Abstract
Background
Virtual reality reflection therapy (VRRT) is a technically enhanced version of the mirror therapy concept. The aim of this study was to investigate whether VRRT could improve the postural balance and gait ability of patients with chronic stroke.
Material/Methods
Twenty-five patients with chronic stroke were randomly allocated into the VRRT group (n=13) and the control group (n=12). The participants in both groups performed a conventional rehabilitation program for 30 minutes. The VRRT group also performed a VRRT program for 30 minutes, five times a week for 4 weeks. The control group performed conventional rehabilitation program and a placebo VRRT program. Outcome measures included Berg Balance Scale (BBS), the Functional Reaching Test (FRT), and the Timed Up and Go (TUG) test (for dynamic balance ability), postural sway (for static balance ability), and 10 meter walking velocity (10 mWV) for gait ability.
Results
There were statistically significant improvements in the VRRT group compared with the control group for BBS, FRT, TUG, postural sway (mediolateral sway distance with eyes open and eyes closed, anteroposterior and total sway distance with eyes open but not with eyes closed), and 10 mWV (p<0.05).
Conclusions
Applying VRRT (even as a home treatment) along with a conventional rehabilitation program for patients with chronic stroke might be even more beneficial than conventional rehabilitation program alone in improving affected lower limb function. Future studies should investigate the effectiveness of VRRT with optimal patient selection, and duration and intensity of training.
MeSH Keywords: Gait, Mirror Neurons, Postural Balance, Stroke
Background
For people with stroke, paralysis and muscle weakness in lower limbs can lead to balance and mobility disorders. This ultimately leaves people with stroke restricted in their activities of daily living [1]. Although there have been many different therapeutic approaches to improving balance and gait, functional electrical stimulation [2], virtual reality training [3], whole body vibration training [4], treadmill training [5], and robot-assisted training [6] have received the most attention. Most of these treatments have proven to be effective interventions, but are not in general use due to the cost of the required equipment [7] and the labor-intensive one-on-one nature of the treatments [8]. Thus, the need for rehabilitation methods using inexpensive equipment and consisting of an independent training program has become apparent [9].
Mirror therapy has been discussed as an answer to these challenges. Mirror therapy uses visual illusion, where patients perceive the reflected image of their unaffected limb as the affected one [10]. It was originally developed to reduce phantom limb pain [11], but was later applied to studies for people with stroke. As a result, improvement of upper extremity function in those patients has been reported [10,12,13].
Two mechanisms explain the effectiveness of mirror therapy. During the mirror therapy, decreased or absent proprioceptive input is compensated through receipt of a normal visual feedback by looking at the image in the mirror [12] and mirror neurons involved in learning new skills and in sensory integration are activated. Through this process, patients can regain much of the same functions on the affected side [14,15].
Mirror therapy has the advantages of both visual feedback training and imagery training [9,10], and has the same effect as bilateral movement training [13]. However, most research on mirror therapy has been on upper limb rehabilitation [9,11–13,16,17], and currently only one study on lower limb rehabilitation has been published [18]. Since this lower limb research was a result of mirror therapy with simple ankle exercises, it reported no improvement on complex performance like walking [18]. So, further research on lower limb rehabilitation with multiple tasks is needed.
Most people with stroke develop trunk asymmetry due to paralysis. Such asymmetry is a major obstacle to improving postural balance and gait [19]. However, during mirror therapy, patients have to bend their body toward the unaffected side to look at the image reflected in the mirror. This leads to yet more asymmetric posture and causes the neck to deviate away from the midline. Therefore, patients receive distorted visual information and cannot maintain correct balance [20]. If patients or therapists tilt the mirror to resolve such imbalance, the image in the mirror is distorted and no visual illusion effect can be expected.
The current study explored a new rehabilitation intervention to resolve head and trunk asymmetry and measure the effects of this new intervention. Using virtual reality reflection therapy (VRRT) (which applies the principles of mirror therapy), movements of the unaffected side were filmed with a camcorder, allowing a patient to look at the projected image on the monitor above their affected limb. This method used visual illusion to provide a wider field of vision to see both limbs from the same angle, and thereby avoid inducing asymmetrical posture. The aim of the present study was to explore the effect of VRRT on gait and balance in people with stroke.
Material and Methods
Participants
Participants were in-patients who had suffered a stroke at least six months previously, and were selected from K Rehabilitation Center in Gyeonggi-do, South Korea. Participants were included in the study if they (1) were able to understand and follow simple verbal instructions; (2) had a Mini Mental State Examination (MMSE) score over 21; (3) had a Brunnstrom score between stage I and IV; (4) had no apraxia or hemineglect and; (5) had no orthopedic and neurologic conditions such as fractures and digital neuropathy on their lower extremities. Prior to inclusion, each participant gave their written informed consent to participate in the study. The principles of the Declaration of Helsinki were followed, and the study was approved in advance by the Institutional Review Board of the University of Sahmyook.
Procedure
Of the 46 contacted individuals, nine did not meet the study criteria, and seven declined to participate. Thirty participants were randomly assigned to either the VRRT group or the control groups, with 15 patients in each group. Random allocation software was used to minimize selection bias [21]. Both the VRRT group and the control group participated in a conventional stroke rehabilitation program, 30 minute a day, five days a week, for four weeks. The conventional rehabilitation program is patient-specific and consists of neurodevelopmental treatment, physical therapy, occupational therapy, and speech therapy (if needed) [22]. Participants in the VRRT group additionally received VRRT program, 30 minutes a day, five days a week, for four weeks. The control group performed the placebo VRRT program for the same duration. The changes in dynamic balance ability, static balance ability, and gait ability were assessed before and after the intervention. The tests were performed by the trained assessors, and the assessors were blinded to the participants’ groups. During the 4-week intervention period, two participants were suddenly discharged from the hospital, and three participants complained of dizziness for a total of five participants dropping out of the study. Thus, 13 patients in the VRRT group and 12 patients in the control group finished the study. Figure 1 describes participation throughout the trial. The baseline characteristics are presented in Table 1.
Figure 1.

Flow diagram based on CONSORT.
Table 1.
General characteristics of participants.
| Characteristics | VRRT group (n=13) | Control group (n=12) | p |
|---|---|---|---|
| Gender (male/female) | 8/5 | 7/5 | ns |
| Affected side (right/left) | 7/6 | 5/7 | ns |
| Lesion type (hemorrhage/ischemia) | 5/8 | 4/8 | ns |
| Age (year) | 57.31±10.53 | 54.42±11.44 | ns |
| Height (cm) | 164.31±7.23 | 165.50±9.87 | ns |
| Weight (kg) | 63.31±8.06 | 61.50±9.37 | ns |
| Duration of stroke (month) | 12.54±4.14 | 13.58±5.28 | ns |
Values are expressed as mean ± standard deviation or frequency. VRRT – virtual reality reflection therapy; ns – not significant.
VRRT methods
VRRT is an exercise that can be safely applied to people with stroke. Participants sit on a mat without back support, with both feet on the floor so that the distance between the inside of each heel is 8.4 inch (21.3 cm), and the outside of the big toes are at a 9° of hallux valgus angle. The patients’ pelvic anterior must be tilted, and the hip, knee, and ankle joints must be flexed so that the trunk is asymmetrical or the weight will be shifted toward the affected area of the participants while sitting independently.
In this study, participants in the VRRT group placed their affected lower limb into the VRRT box to observe the projected movement of the unaffected lower limb without visual asymmetry causing tilting of the head and trunk. The unaffected lower limb of each participant was placed so that the center of the camera was over the limb. Participants then adjusted the lower extremities so that the image was projected in the location of the affected lower extremities. When the program started, the participants were asked to watch the movements of the lower limbs on the monitor only.
They were then asked to move their unaffected lower limb at a comfortable speed. During the 4-week test period, three sets of 10 repetitions per motion were conducted for 30 minutes a day, five days a week. Either the caregiver or the participants, under observation of the guardian, could intervene in the program, and a checklist on the back of the equipment was used to ensure each step was completed. The control group had the same program as the VRRT group, however,. one difference was that the participants in the control group did not see their unaffected lower limb displayed on the monitor, but instead saw them under the camera. A LCD monitor (width 23 cm × length 30 cm × height 75 cm), with a thickness of 3 cm, was used for this activity (Figure 2).
Figure 2.
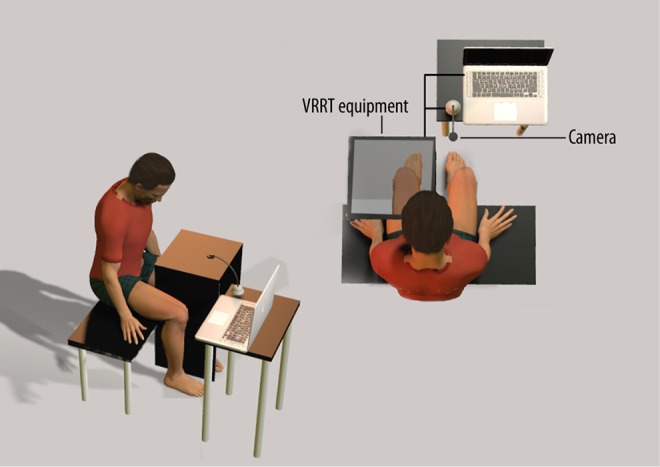
Setting for virtual reality reflection therapy.
VRRT program
The VRRT program used in our study was a modified version of the program presented by Sutbeyaz et al. [18]. The first week was spent adapting to the VRRT, and every week thereafter, the level of tasks was consistently elevated to encourage the participants to take more interest in them.
During the first week, participants were given an opportunity to feel that the movements of the unaffected lower limb projected on the monitor were those of the affected limb. They were encouraged to only observe the dorsiflexion and plantarflexion (lifting of the heel) of the unaffected ankle; adduction and abduction of forefoot and rear foot; and adduction and abduction of the hip (moving the knees inward and outward). In the second week, the participants observed the motions they made in the first week and mimicked the movements of the unaffected lower limb on the monitor with the affected lower limb. In the third week, two motions were combined for complexity. The participants were asked to watch the movements of the dorsiflexion, adduction and abduction of the unaffected ankle; plantar flexion, adduction and abduction of the ankle; and adduction and abduction of the hip of the unaffected lower limb displayed on the monitor and to mimic them with the affected lower limb. The fourth week’s motions were more complex, and involved different tasks. A remote control with up and down buttons was placed on the floor. Participants were asked to press each button with their unaffected foot during dorsiflexion, and adduction and abduction of the unaffected ankle. They then placed their feet over the 10 cm box located in the front, and were asked to tap the unaffected foot on the floor 10 times quickly. The participants then looked at the movements of the unaffected limb on the monitor and followed the adduction and abduction with the affected limb.
Measurements
To compare dynamic balance ability before and after the intervention, the Berg Balance Scale (BBS), the Functional Reaching Test (FRT), and the Timed Up and Go (TUG) test were assessed. The BBS is used to assess the balance of older people and people with neurological disorders who are at high risk of falling. It is a functional balance test that considers three aspects: maintenance of posture, postural control through voluntary exercise, and reaction to external stimulus. The balance assessment consists of 14 items performed in a standard order. Each item is scored on a scale of 0 to 4, with the total score out of 56 [23]. The FRT requires participants to stand with their side 10 cm from the wall, with fists clenched and shoulders flexed at 90°. Then they stretch their arms forward as far as possible, parallel to the floor, and the distance to the metacarpophalangeal joint is measured. In this experiment, a digital laser meter (DLE50, Bosch, Germany) was used to measure the distance between the initial arm location and the outstretched location for greater accuracy. The TUG was used to assess balance in older people, with participants scoring from 0 to 5 on each item of the test. Podsiadlo et al. [24] modified the test to measure the timed element only. Participants sit in a chair with armrests, and, upon hearing the command “Go”, get up from the chair, walk to a 3 m mark ahead, return to the chair and sit down, with measurements taken of the time it took each participant to complete the task.
The static balance ability of the participants was assessed by using a zebris force platform (PDM Multifunction Force Measuring Plate, Zebris, Germany). The plate is a 32×47 cm matrix with 1,504 pressure sensors (one pressure sensor per 1 cm2). The sensors measure the static and dynamic pressure at standing or walking. The participants were asked to stand barefoot on the force platform, with their feet comfortable situated. The location of the feet were marked to ensure that they were positioned in the same place during reassessment. The arms were relaxed and down at the participant’s sides. For testing with their eyes open, they were asked to stare at a 15 cm diameter dot located 3 m in front of them. For testing with their eyes closed, they wore eye covers to completely cut off the light, and were asked not to open their eyes during the measurement. They also used earplugs for 30 seconds so they could concentrate on the measurement activity [25]. The force platform system measures the medial-lateral, anterior-posterior, and total sway distance during the standing position. Data was collected or the three measurements with a rest of 3 minutes between each measurement to minimize muscle fatigue.
To assess gait ability, velocity was calculated by measuring the time it took to move 10 m at maximum speed [26]. Participants were asked to walk 14 m (to exclude acceleration and deceleration), and the speed for the 10 m distance in between was measured [27].
Statistical analysis
Descriptive statistics were used to summarize baseline characteristics. The Shapiro-Wilk test was used to test the variables for normality. The Fisher’s exact test was used for comparison of categorical dependent variables between the groups. The independent t-test was performed to identify differences between groups. Comparisons between pre-and post-treatment data within each group were analyzed using a paired t-test. SPSS version 19.0 for Windows was used to perform all analyses and p values <0.05 were regarded as significant.
Results
Results for outcome measures are shown in Table 2. In the change of BBS scores, both the VRRT and the control group displayed significant improvements after the intervention, and the improvement was significantly better in the VRRT group than in the control group (p<0.05). FRT, TUG, and 10 m WV showed significant improvements compared to baseline in the VRRT group (p<0.05), but not in the control group. FRT, TUG, and 10 m WV showed significant improvements in the VRRT group compared to the control group. In the changes of postural sway distance, all conditions with eyes open and the medial-lateral sway with eyes closed showed significant improvement in the VRRT group (p<0.05), but not in the control group. In addition, the anterior-posterior sway and medial-lateral sway distance with eyes open showed significant improvements in the VRRT group compared to the control group.
Table 2.
Comparison of outcome measures within group and between groups.
| Variables | VRRT group (n=13) | Control group (n=12) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Values | Pre | Post | Values | |
| Dynamic balance ability | ||||||
| BBS (score) | 45.46±4.12 | 49.08±2.72* | 3.62±1.85** | 44.75±3.02 | 46.08±2.97* | 1.33±1.72 |
| FRT (mm) | 194.16±58.89 | 200.83±58.83* | 5.14±3.57** | 197.10±71.07 | 196.13±70.90 | −0.81±1.89 |
| TUG (sec) | 21.82±5.70 | 18.01±3.70* | −3.80±3.72** | 20.39±4.11 | 19.30±3.72 | −1.09±2.23 |
| Static balance ability (cm) | ||||||
| EO-APS | 38.68±4.76 | 31.59±2.30* | −7.09±5.48** | 37.93±3.16 | 37.58±3.81 | −0.35±1.91 |
| EO-MLS | 35.41±3.31 | 33.51±2.91* | −1.91±1.32 | 34.78±3.74 | 33.19±4.47 | −1.59±3.55 |
| EO-TS | 52.16±5.97 | 49.27±6.71* | −2.89±2.98** | 51.30±5.93 | 50.94±3.97 | −0.36±2.55 |
| EC-APS | 56.80±8.43 | 55.40±9.12 | −1.40±3.67 | 60.86±14.67 | 60.87±15.28 | 0.01±7.61 |
| EC-MLS | 50.18±5.69 | 47.31±5.83* | −2.86±2.09 | 52.65±13.56 | 53.50±10.65 | 0.85±7.10 |
| EC-TS | 84.36±8.16 | 82.93±7.11 | −1.44±3.92 | 85.40±19.34 | 84.60±20.84 | −0.80±3.40 |
| Gait ability (m/s) | ||||||
| 10 mWV | 0.60±0.12 | 0.71±0.11* | 0.11±0.06** | 0.66±0.16 | 0.69±0.15 | 0.02±0.05 |
Values are expressed as mean ± standard deviation. VRRT – virtual reality reflection therapy; BBS – Berg balance scale; FRT – functional reach test; TUG – timed up and go test; EO – eyes open; EC – eyes closed; APS – anterior-posterior sway distance; MLS – medial-lateral sway distance; TS – total sway distance; 10 mWV – 10 m walking velocity.
Means significant difference within group.
Means significant differences between group.
Discussion
Most studies of mirror therapy have been on upper extremity rehabilitation. There are only two mirror therapy studies on lower extremity rehabilitation. One is a case study of patients with complex regional pain syndrome [28] and the other is a randomized controlled trial (RCT) of people with stroke [18]. In the study of people with stroke, motor recovery (Brunnstrom stages), spasticity (Modified Ashworth Scale), gait ability (Functional Ambulation Categories) and motor function (motor items of FIM instrument) were evaluated. The results of the RCT showed significant improvement in motor recovery and motor function but failed to show significant improvement in spasticity and gait ability [18]. Sutbeyaz et al. [18] posit that the reason for the lack of improvement in spasticity was due to the complex pathophysiology of spasticity, while the lack of improvement in gait ability was attributed to the simpler ankle movements (dorsiflexion, plantarflexion) that don’t activate brain reorganization. Thus, muscle strength, coordination, balance, and endurance need to be improved for normal walking and task-specific protocols, and intensive training will help realize improvement in these areas.
Our study endeavored to use task-focused training rather than simple exercises (dorsiflexion, plantarflexion). Participants were given step-by-step exercise to move the different joints required for walking. As a result, both gait velocity and cadence increased, unlike in previous reported research. Therefore, these results suggest the VRRT can be used to improve gait ability.
The exercise program in our study included several elements to improve gait ability for people with stroke. For example, putting one foot on a box induced dorsiflexion; pushing switches on a remote control with the feet(used in the swing phase) enhanced inversion and eversion, while pressing the floor with the feet improved push-off motion in the stance phase. Unfortunately, in our study there were many more open kinetic chain movements than close kinetic chain movements.
Carey et al. [29] stated that important variables for reorganization of the brain include cognitive elements, functional specificity, and performance of complex tasks. More complex movements activate the prefrontal cortex, the primary motor cortex, the supplementary motor area, and the cerebellum [30]. We consider the complex exercise resulting from performance of various tasks in our study to have an effect on brain reorganization.
The reason that most mirror therapy has been restricted to the upper extremity can be explained by the way in which the mirror component was applied. Mirror therapy with a small mirror (40×70 cm) forces movements to be simple and makes it difficult to improve complex functions such as walking [18]. One problem with mirror therapy when used for the lower extremities is that patients have to bend towards their unaffected side to look at the image in the mirror, which is counteractive to common weight support training. In addition, this asymmetric neck posture distorts visual information and the sense of equilibrium. This distortion can lead to additional balance problems [20].
McCabe et al. [28] used a mirror longer than the participant’s leg for total lower extremity movement for people with complex regional pain syndrome. Using this longer mirror method for people with stroke may result in greater trunk asymmetry. VRRT involves setting up a monitor above the affected side to act like a mirror, with the advantage that participants can look down comfortably at the horizontal surface without bending their bodies and thus distorting both their visual field and vestibular system.
Staines et al. mentioned that bilateral movements of the limbs activate the primary motor cortex area in the damaged hemisphere [31] and bilateral movements of the limbs results in significantly greater improvement to motor function and balance over unilateral movements [32–34]. In addition, symmetric information from both hands during VRRT maximizes the effect of bilateral movement training [13].
The results of our study showed significant improvement in balance. Increased scores for participants on BBS (a dynamic balance assessment tool [35]), showed improvement in range of motion and functional activity. The FRT, a dynamic balance and flexibility test, showed an expansion of the limit of stability. The TUG, a dynamic balance test, showed improvement in daily living activities and functions.
Hlavackova et al. [36] provided visual information in the mirror in front of participants who had lower limb amputations and measured postural sway, which was significantly less than participants who had no visual information in the mirror. This was reportedly due to the visual feedback replacing lost somatosensory information from amputation.
In our study, visual illusion was induced by the use of VRRT (over the course of four weeks), maximized through graduated exercise of the affected lower limb, and was considered, in conjunction with proprioceptive information, as the reason for decreased postural sway.
Sousa et al. [37] found that since people with stroke have a loss of proprioception and weak muscle strength on their affected side, they cannot shift their whole body weight to regain symmetric posture. Because of this, such people are unstable when standing or walking. In our study, we measured postural sway with the eyes closed and found significant differences between the two groups in terms of proprioception improvement.
Hip and ankle joints play important roles in physical stability [38]. Study result of muscle strength comparison between people who had experienced a fall and those who had not found a significant difference in hip extensions and ankle dorsiflexions [39]. Thus, loss of balance and weak ankle muscles are likely closely related [40].
Participants in this study engaged in repetitive exercise for their affected lower limb while watching the movements from their unaffected side on the monitor. Even though the exercise did not directly engage the ankle joint, various tasks improved ankle strength and control. Consequently, dynamic balance ability was a result.
Our study had some limitations. First, reorganization of the brain could not be confirmed because functional magnetic resonance imaging was not used. Second, since only people with chronic stroke were involved in our study, further research involving people with stroke at the acute and subacute stages is needed.
Conclusions
Our study confirmed the beneficial effects of VRRT on balance and gait ability in people with chronic stroke. Further work will be required to develop programs that take advantage of the benefits of VRRT and determine the impact on rehabilitation in people with acute and subacute stroke.
Footnotes
Source of support: Departmental sources
References
- 1.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:27–32. doi: 10.1016/s0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 2.Daly JJ, Zimbelman J, Roenigk KL, et al. Recovery of coordinated gait: randomized controlled stroke trial of functional electrical stimulation (FES) versus no FES, with weight-supported treadmill and over-ground training. Neurorehabil Neural Repair. 2011;25:588–96. doi: 10.1177/1545968311400092. [DOI] [PubMed] [Google Scholar]
- 3.Yang S, Hwang WH, Tsai YC, et al. Improving balance skills in patients who had stroke through virtual reality treadmill training. Am J Phys Med Rehabil. 2011;90:969–78. doi: 10.1097/PHM.0b013e3182389fae. [DOI] [PubMed] [Google Scholar]
- 4.Brogardh C, Flansbjer UB, Lexell J. No specific effect of whole-body vibration training in chronic stroke: A double-blind randomized controlled study. Arch Phys Med Rehabil. 2012;93:253–58. doi: 10.1016/j.apmr.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Hoyer E, Jahnsen R, Stanghelle JK, Strand LI. Body weight supported treadmill training versus traditional training in patients dependent on walking assistance after stroke: A randomized controlled trial. Disabil Rehabil. 2012;34:210–19. doi: 10.3109/09638288.2011.593681. [DOI] [PubMed] [Google Scholar]
- 6.Fisher S, Lucas L, Thrasher TA. Robot-assisted gait training for patients with hemiparesis due to stroke. Top Stroke Rehabil. 2011;18:269–76. doi: 10.1310/tsr1803-269. [DOI] [PubMed] [Google Scholar]
- 7.Mehrholz J, Hadrich A, Platz T, et al. Electromechanical and robot-assisted arm training for improving generic activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev. 2012;6:CD006876. doi: 10.1002/14651858.CD006876.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Hu XL, Tong KY, Song R, Zheng XJ, Leung WW. A comparison between electromyography-driven robot and passive motion device on wrist rehabilitation for chronic stroke. Neurorehabil Neural Repair. 2009;23:837–46. doi: 10.1177/1545968309338191. [DOI] [PubMed] [Google Scholar]
- 9.Yavuzer G, Selles R, Sezer N, et al. Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2008;89:393–98. doi: 10.1016/j.apmr.2007.08.162. [DOI] [PubMed] [Google Scholar]
- 10.Stevens JA, Stoykov ME. Using motor imagery in the rehabilitation of hemiparesis. Arch Phys Med Rehabil. 2003;84:1090–92. doi: 10.1016/s0003-9993(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996;263:377–86. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- 12.Altschuler EL, Wisdom SB, Stone L, et al. Rehabilitation of hemiparesis after stroke with a mirror. Lancet. 1999;353:2035–36. doi: 10.1016/s0140-6736(99)00920-4. [DOI] [PubMed] [Google Scholar]
- 13.Stevens JA, Stoykov ME. Simulation of bilateral movement training through mirror reflection: A case report demonstrating an occupational therapy technique for hemiparesis. Top Stroke Rehabil. 2004;11:59–66. doi: 10.1310/GCFE-QA7A-2D24-KHRU. [DOI] [PubMed] [Google Scholar]
- 14.Funase K, Tabira T, Higashi T, et al. Increased corticospinal excitability during direct observation of self-movement and indirect observation with a mirror box. Neurosci Lett. 2007;419:108–12. doi: 10.1016/j.neulet.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Small SL, Buccino G, Solodkin A. The mirror neuron system and treatment of stroke. Dev Psychobiol. 2012;54:293–310. doi: 10.1002/dev.20504. [DOI] [PubMed] [Google Scholar]
- 16.Lee MM, Cho HY, Song CH. The mirror therapy program enhances upper-limb motor recovery and motor function in acute stroke patients. Am J Phys Med Rehabil. 2012;91:689–96. doi: 10.1097/PHM.0b013e31824fa86d. quiz 697–700. [DOI] [PubMed] [Google Scholar]
- 17.Michielsen ME, Selles RW, Van Der Geest JN, et al. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: A phase II randomized controlled trial. Neurorehabil Neural Repair. 2011;25:223–33. doi: 10.1177/1545968310385127. [DOI] [PubMed] [Google Scholar]
- 18.Sutbeyaz S, Yavuzer G, Sezer N, Koseoglu BF. Mirror therapy enhances lower-extremity motor recovery and motor functioning after stroke: A randomized controlled trial. Arch Phys Med Rehabil. 2007;88:555–59. doi: 10.1016/j.apmr.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 19.Ikai T, Kamikubo T, Takehara I, Nishi M, Miyano S. Dynamic postural control in patients with hemiparesis. Am J Phys Med Rehabil. 2003;82:463–69. quiz 470–72, 84. [PubMed] [Google Scholar]
- 20.Marque P, Felez A, Puel M, et al. Impairment and recovery of left motor function in patients with right hemiplegia. J Neurol Neurosurg Psychiatry. 1997;62:77–81. doi: 10.1136/jnnp.62.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4:26. doi: 10.1186/1471-2288-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham JV, Eustace C, Brock K, et al. The Bobath concept in contemporary clinical practice. Top Stroke Rehabil. 2009;16:57–68. doi: 10.1310/tsr1601-57. [DOI] [PubMed] [Google Scholar]
- 23.Berg K, Wood-Dauphinee S, Williams JI. The Balance Scale: Reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995;27:27–36. [PubMed] [Google Scholar]
- 24.Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–48. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 25.Laufer Y, Sivan D, Schwarzmann R, Sprecher E. Standing balance and functional recovery of patients with right and left hemiparesis in the early stages of rehabilitation. Neurorehabil Neural Repair. 2003;17:207–13. doi: 10.1177/0888439003259169. [DOI] [PubMed] [Google Scholar]
- 26.Wade DT, Wood VA, Hewer RL. Recovery after stroke – the first 3 months. J Neurol Neurosurg Psychiatry. 1985;48:7–13. doi: 10.1136/jnnp.48.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dean CM, Richards CL, Malouin F. Task-related circuit training improves performance of locomotor tasks in chronic stroke: A randomized, controlled pilot trial. Arch Phys Med Rehabil. 2000;81:409–17. doi: 10.1053/mr.2000.3839. [DOI] [PubMed] [Google Scholar]
- 28.Mccabe CS, Haigh RC, Ring EF, et al. A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1) Rheumatology (Oxford) 2003;42:97–101. doi: 10.1093/rheumatology/keg041. [DOI] [PubMed] [Google Scholar]
- 29.Carey JR, Bhatt E, Nagpal A. Neuroplasticity promoted by task complexity. Exerc Sport Sci Rev. 2005;33:24–31. [PubMed] [Google Scholar]
- 30.Jeannerod M. Mental imagery in the motor context. Neuropsychologia. 1995;33:1419–32. doi: 10.1016/0028-3932(95)00073-c. [DOI] [PubMed] [Google Scholar]
- 31.Staines WR, Mcilroy WE, Graham SJ, Black SE. Bilateral movement enhances ipsilesional cortical activity in acute stroke: A pilot functional MRI study. Neurology. 2001;56:401–4. doi: 10.1212/wnl.56.3.401. [DOI] [PubMed] [Google Scholar]
- 32.Cauraugh JH, Kim S. Two coupled motor recovery protocols are better than one: Electromyogram-triggered neuromuscular stimulation and bilateral movements. Stroke. 2002;33:1589–94. doi: 10.1161/01.str.0000016926.77114.a6. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham CL, Stoykov ME, Walter CB. Bilateral facilitation of motor control in chronic hemiplegia. Acta Psychol (Amst) 2002;110:321–37. doi: 10.1016/s0001-6918(02)00040-9. [DOI] [PubMed] [Google Scholar]
- 34.Whitall J, Mccombe Waller S, et al. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000;31:2390–95. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- 35.Walker C, Brouwer BJ, Culham EG. Use of visual feedback in retraining balance following acute stroke. Phys Ther. 2000;80:886–95. [PubMed] [Google Scholar]
- 36.Hlavackova P, Fristios J, Cuisinier R, et al. Effects of mirror feedback on upright stance control in elderly transfemoral amputees. Arch Phys Med Rehabil. 2009;90:1960–63. doi: 10.1016/j.apmr.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Sousa CO, Barela JA, Prado-Medeiros CL, et al. Gait training with partial body weight support during overground walking for individuals with chronic stroke: A pilot study. J Neuroeng Rehabil. 2011;8:48. doi: 10.1186/1743-0003-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Runge CF, Shupert CL, Horak FB, Zajac FE. Ankle and hip postural strategies defined by joint torques. Gait Posture. 1999;10:161–70. doi: 10.1016/s0966-6362(99)00032-6. [DOI] [PubMed] [Google Scholar]
- 39.Daubney ME, Culham EG. Lower-extremity muscle force and balance performance in adults aged 65 years and older. Phys Ther. 1999;79:1177–85. [PubMed] [Google Scholar]
- 40.Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci. 1995;50(Spec):64–67. doi: 10.1093/gerona/50a.special_issue.64. [DOI] [PubMed] [Google Scholar]


