Abstract
Background
The aim of this study was to investigate the proliferation, differentiation, and tube formation of human outgrowth endothelial progenitor cells (OECs) cultured with porous demineralized bone matrix (DBM) under a dynamic perfusion system in vitro.
Material/Methods
OECs were isolated, expanded, characterized, eGFP-transfected and seeded on DBM scaffold and cultured under static or dynamic perfusion conditions, and continuously observed under fluorescence microscope. DBM scaffolds were harvested on day six for RT-PCR and western blot assay to analyze the mRNA and protein expression level of CD34, VE-cadherin, and VEGF. Scanning electron microscope (SEM) was used to observe the tube formation of OECs seeded on DBM scaffolds.
Results
The results showed the cell density of OECs on DBM was higher when exposed to shear stress generated by a dynamic perfusion system. Shear stress also markedly increased the expression level of VE-cadherin and VEGF and decreased the expression of CD34, at both mRNA and protein levels. SEM showed that the shear-stressed OECs formed tube-like structures inside the pores of DBM scaffolds.
Conclusions
A dynamic perfusion system can be used as an innovative method for the rapid vascularization in tissue engineering, which can accelerate the proliferation and differentiation of OECs and the vascularization of implanted scaffolds.
MeSH Keywords: Bone Matrix; Neovascularization, Physiologic; Stem Cells; Tissue Engineering
Background
Currently, the repair of large bone defects remains a major clinical orthopedic challenge. Tissue engineering strategies have been employed to develop bone grafts for patients lacking sufficient autologous bones for grafting. One of the most widely researched tissue engineering approaches for bone grafts has been the in vitro culture of a three dimensional (3D) scaffolding material, seeded with autologous cells, such as osteoblast and mesenchymal stem cells, followed by implantation in the patient. However, oxygen and nutrient transport from blood vessels of the surrounding host tissue is limited to a diffusion distance of 100 μm to 200 μm [1,2], thus supplying only the cells on the surface of the bone scaffold material, which has been identified as one reason for implant failure and is currently acknowledged as the major challenge in tissue engineering.
Several strategies are being developed to promote rapid vascularization of large bone scaffold material, including in vivo and in vitro pre-vascularization. Regardless of the approach adopted to accelerate vascularization, all of the strategies will directly or indirectly include endothelial cells (ECs). Studies have indicated transplanted ECs can form new blood vessels and do so in conjunction with host ECs [3–5]. Despite such promising findings, the clinical application of the ECs-seeded grafts is hampered by the lack of a convenient source of ECs [6]. During the last decade, the discovery of outgrowth endothelial cells (OECs) – a subpopulation of diverse progeny of endothelial progenitor cells (EPCs) derived from circulating progenitor cells in peripheral blood and umbilical cord blood – has opened up new vistas to possible rapid vascularization with these cells [7]. The features of OECs that make them well-suited for tissue engineering include: (1) robust proliferation rate, (2) high expansion potential, and (3) simple sourcing via venipuncture [8]. The high proliferation rate and expansion potential of OECs allows just a very few colonies to be quickly expanded to the high cell numbers required for tissue engineering applications. In addition, venipuncture is considerably less invasive than harvesting ECs from excised tissue. Consequently, there have been several studies describing the use of OECs for bone tissue engineering applications [9,10], including observations that OECs exhibit many of the same cellular markers and functions as vascular ECs [11,12]. Very little is known about the role of shear stress on the OECs’ capacity for proliferation, differentiation, modulation of angiogenesis related factors, and tube formation, despite shear stress having been proven to accelerate the proliferation, differentiation, and capillary-like tube formation of EPCs [13,14].
The aim of our study was to test and verify whether shear stress influences proliferation, differentiation, and tube formation of OECs on a demineralized bone matrix (DBM). In this study, we chose to load DBM under shear stress using a specific dynamic perfusion system, in which samples could be subjected to simulated capillary flow and controlled levels of shear stress.
Material and Methods
Isolation and expansion of OECs
OECs were isolated and expanded according to the protocol described by Martin-Ramirez et al., 2012 [15]. Briefly, mononuclear cells (MNC) were isolated from buffy coats from one healthy volunteer’s peripheral blood after dilution with phosphate-buffered saline (PBS) by density gradient centrifugation in 1.077 g/mL Histopaque solution (Sigma-Aldrich), then washed several times with PBS, and cultured in EGM-2 SingleQuots Kit medium (Lonza) with 10% fetal bovine serum (GIBCO), on collagen type I (Lonza) coated 25 cm2 cell culture flask. After 24 hours of culture, non-adherent cells were discarded. Cells were fed with fresh medium every second day. The morphological changes of the adherent cells was monitored daily. Single colonies of OECs, appearing with cobblestone-like morphology after two to three weeks in culture, were selected, trypsinized, and expanded over several passages by using standard cell culture procedures. Cells were used at passages four through six.
Characterization of OECs
Flow cytometry analysis
Cultured cells were detached in non-enzymatic cell dissociation medium (Sigma-Aldrich) to preserve cell membrane markers. Cells were incubated for 30 minutes at 4 °C with monoclonal antibodies against CD34-PE, VEGFR2-FITC, and CD133-PE (eBioscience) according to manufacturer’s protocol. Quantitative fluorescence analysis was performed using a fluorescence-activated cell sorting (FACS) flow cytometer and WinMDI software (Becton Dickinson).
DIL-acLDL uptake and binding of FITC-Lectin assay
Cells were incubated with DiI-labeled Ac-LDL (Invitrogen) at a concentration of 10 μg/mL in culture medium for one hour at 37°C and then washed with medium. In parallel, cells were incubated with FITC-conjugated lectin at a concentration of 10 μg/mL. Finally, cells were examined by fluorescence microscopy (Olympus BX 51).
Tube formation assay
Matrigel (BD Biosciences) at 50 μL/well was laid into 96-well plates to solidify. OECs were resuspended in 200 μL of EBM-2 without EGM-2 SingleQuots supplements and then plated on solidified Matrigel. After 18 hours, tube images were obtained by using an inverted microscope (Olympus BX 51).
Lentivirus-mediated enhanced green fluorescent protein transfection to OECs
OECs were transfected with lentivirus-mediated enhanced green fluorescent protein (lenti-eGFP) to permit continuous observation after being seeded on DBM scaffold in vitro. OECs at approximately 80% confluence were washed with PBS three times and exposed to 100 μL of lenti-eGFP (multiplicity of infection, 50, genomeditech) [16]. After six hours of incubation, the cells were washed with PBS and new medium was applied. After 48 hours, the cells were observed under a fluorescent microscope to determine the transfection efficiency; the proliferative activity was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay (Sigma) and compared to non-transfected cells (control group).
Establishment of dynamic perfusion culture system
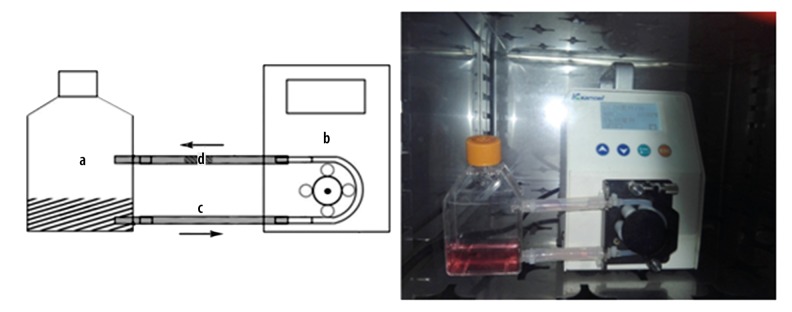
OECs were statically seeded on DBM (OsteoRad) at a density of 2×107 cells/mL and cultured for six hours after cell attachment was completed. Before being implanted into the dynamic perfusion culture system, the complexes were randomly divided into dynamic perfusion culture group (n=6) and static culture group (n=6). The dynamic perfusion culture system created in our laboratory consisted of a special culture flask (Figure 1A), a digital flow-control peristaltic pump (Figure 1B) and a transparent silicone pipe system to permit continuous fluorescent microscope observation of OECs (Figure 1C). The culture medium was added into the flask and pumped at a flow rate of 10 mL/minute. The flow rate needed for cell survival was estimated based on cell mass and reported values for nutrient consumption. The entire perfusion system was sterilized by irradiation and maintained at 37°C with 5% CO2 supplementation. When it came time to continuously observe OECs seeded on DBM scaffold, the clip of pump was loosened, the flask with silicone pipe system was removed and the cells were observed under fluorescent microscope. Cell density and projected cell area was calculated by measuring the numbers and areas of fluorescent cells attached on the surface of DBM scaffold per field using image analyzer software (ImageJ 1.34s).
Figure 1.
Dynamic perfusion culture system (a): Special culture flask; (b): Digital flow-control peristaltic pump; (c): Transparent silicone tube; (d): OECs-DBM scaffold complex.
Four visual fields were randomly selected and counted for each sample.
RNA extraction and quantitative reverse transcriptase real-time PCR
After six days of culture, the cells were harvested from DBM scaffolds in both groups for real-time PCR assay. Total RNA was extracted from cells and treated with DnaseI using the RNeasy Micro kit (Qiagen); 5 μL of total RNA was used for reverse transcription using the ImPromII Reverse Transcription System and Oligo-d(T15) Primers (Promega). Negative controls were processed in parallel without reverse transcriptase. Relative mRNA levels of CD34/VE-cadherin/VEGF were quantified by quantitative reverse transcriptase real-time PCR (QRT-PCR) using cDNA obtained from the reverse transcription reactions as template, with a StepOne instrument (Applied Biosystems) and SYBR-Green PCR Master Mix (Applied Biosystems). The total volume of the reaction was 10 μL. Data were analyzed using the ΔΔCT method and Prism software (Applied Biosystems), according to the manufacturer’s instructions. Human β-actin expression was used as the housekeeping gene control. Individual samples were subjected to duplicate or triplicate QRT-PCR reactions and ΔCT values calculated. The primer sequences are presented in Table 1.
Table 1.
Primer sequences and product size of genes.
| Gene | Primer sequences (5′-3′) | Product size |
|---|---|---|
| CD34 | F: caccctgtgtctcaacatgg | 191 bp |
| R: ggcttcaaggttgtctctgg | ||
| VE-cadherin | F: tgcccagaaaatgaaaaagg | 200 bp |
| R: gtgtatgtggcaatgcgttc | ||
| VEGF | F: cccactgaggagtccaacat | 173 bp |
| R: aaatgctttctccgctctga | ||
| β-actin | F: gctcttttccagccttcctt | 187 bp |
| R: gagccagagcagtgatctcc |
Western blot analysis
After six days of culture, cells were harvested from the DBM scaffolds of both groups for western blot analysis. Cells were washed with cold PBS and resuspended in RIPA Lysis Buffer containing protease inhibitor cocktail (Santa Cruz Biotech). Lysates were centrifuged at 26,000 g for 30 minutes, and the supernatants were mixed with NuPAGE LDS Sample Buffer (Invitrogen) adding 10% β-mercaptoethanol for SDS-PAGE. Gels were transferred to Immobilon polyvinylidene difluoride membranes (Millipore), and the membranes were blocked in PBS with 3% bovine serum albumin (BSA) and 0.1% NaN3 and incubated overnight at 4°C with the antibodies against VEGF, CD34, and VE-cadherin (Sigma). The membranes were then washed with PBS with 0.05% Tween 20 and incubated with IgG horseradish-peroxidase conjugated antibody (GE Healthcare), and the blots were developed with an enhanced chemiluminescence kit (Millipore) and visualized by Cool Saver (ATTO) and CS Analyzer (ATTO). The intensity of each developed band was measured using an image analyzer software (ImageJ 1.34s).
SEM observation
After samples were cultured for six days, the scaffolds were harvested and soaked in 2.5% glutaraldehyde overnight, and an alcohol gradient dehydration was prepared for the SEM observation. The samples were sliced into two convenient pieces for observing the inside pores and both surface sides of scaffolds. The samples were sputter-coated with gold and examined under a S-3000N scanning electron microscope (Hitachi).
Statistical analysis
All measurement data are expressed as mean ±SD. The two groups were compared using a Student t-test. Comparisons of statistical data among multiple groups were performed using one-way ANOVA analysis. p values <0.05 were considered statistically significant. All statistical calculations were performed with SPSS v 20.0 software.
Results
OECs isolation and characterization
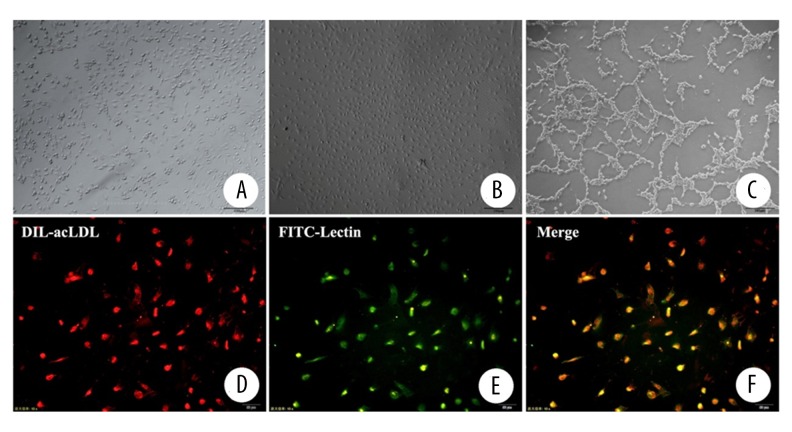
After cultured for 24 hours, the adherent cells exhibited a heterogeneous morphology. Many round, monocyte-like cells were seen as well as a number of well-spread flattened cells that were mixed with spindle-like cells (Figure 2A). The adherent cells exhibited characteristic cobblestone morphology and rapid proliferated after cultured for approximately three weeks (Figure 2B). Flow cytometry analysis showed that OECs were strongly positive for CD34 and CD133 with >90% of the cells positive for each of these markers. OECs were weakly positive for VEGR2. The tube formation assay on Matrigel showed that OECs made capillary formation on Matrigel successfully (Figure 2C). OECs isolated from peripheral blood were characterized by DiI-Ac-LDL uptake and the binding of FITC-lectin. Nearly all of the adherent cells (>95%) after one week in culture showed positive results in the LDL-uptake assays (Figure 2D) and in their capacity to bind lectin (Figure 2E).
Figure 2.
Culture of OECs, tube formation assay, DIL-acLDL uptake and binding of FITC-lectin assay (A): Culture for 24 hours (scale bar=100 μm); (B): Culture for 21 days (scale bar=100 μm); (C): Tube formation assay (scale bar=100 μm); (D): DIL-acLDL uptake (scale bar=50 μm); (E): Binding of FITC-lectin (scale bar=50 μm); (F): Merge of figures (D, E).
Lentivirus-mediated eGFP transfection to OECs
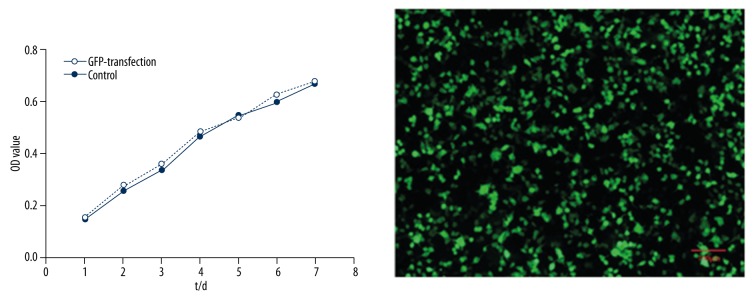
After 48 hours, the expression of eGFP was detected under the fluorescent microscope. About 91±4.5% of the cells were positive. The fluorescence expression was strong and sustained for at least two week without diminishing. At this time point, greater than 90% of the cells were still positive. The MTT assay showed that the in vitro proliferative activity of eGFP-transfected OECs was similar to that of non-transfected OECs (control group) (p>0.05, Figure 3).
Figure 3.
The influence of eGFP-transfection and control group on cell proliferation and eGFP-transfected OECs imaging (scale bar=100 μm).
Gene expression analysis in OECs under static culture and dynamic perfusion culture
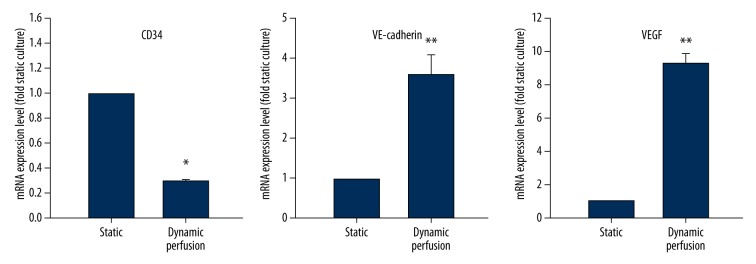
Expression levels of CD34/VE-cadherin/VEGF mRNA in cells under the dynamic perfusion culture (n=4) were compared to static culture group (n=4). For the dynamic perfusion culture group, a 0.29±0.02 fold reduction in expression of CD34 mRNA was observed (p<0.05). Expression of VE-cadherin and VEGF mRNA were increased significantly in the dynamic perfusion culture group (3.55±0.53 fold and 9.32±0.30 fold, respectively) (p<0.01) (Figure 4).
Figure 4.
The influence of dynamic perfusion culture on CD34/VE-cadherin/VEGF mRNA expression (* p<0.05, ** p<0.01). Results are expressed as fold change compared to static culture.
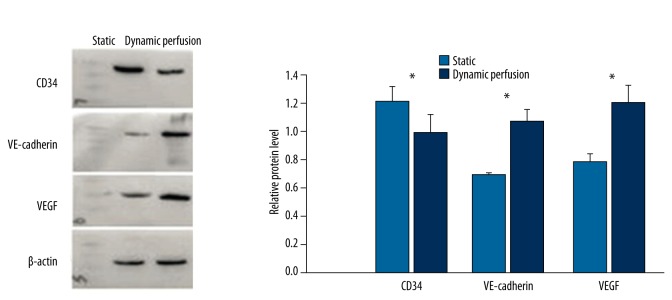
Western blot analysis of OECs under static culture and dynamic perfusion culture
Relative expression level of CD34, VE-cadherin, and VEGF protein were 1.216±0.105, 0.693±0.011, and 0.784±0.051 in the static culture group (n=4), Relative expression level of CD34, VE-cadherin, and VEGF protein were 0.992±0.133, 1.074±0.080, and 1.202±0.126 in the dynamic perfusion culture group (n=4). Compared to the static culture group, the VE-cadherin and VEGF protein expression levels were significantly elevated (p<0.05), while the dynamic perfusion culture significantly reduced the expression of CD34 (p<0.05) (Figure 5).
Figure 5.
Expression of CD34, VE-cadherin, and VEGF protein under static culture and dynamic perfusion culture. (* p<0.05)
Continuous fluorescent observation of OECs seeded on DBM scaffolds
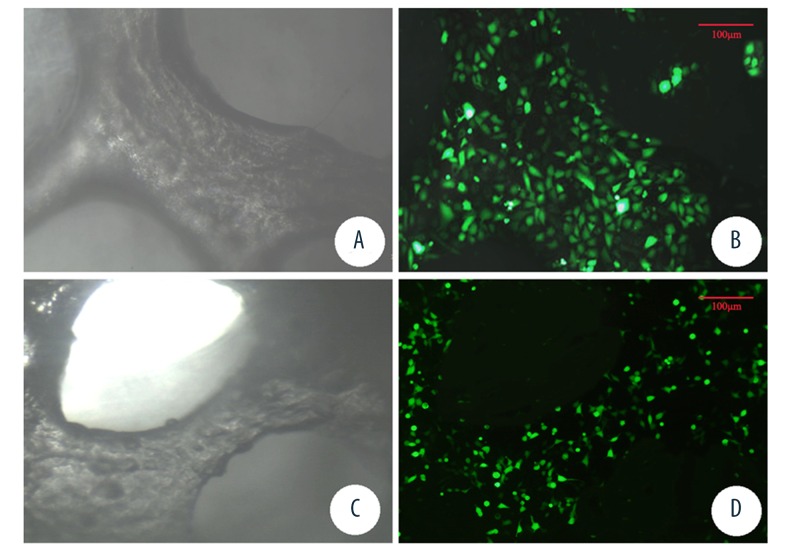
The eGFP transfected OECs and DBM scaffold complex were observed under fluorescent microscope every day. The density of fluorescent cells increased gradually with the processing of cultures in both groups. After cultured for six days, cell density of the dynamic perfusion culture group was significantly higher than the static culture group (83.22±10.28 cells/unit area and 59.15±13.18 cells/unit area, respectively, p<0.05). Additionally, the projected cell area in the dynamic perfusion culture group was slightly larger than that of the static culture group (p<0.05) (Figure 6).
Figure 6.
Light microscope and fluorescence microscope observation of DBM scaffolds under dynamic perfusion culture (A, B) and static culture (C, D) (scale bar=100 μm).
SEM observation of scaffold
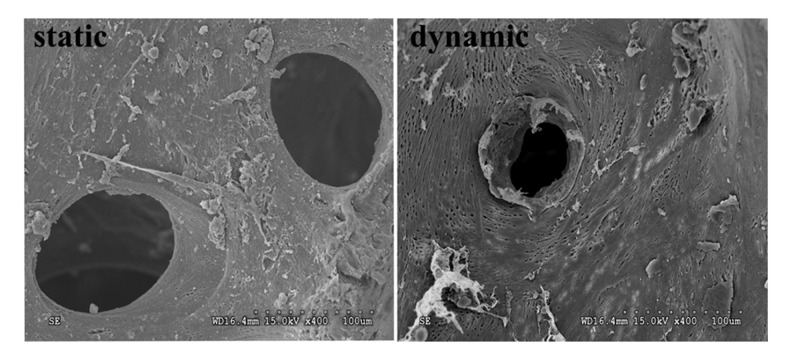
DBM scaffolds were harvested on day six for SEM observation. The scaffolds under dynamic perfusion culture (n=4) were observed to form a microvessel-like structure in most of the pores, but no similar structures were found in the scaffolds under static culture (n=4) (Figure 7).
Figure 7.
SEM of DBM scaffolds under static culture and dynamic perfusion culture.
Discussion
“In tissue engineering, pre-vascularization of tissue constructs represents a promising approach to guarantee their rapid and sufficient vascularization after implantation into a host defect by the process of inosculation” [17]. Currently in vivo pre-vascularization is performed to allow for vascularization of bone scaffolds. Firstly, the bone scaffolds are implanted in axially vascularized tissue (i.e., in subcutaneous, intramuscular, or intraperitoneal sites), where microvascular network formation occurs within the scaffolds within several weeks. The scaffolds are then harvested and transferred as free bone flap to the bone defect site, where the vascular axis is connected via microsurgical vascular anastomosis techniques, resulting in instantaneous perfusion of the entire scaffolds. Several reported drawbacks to this technique include the requirement for two surgeries, cost, formation of a random vascularization pattern, degree of vascularization based on host tissue vascularity, as well as donor site morbidity [18]. Current in vitro pre-vascularization strategies involve the prior seeding and co-culture of endothelial cells or their precursors and osteogenic cells in bone scaffolds in vitro [5,19]. The formation of premature vessels by the endothelial cells or their precursors in vitro, may later mature and anastomose with the host vasculature upon implantation. With this method, anastomoses occurs more quickly in comparison to non-pre-vascularized scaffolds, as host vessels only need to grow into the outer region of the constructs to meet the pre-vascular structures [20]. Primary advantages of this approach include the responsive nature of delivery and the ability of cells to synthesize the desired factors, eliminating the need for a large depot, and decreasing the time needed for vascularization from weeks to days. In our study, we demonstrated that in vitro vascularization of DBM scaffolds can be promoted by seeding of OECs and the use of a dynamic perfusion culture system. The results showed that the shear stress generated by a dynamic perfusion system can promote the proliferation, differentiation, and 3D vessel tube formation of OECs in DBM scaffolds.
Previous studies on EPCs or mature ECs response to shear stress were mostly focus on unidirectional or bidirectional flow influence on cell morphology, proliferation, and gene expression. Whether or not dynamic perfusion shear stress can influence the proliferation, differentiation, and tube formation of OECs seeding on DBM scaffolds is still unclear. When exposed to unidirectional flow shear stress, EPCs and mature ECs elongate and orient along the direction of flow, whereas, cells exposed to bidirectional shear stress show a random distribution [14,21]. In our study, a random distribution of OECs was observed, although the DBM scaffold was loading in an unidirectional flow generated by the dynamic perfusion system. Because the pore structure inside the scaffold was complicated and orderless, the unidirectional flow was converted to turbulent flow when it went through the scaffold. No previous studies have described the flow influence on the projected cell area of OECs. We speculate that the larger cell area in the dynamic perfusion culture group may be due to stimulation of shear stress; cell attachment appeared to be enhanced in the presence of shear stress; and cells were flatten, making the projected cell area larger, however, this speculation needs further study to confirmed. The results of another study suggest that shear stress stimulates EPCs proliferation [14]. This study’s results were similar to our study’s results using OECs cultured on DBM scaffold. Other studies have shown that shear stress promotes EPCs differentiation into a mature endothelial phenotype [22]. Our findings suggested that OECs, similarly to EPCs, responded to shear stress and upregulated the expression level of VE-cadherin and VEGF, but downregulated the expression level of CD34, at both the mRNA and protein levels. Along with the dynamic perfusion culture process, OECs lost immature markers like CD34 and acquired other endothelial markers, such as VE-cadherin. Our findings suggest that shear stress induced OECs differentiated into endothelial cells and increased the surface expression level of endothelial marker proteins by affecting those genes. VE-cadherin is a component of endothelial cell-to-cell adherens junctions, and it has a key role in the maintenance of vascular integrity. In the adult, it controls vascular permeability and inhibits unrestrained vascular growth [23]. Upregulation of VE-cadherin may be attributed to the formation of tube-like structures inside the pores of DBM scaffolds in SEM observation. Upregulation of VEGF demonstrated the contribution of OECs as a source of proangiogenic cytokines on 3D endothelial network formation. Recent study findings recognized that the concentration profile of extracellular growth factors, such as VEGF and basic fibroblast growth factor (bFGF), plays an important role in regulating the formation of 3D microvascular networks in vivo [24]. Our findings indicated that 3D microvessel formation in vitro can also be controlled by the concentration of extracellular growth factors generated by cells.
In contrast to common in vitro or in situ pre-vascularization strategies, our novel approach has the major advantage that microvessels can be seeded into porous scaffolds by dynamic perfusion culture of OECs within a short time period. This pre-vascularization method may be transferable to other 3D porous scaffold types, like tissue engineering skin scaffolds.
Conclusions
Shear stress generated by a dynamic perfusion system can be used as an innovative method for the rapid vascularization in tissue engineering, which can accelerate the proliferation and differentiation of OECs and the vascularization of implanted scaffolds.
Footnotes
Source of support: National Natural Science Foundation of China (81360274)
References
- 1.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008;26(8):434–41. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154(2):375–84. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nor JE, Mitra RS, Sutorik MM, et al. Thrombospondin-1 induces endothelial cell apoptosis and inhibits angiogenesis by activating the caspase death pathway. J Vasc Res. 2000;37(3):209–18. doi: 10.1159/000025733. [DOI] [PubMed] [Google Scholar]
- 5.Buschmann J, Welti M, Hemmi S, et al. Three-dimensional co-cultures of osteoblasts and endothelial cells in DegraPol foam: histological and high-field magnetic resonance imaging analyses of pre-engineered capillary networks in bone grafts. Tissue Eng Part A. 2011;17(3–4):291–99. doi: 10.1089/ten.TEA.2010.0278. [DOI] [PubMed] [Google Scholar]
- 6.Kim S, von Recum H. Endothelial stem cells and precursors for tissue engineering: cell source, differentiation, selection, and application. Tissue Eng Part B Rev. 2008;14(1):133–47. doi: 10.1089/teb.2007.0304. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105(1):71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaushal S, Amiel GE, Guleserian KJ, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7(9):1035–40. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghanaati S, Fuchs S, Webber MJ, et al. Rapid vascularization of starch-poly(caprolactone) in vivo by outgrowth endothelial cells in co-culture with primary osteoblasts. J Tissue Eng Regen Med. 2011;5(6):e136–43. doi: 10.1002/term.373. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs S, Ghanaati S, Orth C, et al. Contribution of outgrowth endothelial cells from human peripheral blood on in vivo vascularization of bone tissue engineered constructs based on starch polycaprolactone scaffolds. Biomaterials. 2009;30(4):526–34. doi: 10.1016/j.biomaterials.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 11.Hristov M, Weber C. Endothelial progenitor cells: Characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8(4):498–508. doi: 10.1111/j.1582-4934.2004.tb00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: Isolation and characterization. Trends Cardiovasc Med. 2003;13(5):201–6. doi: 10.1016/s1050-1738(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 13.Obi S, Yamamoto K, Shimizu N, et al. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. J Appl Physiol (1985) 2009;106(1):203–11. doi: 10.1152/japplphysiol.00197.2008. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto K, Takahashi T, Asahara T, et al. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol (1985) 2003;95(5):2081–88. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- 15.Martin-Ramirez J, Hofman M, van den Biggelaar M, et al. Establishment of outgrowth endothelial cells from peripheral blood. Nat Protoc. 2012;7(9):1709–15. doi: 10.1038/nprot.2012.093. [DOI] [PubMed] [Google Scholar]
- 16.Meng QY, Li XQ, Yu XB, et al. Transplantation of VEGF165-gene-transfected endothelial progenitor cells in the treatment of chronic venous thrombosis in rats. Chin Med J (Engl) 2010;123(4):471–77. [PubMed] [Google Scholar]
- 17.Laschke MW, Vollmar B, Menger MD. Inosculation: Connecting the life-sustaining pipelines. Tissue Eng Part B Rev. 2009;15(4):455–65. doi: 10.1089/ten.TEB.2009.0252. [DOI] [PubMed] [Google Scholar]
- 18.Cassell OC, Hofer SO, Morrison WA, Knight KR. Vascularisation of tissue-engineered grafts: The regulation of angiogenesis in reconstructive surgery and in disease states. Br J Plast Surg. 2002;55(8):603–10. doi: 10.1054/bjps.2002.3950. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Wang Z. Influence of mesenchymal stem cells with endothelial progenitor cells in co-culture on osteogenesis and angiogenesis: An in vitro study. Arch Med Res. 2013;44(7):504–13. doi: 10.1016/j.arcmed.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Laschke MW, Mussawy H, Schuler S, et al. Promoting external inosculation of pre-vascularised tissue constructs by pre-cultivation in an angiogenic extracellular matrix. Eur Cell Mater. 2010;20:356–66. doi: 10.22203/ecm.v020a29. [DOI] [PubMed] [Google Scholar]
- 21.Mazzolai L, Bouzourene K, Hayoz D, et al. Characterization of human late outgrowth endothelial progenitor-derived cells under various flow conditions. J Vasc Res. 2011;48(5):443–51. doi: 10.1159/000324844. [DOI] [PubMed] [Google Scholar]
- 22.Egorova AD, DeRuiter MC, de Boer HC, et al. Endothelial colony-forming cells show a mature transcriptional response to shear stress. In Vitro Cell Dev Biol Anim. 2012;48(1):21–29. doi: 10.1007/s11626-011-9470-z. [DOI] [PubMed] [Google Scholar]
- 23.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013;26(5):441–54. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Ruhrberg C, Gerhardt H, Golding M, et al. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16(20):2684–98. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]