprostane, also showed no associations with either skin AF or CACS in this study. These results may suggest the development of macrovascular complications are affected by long-term hyperglycemia-induced tissue AGE accumulation rather than current increases in markers of AGE and oxidative stress in plasma.
The major limitation of this study is its cross-sectional design, raising the possibility that our results show only associations. However, coronary artery calcification as assessed by MDCT is regarded as an excellent surrogate marker of cardiovascular mortality in Japanese subjects5), such that the observed cross-sectional associations in our living study participants still have important clinical implications. Second, the instrument used, the AGE reader, cannot be applied to subjects with dark skin because of the high absorption grade of the excited light. To address this problem, we excluded patients with skin reflectance below 6% in accordance with a recent report on a non-Caucasian population31). Although Meerwaldt et al. demonstrated significant correlations of skin AF with tissue levels of Nε-(carboxymethyl) lysine, Nε-(carboxyethyl) lysine, and pentosidine14, 41), we did not directly perform histological examinations of skin accumulation of AGE in our subjects. Moreover, the detection capability of skin AGE accumulation by the AGE reader is limited because some types of AGE are not fluorescent. A critical issue of our study is the lack of measurement for serum concentrations of fluorescent AGE, such as pyrraline and pentosidine. In particular, pentosidine is considered as one of the major components of AGEs, leading to vascular complication. In a previous report with Japanese subjects, high serum concentration of pentosidine was closely associated with both increasing arterial stiffness and thickening carotid IMT42). We will try to clarify the relationships among diabetic complications, skin AF values, and serum concentration of fluorescent AGE in a further study. Third, our sample size was too small to allow sufficiently powered statistical analyses to be performed. Furthermore, a recent study showed that CAC scoring employing a combination that includes CAC density would increase the predictive values for CHD43). This possibility merits further examination.
Conclusion
Skin AF results positively correlated with CACS in Japanese subjects with type 2 diabetes. This observation indicates tissue AGE accumulation plays a role in the pathogenesis of diabetic macrovascular disease. Skin AF measurements may be useful for assessing the severity of diabetic complications in Japanese patients.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (24591107) to Y.I., from the Japan Society for the Promotion of Science and a Grant-in-Aid for Strategic Medical Science Research (S1491001, 2014–2018) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of Interest
Dr. Yasushi Ishigaki has received lecture fees from Astellas Pharma Inc., Novartis Pharma K.K., Novo Nordisk Pharma Ltd., Kowa Pharmaceutical Co., Ltd., Sanofi Co., Ltd., Mitsubishi Tanabe Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., and MSD Co., Ltd. and scholarship grants from Mitsubishi Tanabe Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd. and MSD Co., Ltd. Dr. Jo Satoh has received lecture fees from Astellas Pharma Inc., Astra Zeneca Co., Ltd., Dainippon-Sumitomo Pharma Co., Ltd., Sanofi Co., Ltd., Mitsubishi Tanabe Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., and MSD Co., Ltd.
References
- 1). Kannel WB, McGee DL: Diabetes and cardiovascular disease. The Framingham study. JAMA 1979; 241: 2035-2038 [DOI] [PubMed] [Google Scholar]
- 2). Doi Y, Ninomiya T, Hata J, Fukuhara M, Yonemoto K, Iwase M, Iida M, Kiyohara Y: Impact of glucose tolerance status on development of ischemic stroke and coronary heart disease in a general Japanese population: the Hisayama study. Stroke 2010; 41: 203-209 [DOI] [PubMed] [Google Scholar]
- 3). Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA: Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008; 358: 1336-1345 [DOI] [PubMed] [Google Scholar]
- 4). Elkeles RS: Coronary artery calcium and cardiovascular risk in diabetes. Atherosclerosis 2010; 210: 331-336 [DOI] [PubMed] [Google Scholar]
- 5). Yamamoto H, Kitagawa T, Kihara Y: Clinical implications of the coronary artery calcium score in Japanese patients. J Atheroscler Thromb 2014; 21: 1101-1108 [DOI] [PubMed] [Google Scholar]
- 6). Ono M, Takebe N, Oda T, Nakagawa R, Matsui M, Sasai T, Nagasawa K, Honma H, Kajiwara T, Taneichi H, Takahashi Y, Takahashi K, Satoh J: Association of coronary artery calcification with MDA-LDL-C/LDL-C and urinary 8-isoprostane in Japanese patients with type 2 diabetes. Intern Med 2014; 53: 391-396 [DOI] [PubMed] [Google Scholar]
- 7). Sasai T, Takebe N, Ono M, Matsui M, Honma H, Fujiwara F, Kajiwara T, Taneichi H, Takahashi K, Satoh J: Coronary artery calcification to assess coronary heart disease risk inJapanese patient with type 2 diadetes. J Iwate Med Assoc 2012; 64: 363-369 [Google Scholar]
- 8). Beisswenger PJ, Makita Z, Curphey TJ, Moore LL, Jean S, Brinck-Johnsen T, Bucala R, Vlassara H: Formation of immunochemical advanced glycosylation end products precedes and correlates with early manifestations of renal and retinal disease in diabetes. Diabetes 1995; 44: 824-829 [DOI] [PubMed] [Google Scholar]
- 9). Yamagishi S, Imaizumi T: Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des 2005; 11: 2279-2299 [DOI] [PubMed] [Google Scholar]
- 10). Myint KM, Yamamoto Y, Sakurai S, Harashima A, Watanabe T, Li H, Takeuchi A, Yoshimura K, Yonekura H, Yamamoto H: Blockade of diabetic vascular injury by controlling of AGE-RAGE system. Curr Drug Targets 2005; 6: 447-452 [DOI] [PubMed] [Google Scholar]
- 11). Hanssen NM, Engelen L, Ferreira I, Scheijen JL, Huijberts MS, van Greevenbroek MM, van der Kallen CJ, Dekker JM, Nijpels G, Stehouwer CD, Schalkwijk CG: Plasma levels of advanced glycation endproducts Nepsilon-(carboxymethyl)lysine, Nepsilon-(carboxyethyl)lysine, and pentosidine are not independently associated with cardiovascular disease in individuals with or without type 2 diabetes: the Hoorn and CODAM studies. J Clin Endocrinol Metab 2013; 98: E1369-1373 [DOI] [PubMed] [Google Scholar]
- 12). Monnier VM, Bautista O, Kenny D, Sell DR, Fogarty J, Dahms W, Cleary PA, Lachin J, Genuth S: Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes 1999; 48: 870-880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Hricik DE, Wu YC, Schulak A, Friedlander MA: Disparate changes in plasma and tissue pentosidine levels after kidney and kidney-pancreas transplantation. Clin Transplant 1996; 10: 568-573 [PubMed] [Google Scholar]
- 14). Meerwaldt R, Graaff R, Oomen PH, Links TP, Jager JJ, Alderson NL, Thorpe SR, Baynes JW, Gans RO, Smit AJ: Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004; 47: 1324-1330 [DOI] [PubMed] [Google Scholar]
- 15). Meerwaldt R, Hartog JW, Graaff R, Huisman RJ, Links TP, den Hollander NC, Thorpe SR, Baynes JW, Navis G, Gans RO, Smit AJ: Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol 2005; 16: 3687-3693 [DOI] [PubMed] [Google Scholar]
- 16). Arsov S, Graaff R, van Oeveren W, Stegmayr B, Sikole A, Rakhorst G, Smit AJ: Advanced glycation end-products and skin autofluorescence in end-stage renal disease: a review. Clin Chem Lab Med 2014; 52: 11-20 [DOI] [PubMed] [Google Scholar]
- 17). Meerwaldt R, Lutgers HL, Links TP, Graaff R, Baynes JW, Gans RO, Smit AJ: Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care 2007; 30: 107-112 [DOI] [PubMed] [Google Scholar]
- 18). Conway B, Edmundowicz D, Matter N, Maynard J, Orchard T: Skin fluorescence correlates strongly with coronary artery calcification severity in type 1 diabetes. Diabetes Technol Ther 2010; 12: 339-345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Gerrits EG, Lutgers HL, Kleefstra N, Graaff R, Groenier KH, Smit AJ, Gans RO, Bilo HJ: Skin autofluorescence: a tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care 2008; 31: 517-521 [DOI] [PubMed] [Google Scholar]
- 20). Tanaka K, Tani Y, Asai J, Nemoto F, Kusano Y, Suzuki H, Hayashi Y, Asahi K, Nakayama M, Miyata T, Watanabe T: Skin autofluorescence is associated with severity of vascular complications in Japanese patients with Type 2 diabetes. Diabet Med 2012; 29: 492-500 [DOI] [PubMed] [Google Scholar]
- 21). Sugisawa E, Miura J, Iwamoto Y, Uchigata Y: Skin autofluorescence reflects integration of past long-term glycemic control in patients with type 1 diabetes. Diabetes Care 2013; 36: 2339-2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). de Ranitz-Greven WL, Bos DC, Poucki WK, Visser GH, Beulens JW, Biesma DH, de Valk HW: Advanced glycation end products, measured as skin autofluorescence, at diagnosis in gestational diabetes mellitus compared with normal pregnancy. Diabetes Technol Ther 2012; 14: 43-49 [DOI] [PubMed] [Google Scholar]
- 23). Yue X, Hu H, Koetsier M, Graaff R, Han C: Reference values for the Chinese population of skin autofluorescence as a marker of advanced glycation end products accumulated in tissue. Diabet Med 2011; 28: 818-823 [DOI] [PubMed] [Google Scholar]
- 24). Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R: Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15: 827-832 [DOI] [PubMed] [Google Scholar]
- 25). Okimoto H, Ishigaki Y, Koiwa Y, Hinokio Y, Ogihara T, Suzuki S, Katagiri H, Ohkubo T, Hasegawa H, Kanai H, Oka Y: A novel method for evaluating human carotid artery elasticity: possible detection of early stage atherosclerosis in subjects with type 2 diabetes. Atherosclerosis 2008; 196: 391-397 [DOI] [PubMed] [Google Scholar]
- 26). Tanaka K, Nakayama M, Kanno M, Kimura H, Watanabe K, Tani Y, Kusano Y, Suzuki H, Hayashi Y, Asahi K, Sato K, Miyata T, Watanabe T: Skin autofluorescence is associated with the progression of chronic kidney disease: a prospective observational study. PLoS One 2013; 8: e83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Kunita E, Yamamoto H, Kitagawa T, Ohashi N, Oka T, Utsunomiya H, Urabe Y, Tsushima H, Awai K, Budoff MJ, Kihara Y: Prognostic value of coronary artery calcium and epicardial adipose tissue assessed by non-contrast cardiac computed tomography. Atherosclerosis 2014; 233: 447-453 [DOI] [PubMed] [Google Scholar]
- 28). Bos DC, de Ranitz-Greven WL, de Valk HW: Advanced glycation end products, measured as skin autofluorescence and diabetes complications: a systematic review. Diabetes Technol Ther 2011; 13: 773-779 [DOI] [PubMed] [Google Scholar]
- 29). Lutgers HL, Graaff R, deVries R, Smit AJ, Dullaart RP: Carotid artery intima media thickness associates with skin autofluoresence in non-diabetic subjects without clinically manifest cardiovascular disease. Eur J Clin Invest 2010; 40: 812-817 [DOI] [PubMed] [Google Scholar]
- 30). den Dekker MA, Zwiers M, van den Heuvel ER, de Vos LC, Smit AJ, Zeebregts CJ, Oudkerk M, Vliegenthart R, Lefrandt JD, Mulder DJ: Skin autofluorescence, a noninvasive marker for AGE accumulation, is associated with the degree of atherosclerosis. PLoS One 2013; 8: e83084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Wang AY, Wong CK, Yau YY, Wong S, Chan IH, Lam CW: Skin autofluorescence associates with vascular calcification in chronic kidney disease. Arterioscler Thromb Vasc Biol 2014; 34: 1784-1790 [DOI] [PubMed] [Google Scholar]
- 32). Tanaka K, Tani Y, Asai J, Nemoto F, Kusano Y, Suzuki H, Hayashi Y, Asahi K, Katoh T, Miyata T, Watanabe T: Skin autofluorescence is associated with renal function and cardiovascular diseases in pre-dialysis chronic kidney disease patients. Nephrol Dial Transplant 2011; 26: 214-220 [DOI] [PubMed] [Google Scholar]
- 33). Kimura H, Tanaka K, Kanno M, Watanabe K, Hayashi Y, Asahi K, Suzuki H, Sato K, Sakaue M, Terawaki H, Nakayama M, Miyata T, Watanabe T: Skin autofluorescence predicts cardiovascular mortality in patients on chronic hemodialysis. Ther Apher Dial 2014; 18: 461-467 [DOI] [PubMed] [Google Scholar]
- 34). Miyata T, Wada Y, Cai Z, Iida Y, Horie K, Yasuda Y, Maeda K, Kurokawa K, van Ypersele de Strihou C: Implication of an increased oxidative stress in the formation of advanced glycation end products in patients with endstage renal failure. Kidney Int 1997; 51: 1170-1181 [DOI] [PubMed] [Google Scholar]
- 35). Tanaka K, Katoh T, Asai J, Nemoto F, Suzuki H, Asahi K, Sato K, Sakaue M, Miyata T, Watanabe T: Relationship of skin autofluorescence to cardiovascular disease in Japanese hemodialysis patients. Ther Apher Dial 2010; 14: 334-340 [DOI] [PubMed] [Google Scholar]
- 36). Matsumoto T, Tsurumoto T, Baba H, Osaki M, Enomoto H, Yonekura A, Shindo H, Miyata T: Measurement of advanced glycation endproducts in skin of patients with rheumatoid arthritis, osteoarthritis, and dialysis-related spondyloarthropathy using non-invasive methods. Rheumatol Int 2007; 28: 157-160 [DOI] [PubMed] [Google Scholar]
- 37). Vouillarmet J, Maucort-Boulch D, Michon P, Thivolet C: Advanced glycation end products assessed by skin autofluorescence: a new marker of diabetic foot ulceration. Diabetes Technol Ther 2013; 15: 601-605 [DOI] [PubMed] [Google Scholar]
- 38). Kouidrat Y, Amad A, Desailloud R, Diouf M, Fertout E, Scoury D, Lalau JD, Loas G: Increased advanced glyca tion end-products (AGEs) assessed by skin autofluorescence in schizophrenia. J Psychiatr Res 2013; 47: 1044-1048 [DOI] [PubMed] [Google Scholar]
- 39). Spauwen PJ, van Eupen MG, Kohler S, Stehouwer CD, Verhey FR, van der Kallen CJ, Sep SJ, Koster A, Schaper NC, Dagnelie PC, Schalkwijk CG, Schram MT, van Boxtel MP: Associations of advanced glycation end-products with cognitive functions in individuals with and without type 2 diabetes: the maastricht study. J Clin Endocrinol Metab 2015; 100: 951-960 [DOI] [PubMed] [Google Scholar]
- 40). Yamagishi S, Nakamura K, Matsui T: Potential utility of telmisartan, an angiotensin II type 1 receptor blocker with peroxisome proliferator-activated receptor-gamma (PPARgamma)- modulating activity for the treatment of cardiometabolic disorders. Curr Mol Med 2007; 7: 463-469 [DOI] [PubMed] [Google Scholar]
- 41). Meerwaldt R, Links T, Graaff R, Thorpe SR, Baynes JW, Hartog J, Gans R, Smit A: Simple noninvasive measurement of skin autofluorescence. Ann N Y Acad Sci 2005; 1043: 290-298 [DOI] [PubMed] [Google Scholar]
- 42). Yoshida N, Okumura K, Aso Y: High serum pentosidine concentrations are associated with increased arterial stiffness and thickness in patients with type 2 diabetes. Metabolism 2005; 54: 345-350 [DOI] [PubMed] [Google Scholar]
- 43). Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA: Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2014; 311: 271-278 [DOI] [PMC free article] [PubMed] [Google Scholar]


