Abstract
Aims: Phospholipase A2 receptor 1 (PLA2R) has multiple biological functions other than functioning as a receptor for secretory PLA2s. Two nonsynonymous polymorphisms in the C-type lectin-like domains (CTLD) 1 of PLA2R gene have been associated with idiopathic membranous nephropathy. This study examined whether the same PLA2R polymorphisms may alter functions of PLA2R in cells expressing the variant PLA2R. In addition, the clinical relevance of the experiment was examined.
Methods: Two nonsynonymous polymorphisms (T/C at rs3749117 and G/C at rs35771982) in CTLD1 of PLA2R gene were completely linked. HEK293 cells expressing human wild-type PLA2R (T at rs3749117 and G at rs35771982) or human mutant PLA2R that had double mutations (C at rs3749117 and C at rs35771982) were constructed.
Results: HEK293 cells expressing mutant PLA2R had lower migratory and proliferative responses to collagen I compared with cells expressing wild-type PLA2R. In 580 male patients, PLA2R gene polymorphisms were associated with an increase in maximum intima-media thickness (maxIMT) of the carotid artery. The multivariate regression model showed that PLA2R gene polymorphisms were a risk factor of an increased maxIMT that was independent of conventional risk factors (OR = 1.93, 95% CI: 1.17–3.19, p < 0.01).
Conclusions: The nonsynonymous common variants of PLA2R gene altered biological functions in cells expressing variant PLA2R. PLA2R gene polymorphisms present a genetic risk of an increased IMT of the carotid artery in male. The functional changes in the variant PLA2R may potentially be responsible for its association with an increased IMT of the carotid artery.
Keywords: Gene polymorphism, Phospholipase A2 receptor, Carotid artery, Atherosclerosis
Introduction
Phospholipase A2 receptor 1 (PLA2R), an M-type PLA2 receptor, is a type I transmembrane glycoprotein with a molecular mass of 180 kDa1–4). It is composed of an extracellular portion consisting of an N-terminal cysteine-rich domain (CRD), a fibronectin-like type II (FNII) domain, a tandem repeat of 8 C-type lectin-like domains (CTLD), and a short intra-cellular C-terminal region (Fig. 1)1–4).
Fig. 1.
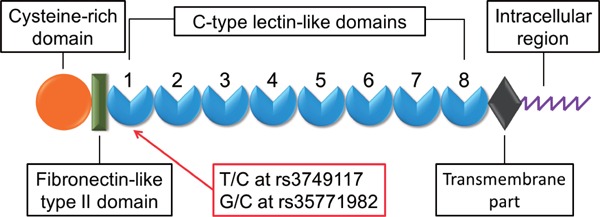
Schematic representation of T/C at rs3749117 and G/C at rs35771982 polymorphisms located in the C-type lectin-like domains (CTLD) 1 of PLA2R gene
The murine secretory phospholipase A2 (sPLA2), including sPLA2-IB, -IIA, -IIE, -IIF and -X, acts as a ligand for murine PLA2R2, 3, 5). Murine PLA2R functions as a clearance receptor through internalization and degradation of bound sPLA2s5–8). In contrast, there are no reports showing that human sPLA2s bind to human PLA2R with high affinity. Thus, there is species-specificity in the ligand affinity of sPLA2 for PLA2R9).
Bernard et al. recently showed that human PLA2R promotes senescence in fibroblasts and induces cancer cell death through a JAK2-mediated mechanism10, 11). We have recently shown that the extracellular portion of mouse PLA2R interacts with that of integrin β1 through collagen type I and that PLA2R induces collagen-dependent migration and proliferation of myofibroblasts through an integrin β1-mediated intracellular signaling mechanism9, 12). Our group and other researchers showed that collagen I binds to the FNII domain of PLA2R9, 12, 13) and that the adjacent CTLD1 region modulates the collagen binding activity of FNII domain9). We showed that PLA2R played a crucial role in healing post-MI myocardium12). Thus, PLA2R appears to have multiple biological functions in a sPLA2-dependent or -independent fashion.
PLA2R was identified as a major antigen in idiopathic membranous nephropathy (IMN)14). Circulating antibodies to PLA2R are present in 70% of patients with IMN14). The anti-PLA2R1 antibody is likely to recognize the N-terminal region of PLA2R, consisting of the CRD, FNII domain, and CTLD 1 and 215, 16). Furthermore, two nonsynonymous polymorphisms (rs3749117 and rs35771982) in the CTLD1 domain of the PLA2R gene have been linked to the occurrence of IMN in patients (Fig. 1)17, 18). It has been suggested that two amino acid substitutions (Met292Val encoded by SNP rs3749117 and His300Asp encoded by rs35771982) in CTLD1 may change its configuration, facilitating antigenicity of the N-terminal region of the variant PLA2R17, 18).
It is unknown whether these nonsynonymous polymorphisms in CTLD1 of the PLA2R gene alter the biological functions of PLA2R. Thus, this study aimed to determine whether these polymorphisms in CTLD1 modulate the binding activity and the migratory and proliferative responses to collagen in cells expressing the human variant PLA2R. To determine the clinical relevance of this experiment, we tested the possible association of these polymorphisms in CTLD1 of the PLA2R gene with peripheral vascular parameters including intima-media thickness (IMT) of the carotid artery, pulse wave velocity (PWV), and ankle-brachial index (ABI) in humans.
Materials and Methods
Reagents
Human collagen type I from placenta was purchased from BD Biosciences (Bedford, MD, USA). Antibody against CTLD5–6 of human PLA2R was purchased from GeneTex (Irvine, CA, USA). Sodium [125I]-iodine (carrier-free, 3.7 GBq/mL) was purchased from Perkin Elmer (Boston, MA, USA).
Generation of cDNA Constructs Encoding Human Wild-Type and Mutant PLA2R
Human PLA2R wild-type plasmid (NM_001195641) was purchased from Origene (Rockville, MD, USA). The PLA2R cDNA was removed from the original vector and inserted into the pBA po-CMV vector. Mutations inCTLD1 of wild-type PLA2R were made using a QuikChange II Site-Directed Mutagenesis Kit and primers from Agilent (Santa Clara, CA, USA). The mutagenesis primers are described in Supplemental Table 1. pcDNAs of all constructs were transformed into Escherichia coli One Shot TOP10 cells from Invitrogen (Carlsbad, CA, USA) and purified using a QIAfilter Plasmid Kit from QIAGEN (Valencia, CA, USA). Further, the DNA sequences were checked to exclude sequence errors.
Supplemental Table 1. Primers used for point mutagenesis of T/C at rs3749117 and G/C at rs35771982 of PLA2R gene.
| Forward | Reverse | |
|---|---|---|
| rs3749117 (T/C) | 5′AGTGGAGGTGTGGGTGGGCCTCAATCAGC3′ | 5′GCTGATTGAGGCCCACCCACACCTCCACT3′ |
| rs35771982 (G/C) | 5′ATCAGCTGGATGAAGACGCTGGCTGGCAG3′ | 5′CTGCCAGCCAGCGTCTTCATCCAGCTGAT3′ |
Cell Lines and Transfection
The human embryonic kidney cell line (HEK293) (ECACC No. EC85120602) was maintained in Dulbecco's modified Eagle's medium (DMEM) (Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Life Technologies). Lipofectamine 2000 (Life Technologies) was used to mediate transient transfection according to the manufacturer's protocol9).
Cell Migration Assay
Migration assays were performed using modified Boyden chamber with polycarbonate filters (pore size, 8 µm) (Corning, NY, USA) for 24-well trays in which the bottom surface was precoated with collagen I (10 µg/mL) (BD Biosciences, MD, USA) or PBS as a vehicle control for 1 h9, 12). Cells (2 × 105) were suspended in serum-free DMEM containing 0.5% BSA and placed in the upper chamber. Incubation lasted 3 h at 37°C in 5% CO2. On incubation, cells on the upper surface were removed by scraping, and cells adherent to the bottom surface of the filter were fixed in methanol and stained with a Diff Quik kit (Sysmex, Hyogo, Japan) for counting. The number of migrating cells was determined by counting 5 fields randomly selected from each filter with an optical microscope at a magnification of 100×. Each experiment was per-formed in triplicate and migration was expressed as the average number of total cells counted per field.
Proliferation Assay
Proliferation of transfected HEK293 cells (as determined by DNA synthesis) was measured by a colorimetric bromodeoxyuridine (BrdU) assay kit from Roche (Mannheim, Germany) according to the manufacturer's protocol. Transfected HEK293 cells were seeded in a 96-well plate coated with collagen I or PBS (as a control) (2 × 104 cells/well). The BrdU reagent was added for 2 h according to the provided protocol. Proliferation was measured by light absorbance at 370/492 nm.
Binding Assay
Collagen I in Tris buffer (25 mM Tris-HCl, pH 7.5, 0.4 mM NaCl) was labeled with 30 MBq of Na125I (Perkin Elmer, MA, USA) in precoated iodination tubes (Thermo Scientific, MA, USA), as described in our previous report9). For binding assays, transfected HEK293 cells were incubated on 24-well plates with the indicated concentrations of 125I-labeled collagen I in the absence or presence of a 50-fold excess of unlabeled collagen I for 2 h at 4°C. After the reaction, the cells were washed 3 times with ice-cold PBS, and the cell-associated radioactivity was measured with a gamma counter. The specific binding was defined as the difference between binding in the presence and absence of the unlabeled collagen I, which was the difference between the total binding and non-specific binding.
Sources of Cultures of Human Umbilical Vein Endothelial Cells and Human Vascular Smooth Muscle Cells
Human umbilical vein endothelial cells (HUVECs) were obtained from ATCC (Manassas, VA, USA) and cultured with F-12K Nutrient Mixture (ATCC) supplemented with 10% FBS, 0.03 mg/dL endothelial cell growth supplement (Sigma, St. Louis, MO, USA), and 0.5 mg/dL heparin (Sigma). Human vascular smooth muscle cells (SMCs) were from Lonza (Walkersville, MD, USA) and cultured with DMEM supplemented with 10% FBS. HUVECs and human vascular SMCs at confluent condition were used for the measurement of PLA2R mRNA expression.
Measurements of mRNA
The mRNA expression levels were quantified with a real-time two-step reverse transcriptase polymerase chain reaction (PCR) assay using TaqMan chemistry and a 7500 Real-Time PCR System (Applied Biosystems, CA, USA). The primers were predesigned by TaqMan Gene Expression Assays (Applied Biosystems) (Hs00234853_m1).
Western Blotting Analysis
Immunoblotting analyses were performed to detect human PLA2R protein expression in transfected cells using polyclonal antibody against CTLD5–6 of human PLA2R (GeneTex). Protein (10 µg) from the extracts of transfected cells were separated by SDSPAGE and transferred to a polyvinylidene difluoride membrane. The membrane was treated with blocking buffer and incubated overnight at 4°C with the antibody against human PLA2R.
Flow Cytometric Analysis
Flow cytometry was performed using the FACS-Caliber. Transfected HEK293 cells were suspended (2×106 cells/tube) in PBS/0.1% FBS/0.09% NaN3 and incubated with the antibody against CTLD5–6 of human PLA2R (GeneTex)for 30 min at 4°C. After the washing step in PBS/0.1% FBS/0.09% NaN3, the cells were incubated with anti-goat IgG-Alexa 647 antibody (1:100 dilution) (Life Technologies). We set an isotype-matched IgG as a control.
Patients for Clinical Study
The clinical study screened 941 consecutively-enrolled patients with chest pain who underwent cardiac catheterization at Yamanashi University Hospital from April 2002 to March 2011. The patients had routine non-invasive vascular measurements including intima-media thickness (IMT) of the carotid artery, brachial-ankle pulse wave velocity (baPWV), and ankle-brachial index (ABI). This study excluded 16 patients who had none of these vascular measurements; thus, 925 patients (580 men) were included in this study. The characteristics of the patients are shown in Table 1. The patients provided written informed consent for the study before enrollment. The study was approved by the ethics committee of Yamanashi University Hospital. The investigation conformed to the principles outlined in the 1975 Declaration of Helsinki.
Table 1. Baseline characteristics of the enrolled patients.
| Total patients (n = 925) | Men (n = 580) | Women (n = 345) | |
|---|---|---|---|
| Age, (y) | 64.4 (57.3–74.0) | 57.3 (55.4–72.8) | 69.7 (61.9–75.5) |
| Smoking, n (%) | 90 (9.7) | 82 (14.1) | 8 (2.3) |
| DM, n (%) | 300 (32.4) | 209 (36.0) | 91 (26.4) |
| Hypertension, n (%) | 473 (51.1) | 295 (50.9) | 178 (51.6) |
| BMI, (kg/m2) | 23.8 ± 3.4 | 24.0 ± 3.1 | 23.6 ± 3.8 |
| MI, n (%) | 265 (28.6) | 198 (34.1) | 67 (19.4) |
| Stroke, n (%) | 63 (6.8) | 42 (7.2) | 21 (6.1) |
| HbA1c, (%) | 5.6 (5.2–6.2) | 5.6 (5.3–6.4) | 5.6 (5.2–6.0) |
| CRP, (mg/L) | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) |
| eGFR, (ml/min/1.73 m2) | 69.1 (52.4–90.6) | 73.4 (55.8–95.5) | 62.0 (47.9–79.9) |
| TG, (mg/dL) | 121.0 (88.0–167.0) | 126.0 (93.0–172.0) | 111.0 (80.5–147.0) |
| HDL-C, (mg/dL) | 47.0 (39.0–57.0) | 45.0 (37.0–54.0) | 53.0 (43.0–66.0) |
| LDL-C, (mg/dL) | 120.6 (98.0–143.8) | 116.2 (95.8–142.6) | 123.3 (102.6–147.2) |
| Non HDL-C, (mg/dL) | 147.0 (121.0–174.0) | 146.0 (119.0–174.0) | 149.5 (125.0–174.0) |
| maxIMT, (mm) | 0.9 (0.7–1.4) | 0.9 (0.7–1.5) | 0.9 (0.7–1.4) |
| ABI | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) |
| baPWV, (m/sec) | 16.2 (13.9–19.0) | 15.9 (13.6–18.8) | 16.6 (14.7–19.8) |
Data given as mean ± SD, median (IQR) or n (%).
ABI, ankle-brachial index; baPWV, brachial-ankle pulse wave velocity; BMI, body mass index; CRP, C-reactive protein; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; maxIMT, maximum intima-media thickness; LDL-C, low-density lipoprotein cholesterol; MI, history of previous myocardial infarction; stroke, history of stroke; TG, triglyceride.
Genotyping
Genomic DNA was extracted from whole blood samples using a DNA Extractor WB-Rapid Kit (Wako, Osaka, Japan) or a Quick Gene DNA Whole Blood Kit (Fujifilm, Tokyo, Japan) with a Quick Gene-800 (Fujifilm) according to the manufacturer's protocol.
The polymorphic regions were amplified with a Taqman SNP genotyping assay with a 7500 Real-Time PCR System using 10 ng genomic DNA in a 20 µL reaction mixture containing 2× FastStart Universal Probe Master (Roche, Mannheim, Germany) and forward and reverse primers, encompassing the polymorphic sites (T/C at rs3749117 and G/C at rs35771982). The primers were predesigned by Taq-Man Gene Expression Assays (Applied Biosystems) (Assay IDs, C_25805618_10 and C_25621628_10, respectively). The amplification protocol consisted of initial denaturation at 95°C for 2 min, followed by 35 cycles of denaturation at 95°C for 30 sec, annealing for 30 sec at the appropriate temperature for the primer pair, extension at 72°C for 30 sec, and final extension at 72°C for 5 min.
Measurement of Intima-Media Thickness of the Carotid Artery
Carotid ultrasound was carried out using an 11.0 MHz, linear-array transducer (SONOS-5500; Phillips, Andover, MA, USA) according to our previous reports19–21). Briefly, 2 well-trained researchers performed all the carotid scans without any information on the patients' clinical characteristics. Longitudinal images of each carotid artery were used to measure intima-media thickness (IMT), defined as the distance from the leading edge of the lumen-intima interface to the leading edge of the media-adventitia interface. Maximum IMT (maxIMT) was defined as the greatest IMT in the 6 carotid arteries including the common carotid artery, bifurcation, and proximal internal carotid artery of right and left sides. The cutoff value of 1.1 mm was derived from previous reports19–22). In our study, the mean biases of the inter- and intraobserver for repeated measurements of maxIMT in 20 patients extracted at random were 0.02 ± 0.24 mm and 0.01 ± 0.17 mm, respectively.
Measurement of Brachial-Ankle Pulse Wave Velocity
We measured brachial-ankle pulse wave velocity (baPWV) by a volume plethysmographic method using an automatic waveform analyzer (VP-1000; Colin Co. Ltd., Komaki, Japan), according to previous reports21, 23). baPWV was calculated by measuring the time for the pulse wave to travel between the brachial and posterior tibial arteries. The mean value of the left and right baPWV was used in the statistical analyses. Inter- and intra-observer variabilities for repeated baPWV measurements in 20 patients on separate days were 0.62 ± 0.31 m/sec and 0.53 ± 0.26 m/sec, respectively. The 75th percentile value of baPWV (19 m/sec) in the distribution of baPWV value in the study patient population was selected as a cutoff in this study.
Measurement of Ankle-Brachial Index
Systolic blood pressure was measured in all 4 extremities using an oscillometric device (VP-1000; Colin Co. Ltd., Komaki, Japan). Resting ankle-brachial index (ABI) was calculated by dividing the higher value of the 2 ankle systolic blood pressures in each leg (dorsalis pedis or posterior tibial artery) by the higher value of systolic blood pressures in the 2 brachial arteries. The selected ABI was the lower of the values for the left and right legs. The inter- and intra-observer variability for the repeated measurements of ABI in 20 patients on separate days was 0.011 ± 0.10 and 0.008 ± 0.08, respectively. The 25th percentile value of ABI (0.88) in the distribution of ABI value in the study patient population was selected as a cutoff for analysis in this study.
Statistical Analysis
All descriptive data are expressed as either means ± SD or median, or frequency (%) as indicated. The Shapiro–Wilk test showed that age, estimated glomerular filtration rate (eGFR), maxIMT, ABI, baPWV, and levels of HbA1c, C-reactive protein, triglycerides, HDL-cholesterol (C), LDL-C, and non HDL-C were not distributed normally. Therefore, these variables were expressed as the median and interquartile range (25th and 75th percentiles). Statistical relationships were assessed on univariate or multivariate logistic analysis. Multivariate logistic regression analysis was conducted using confounders that were significant in the univariate model. Univariate and multivariate logistic regression analysis examined 1-SD changes in continuous variables. The presence of dichotomous variables was coded as 1 and the absence as 0. D’ statistics and correlation coefficient (r statistics) were used to investigate the linkage disequilibrium between T/C at rs3749117 and G/C at rs35771982 polymorphisms of PLA2R. To assess the effect of different alleles on the vascular parameters, logistic regression analysis was employed in 5 inheritance models: codominant, dominant, recessive, over-dominant, and log-additive.
All probabilities were expressed as two-tailed, with statistical significance inferred at p < 0.05. All confidence intervals were computed at the 95% level. Statistical analysis was conducted using STATA 10.0 (StataCorp, College Station, TX, USA).
Results
Human PLA2R Expression in Transfected HEK293 Cells
As previously reported17, 18), two nonsynonymous polymorphisms (T/C at rs3749117 and G/C at rs35771982) were identified in the CTLD1 region of the PLA2R gene in the study patient population (Fig. 1). The two polymorphisms were completely linked (D' statistics = 1, r statistics = 1). Thus, we produced HEK293 cells expressing human wild-type PLA2R (T at rs3749117 and G at rs35771982) or mutant PLA2R that had double mutations in CTLD1 of human PLA2R (C at rs3749117 and C at rs35771982). In immunoblotting experiments, the apparent molecular weights of wild-type and mutant human PLA2Rs were consistent with their calculated molecular weights (Fig. 2A). The extent of wild-type and mutant type PLA2R protein expression was similar (Fig. 2B). Flow cytometric analysis confirmed wild-type and mutant PLA2R cell surface expression (Fig. 2C). The mean fluorescence intensity (MFI) was similar in cells expressing wild-type and mutant type PLA2R (Fig. 2D). HEK293 cells expressed only faint levels of mRNA of endogenous PLA2R (per GAPDH mRNA expression; 4 × 10−4 at 35 PCR cycles). After transfection with a construct encoding wild-type PLA2R, PLA2R mRNA and cell surface protein expression increased approximately 6 × 103-fold compared with transfection with an empty vector (data not shown).
Fig. 2.
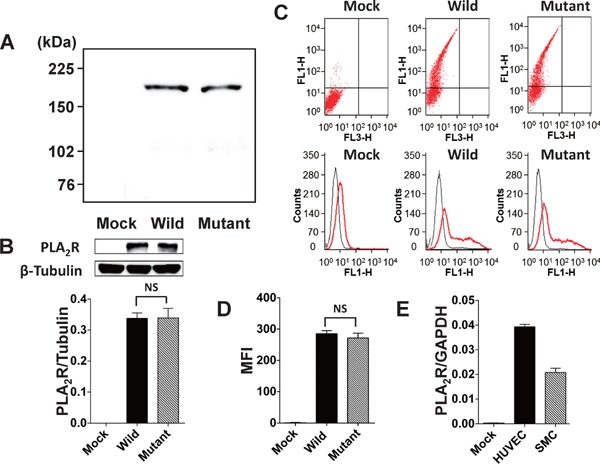
Wild-type and mutant human PLA2R protein expression in transfected HEK293 cells
(A) Western blot analysis of wild-type and mutant PLA2R expressed in HEK293 cells using polyclonal antibody for against CTLD5–6 of human PLA2R. The apparent molecular weights of wild-type and mutant PLA2R are consistent with their calculated molecular weights, indicating efficient translation. No protein band was detected in control mock-transfected cells (mock). (B) Quantitative analysis of the immunoblotting. Protein expression levels of wild-type and mutant PLA2R were similar in HEK293 cells transfected with the respective genotypes. (C) Flow cytometric analyses of wild-type and mutant PLA2R cell surface expression in HEK293 cells transfected with a cDNA construct encoding wild-type and mutant PLA2R using polyclonal antibody against CTLD5–6 of human PLA2R. Upper panels, representative dot plots of suspensions of HEK293 cells transfected with wild-type or mutant PLA2R; lower panels, representative histograms studying PLA2R expression on cells transfected with the respective PLA2R genotypes. (D) Comparison of mean fluorescence intensity (MFI) for PLA2R expression in HEK293 cells transfected with wild-type or mutant PLA2R. (E) PLA2R expression in cultures of human umbilical vein endothelial cells (HUVECs), human vascular smooth muscle cells (SMCs), and mock. The mRNA expression levels were quantified with a real-time two-step reverse transcriptase PCR assay. n = 7 in each experiment. NS, statistically not significant.
Migration Assay
In the Boyden chamber experiments, HEK293 cells migrated on filters precoated with human collagen I, but not on plain membranes (PBS coating, as a control), regardless of the PLA2R expression (Fig. 3A). Cells expressing wild-type PLA2R showed greater migration on filters with collagen I than the control mock-transfected cells (mock) (Fig. 3A). In contrast, cells expressing double mutant PLA2R showed lower migratory responses to collagen I than mock.
Fig. 3.
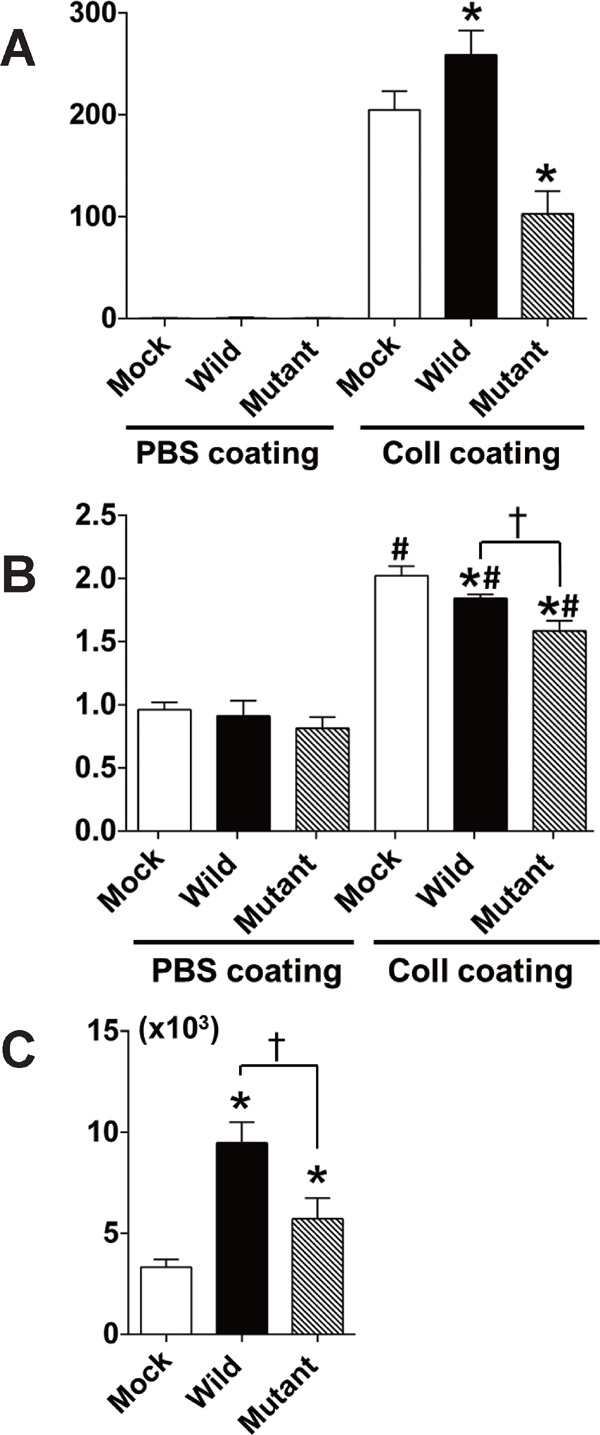
Comparison of migration, proliferation, and binding to human collagen I between HEK293 cells expressing wild-type PLA2R and double mutant PLA2R (C at rs3749117 and C at rs35771982)
(A) Migration of HEK293 cells expressing wild-type or double mutant type of PLA2R on filter membranes coated with PBS (as a control) or collagen I (Coll) in Boyden chamber experiments. (B) Proliferation (measured by light absorbance at 370/492 nm) of cells expressing wild-type or double mutant type of PLA2R on dishes coated with PBS or collagen I (Coll). (C) Binding activity to collagen I in cells expressing wild-type or double mutant type of PLA2R. Cells were incubated with 125I-labeled collagen I at 4°C in the absence or presence of a 50-fold excess amount of unlabeled collagen I and washed with cold PBS. The radioactivity of collagen I bound to cells (counts per minute in 100 µg of protein) is shown after correcting for nonspecific binding. * p < 0.05 in comparison with mock; † p < 0.05 between wild-type; and double mutant type of PLA2R. # < 0.05 in comparison with respective genotypes of cells on PBS-coated dishes. n = 6 for each experiment.
Proliferation Assay
Proliferative response was increased in all genotypes of the transfected cells on the collagen I-coated dishes compared with the respective genotypes of cells on dishes coated with PBS as a control (Fig. 3B). Cells expressing wild-type or double mutant PLA2R showed a decrease in proliferative response to collagen I compared with mock (Fig. 3B). Cells expressing double mutant PLA2R showed a greater decrease in proliferative response to collagen I than those expressing wild-type PLA2R. The proliferative response on PBS-coating dishes was similar for wild-type and mutant PLA2R or mock (Fig. 3B).
Binding Assay
As shown in Fig. 3C, HEK293 cells expressing either wild-type or double mutant PLA2R showed increased binding to human collagen I compared with mock. Cells expressing double mutant PLA2R had less binding to collagen I compared with those expressing wild-type PLA2R (Fig. 3C).
Migratory, Proliferative and Binding Activities in Cells Expressing Single Mutant PLA2R
We produced HEK293 cells expressing mutant PLA2R that had a single mutation in CTLD1 of human PLA2R (either C at rs3749117 [Singl Mut1] or C at rs35771982 [Singl Mut2]). HEK293 cells expressing single mutant PLA2R at either C at rs3749117 or C at rs35771982 showed lower migratory (Fig. 4A), proliferative (Fig. 4B), and binding activity (Fig. 4C) compared with those expressing wild-type PLA2R.
Fig. 4.
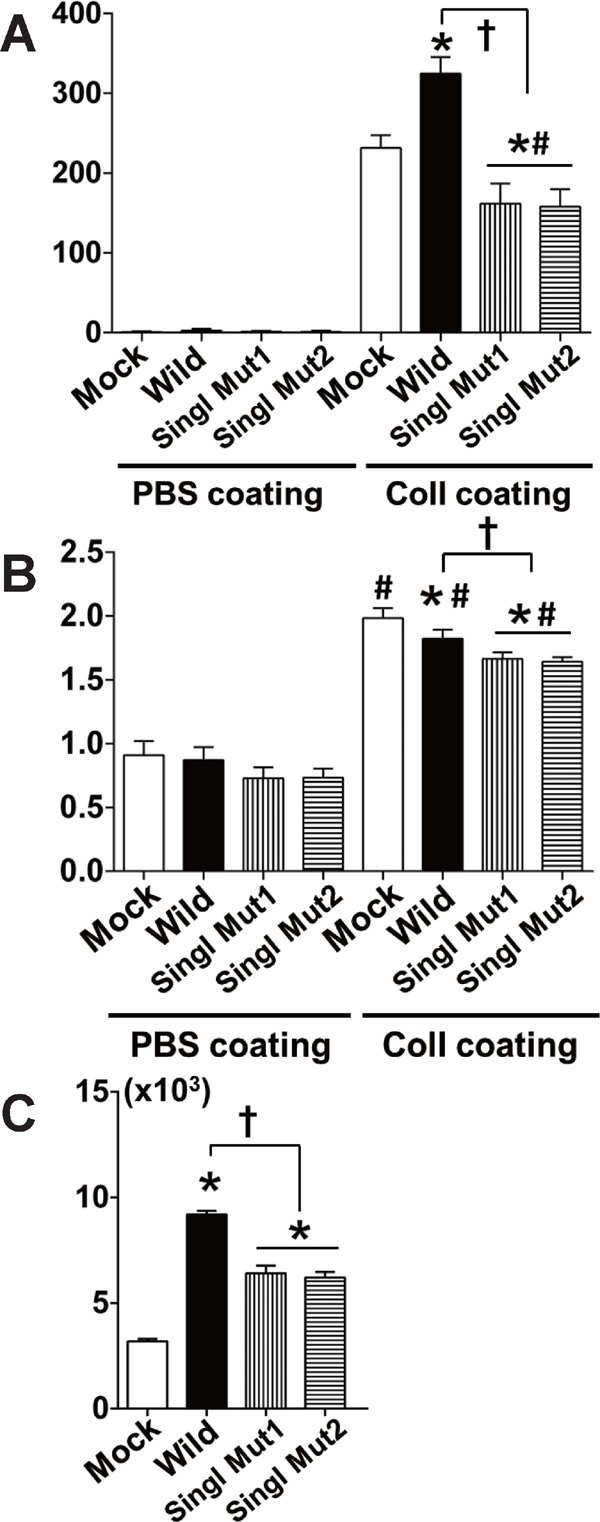
Comparison of migration, proliferation, and binding to human collagen I between HEK293 cells expressing wild-type PLA2R and single mutant PLA2R (either C at rs3749117 [Singl Mut1] or C at rs35771982 [Singl Mut2])
(A) Migration of HEK293 cells expressing wild-type or single mutant type of PLA2R. (B) Proliferation of cells expressing wild-type or single mutant type of PLA2R. (C) Binding activity to collagen I in cells expressing wild-type or single mutant type of PLA2R. * p < 0.05 in comparison with mock; † p < 0.05 between wild type; and single mutant type of PLA2R. # <0.05 in comparison with respective genotypes of cells on PBS-coated dishes. n = 6 for each experiment.
PLA2R Expression in Cultures of HUVECs and human vascular SMCs
We examined PLA2R mRNA expression in cells from human vessels. As shown in Fig. 2E, PLA2R was substantially expressed in cultures of HUVECs and human vascular SMCs. The extent of PLA2R expression in cultures of HUVECs and human vascular SMCs was approximately 100 times less than that in the HEK293 cells overexpressing wild-type PLA2R (data not shown).
Relation between PLA2R Gene Polymorphism and maxIMT of the Carotid Artery, baPWV, or ABI in Humans
As previously reported17, 18), two polymorphisms (T/C at rs3749117 and G/C at rs35771982) were identified in the CTLD1 region of the PLA2R gene (Fig. 1). The 2 polymorphisms were completely linked (D' statistics = 1, r statistics = 1). The distribution of the genotypes was consistent with a Hardy-Weinberg equilibrium in the population (exact test, p = 0.79). Because the 2 polymorphisms were completely linked, we chose the T/C at rs3749117 polymorphism for further statistical analyses. Logistic regression analysis showed that there were significant relationships between PLA2R polymorphism and the increased maxIMT of the carotid artery in codominant and recessive models in a subgroup of male patients, but not female patients and total patients (Table 2, Supplemental Tables 2 and 3). Representative ultrasound images of the carotid artery are shown in Fig. 5. There was no significant relationship between PLA2R polymorphism and baPWV or ABI in either total patients or the subgroups of male or female patients (Supplemental Tables 4–9). We determined whether PLA2R polymorphism could be a risk for increased maxIMT of the carotid artery in the male subgroup. In multivariate logistic regression, the CC genotype at rs3749117 of PLA2R gene was a significant risk factor of an increased maxIMT of the carotid artery that was independent of other conventional risk factors (OR = 1.93, 95% CI: 1.17–3.19, p < 0.01) (Table 3).
Table 2. Relationships between T/C genotypes at rs3749117 of PLA2R gene and an increased maxIMT in male patients.
| Genotypes | maxIMT < 1.1 mm | maxIMT ≥ 1.1 mm | OR (95% CI) | p | |
|---|---|---|---|---|---|
| Codominant | T/T C/T C/C |
98 (37.0) 138 (52.1) 29 (10.9) |
113 (35.9) 143 (45.4) 59 (18.7) |
1.00 0.90 (0.63–1.29) 1.76 (1.05–2.97) |
0.026 |
| Dominant | T/T C/T-C/C |
98 (37.0) 167 (63.0) |
113 (35.9) 202 (64.1) |
1.00 1.05 (0.75–1.47) |
0.78 |
| Recessive | T/T-C/T C/C |
236 (89.1) 29 (10.9) |
256 (81.3) 59 (18.7) |
1.00 1.88 (1.38–2.86) |
0.0085 |
| Over-dominant | T/T-C/C C/T |
127 (47.9) 138 (52.1) |
172 (54.6) 143 (45.4) |
1.00 0.77 (0.55–1.06) |
0.11 |
| Log-additive | --- | --- | --- | 1.21 (0.95–1.54) | 0.12 |
Data given as n (%). CI, confidence interval; OR, odd ratio. Other abbreviations as in Table 1.
Supplemental Table 2. Relationships between T/C genotypes at rs3749117 of PLA2R gene and an increased maxIMT of the carotid artery in total patient population.
| Genotypes | maxIMT < 1.1 mm | maxIMT ≥ 1.1 mm | OR (95% CI) | p | |
|---|---|---|---|---|---|
| Codominant | T/T C/T C/C |
152 (33.8) 227 (50.4) 71 (15.8) |
170 (35.8) 215 (45.3) 90 (18.9) |
1.00 0.85 (0.64–1.13) 1.13 (0.77–1.66) |
0.23 |
| Dominant | T/T C/T-C/C |
152 (33.8) 298 (66.2) |
170 (35.8) 305 (64.2) |
1.00 0.92 (0.70–1.20) |
0.52 |
| Recessive | T/T-C/T C/C |
379 (84.2) 71 (15.8) |
385 (81.0) 90 (18.9) |
1.00 1.25 (0.89–1.76) |
0.20 |
| Over-dominant | T/T-C/C C/T |
223 (49.6) 227 (50.4) |
260 (54.7) 215 (45.3) |
1.00 0.81 (0.63–1.05) |
0.11 |
| Log-additive | --- | --- | --- | 1.02 (0.85–1.23) | 0.80 |
Data given as n (%). CI, confidence interval; OR, odd ratio. Other abbreviations as in Table 1.
Fig. 5.
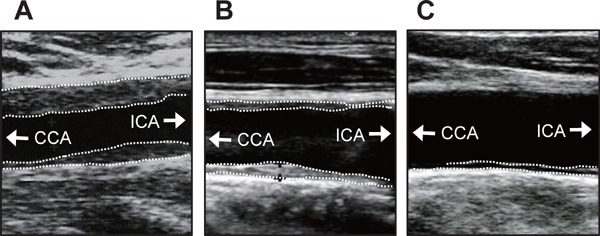
Representative ultrasonic images of carotid arteries in patients with C/C (A), C/T (B), or T/T (C) at rs3749117
CCA = common carotid artery; ICA = internal carotid artery. White dotted lines indicate margin of plaque or IMT of the carotid arteries
Supplemental Table 4. Relationships between T/C genotypes at rs3749117 of PLA2R gene and an increased baPWV (≥ 19 m/sec) in total patient population.
| Genotypes | baPWV < 19 m/sec | baPWV ≥ 19 m/sec | OR (95% CI) | p | |
|---|---|---|---|---|---|
| Codominant | T/T C/T C/C |
245 (35.0) 341 (48.6) 115 (16.4) |
77 (34.4) 101 (45.1) 46 (20.5) |
1.00 0.94 (0.67–1.32) 1.27 (0.83–1.95) |
0.35 |
| Dominant | T/T C/T-C/C |
245 (35.0) 456 (65.0) |
77 (34.4) 147 (65.6) |
1.00 1.03 (0.75–1.41) |
0.87 |
| Recessive | T/T-C/T C/C |
586 (83.6) 115 (16.4) |
178 (79.5) 46 (20.5) |
1.00 1.32 (0.90–1.93) |
0.16 |
| Overdominant | T/T-C/C C/T |
360 (51.4) 341 (48.6) |
123 (54.9) 101 (45.1) |
1.00 0.87 (0.64–1.17) |
0.35 |
| Log-additive | --- | --- | --- | 1.10 (0.89–1.36) | 0.38 |
Data given as n (%). CI, confidence interval; OR, odd ratio. Other abbreviations as in Table 1.
Supplemental Table 9. Relationships between T/C genotypes at rs3749117 of PLA2R gene and a decreased ABI (<0.88) in female patients.
| Genotypes | ABI ≥ 0.88 | ABI < 0.88 | OR (95% CI) | p | |
|---|---|---|---|---|---|
| Codominant | T/T C/T C/C |
76 (30.5) 121 (48.6) 52 (20.9) |
38 (39.6) 37 (38.5) 21 (21.9) |
1.00 0.61 (0.36–1.05) 0.81 (0.43–1.53) |
0.19 |
| Dominant | T/T C/T-C/C |
76 (30.5) 173 (69.5) |
38 (39.6) 58 (60.4) |
1.00 0.67 (0.41–1.09) |
0.11 |
| Recessive | T/T-C/T C/C |
197 (79.1) 52 (20.9) |
75 (78.1) 21 (21.9) |
1.00 1.06 (0.60–1.88) |
0.84 |
| Overdominant | T/T-C/C C/T |
128 (51.4) 121 (48.6) |
59 (61.5) 37 (38.5) |
1.00 0.66 (0.41–1.07) |
0.09 |
| Log-additive | --- | --- | --- | 0.86 (0.62–1.19) | 0.35 |
Data given as n (%). CI, confidence interval; OR, odd ratio. Other abbreviations as in Table 1.
Table 3. Univariate and multivariate logistic regression analysis of association of C/C genotype at rs3749117 of PLA2R gene and cardiovascular risk factors with an increased maxIMT of the carotid artery in male patients.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Smoking | 0.82 | 0.51–1.30 | 0.40 | --- | --- | --- |
| DM | 1.88 | 1.32–2.66 | <0.01 | 1.59 | 0.97–2.61 | 0.06 |
| Hypertension | 1.89 | 1.36–2.54 | <0.01 | 1.69 | 1.19–2.39 | <0.01 |
| BMI | 1.02 | 0.96–1.07 | 0.53 | --- | --- | --- |
| HbA1c | 1.27 | 1.10–1.46 | <0.01 | 1.11 | 0.92–1.32 | 0.28 |
| CRP | 1.04 | 0.95–1.14 | 0.396 | --- | --- | --- |
| eGFR | 0.97 | 0.96–0.98 | <0.01 | 0.98 | 0.97–0.98 | <0.01 |
| TG | 0.99 | 0.99–1.00 | 0.530 | --- | --- | --- |
| HDL-C | 0.99 | 0.98–1.01 | 0.270 | --- | --- | --- |
| LDL-C | 1.00 | 0.99–1.01 | 0.081 | --- | --- | --- |
| Non HDL-C | 1.00 | 0.99–1.00 | 0.193 | --- | --- | --- |
| C/C | 1.88 | 1.38–2.86 | <0.01 | 1.93 | 1.17–3.19 | <0.01 |
CI, confidence interval; OR, odd ratio. C/C, C/C genotype at rs3749117 of PLA2R gene. Other abbreviations as in Table 1.
Discussion
The present study showed that two nonsynonymous polymorphisms (T/C at rs3749117 and G/C at rs35771982) in CTLD1 of the PLA2R gene were completely linked. Our group and other researchers have previously shown that the FNII domain of PLA2R was responsible for binding of PLA2R to collagen I and collagen-dependent migration and prolifera-tion9, 12, 13). In addition, we demonstrated that the neighboring CTLD1 may modulate the collagen binding activity of the FNII domain9). The combination of two amino acid substitutions (Met292Val encoded by SNP rs3749117 and His300Asp encoded by rs35771982) could lead to a conformational change in CTLD1. The conformational change in CTLD1 may consequently reduce collagen binding activity of the FNII domain and the migratory and proliferative responses of cells expressing the variant type of human PLA2R.
The present study was the first to show that PLA2R gene polymorphisms were a genetic risk of an increased maxIMT of the carotid artery. PLA2R is expressed in HUVECs and human vascular SMCs. Vascular endothelial cells play a critical role in the restoration of vascular injury that could lead to development of atherosclerosis24). Endothelial cells expressing variant PLA2R may have a reduced ability to restore injured arteries because these endothelial cells may be dysfunctional as suggested by HEK293 cells transfected with the variant PLA2R gene. A recent report showed that senescence of vascular SMCs promoted atherosclerosis25). Senescence of vascular SMCs may have a role in the increased IMT of the carotid artery in patients with the variant PLA2R gene because vascular SMCs expressing variant PLA2R may have a reduced proliferative activity as suggested by our observation using HEK293 cells. These scenarios may explain the higher prevalence of an increased maxIMT of the carotid artery in male patients with variant PLA2R compared with those with wild-type PLA2R. The extent of PLA2R expression in cultures of HUVECs and human vascular SMCs was approximately 100 times less than that in the HEK293 cells expressing wild-type PLA2R. Whether PLA2R may be expressed in endothelial cells and vascular SMCs in the arterial walls with high enough extent to exert a physiological role is yet unclear.
A previous report showed that frequencies of each genotype of the polymorphism (rs3749117) were 47.9% in T/T genotype, 44.2% in C/T, and 7.8% in C/C in patients without membrane nephropathy in the Han population26). Thus, there was similar tendency of genotype frequencies in the polymorphism (rs3749117) between Han population and the present Japanese population. In addition, that previous report showed very similar frequencies between T/T and G/G, T/C and G/C, and C/C and C/C genotypes of rs3749117 and rs35771982, respectively26), suggesting that the two polymorphisms may also be linked in the Han population. Antiatherosclerotic effects of estrogen may confound the association between PLA2R gene polymorphisms and an increased maxIMT of the carotid artery in female patients, which may explain the inability to demonstrate an association in female patients and within the total patient population. A decrease of ABI reflects a late stage of atherosclerosis27). PWV reflects arterial stiffening that is increased by adverse structural and functional alterations in the vascular wall27). These arterial tests evaluate different stages of arterial damage at different arterial sites21). These may account for the absence of an association between PLA2R gene polymorphisms and baPWV or ABI in the present study. The inverse association between an estimated glomerular filtration rate (eGFR) and IMT of the carotid artery is consistent with the data in a previous report28) that showed renal dysfunction is associated with carotid atherosclerosis.
Previous studies showed that patients with IMN were associated with the same nonsynonymous variants (Met292Val or His300Asp) in the CTLD1 of PLA2R gene with regard to an increased maxIMT of the carotid artery in the present study17, 18). It is hypothesized that the amino acid substitution Met292Val or His300Asp could lead to a conformational change in CTLD1 of PLA2R, resulting in its function as an antigen17, 18).
The present study was the first to show that the two variants were completely linked. It may be expected that simultaneous substitution of Met292Val and His300Asp in PLA2R could induce a conformational change with greater extent than single substitution of each variant. However, HEK293 cells expressing single mutant PLA2R similarly decreased the bio-logical responses similar to those expressing double mutant PLA2R. The mechanisms underlying the association between these gene variants and functional changes in cells expressing these variants remain largely undefined. We cannot exclude the possibility that this polymorphism is a marker for another functional variant within this or adjacent genes. A recent paper failed to show a greater binding of the variant PLA2R to the autoantibody in IMN patients than wild-type PLA2R15). In that experiment, the variant PLA2R was prepared by individual substitution of Met-292 with Val or of His-300 with Asp in wild-type PLA2R15). Whether simultaneous substitution of Met292Val and His300Asp in PLA2R shows a greater antigenicity of these variants than wild-type PLA2R is yet unclear.
The present study showed that human PLA2R inhibited the cells' proliferative response independent of its gene polymorphisms. This observation is in agreement with a previous report11) showing that human PLA2R promoted senescence in fibroblasts. In contrast, mouse PLA2R increased the proliferative response to collagen I9, 12). The reason for this discrepancy in the proliferative response between human and mouse PLA2R is unknown. Moreover, higher binding activity to collagen was associated with lower proliferative response in the cells expressing wild-type human PLA2R compared with mock cells. Thus, human PLA2R-mediated migratory and proliferative response may not necessarily depend on collagen binding. Although the exact mechanisms for human PLA2R-mediated biological responses remain unknown, human PLA2R promotes senescence in fibroblasts and induces cancer cell death through a JAK2-mediated mechanism10, 11).
Conclusions
Two nonsynonymous common variants (Met292Val at rs3749117 and His300Asp at rs35771982) in the CTLD1 of the PLA2R gene present a genetic risk for an increase in IMT of the carotid artery. These variants in the PLA2R gene were completely linked. The simultaneous substitution of the two amino acids (Met292Val and His300Asp) in PLA2R inhibited binding, migratory, and proliferative responses to collagen I in cells expressing the variant PLA2R. The functional changes associated with the amino acid substitutions in the variant PLA2R may be potentially responsible for the association between an increase in IMT of the carotid artery and polymorphisms in the PLA2R gene.
Acknowledgements
This work was supported by AMED-CREST, and JSPS KAKENHI Grant Number B2-19390209 and B-22390158. We gratefully acknowledge the technical assistance of C. Komatsu, M. Yoda and C. Obata.
Conflict of Interest
We have no conflict of interest and no financial disclosure to declare in conjunction with the publication of this work.
Supplemental Table 3. Relationships between T/C genotypes at rs3749117 of PLA2R gene and an increased maxIMT in female patients.
| Genotypes | maxIMT < 1.1 mm | maxIMT ≥ 1.1 mm | OR (95% CI) | p | |
|---|---|---|---|---|---|
| Codominant | T/T C/T C/C |
55 (29.6) 91 (48.9) 40 (21.5) |
59 (37.1) 67 (42.1) 33 (20.8) |
1.00 0.69 (0.42–1.11) 0.77 (0.43–1.39) |
0.31 |
| Dominant | T/T C/T-C/C |
55 (29.6) 131 (70.4) |
59 (37.1) 100 (62.9) |
1.00 0.71 (0.45–1.12) |
0.14 |
| Recessive | T/T-C/T TC/C | 146 (78.5) 40 (21.5) |
126 (79.2) 33 (20.8) |
1.00 0.96 (0.57–1.61) |
0.86 |
| Overdominant | T/T-C/C C/T |
95 (51.1) 91 (48.9) |
92 (57.9) 67 (42.1) |
1.00 0.76 (0.50–1.16) |
0.21 |
| Log-additive | --- | --- | --- | 0.85 (0.64–1.14) | 0.29 |
Data given as n (%). CI, confidence interval; OR, odd ratio. Other abbreviations as in Table 1.
Supplemental Table 5. Relationships between T/C genotypes at rs3749117 of PLA2R gene and a decreased ABI (<0.88) in total patient population.
| Genotypes | ABI ≥ 0.88 | ABI <0.88 | OR (95% CI) | p | |
|---|---|---|---|---|---|
| Codominant | T/T C/T C/C |
224 (33.3) 335 (49.8) 114 (16.9) |
98 (38.9) 107 (42.5) 47 (18.6) |
1.00 0.73 (0.53–1.01) 0.94 (0.62–1.43) |
0.13 |
| Dominant | T/T C/T-C/C |
224 (33.3) 449 (66.7) |
98 (38.9) 154 (61.1) |
1.00 0.78 (0.58–1.06) |
0.11 |
| Recessive | T/T-C/T C/C |
559 (83.1) 114 (16.9) |
205 (81.3) 47 (18.6) |
1.00 1.12 (0.77–1.64) |
0.54 |
| Overdominant | T/T-C/C C/T |
338 (50.2) 335 (49.8) |
145 (57.5) 107 (42.5) |
1.00 0.74 (0.56–1.00) |
0.10 |
| Log-additive | --- | --- | --- | 0.92 (0.75–1.14) | 0.45 |
Data given as n (%). CI, confidence interval; OR, odd ratio. Other abbreviations as in Table 1.
Supplemental Table 6. Relationships between T/C genotypes at rs3749117 of PLA2R gene and an increased baPWV (≥ 19 m/sec) in male patients.
| Genotypes | PWV < 19 m/sec | PWV ≥ 19 m/sec | OR(95% CI) | p | |
|---|---|---|---|---|---|
| Codominant | T/T C/T C/C |
163 (37.4) 208 (47.7) 65 (14.9) |
48 (33.3) 73 (50.7) 23 (16.0) |
1.00 1.19 (0.78–1.81) 1.20 (0.68–2.13) |
0.68 |
| Dominant | T/T C/T-C/C |
163 (37.4) 273 (62.6) |
48 (33.3) 96 (66.7) |
1.00 1.19 (0.80–1.78) |
0.38 |
| Recessive | T/T-C/T C/C |
371 (85.1) 65 (14.9) |
121 (84.0) 23 (16.0) |
1.00 1.08 (0.65–1.82) |
0.76 |
| Overdominant | T/T-C/C C/T |
228 (52.3) 208 (47.7) |
71 (49.3) 73 (50.7) |
1.00 1.13 (0.77–1.64) |
0.53 |
| Log-additive | --- | --- | --- | 1.11 (0.85–1.46) | 0.44 |
Data given as n (%). CI, confidence interval; OR, odd ratio. Other abbreviations as in Table 1.
Supplemental Table 7. Relationships between T/C genotypes at rs3749117 of PLA2R gene and a decreased ABI (<0.88) in male patients.
| Genotypes | ABI ≥ 0.88 | ABI < 0.88 | OR (95% CI) | p | |
|---|---|---|---|---|---|
| Codominant | T/T C/T C/C |
156 (34.8) 225 (50.2) 67 (15.0) |
55 (41.7) 56 (42.4) 21 (15.9) |
1.00 0.71 (0.46–1.08) 0.89 (0.50–1.59) |
0.27 |
| Dominant | T/T C/T-C/C |
156 (34.8) 292 (65.2) |
55 (41.7) 77 (58.3) |
1.00 0.75 (0.50–1.11) |
0.15 |
| Recessive | T/T-C/T C/C |
381 (85.0) 67 (15.0) |
111 (84.1) 21 (15.9) |
1.00 1.08 (0.63–1.83) |
0.79 |
| Overdominant | T/T-C/C C/T |
223 (49.8) 225 (50.2) |
76 (57.6) 56 (42.4) |
1.00 0.73 (0.49–1.08) |
0.11 |
| Log-additive | --- | --- | --- | 0.88 (0.66–1.17) | 0.38 |
Data given as n (%). CI, confidence interval; OR, odd ratio. Other abbreviations as in Table 1.
Supplemental Table 8. Relationships between T/C genotypes at rs3749117 of PLA2R gene and an increased baPWV (<19.0 m/sec): in female patients.
| Genotypes | baPWV < 19 m/sec | baPWV ≥ 19 m/sec | OR (95% CI) | p | |
|---|---|---|---|---|---|
| Codominant | T/T C/T C/C |
74 (31.5) 115 (48.9) 46 (19.6) |
40 (36.4) 43 (39.1) 27 (24.6) |
1.00 0.69 (0.41–1.16) 1.09 (0.59–2.00) |
0.22 |
| Dominant | T/T C/T-C/C |
74 (31.5) 161 (68.5) |
40 (36.4) 70 (63.6) |
1.00 0.80 (0.50–1.29) |
0.37 |
| Recessive | T/T-C/T C/C |
189 (80.4) 46 (19.6) |
83 (75.5) 27 (24.6) |
1.00 1.34 (0.78–2.30) |
0.30 |
| Overdominant | T/T-C/C C/T |
120 (51.1) 115 (48.9) |
67 (60.9) 43 (39.1) |
1.00 0.67 (0.42–1.06) |
0.09 |
| Log-additive | --- | --- | --- | 1.00 (0.73–1.37) |
0.99 |
Data given as n (%). CI, confidence interval; OR, odd ratio. Other abbreviations as in Table 1.
References
- 1). Lambeau G, Ancian P, Barhanin J, Lazdunski M: Cloning and expression of a membrane receptor for secretory phospholipases A2. J Biol Chem, 1994; 269: 1575-1578 [PubMed] [Google Scholar]
- 2). Ishizaki J, Hanasaki K, Higashino K, Kishino J, Kikuchi N, Ohara O, Arita H: Molecular cloning of pancreatic group I phospholipase A2 receptor. J Biol Chem, 1994; 269: 5897-5904 [PubMed] [Google Scholar]
- 3). Rouault M, Le Calvez C, Boilard E, Surrel F, Singer A, Ghomashchi F, Bezzine S, Scarzello S, Bollinger J, Gelb MH, Lambeau G: Recombinant production and properties of binding of the full set of mouse secreted phospholipases A2 to the mouse M-type receptor. Biochemistry, 2007; 46: 1647-1662 [DOI] [PubMed] [Google Scholar]
- 4). East L, Isacke CM: The mannose receptor family. Biochim Biophys Acta, 2002; 1572: 364-386 [DOI] [PubMed] [Google Scholar]
- 5). Yokota Y, Notoya M, Higashino K, Ishimoto Y, Nakano K, Arita H, Hanasaki K: Clearance of group X secretory phospholipase A2 via mouse phospholipase A2 receptor. FEBS Lett, 2001; 509: 250-254 [DOI] [PubMed] [Google Scholar]
- 6). Zvaritch E, Lambeau G, Lazdunski M: Endocytic properties of the M-type 180-kDa receptor for secretory phospholipases A2. J Biol Chem, 1996; 271: 250-257 [DOI] [PubMed] [Google Scholar]
- 7). Tamaru S, Mishina H, Watanabe Y, Watanabe K, Fujioka D, Takahashi S, Suzuki K, Nakamura T, Obata JE, Kawabata K, Yokota Y, Murakami M, Hanasaki K, Kugiyama K: Deficiency of phospholipase A2 receptor exacerbates ovalbumin-induced lung inflammation. J Immunol, 2013; 191: 1021-1028 [DOI] [PubMed] [Google Scholar]
- 8). Murakami M, Taketomi Y, Miki Y, Sato H, Hirabayashi T, Yamamoto K: Recent progress in phospholipase A2 research: from cells to animals to humans. Prog Lipid Res, 2011; 50: 152-192 [DOI] [PubMed] [Google Scholar]
- 9). Takahashi S, Watanabe K, Watanabe Y, Fujioka D, Nakamura T, Nakamura K, Obata JE, Kugiyama K: C-type lectin-like domain and fibronectin-like type II domain of phospholipase A(2) receptor 1 modulate binding and migratory responses to collagen. FEBS Lett, 2015; 589: 829-835 [DOI] [PubMed] [Google Scholar]
- 10). Vindrieux D, Augert A, Girard CA, Gitenay D, Lallet-Daher H, Wiel C, Le Calvé B, Gras B, Ferrand M, Verbeke S, de Launoit Y, Leroy X, Puisieux A, Aubert S, Perrais M, Gelb M, Simonnet H, Lambeau G, Bernard D: PLA2R1 mediates tumor suppression by activating JAK2. Cancer Res, 2013; 73: 6334-6345 [DOI] [PubMed] [Google Scholar]
- 11). Augert A, Payré C, de Launoit Y, Gil J, Lambeau G, Bernard D: The M-type receptor PLA2R regulates senescence through the p53 pathway. EMBO Rep, 2009; 10: 271-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Mishina H, Watanabe K, Tamaru S, Watanabe Y, Fujioka D, Takahashi S, Suzuki K, Nakamura T, Obata JE, Kawabata K, Yokota Y, Inoue O, Murakami M, Hanasaki K, Kugiyama K: Lack of phospholipase A2 receptor increases susceptibility to cardiac rupture after myocardial infarction. Circ Res, 2014; 114: 493-504 [DOI] [PubMed] [Google Scholar]
- 13). Ancian P, Lambeau G, Lazdunski M: Multifunctional activity of the extracellular domain of the M-type (180 kDa) membrane receptor for secretory phospholipases A2. Biochemistry, 1995; 34: 13146-13151 [DOI] [PubMed] [Google Scholar]
- 14). Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med, 2009; 361: 11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Kao L, Lam V, Waldman M, Glassock RJ, Zhu Q: Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J Am Soc Nephrol, 2015; 26: 291-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Fresquet M, Jowitt TA, Gummadova J, Collins R, O'Cualain R, McKenzie EA, Lennon R, Brenchley PE: Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol, 2015; 26: 302-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, Stephens HA, Laundy V, Padmanabhan S, Zawadzka A, Hofstra JM, Coenen MJ, den Heijer M, Kiemeney LA, Bacq-Daian D, Stengel B, Powis SH, Brenchley P, Feehally J, Rees AJ, Debiec H, Wetzels JF, Ronco P, Mathieson PW, Kleta R: Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med, 2011; 364: 616-626 [DOI] [PubMed] [Google Scholar]
- 18). Liu YH, Chen CH, Chen SY, Lin YJ, Liao WL, Tsai CH, Wan L, Tsai FJ: Association of phospholipase A2 receptor 1 polymorphisms with idiopathic membranous nephropathy in Chinese patients in Taiwan. J Biomed Sci, 2010; 17: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Takiuchi S, Kamide K, Miwa Y, Tomiyama M, Yoshii M, Matayoshi T, Horio T, Kawano Y: Diagnostic value of carotid intima-media thickness and plaque score for predicting target organ damage in patients with essential hypertension. J Hum Hypertens, 2004; 18: 17-23 [DOI] [PubMed] [Google Scholar]
- 20). Nakamura T, Kitta Y, Uematsu M, Sugamata W, Hirano M, Fujioka D, Sano K, Saito Y, Kawabata K, Obata JE, Kugiyama K: Ultrasound assessment of brachial endothelial vasomotor function in addition to carotid plaque echolucency for predicting cardiovascular events in patients with coronary artery disease. Int J Cardiol, 2013; 167: 555-560 [DOI] [PubMed] [Google Scholar]
- 21). Sugamata W, Nakamura T, Uematsu M, Kitta Y, Fujioka D, Saito Y, Kawabata K, Obata JE, Watanabe Y, Watanabe K, Kugiyama K: Combined assessment of flow-mediated dilation of the brachial artery and brachial-ankle pulse wave velocity improves the prediction of future coronary events in patients with chronic coronary artery disease. J Cardiol, 2014; 64: 179-184 [DOI] [PubMed] [Google Scholar]
- 22). Nezu T, Hosomi N, Aoki S, Matsumoto M: Carotid Intima-Media Thickness for Atherosclerosis. J Atheroscler Thromb, 2016; 23: 18-31 [DOI] [PubMed] [Google Scholar]
- 23). Tomiyama H, Yamashina A: Non-invasive vascular function tests: their pathophysiological background and clinical application. Circ J, 2010; 74: 24-33 [DOI] [PubMed] [Google Scholar]
- 24). Deanfield JE, Halcox JP, Rabelink TJ: Endothelial function and dysfunction: testing and clinical relevance. Circulation, 2007; 115: 1285-1295 [DOI] [PubMed] [Google Scholar]
- 25). Wang J, Uryga AK, Reinhold J, Figg N, Baker L, Finigan A, Gray K, Kumar S, Clarke M, Bennett M: Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation, 2015; 132: 1909-1919 [DOI] [PubMed] [Google Scholar]
- 26). Lv J, Hou W, Zhou X, Liu G, Zhou F, Zhao N, Hou P, Zhao M, Zhang H: Interaction between PLA2R1 and HLA-DQA1 variants associates with anti-PLA2R antibodies and membranous nephropathy. J Am Soc Nephrol, 2013; 24: 1323-1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, De Carlo M, Gallino A, Landmesser U, Laurent S, Lekakis J, Mikhailidis DP, Naka KK, Protogerou AD, Rizzoni D, Schmidt-Trucksäss A, Van Bortel L, Weber T, Yamashina A, Zimlichman R, Boutouyrie P, Cockcroft J, O'Rourke M, Park JB, Schillaci G, Sillesen H, Townsend RR: The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis, 2015; 241: 507-532 [DOI] [PubMed] [Google Scholar]
- 28). Mochida T, Nakamura T, Nakamura K, Fujioka D, Saito Y, Obata JE, Watanabe Y, Watanabe K, Kugiyama K: Echolucent Carotid Plaque is Associated with Future Renal Dysfunction in Patients with Stable Coronary Artery Disease. J Atheroscler Thromb, 2015; 22: 685-696 [DOI] [PubMed] [Google Scholar]


