Abstract
Aim: This study aims to determine the association between glucose metabolism and proinflammatory/anti-inflammatory properties of circulating monocytes or those of carotid plaques in patients who underwent carotid endarterectomy.
Methods: Clinical characteristics and expression levels of proinflammatory/anti-inflammatory markers in circulating monocytes/carotid plaques were examined in 12 patients with diabetes and 12 patients without diabetes.
Results: Circulating monocytes from patients with diabetes revealed higher tumor necrosis factor (TNF)-α and lower interleukin (IL)-10 expression levels compared with those from patients without diabetes, which was also observed in carotid plaques from patients with diabetes. Hyperglycemia revealed positive and negative correlations with the ratios of IL-6+ and IL-10+ cells in carotid plaques, respectively. Moreover, we determined a positive correlation between circulating monocytes and carotid plaques with respect to TNF-α and IL-6 expressions.
Conclusions: The inflammatory property of circulating monocytes was associated with that of carotid plaques. Hyperglycemia increased inflammatory properties and decreased anti-inflammatory properties of carotid plaques.
Keywords: Monocytes, Hyperglycemia, Carotid plaque, Diabetes, Atherosclerosis
Introduction
Type 2 diabetes contributes to an increased risk of developing atherosclerotic diseases such as coronary heart disease and stroke1, 2). The Hisayama study demonstrated that diabetes is an independent risk factor for ischemic stroke in the Japanese population3). Therefore, it is of clinical importance to predict and evaluate the onset of stroke in patients with diabetes. The monocyte/macrophage (Mφ) system plays critical roles in the pathogenesis of inflammatory diseases, including atherosclerosis. This system demonstrates at least two distinct phenotypes of differentiation: proinflammatory (M1) and anti-inflammatory (M2)4, 5). M1 Mφ in atherosclerotic plaques secrete inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), and promote plaque development and rupture, thereby contributing to the formation of atherothrombosis6, 7). Regarding the properties of circulating monocytes, this and other studies previously reported the unfavorable inflammatory balance of M1/M2-like phenotypes of circulating monocytes in obese patients with and without diabetes4, 8). This study was designed to elucidate the association of glucose metabolism and inflammatory/anti-inflammatory properties of circulating monocytes with those of carotid plaques in patients who underwent carotid endarterectomy (CEA).
Methods
Subjects
A total of 24 male Japanese patients with advanced carotid plaques who underwent CEA were recruited from the Department of Neurosurgery of our hospital. The study protocol was approved by the Ethics Committee for Human Research at the Kyoto Medical Center (ID: 11-06). Written informed con-sent was obtained from all participants. This study is registered in the University Hospital Medical Information Network Clinical Trial Registry (UMIN-CTR) System (ID: UMIN000019115).
Data Collection and Laboratory Methods
Anthropometric and metabolic parameters were measured as previously described5, 9, 10). The HbA1c levels were measured before the meal on the day prior to CEA. The insulin resistance index was assessed using the homeostasis model assessment of insulin resistance (HOMA-IR). Human circulating monocytes were obtained from blood samples and were analyzed as previously described5, 9, 10). In brief, peripheral blood mononuclear cells were collected from heparin-ized blood samples by density-gradient centrifugation with lymphocyte separation solution (Nacalai Tesque, Kyoto, Japan) before the meal on the day prior to CEA. Circulating monocytes were then isolated by magnetic-activated cell sorting with anti-human CD14 immunomagnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany).
The expression levels of genes of interest were analyzed using quantitative RT-PCR and immunohistochemistry using standard procedures5, 9–11). The primers used in the assays were purchased from Sigma-Aldrich (Tokyo, Japan), and the primer sequences are listed in Supplementary Table 1. The primary antibodies for immunohistochemistry were as follows: anti-TNF-α (R&D Systems, Minneapolis, MN, USA), anti-interleukin (IL)-6 (R&D Systems), antiIL-10 (R&D Systems), anti-major histocompatibility complex class II (MHC-II) (Abcam plc, Cambridge, UK), anti-CD86 (Abcam plc), anti-mannose receptor CD206 (Abcam), and anti-CD3 (Abcam). For quantitative analysis in immunohistochemistry, two areas were randomly selected from each serial section of carotid plaques. The number of immunohistochemically positive cells of interest was divided by the total number of cells obtained by counterstaining with hematoxylin, and the ratio of each positive cell was obtained.
Supplementary Table 1. Primer sequences for quantitative real-time PCR.
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) | Reference |
|---|---|---|---|
| GAPDH | TGCACCACCAACTGCTTAGC | GGATGCAGGGATGATGTTCTG | 1 |
| TNF-α | CGCTCTTCTGCCTGCTGCACTT | TCGGGGTTCGAGAAGATGATCTGAC | This study |
| IL-6 | AAATGCCAGCCTGCTGACGAAG | AACAACAATCTGAGGTGCCCATGCTAC | 2 |
| IL-10 | GTGATGCCCCAAGCTGAGA | CACGGCCTTGCTCTTGTTTT | RTPrimer DB ID: 8230 |
| CD14 | CAGTATGCTGACACGGTCAAGG | ATCTCGGAGCGCTAGGGTTTA | This study |
| CD16 | CGGTGCAGCTAGAAGTCCAT | GGTTGACACTGCCAAACCTTG | This study |
| CD68 | CATCCCCACCTGCTTCTCTC | GAGAATGTCCACTGTGCTGC | This study |
| MHC-II | GCATGGTGTGTCTGAAGCTC | CAGCTCCAAGAAACGTGGTC | 3 |
| CD86 | AGCACAGACACACGGATGAG | AGAGGAGCAGCACCAGAGAG | This study |
| CD206 | CTTTGGACGGATGGACGAGG | GGATTAGTCAAGGAAGGGTCGGA | This study |
| Dectin-1 | TGTGCTGCATCTCCTCCTTG | GGCTGGAAAGAACCCCTGTG | This study |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TNF-α, tumor necrosis factor-a; IL-6, interleukin-6; IL-10, interleukin-10; MHC-II, major histocompatibility complex, class II.
Statistical Analysis
If normally distributed, continuous data were reported as mean ± standard deviation, and if not normally distributed, they were reported as median and interquartile range (IQR). Student's t-test was used for comparisons of the means between the two groups. If data were not normally distributed, the Mann–Whitney U test was used. For comparisons of the median among the ratios of immunohistochemically positive cells, the Wilcoxon signed-rank test was used. Spearman's rank correlation coefficient (ρ) was used to investigate the correlations between proinflammatory/anti-inflammatory parameters (expression levels in circulating monocytes and carotid plaques and percentage of positive cells in the plaque) and metabolic parameters. Moreover, Spearman's partial rank correlation coefficients (ρpa) were obtained by employing medications (calcium antagonist, ACE/ARB, and statins) as control variables. A P value of <0.05 was considered significant. All statistical analyses were performed with SPSS v.22.0 for Windows (IBM Japan, Ltd., Tokyo, Japan).
Results
Of all 24 patients who underwent CEA (12 with diabetes and 12 without diabetes; mean age, 72.3 ± 8.2 years), the serum FPG and HbA1c levels were significantly higher in patients with diabetes than in patients without diabetes (P < 0.001) (Table 1). To elucidate the impact of diabetes on proinflammatory/anti-inflammatory properties of circulating monocytes and carotid plaques, we examined TNF-α, IL-6, and IL-10 expression levels in these cell types and the ratios of cells expressing the cytokines in the plaques (Table 2 and Fig. 1). As shown in Table 2, we found that the relative mRNA level of TNF-α [median, 1.7 (IQR, 1.0–5.6)] was significantly higher and that of IL-10 [0.4 (0.2–0.9)] was significantly lower in circulating monocytes of patients with diabetes than in those of patients without diabetes [TNF-α, 1.0 (0.8–1.7), P = 0.049; IL-10, 1.0 (0.3–1.4), P = 0.025]. With respect to carotid plaques, we found that both the relative mRNA levels of TNF-α [1.7 (1.1–3.3)] and IL-6 [1.8 (1.0–4.1)] were significantly higher in the carotid plaques of patients with diabetes than in those of patients without diabetes [TNF-α, 1.0 (0.7–2.0), P = 0.042; IL-6, 1.0 (0.5–1.1), P = 0.038]. In addition, the percentage of IL-6+ cells [63.2 (40.8–71.1)] was significantly higher and that of IL-10+ cells [58.0 (37.6–65.2)] was significantly lower in carotid plaques of patients with diabetes than in those of patients with diabetes [IL-6+, 42.9 (38.7–54.9), P = 0.040; IL-10+, 70.9 (59.3–83.7), P = 0.019].
Table 1. Clinical characteristics and metabolic parameters of nondiabetic and diabetic patients.
| Nondiabetic patients | Diabetic patients | P-value* | |
|---|---|---|---|
| n (male) | 12 | 12 | |
| Age (years) | 74.2 ± 8.9 | 70.4 ± 7.2 | 0.270 |
| Duration of diabetes (years) | - | 6.5 [2.3–17.5] | - |
| Body weight (kg) | 60.6 ± 9.3 | 63.7 ± 8.4 | 0.392 |
| BMI (kg/m2) | 23.5 ± 3.0 | 23.8 ± 3.1 | 0.772 |
| WC (cm) | 84.9 ± 6.7 | 84.6 ± 9.4 | 0.931 |
| SBP (mmHg) | 133.4 ± 17.3 | 127.3 ± 14.8 | 0.364 |
| DBP (mmHg) | 69.4 ± 13.1 | 71.2 ± 13.8 | 0.753 |
| FPG (mmol/L) | 5.5 ± 0.9 | 7.5 ± 1.4 | <0.001 |
| IRI (pmol/L) | 39.0 [20.3–51.5] | 43.5 [27.8–78.3] | 0.453 |
| HbA1c (%) | 5.6 ± 0.3 | 7.0 ± 0.6 | <0.001 |
| HOMA-IR | 1.9 ± 1.5 | 4.1 ± 4.5 | 0.131 |
| TC (mmol/L) | 4.4 ± 1.1 | 4.1 ± 1.0 | 0.481 |
| TG (mmol/L) | 1.4 [1.1–2.1] | 1.2 [0.8–1.4] | 0.073 |
| HDL-C (mmol/L) | 1.4 ± 0.4 | 1.2 ± 0.3 | 0.278 |
| LDL-C (mmol/L) | 2.5 ± 1.0 | 2.4 ± 0.7 | 0.779 |
| hsCRP (µg/mL) | 1.3 [0.2–4.4] | 2.3 [0.3–3.9] | 0.686 |
| Proportion of patients with/taking: | |||
| hypertension (%) | 83.3 | 100.0 | 0.478 |
| dyslipidemia (%) | 83.3 | 83.3 | >0.999 |
| calcium antagonist (%) | 66.7 | 33.3 | 0.220 |
| ACE/ARB (%) | 41.7 | 91.7 | 0.027 |
| statins (%) | 66.7 | 66.7 | >0.999 |
| antidiabetic medications (%) | 0.0 | 83.3 | - |
Data are shown as the mean ± SD or median [IQR]. BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; IRI, immunoreactive insulin; HbA1c, hemoglobin A1c; HOMA-IR, homeostasis model assessment of insulin resistance; TC, total cholesterol; TG, triglycerides; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; hsCRP, high-sensitive C-reactive protein; ACE, angiotensin-converting enzyme; and ARB, angiotensin II type 1 receptor blocker.
nondiabetic versus diabetic patients
Table 2. Inflammatory/anti-inflammatory properties of monocytes and carotid plaque of nondiabetic and diabetic patients.
| Non-diabetic patients | Diabetic patients | P-value* | |
|---|---|---|---|
| n | 12 | 12 | - |
| mRNA level in monocytes | |||
| TNF-α | 1.0 [0.8–1.7] | 1.7 [1.0–5.6] | 0.049 |
| IL-6 | 1.0 [0.6–1.7] | 0.7 [0.3–1.5] | 0.525 |
| IL-10 | 1.0 [0.3–1.4] | 0.4 [0.2–0.9] | 0.025 |
| CD14 | 1.0 [0.7–1.4] | 1.5 [1.1–1.8] | 0.078 |
| CD16 | 1.0 [0.8–1.4] | 0.8 [0.7–1.5] | 0.862 |
| CD68 | 1.0 [0.8–1.1] | 0.9 [0.8–0.9] | 0.166 |
| mRNA level in plaque | |||
| TNF-α | 1.0 [0.7–2.0] | 1.7 [1.1–3.3] | 0.042 |
| IL-6 | 1.0 [0.5–1.1] | 1.8 [1.0–4.1] | 0.038 |
| IL-10 | 1.0 [0.5–1.4] | 1.4 [0.9–2.0] | 0.149 |
| MHC-II | 1.0 [0.5–1.6] | 0.9 [0.3–1.9] | 0.603 |
| CD86 | 1.0 [0.8–1.8] | 1.0 [0.6–1.6] | 0.908 |
| CD206 | 1.0 [0.6–1.4] | 0.8 [0.4–2.1] | 0.644 |
| Dectin-1 | 1.0 [0.4–2.1] | 1.8 [0.5–6.4] | 0.326 |
| Percent of positive cells in plaque | |||
| TNF-α (%) | 54.2 [44.8–63.9] | 65.5 [52.1–76.1] | 0.193 |
| IL-6 (%) | 42.9 [38.7–54.9] | 63.2 [40.8–71.1] | 0.040 |
| IL-10 (%) | 70.9 [59.3–83.7] | 58.0 [37.6–65.2] | 0.019 |
| MHC-II (%) | 75.4 [66.4–78.8] | 83.1 [73.7–88.1] | 0.094 |
| CD86 (%) | 14.7 [11.9–23.5] | 17.5 [11.4–22.7] | 0.729 |
| CD206 (%) | 32.2 [25.5–42.5] | 37.4 [26.6–40.4] | 0.817 |
| CD3 (%) | 10.3 [8.6–14.0] | 13.0 [8.9–15.0] | 0.299 |
Data are shown as the median [IQR]. The mRNA expression levels of nondiabetic patients are set at 1.0 and relative values are shown.
nondiabetic versus diabetic patients
Fig. 1.
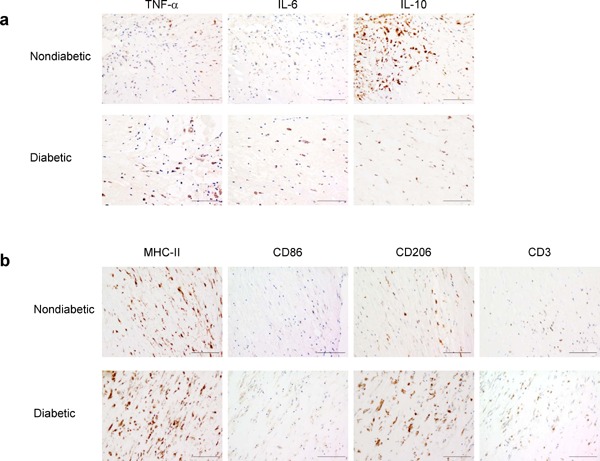
Immunohistochemical analyses of cells expressing proinflammatory/anti-inflammatory cytokines (a), M1/M2 markers, or T-cell markers (b) in carotid plaques of patients without diabetes (upper panels) and thos with diabetes (lower panels) who underwent CEA
Positive cells expressing the cytokine/cell-surface marker of interest were immunohistochemically detected using a specific antibody and a 3, 3′-diaminobenzidine tetrahydrochloride solution, followed by counterstaining with hematoxylin. Magnification, × 200 and scale bars, 100 µm
To further examine the properties of circulating monocytes and carotid plaques, we analyzed the expression levels of cell-surface markers for proinflammatory/anti-inflammatory properties of circulating monocytes/Mφ. Circulating monocytes are classified by the expression levels of markers, such as CD14, CD16, and CD68, thereby reflecting different inflammatory states12, 13). Furthermore, MHC-II, a marker of circulating monocytes/Mφ, and CD86 are upregulated in M1 Mφ, whereas CD206 and Dectin-1 are highly expressed in M2 Mφ14–16). As shown in Table 2 and Fig. 1b, we found no significant difference between patients with and without diabetes with respect to the expression levels of these markers in circulating monocytes and carotid plaques. This differed from that observed for proinflammatory/anti-inflammatory cytokine levels (Table 2 and Fig. 1a). Furthermore, we found that the ratio of cells expressing CD3, a T-cell marker, in plaques was not significantly different between patients with and without diabetes (Table 2). Furthermore, our data revealed that the MHC-II+ ratio was significantly higher than the CD3+ ratio in carotid plaques of both patient without diabetes [75.4 (66.4–78.8) and 10.3 (8.6–14.0), P = 0.002] and those with diabetes [83.1 (73.7–88.1) and 13.0 (8.9–15.0), P = 0.002], thereby suggesting that the Mφ ratio was higher than the T-cell ratio in carotid plaques of both patients.
Next, we examined the correlation between glucose metabolism and inflammatory properties of carotid plaques in all patients who underwent CEA. As shown in Table 3 and Fig. 2, we found that FPG and HbA1c levels displayed significant negative correlations with the IL-10+ ratio in carotid plaques [FPG, ρ = −0.471, P = 0.020 (Fig. 2a); HbA1c, ρ = −0.428, P = 0.037 (Fig. 2b)]. Furthermore, IRI and HOMA-IR revealed significant negative correlations with the IL-10+ ratio in carotid plaques (IRI, ρ = −0.543, P = 0.006; HOMA-IR, ρ = −0.640, P < 0.001) (Table 3). Moreover, as shown in Tables 3 and 4 and Fig. 3, HbA1c levels revealed significant positive correlations with the IL-6+ ratio (ρ = 0.466, P = 0.022) (Table 3 and Fig. 3a) and TNF-α expression levels (ρ = 0.527, P = 0.01) (Table 4 and Fig. 3b) in carotid plaques. The HbA1c level was not significantly correlated with the expression levels of cell-surface markers, such as MHC-II, CD86, CD206, and Dectin-1, and the ratios of MHC-II+, CD86+, CD206+, and CD3+ cells in carotid plaques. In partial rank correlation analyses employing medications as control variables, we observed significant negative correlations between glucose metabolism and the IL-10+ ratio in carotid plaques (FPG, ρpa = −0.488, P = 0.034; IRI, ρpa = −0.540, P = 0.017; HbA1c, ρpa = −0.452, P = 0.048; and HOMA-IR, ρpa = −0.629, P = 0.004), which is similar to the case of single correlation analyses in Table 3 and Fig. 2. Furthermore, we found a significant positive correlation between HbA1c levels and the IL-6+ ratio in carotid plaques (ρpa = 0.606, P = 0.006).
Table 3. Correlations of the positive ratios of cells expressing proinflammatory/anti-inflammatory cytokines in carotid plaque with glucose metabolism or proinflammatory/anti-inflammatory properties of circulating monocytes.
| Percent of positive cells in plaque |
||||||
|---|---|---|---|---|---|---|
| TNF-α |
IL-6 |
IL-10 |
||||
| ρ | P-value | ρ | P-value | ρ | P-value | |
| FPG | 0.191 | 0.371 | 0.346 | 0.098 | −0.471 | 0.020 |
| IRI | −0.066 | 0.759 | −0.030 | 0.891 | −0.543 | 0.006 |
| HbA1c | 0.246 | 0.247 | 0.466 | 0.022 | −0.428 | 0.037 |
| HOMA-IR | −0.014 | 0.947 | 0.087 | 0.686 | −0.640 | <0.001* |
| mRNA level in monocytes | ||||||
| TNF-α | 0.444 | 0.034 | 0.406 | 0.054 | 0.212 | 0.330 |
| IL-6 | 0.309 | 0.142 | 0.148 | 0.491 | −0.130 | 0.544 |
| IL-10 | −0.251 | 0.248 | −0.362 | 0.090 | 0.145 | 0.508 |
FPG, fasting plasma glucose; IRI, immunoreactive insulin; HbA1c, hemoglobin A1c; HOMA-IR, homeostasis model assessment of insulin resistance.
P < = 0.0008.
ρ, Spearman's rank correlation coefficient
Fig. 2.
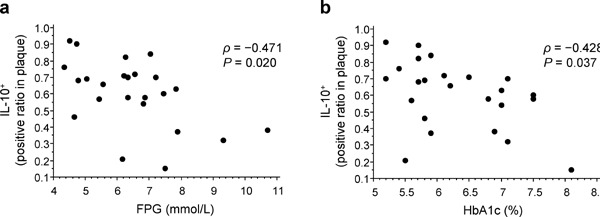
Correlation between glucose metabolism and the IL-10+ ratio in carotid plaques
Scatter plots highlighting the correlation between the IL-10+ ratio in carotid plaques and both FPG (a) and HbA1c (b) levels. ρ, Spearman's rank correlation coefficient
Table 4. Correlations between the expression levels of proinflammatory/anti-inflammatory cytokines in carotid plaque and glucose metabolism or proinflammatory/anti-inflammatory phenotypes of circulating monocytes.
| mRNA level in plaque |
||||||
|---|---|---|---|---|---|---|
| TNF-α |
IL-6 |
IL-10 |
||||
| ρ | P-value | ρ | P-value | ρ | P-value | |
| FPG | 0.185 | 0.399 | 0.219 | 0.304 | 0.259 | 0.222 |
| IRI | −0.041 | 0.854 | −0.256 | 0.228 | 0.241 | 0.256 |
| HbA1c | 0.527 | 0.010 | 0.359 | 0.085 | 0.101 | 0.640 |
| HOMA-IR | −0.027 | 0.904 | −0.110 | 0.607 | 0.238 | 0.264 |
| mRNA level in monocytes | ||||||
| TNF-α | 0.459 | 0.027 | 0.332 | 0.122 | −0.053 | 0.809 |
| IL-6 | 0.091 | 0.680 | 0.427 | 0.037 | −0.102 | 0.636 |
| IL-10 | −0.360 | 0.100 | −0.322 | 0.134 | −0.091 | 0.678 |
FPG, fasting plasma glucose; IRI, immunoreactive insulin; HbA1c, hemoglobin A1c; HOMA-IR, homeostasis model assessment of insulin resistance. ρ, Spearman's rank correlation coefficients
Fig. 3.
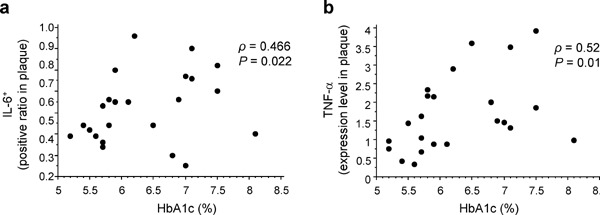
Correlation between HbA1c level with the IL-6+ ratio and TNF-α expression level in carotid plaques
Scatter plots highlighting the correlation between HbA1c level and the IL-6+ ratio (a) or TNF-α expression level (b) in carotid plaques. ρ, Spearman's rank correlation coefficient
We further addressed the association between circulating monocytes and carotid plaques with regard to the proinflammatory/anti-inflammatory cytokine levels in all patients who underwent CEA (Tables 3 and 4). We observed that the TNF-α expression level in circulating monocytes was positively correlated with the TNF-α+ ratio (ρ = 0.444, P = 0.034) (Table 3) and TNF-α expression level (ρ = 0.459, P = 0.027) (Table 4) in carotid plaques. In addition, the IL-6 expression level in circulating monocytes revealed a significant positive correlation with that in carotid plaques (ρ = 0.427, P = 0.037) (Table 4) but not with the IL-6+ ratio in carotid plaques (Table 3). Conversely, no significant correlation was observed with respect to the IL-10 expression level between circulating monocytes and carotid plaques (Tables 3 and 4). Significant correlations between circulating monocytes and carotid plaques regarding inflammatory properties were also obtained by partial rank correlation analyses, wherein medications were control variables.
Finally, we investigated the association between clinical settings and proinflammatory/anti-inflammatory properties of circulating monocytes and carotid plaques. We found that the properties of circulating monocytes and carotid plaques did not significantly correlate with the duration of diabetes (Supplementary Table 2). One of the 12 patients with diabetes had a transient weakness, but all the patients had no paralysis and were able to walk. Regarding the severity of diabetes, we found that the TNF-α expression level was significantly higher in circulating monocytes of patients with diabetes having neuropathy [8 patients, 4.0 (1.8–8.2)] than in those of patients with diabetes not having neuropathy [4 patients, 1.0 (0.9–1.2), P = 0.014]. In addition, the IL-10 expression level was significantly lower in carotid plaques of patients with diabetes having a stroke [6 patients, 0.4 (0.2–0.8)] than in those of patients with diabetes not having a stroke [6 patients, 1.0 (0.7–1.2), P = 0.036]. Consequently, we suggest that inflammatory properties of circulating monocytes and carotid plaques are exacerbated in patients with advanced diabetes.
Supplementary Table 2. Correlations between the proinflammatory/anti-inflammatory phenotypes of circulating monocytes or carotid plaque and the duration of diabetes in diabetic patients (n = 12).
| Duration of diabetes |
||
|---|---|---|
| ρ | P-value | |
| mRNA level in monocytes | ||
| TNF-α | −0.279 | 0.407 |
| IL-6 | −0.060 | 0.854 |
| IL-10 | −0.046 | 0.894 |
| CD14 | −0.298 | 0.346 |
| CD16 | 0.144 | 0.656 |
| CD68 | −0.025 | 0.940 |
| mRNA level in plaque | ||
| TNF-α | −0.324 | 0.331 |
| IL-6 | −0.249 | 0.435 |
| IL-10 | 0.208 | 0.516 |
| MHC-II | −0.225 | 0.483 |
| CD86 | 0.242 | 0.448 |
| CD206 | −0.474 | 0.120 |
| Dectin-1 | 0.021 | 0.948 |
| Percent of positive cells in plaque | ||
| TNF-α | 0.126 | 0.696 |
| IL-6 | 0.175 | 0.585 |
| IL-10 | −0.488 | 0.108 |
| MHC-II | −0.228 | 0.476 |
| CD86 | 0.042 | 0.897 |
| CD206 | 0.119 | 0.712 |
| CD3 | 0.151 | 0.640 |
ρ, Spearman's rank correlation coefficient
Discussion
This study is the first to demonstrate that carotid plaques from patients with diabetes have a higher ratio of inflammatory cells and a lower ratio of anti-inflammatory cells compared with those from patients without diabetes. Furthermore, anti-inflammatory properties of carotid plaques were decreased by hyperglycemia. Moreover, we observed a positive correlation between circulating monocytes and carotid plaques, particularly with regard to inflammatory properties. These findings suggest that hyperglycemia and inflammatory properties of circulating monocytes are closely associated with inflammatory properties of carotid plaques in patients who underwent CEA.
Hyperglycemia causes endothelial cell damage, triggering monocyte recruitment17). In addition, circulating monocytes with inflammatory properties display an intrinsically higher transendothelial migratory capacity than those with anti-inflammatory properties13). Therefore, our findings suggest that circulating monocytes with inflammatory properties infiltrate carotid plaques and differentiate into M1 Mφ more efficiently relative to circulating monocytes with anti-inflammatory properties in patients with hyperglycemia. Alternatively, hyperglycemia may have deleterious impact on IL-10 production in Mφ in carotid plaques. Sato T et al. reported that IL-10 production by Mφ in atherothrombotic plaques is impaired in patients with acute coronary syndrome having diabetes and insulin resistance18). Therefore, hyperglycemia may be involved in the reduced anti-inflammatory properties of Mφ in carotid plaques.
A recent epidemiological study, the Malmo Diet and Cancer study, reported that elevated CD14highCD16− monocytes in a heterogeneous monocyte population can predict cardiovascular events19). On the other hand, Fadini et al.13) reported that the functions of inflammatory and anti-inflammatory monocytes rather than traditional cell-surface markers are associated with microangiopathy in patients with diabetes13). In addition, Neele et al.20) reported that surface markers expressed by Mφ do not reveal the activation status of Mφ20). Similarly, in this study, no significant difference was observed between patients with and without diabetes with regard to the expression levels of cell-surface markers for inflammatory/anti-inflammatory properties in circulating monocytes and carotid plaques. However, data regarding the proinflammatory/anti-inflammatory cytokine expression levels revealed that inflammatory properties of circulating monocytes and carotid plaques were more exacerbated in patients with diabetes than in those without diabetes. Furthermore, we found that these inflammatory properties were aggravated in patients with advanced diabetes. Accordingly, our data reported for the first time that inflammatory properties of circulating monocytes/Mφ were exacerbated in the absence of remarkable changes of cell-surface inflammatory/anti-inflammatory markers in patients with diabetes. Further studies are required to clarify the mechanisms by which the cytokine expression is influenced by diabetes in the absence of concomitant changes of cell-surface markers.
Carotid ultrasonography may be a useful tool for predicting the atherosclerotic status of coronary arteries21); however, the association between circulating monocytes and carotid plaques pertaining inflammatory/anti-inflammatory properties remains unclear. On the basis of the proinflammatory/anti-inflammatory cytokine expression levels, we demonstrated for the first time that inflammatory properties of circulating monocytes are positively correlated with those of carotid plaques. In a previous study involving histological and gene expression analyses of carotid plaques, a higher gene expression level of inflammatory cytokines in carotid plaques was correlated with the morphological features of a more advanced or an unstable condition22). Moreover, the balance between proinflammatory/pro-thrombotic and anti-inflammatory mediators in mononuclear cells of carotid plaques may modulate plaque progression23). Therefore, our findings suggest that inflammatory properties of circulating monocytes are useful as potential novel markers for evaluating atherosclerosis progression and predicting cardiovascular risk, thereby leading to novel therapeutic targets.
This study had some limitations. This study included a cohort comprising 24 males. A study with a larger sample size would further clarify the association between glucose metabolism and proinflammatory/anti-inflammatory properties of circulating monocytes and carotid plaques. Furthermore, a cohort study comprising females would be informative. In this study, the expression of the genes of interest in carotid plaques was analyzed using plaque tissues. Because carotid plaques contain multiple cell types, the difference in mRNA levels, such as IL-10 levels, in carotid plaques between patients with and without diabetes may be vague. Furthermore, Saraiva et al.24) reported several mechanisms of posttranscriptional regulation with an impact on IL-10 mRNA half-life and on the amount of IL-10 secretion in Mφ24). Hence, the inconsistency between immunohistochemical and plaque mRNA data may be attributable to these mechanisms. In addition, a longitudinal study would help to provide novel insights in the correlation between proinflammatory/anti-inflammatory properties of circulating monocytes and/or carotid plaques and prognosis of patients who underwent CEA.
Finally, further investigation in larger cohorts with more extensive phenotypic analyses of circulating monocytes would be expected to elucidate the critical types of circulating monocytes, thereby providing further insights into the diagnostic significance of circulating monocytes in evaluating atherosclerosis and cardiovascular disease progression.
Conclusion
Hyperglycemia and inflammatory properties of peripheral blood monocytes demonstrated positive correlations with inflammatory properties of carotid plaques in patients who underwent CEA. Inflammatory properties of circulating monocytes may be potential novel markers for evaluating atherosclerosis progression.
Acknowledgments
We wish to thank Natsuko Nakajo and Satomi Kikuchi at Kyoto Medical Center, for their technical assistance.
Notice of Grant Support
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of the Japan Ministry of Health, Labour, and Welfare of Japan (to N. S-A. and Y. M.); The 5th. Annual Research Award Grant of Japanese Society of Anti-Aging Medicine (to N. S-A.); and grants from the National Hospital Organization for collaborative clinical research and the Smoking Research Foundation (to N. S-A.).
Conflict of Interest
The authors declare that there is no duality of interest associated with this manuscript.
References
- 1). Satoh-Asahara N, Shimatsu A, Sasaki Y, Nakaoka H, Himeno A, Tochiya M, Kono S, Takaya T, Ono K, Wada H, Suganami T, Hasegawa K, Ogawa Y: Highly purified eicosapentaenoic acid increases interleukin-10 levels of peripheral blood monocytes in obese patients with dyslipidemia. Diabetes Care, 2012; 35: 2631-2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Bruun JM, Lihn AS, Pedersen SB, Richelsen B: Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab, 2005; 90: 2282-2289 [DOI] [PubMed] [Google Scholar]
- 3). Chang YC, Chen TC, Lee CT, Yang CY, Wang HW, Wang CC, Hsieh SL: Epigenetic control of MHC class II expression in tumor-associated macrophages by decoy receptor 3. Blood, 2008; 111: 5054-5063 [DOI] [PubMed] [Google Scholar]
References
- 1). Plutzky J: Macrovascular effects and safety issues of therapies for type 2 diabetes. Am J Cardiol, 2011; 108: 25B-32B [DOI] [PubMed] [Google Scholar]
- 2). Gibbons GW, Shaw PM: Diabetic vascular disease: characteristics of vascular disease unique to the diabetic patient. Semin Vasc Surg, 2012; 25: 89-92 [DOI] [PubMed] [Google Scholar]
- 3). Doi Y, Ninomiya T, Hata J, Fukuhara M, Yonemoto K, Iwase M, Iida M, Kiyohara Y: Impact of glucose tolerance status on development of ischemic stroke and coronary heart disease in a general Japanese population: the Hisayama study. Stroke, 2010; 41: 203-209 [DOI] [PubMed] [Google Scholar]
- 4). Lumeng CN, Bodzin JL, Saltiel AR: Obesity induces a phenotypic switch in adipose tissuemacrophage polarization. J Clin Invest, 2007; 117: 175-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Satoh N, Shimatsu A, Himeno A, Sasaki Y, Yamakage H, Yamada K, Suganami T, Ogawa Y: Unbalanced M1/M2 phenotype of peripheral blood monocytes in obese diabetic patients: effect of pioglitazone. Diabetes Care, 2010; 33: e7. [DOI] [PubMed] [Google Scholar]
- 6). Tatsumi K, Mackman N: Tissue Factor and Atherothrombosis. J Atheroscler Thromb, 2015; 22: 543-549 [DOI] [PubMed] [Google Scholar]
- 7). Komohara Y, Fujiwara Y, Ohnishi K, Shiraishi D, Takeya M: Contribution of Macrophage Polarization to Metabolic Diseases. J Atheroscler Thromb, 2015; [Epub ahead of print] PubMed PMID: [DOI] [PubMed] [Google Scholar]
- 8). Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P: Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation, 2004; 110: 1564-1571 [DOI] [PubMed] [Google Scholar]
- 9). Satoh-Asahara N, Shimatsu A, Sasaki Y, Nakaoka H, Himeno A, Tochiya M, Kono S, Takaya T, Ono K, Wada H, Suganami T, Hasegawa K, Ogawa Y: Highly purified eicosapentaenoic acid increases interleukin-10 levels of peripheral blood monocytes in obese patients with dyslipidemia. Diabetes Care, 2012; 35: 2631-2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Satoh-Asahara N, Sasaki Y, Wada H, Tochiya M, Iguchi A, Nakagawachi R, Odori S, Kono S, Hasegawa K, Shimatsu A: A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism, 2013; 62: 347-351 [DOI] [PubMed] [Google Scholar]
- 11). White MG, Marshall HL, Rigby R, Huang GC, Amer A, Booth T, White S, Shaw JA: Expression of mesenchymal and a-cell phenotypic markers in islet b-cells in recently diagnosed diabetes. Diabetes Care, 2013; 36: 3818-3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC: Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood, 2011; 118: e16-31 [DOI] [PubMed] [Google Scholar]
- 13). Fadini GP, de Kreutzenberg SV, Boscaro E, Albiero M, Cappellari R, Kränkel N, Landmesser U, Toniolo A, Bolego C, Cignarella A, Seeger F, Dimmeler S, Zeiher A, Agostini C, Avogaro A: An unbalanced monocyte polarisation in peripheral blood and bone marrow of patients with type 2 diabetes has an impact on microangiopathy. Diabetologia, 2013; 56: 1856-1866 [DOI] [PubMed] [Google Scholar]
- 14). Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG: Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci, 2009; 29: 13435-13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Mia S, Warnecke A, Zhang XM, Malmström V, Harris RA: An optimized protocol for human M2 macrophages using M-CSF and IL-4/IL-10/TGF-β yields a dominant immunosuppressive phenotype. Scand J Immunol, 2014; 79: 305-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Chistiakov DA, Bobryshev YV, Orekhov AN: Changes in transcriptome of macrophages in atherosclerosis. J Cell Mol Med, 2015; 19: 1163-1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Fadini GP: A reappraisal of the role of circulating (progenitor) cells in the pathobiology of diabetic complications. Diabetologia, 2014; 57: 4-15 [DOI] [PubMed] [Google Scholar]
- 18). Sato T, Kameyama T, Noto T, Inoue H: Impaired macrophage production of anti-atherosclerotic interleukin-10 induced by coronary intraplaque hemorrhage in patients with acute coronary syndrome and hyperglycemia. J Diabetes Complications, 2014; 28: 196-202 [DOI] [PubMed] [Google Scholar]
- 19). Berg KE, Ljungcrantz I, Andersson L, Bryngelsson C, Hedblad B, Fredrikson GN, Nilsson J, Björkbacka H: Elevated CD14++CD16− monocytes predict cardiovascular events. Circ Cardiovasc Genet, 2012; 5: 122-131 [DOI] [PubMed] [Google Scholar]
- 20). Neele AE, Van den Bossche J, Hoeksema MA, de Winther MP: Epigenetic pathways in macrophages emerge as novel targets in atherosclerosis. Eur J Pharmacol, 2015; 763: 79-89 [DOI] [PubMed] [Google Scholar]
- 21). Fujihara K, Suzuki H, Sato A, Kodama S, Heianza Y, Saito K, Iwasaki H, Kobayashi K, Yatoh S, Takahashi A, Yamada N, Sone H, Shimano H: Carotid artery plaque and LDL-to-HDL cholesterol ratio predict atherosclerotic status in coronary arteries in asymptomatic patients with type 2 diabetes mellitus. J Atheroscler Thromb, 2013; 20: 452-464 [DOI] [PubMed] [Google Scholar]
- 22). Puig O, Yuan J, Stepaniants S, Zieba R, Zycband E, Morris M, Coulter S, Yu X, Menke J, Woods J, Chen F, Ramey DR, He X, O'Neill EA, Hailman E, Johns DG, Hubbard BK, Yee Lum P, Wright SD, Desouza MM, Plump A, Reiser V: A gene expression signature that classifies human atherosclerotic plaque by relative inflammation status. Circ Cardiovasc Genet, 2011; 4: 595-604 [DOI] [PubMed] [Google Scholar]
- 23). Sternberg Z, Ghanim H, Gillotti KM, Tario JD, Jr, Munschauer F, Curl R, Noor S, Yu J, Ambrus JL, Sr, Wallace P, Dandona P: Flow cytometry and gene expression profiling of immune cells of the carotid plaque and peripheral blood. Atherosclerosis, 2013; 229: 338-347 [DOI] [PubMed] [Google Scholar]
- 24). Saraiva M, O'Garra A: The regulation of IL-10 production by immune cells. Nat Rev Immunol, 2010; 10: 170-181 [DOI] [PubMed] [Google Scholar]


