Abstract
Background
Serum uric acid (sUA) levels were previously found to be correlated with hypoxic states. We aimed to determine the levels of sUA and sUA/creatinine ratios in stable COPD patients and to evaluate whether sUA level and sUA/creatinine ratio can be used as predictors of exacerbation risk and disease severity.
Material/Methods
This cross-sectional study included stable COPD patients and healthy controls. The sUA levels and sUA/creatinine ratios in each group were evaluated and their correlations with the study parameters were investigated. ROC analyses for exacerbation risk and disease severity were reported.
Results
The study included 110 stable COPD patients and 52 healthy controls. The mean sUA levels and sUA/creatinine ratios were significantly higher in patients with COPD compared to healthy controls. The most common comorbidities in COPD patients were hypertension, diabetes, and coronary artery disease. While sUA levels were significantly higher in patients with hypertension (p=0.002) and malignancy (p=0.033), sUA/creatinine ratios was higher in patients with malignancy (p=0.004). The ROC analyses indicated that sUA/creatinine ratios can be more useful than sUA levels in predicting exacerbation risk (AUC, 0.586 vs. 0.426) and disease severity (AUC, 0.560 vs. 0.475) especially at higher cut-off values, but with low specificity.
Conclusions
Our study suggested that sUA levels and sUA/creatinine ratios increased in patients with stable COPD, especially among patients with certain comorbidities compared to healthy controls. At higher cut-off values, sUA levels and especially sUA/creatinine ratios, might be useful in predicting COPD exacerbation risk and disease severity. Also, their association with comorbidities, especially with malignancy and hypertension, may benefit from further investigation.
MeSH Keywords: Creatinine; Disease Progression; Oxidative Stress; Pulmonary Disease, Chronic Obstructive; Spirometry; Uric Acid
Background
Chronic obstructive pulmonary disease (COPD) is one of the most common preventable diseases and is a leading cause of mortality and morbidity worldwide [1,2].
Because evaluating the severity of COPD only with spirometric criteria limits clinical assessment and management of COPD, combined assessment of disease severity using Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages (A, B, C and D) in stable COPD period is recommended [1]. However, has been reported that the new classification had low reliability and the effect of comorbidities seemed to be independent from COPD severity [3]. In addition, this combined classification was not found to be efficient in determining dyspnea and oxygen desaturation in patients during their daily living activities [4]. Exacerbation of COPD is another factor affecting disease severity and has been defined as an acute event characterized by a worsening of the patient’s respiratory symptoms beyond normal day-to-day variations that leads to a change in medication [1]. Because patient recovery is often not complete after exacerbations, exacerbations have an important predictive role for COPD prognosis [5,6]. The frequency of exacerbations increases with the severity of disease and if a patient has had two or more acute events during the previous year, the patient is considered high risk [7,8].
Serum uric acid (sUA) levels have been found to be a useful marker of hypoxia in patients with COPD exacerbations, as well as in patients with other respiratory diseases such as obstructive sleep apnea syndrome (OSAS) [9,10], and numerous extrapulmonary diseases, especially cardiovascular, renal, and metabolic diseases [11,12]. This role of uric acid (UA) appears to be due to its role as the end product of purine catabolism in which adenosine is degraded due to tissue hypoxia [13]. The relationship between sUA levels and the risk levels of respiratory diseases is, however, controversial. While some researchers have reported negative correlations between sUA levels and the severity of the pulmonary disease [9,14], others have reported positive correlations [10,15]. The level of sUA, sUA/creatinine ratio, and their clinical importance in stable COPD patients are largely unknown. In addition, there are no objective biomarkers of disease activity, disease progression, or risk stratification of patients for imminent exacerbations [16].
In this study, our primary aim was to determine whether sUA levels and sUA/creatinine ratios in patients with stable COPD were different from healthy controls; our secondary aim was to investigate whether these easy-to-measure parameters can be used efficiently to predict patients at risk for exacerbation and/or severity of disease.
Material and Methods
Study design
Cross-sectional.
Study population
Inclusion criteria
Patients with COPD in its stable period who were seen at the outpatient clinic of a teaching and research hospital between August 2014 and April 2015 were included in the study. The control group consisted of the patients who were at least 40 years of age with no known systemic diseases or drug use.
Exclusion criteria
Patients with current COPD exacerbation or having history of exacerbation during the previous four weeks were excluded. Patients with chronic renal failure (serum creatinine levels ≥3 mg/dL or glomerular filtration rate <30 mL/min), gout disease, or those who used any drugs that might affect sUA levels, including allopurinol, febuxostat, probenecid, losartan, fenofibrate, pyrazinamide, ethambutol, cyclosporine, and heparin, were excluded.
Definitions
COPD diagnosis
Using the GOLD criteria, COPD was defined as patients with dyspnea, chronic cough or sputum production, and a history of exposure to risk factors for the disease (tobacco smoke, smoke from home cooking and heating fuels, occupational dusts and chemicals) with a spirometric criterion for airflow limitation (i.e., a post-bronchodilator forced expiratory volume during the first second (FEV1) divided by forced vital capacity (FVC) of <70%) (1).
COPD exacerbation
An exacerbation was defined as an acute event characterized by a worsening of the patient’s respiratory symptoms that was beyond normal day-to-day variations and that led to a change in medication [1]. Change in medication included increased doses and/or frequency of bronchodilators, and added oral or intravenous corticosteroids and/or antibiotics with or without hospitalization [1,17]. If patients had self-reported two or more exacerbations during the previous 12 months, they were classified as “had frequent exacerbations” and “patients with high risk” [1].
Modified Medical Council (mMRC) grade
The mMRC questionnaire was used for assessing the severity of breathlessness [1].
GOLD stage
GOLD stages of all patients with COPD were determined by assessment using symptoms (COPD Assessment Test or CAT score), breathlessness (mMRC grade), spirometric classification, and risk of exacerbations [1]. When there were discrepancies between CAT and mMRC scores, the worse one was used for determining the GOLD stage.
Measurements
A sample of venous blood was taken from each patient for a total blood count (Beckman-Coulter LH780, USA) and routine biochemical analyses (Beckman-Coulter AU480, USA), including glucose, C-reactive protein (CRP), urea, creatinine, and UAs were performed using standard techniques.
Spirometry was performed on all COPD patients (Spire ZAN 100-GPI 3.00 pulmonary spirometer, nSpire Health Inc.) and 20 minutes after the inhalation (using 400 μg salbutamol with a metered-dose inhaler), the measurement was repeated [18]. All pulmonary function tests were performed by the same technician and with patients in a sitting position.
Data
Demographic information (age, gender), body mass index (BMI), smoking status, and any comorbidity were recorded for each patient when admitted to the outpatient clinic. Laboratory parameters (leukocyte, CRP, glucose, urea, creatinine, sUA, sUA/creatinine ratio), spirometric values (FVC, FEV1, FEV1/FVC), mMRC grades, CAT scores, the number of exacerbations during the previous 12 months, GOLD stages, use of long-term oxygen therapy (LTOT), and noninvasive mechanical ventilation (NIMV) use were also recorded.
Statistical analyses
All statistical analyses were carried out using the SPSS software (version: 16.0; SPSS Inc., Chicago, IL, USA). Chi-squared tests were performed for comparing categorical variables between groups, t-tests for normally distributed continuous variables, and non-parametric Mann-Whitney U tests for comparing variables with non-normal distributions. Correlation analyses (Pearson) were performed to detect any associations between continuous variables. Receiver operating characteristic (ROC) curves were used to evaluate the prognostic values of sUA levels and sUA/creatinine ratios for predicting the disease severity (GOLD stage C and D) and frequent exacerbations (two or more in the last year). A p value <0.05 was considered significant.
Ethics
The study was approved by the Local Ethics Committee of the Institution and was conducted in accordance with the ethical principles stated in the Declaration of Helsinki. A patient informed consent form was obtained from each patient included in the study.
Results
A total of 110 patients with COPD in a stable disease period (92 males and 18 females, with a mean age of 65±10 years) and 52 healthy controls (37 males and 15 females, with a mean age of 63±7 years) were included in the study. The characteristics of the patient and control groups are given in Table 1. The patient group had more smoking pack/years than the control group (34.2±24.9 vs. 13.6±17.8, p<0.001). The mean sUA levels and sUA/creatinine ratios were found to be significantly higher in the patient group than in the control group.
Table 1.
Patient and control group comparison.
| Characteristics | Patient group (n=110) n, mean (SD) | Control group (n=52) n, mean (SD) | p |
|---|---|---|---|
| Gender | |||
| Male | 92 | 37 | 0.09 |
| Female | 18 | 15 | |
| Age | 65.4 (9.9) | 62.7 (7.4) | 0.11 |
| Current smoker | 20 (18%) | 14 (27%) | 0.22 |
| Smoking (pack/years) | 34.2 (24.9) | 13.6 (17.8) | 0.000 |
| BMI | 27.0 (6.4) | 27.5 (4.4) | 0.69 |
| CRP | 13.8 (24.2) | 17.4 (19.2) | 0.89 |
| Urea (mg/dL) | 27.1 (16.2) | 31.1 (16.3) | 0.16 |
| Creatinine (mg/dL) | 0.9 (0.4) | 0.8 (0.1) | 0.013 |
| Uric acid (mg/dL) | 5.9 (1.5) | 4.6 (1.2) | 0.000 |
| Uric acid/creatinine ratio | 6.8 (1.5) | 5.9 (1.1) | 0.000 |
BMI – body mass index; CRP – C-reaktive protein; SD – standard deviation.
Detected comorbidities in the patient group were hypertension (n=27, 24.5%), diabetes mellitus (n=14, 12.7%), coronary artery disease (n=14, 12.7%), malignancy (n=4, 3.5%) and cardiac failure (n=3, 2.7%). The mean sUA levels were significantly higher in the cases with a comorbidity, specifically in those who had hypertension or malignancy (p=0.002 and p=0.033, respectively). The patients who had frequent exacerbations, and LTOT or NIMV users had higher mean sUA levels (without statistical significance) but there was no difference between the GOLD stages. The sUA/creatinine ratio was higher in all comorbidities but a statistical significance was observed only for malignancy (p=0.004). Patients who had frequent exacerbations, LTOT or NIMV users, and GOLD stage D patients had higher sUA/creatinine ratios without statistical significance. According to the parameters studied in the patient group, CRP values were not significantly different except for the presence of malignancy (p=0.033) (Table 2).
Table 2.
Comparison of serum uric acid and serum uric acid/creatinine ratio according to the parameters studied in the patient group.
| Variables (n) | Uric acid Mean (SD) | p | Uric acid/creatinine ratio Mean (SD) | p |
|---|---|---|---|---|
| Comorbidity | ||||
| Yes (47) | 6.3 (1.5) | 0.021 | 7.1 (1.8) | 0.11 |
| No (58) | 5.6 (1.5) | 6.8 (1.6) | ||
| Frequent exacerbations | ||||
| Yes (34) | 6.0 (1.7) | 0.23 | 7.1 (1.3) | 0.16 |
| No (67) | 5.6 (1.2) | 6.6 (1.6) | ||
| GOLD Stage | ||||
| A (13) | 6.2 (2.1) | 0.78 | 6.4 (1.6) | 0.66 |
| B (36) | 5.9 (1.3) | 6.8 (1.8) | ||
| C (9) | 6.0 (1.7) | 6.8 (1.6) | ||
| D (46) | 6.1 (1.6) | 7.0 (1.3) | ||
| LTOT use | ||||
| Yes (19) | 5.9 (1.5) | 0.74 | 7.4 (1.6) | 0.09 |
| No (87) | 5.8 (1.6) | 6.7 (1.5) | ||
| NIMV use | ||||
| Yes (6) | 5.9 (1.5) | 0.08 | 7.1 (2.3) | 0.67 |
| No (99) | 4.9 (1.8) | 6.8 (1.5) | ||
GOLD – Global Initiative for Chronic Obstructive Lung Disease, LTOT – long term oxygen therapy, NIMV – non-invasive mechanical ventilation; SD – standard deviation.
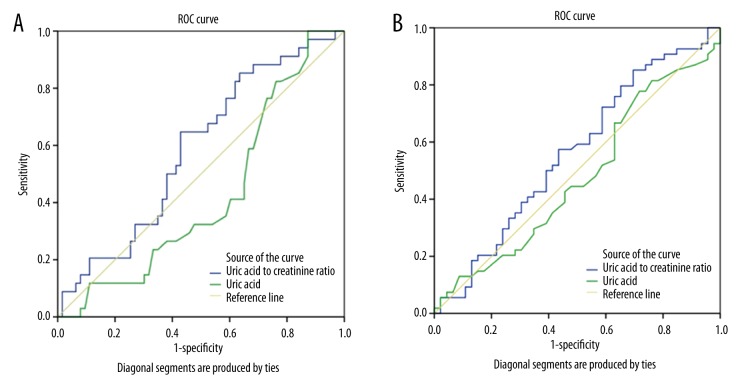
Although there was no correlation between sUA levels and all the parameters studied, there was a positive correlation between sUA/creatinine ratios and smoking pack/years (r=0.21, p=0.038), BMI (r=0.35, p=0.004), and CAT scores (r=0.31, p=0.007); and a negative correlation between sUA/creatinine ratios and FVC (r=−0.25, p=0.017). The area under the curve (AUC) obtained from the ROC analyses showed low performance of sUA levels and sUA/creatinine ratios for frequent exacerbation (0.426 vs. 0.586) and the disease severity state (0.475 vs. 0.560). When comparing two parameters, the ROC analyses indicated that sUA/creatinine ratio was more specific than sUA level alone with a similar sensitivity at higher cut-off values and more sensitive with similar specificity at lower cut-off values (Tables 3, 4) for both exacerbation (Figure 1A) and severity of disease (Figure 1B).
Table 3.
Cut off values of uric acid and uric acid to creatinine ratio for frequent exacerbation state.
| Values | Specificity (%) | Sensitivity (%) |
|---|---|---|
| Uric acid* | ||
| 4.35 | 13.4 | 91.2 |
| 4.95 | 28.4 | 70.6 |
| 5.15 | 32.8 | 58.8 |
| 6.00 | 52.2 | 32.4 |
| 6.95 | 70.1 | 11.8 |
| 8.30 | 91.0 | 2.9 |
| Uric acid/creatinine ratio** | ||
| 5.38 | 22.2 | 91.2 |
| 6.40 | 41.3 | 70.6 |
| 7.23 | 61.9 | 50.0 |
| 7.44 | 71.4 | 32.4 |
| 8.51 | 92.1 | 14.7 |
AUC: 0.426, 95%CI: 0.312–0.541;
AUC: 0.586, 95%CI: 0.470–0.702.
AUC – area under the curve, CI – confidence interval.
Table 4.
Cut-off values of uric acid and uric acid to creatinine ratio for severe disease (GOLD C,D) state.
| Values | Specificity (%) | Sensitivity (%) |
|---|---|---|
| Uric acid* | ||
| 4.00 | 4.1 | 90.9 |
| 4.95 | 28.6 | 72.7 |
| 5.55 | 40.8 | 50.9 |
| 6.85 | 71.4 | 21.8 |
| 8.10 | 91.8 | 7.3 |
| 8.30 | 91.0 | 2.9 |
| Uric acid/creatinine ratio** | ||
| 5.32 | 19.6 | 90.7 |
| 6.39 | 41.3 | 70.4 |
| 7.17 | 60.9 | 50.0 |
| 7.44 | 71.7 | 35.2 |
| 9.42 | 91.3 | 5.6 |
AUC: 0.475, 95%CI: 0.361–0.590;
AUC: 0.560, 95%CI: 0.446–0.675.
AUC – area under the curve, CI – confidence interval.
Figure 1.
(A) ROC curves for uric acid and uric acid to creatinine ratio by frequent exacerbation. (B) ROC curves for uric acid and uric acid to creatinine ratio by disease severity (GOLD C,D).
Discussion
Our findings showed that both the sUA levels and sUA/creatinine ratios were higher in patients with COPD compared to healthy controls. Although CRP is one of the well-studied biomarker for patients with COPD [19], there was no difference between the groups in our study. The sUA levels and sUA/creatinine ratios were higher in patients with comorbidities; in addition, the sUA levels in hypertension and both the sUA levels and sUA/creatinine ratios in patients with malignancy were significantly higher. However, sUA/creatinine ratios had better correlation with some parameters associated with COPD, including smoking pack/year, CAT score, and FVC. Moreover, the sUA/creatinine ratio was more sensitive and specific than sUA level for evaluating exacerbation risk and severity of disease.
Uric acid is produced from the metabolic conversion of both exogenous purines (present in fatty meat, organ meats, and seafood) and endogenous purines [14]. Tissue hypoxia increases the catabolism of purines and leads to increased levels of uric acid [9,14]. Diet (intake of purines), exercise, and BMI also affect sUA levels [9,11,14,20]. Correspondingly, we detected positive correlation between sUA/creatinine ratios and BMI. Since sUA is found in urinary excretion, and various systemic diseases may affect sUA levels, we also analyzed sUA values corrected based on creatinine levels. We observed significantly different serum creatinine values between our patient group and control group. In addition, about half of the patients had a comorbidity, and sUA levels were found to be related to the presence of comorbidity, specifically hypertension and malignancy. However, the sUA/creatinine ratio was only higher in patients with a malignant disease. We also, found that the sUA/creatinine ratio was correlated with smoking pack/years, suggesting that this ratio might be a more reliable parameter than sUA level for COPD patients who have a smoking history and concomitant comorbid disease.
There are few studies investigating the relationship between sUA levels and COPD, and the results of these studies appear to be inconsistent with one another. In some studies, increased sUA levels indicated worse conditions. For example, Bartziokas et al. determined that sUA was associated with increased acute exacerbation of COPD, hospitalization, and 30-day mortality in 314 COPD patients with acute exacerbation; and based on these findings, they concluded that systemic UA levels are associated with oxidative stress and inflammation in vivo [15]. In another study of 40 patients, salivary UA concentrations were found to be two times higher in COPD patients than in patients without COPD [21]. In contrast, Nicks et al. found that COPD patients with GOLD stages 1 and 2 (according to spirometric measurements) had higher levels of the UA than did severe COPD patients (GOLD stages 3–4). The authors concluded that a higher level of UA was associated with better lung function [9]. Moreover, another study reported that low levels of sUA were associated with higher rates of COPD and lung cancer in current smokers [22]. In our study, both sUA levels and sUA/creatinine ratios were significantly higher in COPD patients than in healthy controls. It has been suggested that sUA levels increase in the presence of persistent systemic inflammation caused by COPD [23]. Interestingly, sUA levels and sUA/creatinine ratios were not associated with the severity of COPD in our study. This may be due to the unequal numbers of patients in the COPD and control groups, and the low number of patients. However, the increase of the sUA/creatinine ratio based on the GOLD stages was interpreted as remarkable.
Studies of the relationship between the sUA/creatinine ratio and respiratory diseases have mostly involved patients with OSAS and/or COPD who needed LTOT or NIMV. In a study of 91 outpatients with COPD receiving LTOT during a mean follow-up period of 31 months, mortality was found to be significantly higher for patients with high sUA/creatinine ratios [24]. In a study of COPD and OSAS groups, the overnight change in the sUA/creatinine ratio was found to be positive for both groups, when compared with normal patients [25]. In a study of 29 patients with chronic respiratory failure treated with NIMV, patients who showed a decrease in the sUA/creatinine ratio after applying NIMV suffered fewer chronic respiratory failure exacerbations than did patients who did not show a decrease in the sUA/creatinine ratio. Based on this finding, the investigators concluded that the sUA/creatinine ratio may be a valuable predictor for prognosis [10]. In our study, although not statistically significant, patients with frequent exacerbations or required LTOT and NIMV support had higher sUA levels and sUA/creatinine ratios than those who they did not require support. On the other hand, the number of patients receiving NIMV and LTOT wasrelatively low (n=6 and n=18, respectively) and we propose that a larger group of patients is needed for further evaluation.
A study of OSAS patients reported lower FVC and FEV1 in patients with hyperuricaemia (UA >5.7 mg/dL) [26]. Significant inverse correlations between spirometric parameters and sUA levels were also observed in female patients who were at least 40 years old [6]. We found no association between sUA and spirometric values. Difference in study populations may explain our results. Garcia-Pachon et al. found that in 58 patients with stable COPD, the sUA/creatinine ratio was inversely correlated with FVC and FEV1, but positively correlated with mMRC grade. At the same time, the sUA level did not correlate with these parameters except for FVC [27]. Correspondingly, we detected a negative correlation between FVC and the sUA/creatinine ratio. Increase in symptoms of patients determined by CAT score was correlated with sUA/creatinine ratio. On the other hand, both FVC and CAT scores were not found to be associated with the sUA levels suggesting that sUA/creatinine ratio is a more informative parameter than sUA values.
According to our best knowledge, there is no previous study evaluating sensitivity and specificity of sUA levels or sUA/creatinine ratio as a biomarker in the evaluation of COPD exacerbation risk and in determining severity of disease. One of limitation of our study was that it was a single-center, tertiary-care hospital study that could have resulted in an unequal numbers of patients in the different GOLD patient groups. Other limitations include: small numbers of female patients, and small numbers of LTOT and NIMV users that might under represent the overall COPD population.
Conclusions
Serum UA levels and sUA/creatinine ratios were higher in stable COPD patients compared to healthy controls; and sUA/creatinine ratio appears to be a more valuable parameter than the sUA level. Although both parameters showed low diagnostic performance, they had higher sensitivity but lower specificity at higher cut-off values in predicting patients at risk for exacerbation and severity of disease. Also, their association with comorbidities, especially with malignancy and hypertension, may be of value for further investigation.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD), Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2014
- 2.Fischer BM, Voynow JA, Ghio AJ. COPD: balancing oxidants and antioxidants. Int J Chron Obstruct Pulmon Dis. 2015;10:261–76. doi: 10.2147/COPD.S42414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodrigo GJ, Soler-Cataluna JJ, Solanes I, et al. Assessment of the internal structure of GOLD 2011 system. Pulm Pharmacol Ther. 2015;30:87–92. doi: 10.1016/j.pupt.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Barusso MS, Gianjoppe-Santos J, Basso-Vanelli RP, et al. Limitation of activities of daily living and quality of life based on COPD combined classification. Respir Care. 2015;60:388–98. doi: 10.4187/respcare.03202. [DOI] [PubMed] [Google Scholar]
- 5.Halpin DMG, Decramer M, Celli B, Kestern S, et al. Risk of nonlower respiratory serious adverse events following COPD exacerbations in the 4-year UPLIFT (R) trial. Lung. 2011;189:261–68. doi: 10.1007/s00408-011-9301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkinson TM, Donaldson GC, Hurst JR, et al. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169:1298–303. doi: 10.1164/rccm.200310-1443OC. [DOI] [PubMed] [Google Scholar]
- 7.Burge S, Wedzicha JA. COPD exacerbations definitions and classifications. Eur Respir J. 2003;41:46–53. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- 8.Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–22. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 9.Nicks ME, O’Brien MM, Bowler RP. Plasma antioxidants are associated with impaired lung function and COPD exacerbations in smokers. COPD. 2011;8:264–69. doi: 10.3109/15412555.2011.579202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadowaki T, Hamada H, Yokoyama A, et al. Significance of serum uric acid in patients with chronic respiratory failure treated with non-invasive positive pressure ventilation. Intern Med. 2007;46:691–97. doi: 10.2169/internalmedicine.46.6120. [DOI] [PubMed] [Google Scholar]
- 11.Kutzing MK, Firestein BL. Altered uric acid levels and disease states. J Pharmacol Exp Ther. 2008;324:1–7. doi: 10.1124/jpet.107.129031. [DOI] [PubMed] [Google Scholar]
- 12.Akkasilpa S, Avihingsanon Y, Hanvivadhanakul P, Wonchinsri J. Clinical manifestations of patients with hyperuricemia. J Med Assoc Thai. 2004;87(Suppl 2):41–44. [PubMed] [Google Scholar]
- 13.Aida Y, Shibata Y, Osaka D, et al. The relationship between serum uric acid and spirometric values in participants in a health check: The Takahata Study. Int J Med Sci. 2011;8:470–78. doi: 10.7150/ijms.8.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang DH, Ha SK. Uric acid puzzle: Dual role as anti-oxidant and pro-oxidant. Electrolyte Blood Press. 2014;12:1–6. doi: 10.5049/EBP.2014.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartziokas K, Papaioannou AI, Loukides S, et al. Serum uric acid as a predictor of mortality and future exacerbations of COPD. Eur Respir J. 2014;43:43–53. doi: 10.1183/09031936.00209212. [DOI] [PubMed] [Google Scholar]
- 16.Sin DD, Zsuzsanna H, DeMarco ML, et al. Biomarker development for chronic obstructive pulmonary disease from discovery to clinical implementation. Am J Respir Crit Care Med. 2015;192:1162–70. doi: 10.1164/rccm.201505-0871PP. [DOI] [PubMed] [Google Scholar]
- 17.Yilmazel Ucar E, Araz O, et al. Two different dosages of nebulized steroid versus parenteral steroid in the management of COPD exacerbations: A randomized control trial. Med Sci Monit. 2014;20:513–20. doi: 10.12659/MSM.890210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spirometry for health care providers, Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2010
- 19.Tkacova R, McWilliams A, Lam S, Sin Don D. Integrating lung and plasma expression of pneumo-proteins in developing biomarkers in COPD: A case study of surfactant protein D. Med Sci Monit. 2010;16(11):CR540–44. [PubMed] [Google Scholar]
- 20.Desideri G, Castaldo G, Lombardi A, et al. Is it time to revise the normal range of serum uric acid levels? Eur Rev Med Pharmacol Sci. 2014;18:1295–306. [PubMed] [Google Scholar]
- 21.Yigla M, Berkovich Y, Nagler RM. Oxidative stress indices in COPD broncho-alveolar lavage and salivary analysis. Arch Oral Biol. 2007;52:36–43. doi: 10.1016/j.archoralbio.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Horsfall LJ, Nazareth I, Petersen I. Serum uric acid and the risk of respiratory disease: A population-based cohort study. Thorax. 2014;69:1021–26. doi: 10.1136/thoraxjnl-2014-205271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Xie M, He X, Gao H. Activity of sputum p38 MAPK is correlated with airway inflammation and reduced FEV1 in COPD patients. Med Sci Monit. 2013;19:1229–35. doi: 10.12659/MSM.889880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato N, Kurashima K, Ubukata M, et al. Prognostic significance of serum uric acid in patients with chronic obstructive pulmonary disease receiving home oxygen therapy. Nihon Kokyuki Gakkai Zasshi. 2003;41:74–80. [PubMed] [Google Scholar]
- 25.Braghiroli A, Sacco C, Erbetta M, et al. Overnight urinary uric acid: Creatinine ratio for detection of sleep hypoxemia. Validation study in chronic obstructive pulmonary disease and obstructive sleep apnea before and after treatment with nasal continuous positive airway pressure. Am Rev Respir Dis. 1993;148:173–78. doi: 10.1164/ajrccm/148.1.173. [DOI] [PubMed] [Google Scholar]
- 26.Pływaczewski R, Bednarek M, Jonczak L, et al. Hyperuricemia in females with obstructive sleep apnoea. Pneumonol Alergol Pol. 2006;74:159–65. [PubMed] [Google Scholar]
- 27.Garchia-Pachon E, Padilla-Navas I, Shum C. Serum uric acid to creatinine ratio in patients with chronic obstructive pulmonary disease. Lung. 2007;185:21–24. doi: 10.1007/s00408-006-0076-2. [DOI] [PubMed] [Google Scholar]