Abstract
Background
Angiogenesis plays a significant role in complex inflammatory and angiogenic processes and is also involved in multiple myeloma (MM) pathogenesis. IL-37 is a proinflammatory cytokine in antitumor activity. Our purpose was to evaluate the IL-37 clinical significance on MM.
Material/Methods
We measured serum levels of IL-37 in 45 patients with different stages of MM and 30 healthy control subjects and correlated IL-37 with numerous cytokines, such as angiogenesis factors including vascular endothelial growth factor (VEGF) and angiotensin-2 (Ang-2). We also measured the tube formation of human umbilical vein endothelial cells (HUVECs) after pretreatment with recombinant human IL-37 (rhIL-37).
Results
Serum IL-37 level was lower in the patients with MM than in the healthy control subjects, whereas VEGF and Ang-2 levels were higher, depending on International Staging System stage. Serum IL-37 level had a negative correlation to VEGF and Ang-2 levels, and VEGF had a positive correlation to Ang-2 level. The tube formation of HUVECs was suppressed by the rhIL-37 pretreatment.
Conclusions
Our results indicate that serum level of IL-37 plays a part in the pathophysiology of MM progression. Therefore, IL-37 serum level may be a biomarker for disease stage and angiogenesis processes.
MeSH Keywords: Interleukins, Multiple Myeloma, Vascular Endothelial Growth Factors
Background
Multiple myeloma (MM) is a clonal B-cell dyscrasia characterized by the accumulation of monotypic paraprotein-secreting cells in the bone marrow (BM), in close contact with adjacent cells in its microenvironment [1]. MM derives from various complex inflammatory and angiogenic processes [2].
A recent study reported that angiogenesis plays a significant role in the pathogenesis of MM [3]. Plasma cells secrete a well-known angiogenic cytokine, vascular endothelial growth factor (VEGF), in response to stimulation of inflammatory factors, such as interleukin (IL)-6, IL-10, IL-17, and IL-20 [4–7]; on the other hand, microvascular endothelial cells (ECs) and BM stromal cells secrete other potent growth factors for malignant plasma cells, thus regulating VEGF stimulation [8,9]. Another angiogenic cytokine, angiopoietin-2 (Ang-2), prevents Tie-2 binding and leads to vessel instability, associated with sprouting angiogenesis [10]. These accumulated data suggest that angiogenesis is tightly controlled by angiogenic cytokines and inflammatory factors in MM.
IL-37 is a natural suppressor of innate inflammatory and immune responses. IL-37 protein is associated with plasma cells and constitutively expressed in the cytoplasm of monocytes and peripheral blood mononuclear cells [11]. IL-37 was reported to markedly inhibit the migration and proliferation and promote apoptosis in renal cell carcinoma [12]. Moreover, IL-37 prevented the pathogenesis of malignant B-cell neoplasms and NRasV12-mediated oncogenesis [13]. However, whether IL-37 is involved in the progression of MM remains unknown.
The aim of this study was to measure serum levels of IL-37 in patients in different stages of MM and to correlate these levels with VEGF and Ang-2 in order to investigate their clinical significance.
Material and Methods
Patients
The original research was approved by the medical ethics committee of our hospital, and every patient provided written informed consent. A total of 45 newly diagnosed patients with MM were included in this study. We excluded individuals with hypertension, those with diabetes, and those who received any other surgery. The stage of MM was classified according to the International Staging System (ISS). Age- and sex-matched healthy individuals were used as control subjects. All participants in this study were from Binzhou People’s Hospital (China), since January 2011. The study protocol was approved by the institutional ethics committee [2011060]. The characteristics of subjects enrolled in the study are shown in Table 1.
Table 1.
Demographic and clinical data for patients with multiple myeloma and control subjects.
| Patients | Control people | |
|---|---|---|
| Total | 45 | 30 |
| Male/Female | 21/24 | 17/13 |
| Age (year) mean (range) | 62 (44–75) | 65 (42–84) |
| ISS | ||
| Stage I | 12 | – |
| Stage II | 18 | – |
| Stage III | 15 | – |
ISS – international staging system.
Cytokine measurements
All blood samples were collected, mixed with EDTA, centrifuged at 3000 rpm for 15 min at room temperature, then stored at −80°C and analyzed at the end of the collection. The detection of IL-37, VEGF, and Ang-2 in the serum was performed by ELISA (R&D Systems, CA, USA), according to the operating manual.
Tube formation
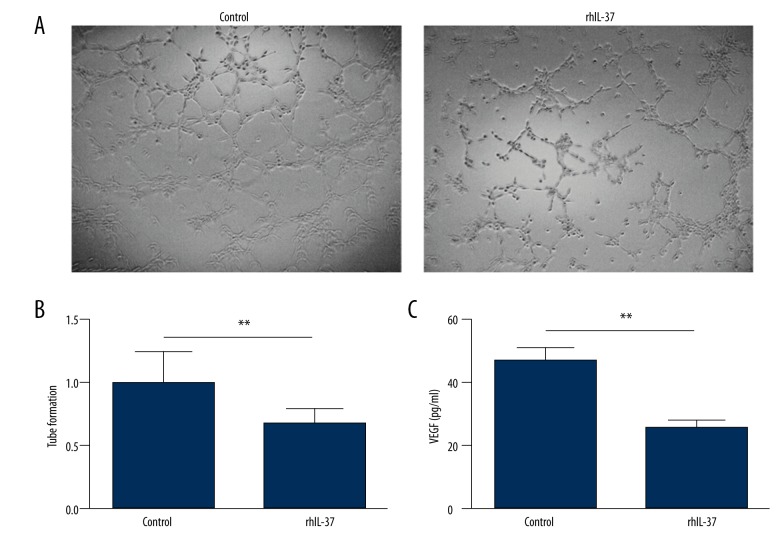
The tube formation of vascular-like structures was determined by human umbilical vein ECs (HUVECs) on Matrigel (BD Biosciences, Franklin, NJ, USA) [14]. Onto a 48-well plate coated with 150 μL Matrigel, 4×104 HUVECs were planted. Cells were treated with recombinant human IL-37 (rhIL-37; 100 ng/mL) or phosphate buffer solution and then were maintained at 37°C, 5% CO2, for 6 h. Tubes were defined as straight cellular extensions joining 2 cell masses; 3 random digital images (200×) were counted for each well.
Statistical analysis
Results are expressed as mean ±SD. Graphs were drawn using GraphPad Prism software. One-way analysis of variance, followed by t test, was used to examine differences between stages. Correlations between various measured parameters were calculated by the Spearman’s rank correlation coefficient. P<0.05 was considered statistically significant.
Results
Serum levels of IL-37 decreased in active patients with MM compared with healthy control subjects, whereas VEGF and Ang-2 increased, as shown in Table 2. Table 3 shows the values in each ISS stage; it also shows that VEGF and Ang-2 increased and IL-37 decreased in parallel with disease stage (P<0.001 for all cases). Significant negative correlations were found between serum levels of IL-37 with VEGF (r=0.337; Figure 1) and Ang-2 (r=0.306; P<0.05; Figure 2), and a positive correlation was found between VEGF and Ang-2 (r=0.6458; P<0.001; Figure 3). The effects of rhIL-37 on tube formation are shown in Figure 3A and 3B. Compared with the control (1.00±0.24), rhIL-37 pretreatment significantly decreased tube formation in HUVECs (0.67±0.12) and significantly reduced supernatant VEGF levels in HUVECs (Figure 3C).
Table 2.
Serum levels of interleukin-37, vascular endothelial growth factor, and angiopoietin-2 in patients with multiple myeloma and in healthy control subjects.
| IL-37 (pg/ml) | VEGF (pg/ml) | Ang-2 (pg/ml) | |
|---|---|---|---|
| Controls | 150.42±15.45 | 335.15±74.64 | 250.77±101.23 |
| MM patients | 58.89±33.85 | 239.93±105.49 | 630.97±364.82 |
| P value | <0.05 | <0.05 | <0.05 |
Table 3.
Serum levels of interleukin-37, vascular endothelial growth factor, and angiopoietin-2 in different myeloma stages.
| IL-37 (pg/ml) | VEGF (pg/ml) | Ang-2 (pg/ml) | |
|---|---|---|---|
| Stage I | 81.01±25.49 | 137.233±49.02 | 239.42±65.07 |
| Stage II | 67.64±33.97 | 210.15±50.14 | 612.79±135.68 |
| Stage III | 30.70±17.90 | 357.83±70.45 | 966.04±374.83 |
| P value | <0.01 | <0.01 | <0.01 |
Figure 1.
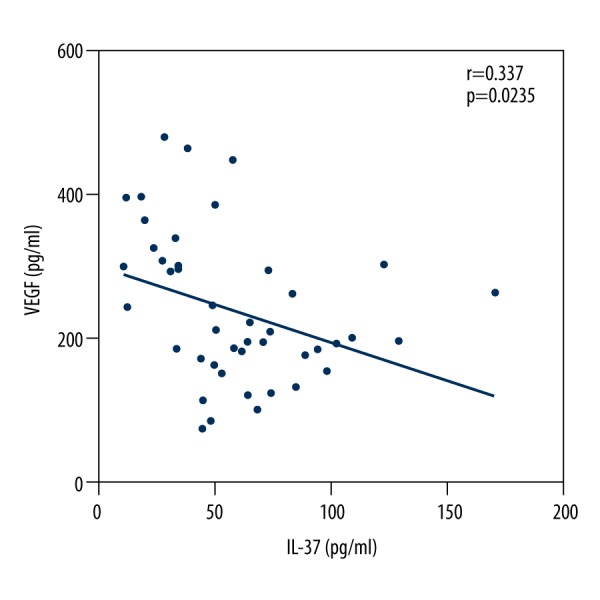
Negative correlation between serum levels of interleukin-37 with vascular endothelial growth factor in patients with multiple myeloma.
Figure 2.
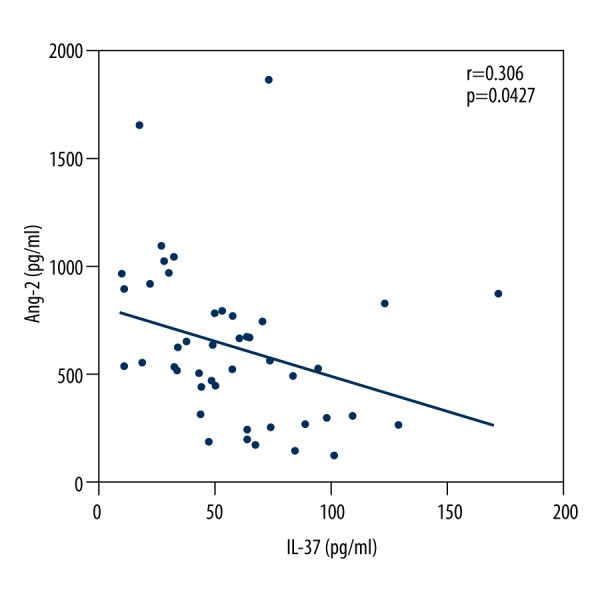
Negative correlation between serum levels of interleukin-37 with angiopoietin-2 in patients with multiple myeloma.
Figure 3.
Interleukin-37 (IL-37) suppressed tube formation in human umbilical vein endothelial cells (HUVECs). (A) Representative pictures of control and recombinant human IL-37 (rhIL-37) pretreatments. (B) Quantitative analysis of tube formation. Results are expressed relative to the control. (C) Supernatant vascular endothelial growth factor level from HUVECs pretreated with rhIL-37 or control. **P < 0.01; n=3.
Discussion
More and more evidence indicates that BM angiogenesis is crucial to MM pathogenesis and that the angiogenic and antiangiogenic switch contributes to MM progression [15,16]. The identification and specific contribution of proangiogenic molecules to be used as therapeutic targets and/or reliable angiogenesis biomarkers in MM is still unresolved [17]. In this article, we show that IL-37 correlates significantly with important angiogenetic factors, suggesting that IL-37 may be a biomarker for MM disease stage and angiogenesis processes.
We first determined serum IL-37, VEGF, and Ang-2 levels in patients with MM at different stages of disease and in healthy control subjects. We found that, compared with healthy control subjects, serum levels of IL-37 decreased in patients with MM, whereas VEGF and Ang-2 levels increased, depending on ISS stage. These data support a putative value of these molecules in the MM disease process.
IL-37 is a newly identified member of the IL-1 family, which are proinflammatory cytokines demonstrating various anti-inflammatory and immunosuppressive properties in response to infection and inflammation [17]. Recently, IL-37 emerged as a fundamental anti-inflammatory cytokine, favoring tumor escape from immune surveillance [18]. High expression of IL-37, which may mediate antitumor immunity by regulating natural killer cell activity, was associated with better overall survival in hepatocellular carcinoma [19]. The ectopic selection of IL-37 preserved the function of B-cell progenitors and prevented NRasV12-mediated oncogenesis in aging [13]. These results all suggest the essential role played by IL-37 in the tumor microenvironment. Through this research, we verified that serum IL-37 levels in patients with MM were lower than in healthy control subjects and that levels decreased with advancing disease.
Upon activation by MM plasma cells and mesenchymal stromal cells, macrophages can release growth factors, proteolytic enzymes, cytokines, and inflammatory mediators that promote plasma cell growth and survival. Indeed, these macrophages are essential for the induction of an angiogenic response through vasculogenic mimicry, and this ability proceeds in step with progression of the plasma cell tumors [20]. Teng et al. observed reduced systemic levels of IL-10 and local interferon-gamma gene transcripts in keratin-14 VEGF-A transgenic mice treated with plasmid coding human IL-37 sequence-formulated cationic liposomes [21]. In MM, plasma cells secrete various angiogenic cytokines, such as VEGF, matrix metalloproteinase-2, and fibroblast growth factor-2, which in turn cause inflammatory cells to secrete other angiogenic factors. All these factors enlist and activate MM-associated macrophages to cooperate with other cells in angiogenesis [22]. BM sera with high Ang-2 levels specifically contributed to EC activation, suggesting that Ang-2 produced in the BM may contribute to MM angiogenesis [17]. In our study, we found that both VEGF and Ang-2 were significantly higher in patients with MM than in healthy control subjects and that these values increased in parallel with disease progression. We also confirmed elevated IL-37 levels in MM significant negative correlations with VEGF and Ang-2. In view of these facts, we further investigated whether angiogenesis could be weakened by the increased expression of IL-37. We found that the tube formation of HUVECs, which was also suppressed by rhIL-37 pretreatment, may be involved in VEGF secretion. These results suggest that IL-37 may play an important role in angiogenesis during MM progression.
Conclusions
Our results show that in patients with MM, serum VEGF and Ang-2 levels increase and IL-37 level decreases with disease progression. Moreover, IL-37 level has a strongly negative correlation to Ang-2 and VEGF levels. Pretreatment with rhIL-37 promoted tube formation in HUVECs. These results suggest that serum levels of IL-37 play a part in the pathophysiology of MM progression. Therefore, IL-37 could serve as a biomarker of the disease state and angiogenesis processes in MM.
Footnotes
Source of support: Departmental sources
Conflict of interest statement
None of the authors have any conflicts of interest to report.
References
- 1.Accardi F, Toscani D, Bolzoni M, et al. Mechanism of action of bortezomib and the new proteasome inhibitors on myeloma cells and the bone microenvironment: impact on myeloma-induced alterations of bone remodeling. Biomed Res Int. 2015;2015:172458. doi: 10.1155/2015/172458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauta VM. A review of the cytokine network in multiple myeloma: Diagnostic, prognostic, and therapeutic implications. Cancer. 2003;97:2440–52. doi: 10.1002/cncr.11072. [DOI] [PubMed] [Google Scholar]
- 3.Gkotzamanidou M, Christoulas D, Souliotis VL, et al. Angiogenic cytokines profile in smoldering multiple myeloma: no difference compared to MGUS but altered compared to symptomatic myeloma. Med Sci Monit. 2013;19:1188–94. doi: 10.12659/MSM.889752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthes T, Manfroi B, Zeller A, et al. Autocrine amplification of immature myeloid cells by IL-6 in multiple myeloma-infiltrated bone marrow. Leukemia. 2015;29:1882–90. doi: 10.1038/leu.2015.145. [DOI] [PubMed] [Google Scholar]
- 5.Alexandrakis MG, Goulidaki N, Pappa CA, et al. Interleukin-10 induces both plasma cell proliferation and angiogenesis in multiple myeloma. Pathol Oncol Res. 2015;21:929–34. doi: 10.1007/s12253-015-9921-z. [DOI] [PubMed] [Google Scholar]
- 6.Alexandrakis MG, Pappa CA, Kokonozaki M, et al. Circulating serum levels of IL-20 in multiple myeloma patients: Its significance in angiogenesis and disease activity. Med Oncol. 2015;32:42. doi: 10.1007/s12032-015-0488-z. [DOI] [PubMed] [Google Scholar]
- 7.Lemancewicz D, Bolkun L, Jablonska E, et al. The role of Interleukin-17A and Interleukin-17E in multiple myeloma patients. Med Sci Monit. 2012;18(1):BR54–59. doi: 10.12659/MSM.882204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vacca A, Ria R, Ribatti D, et al. A paracrine loop in the vascular endothelial growth factor pathway triggers tumor angiogenesis and growth in multiple myeloma. Haematologica. 2003;88:176–85. [PubMed] [Google Scholar]
- 9.Dankbar B, Padro T, Leo R, et al. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood. 2000;95:2630–36. [PubMed] [Google Scholar]
- 10.Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771–80. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 11.Boraschi D, Lucchesi D, Hainzl S, et al. IL-37: A new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw. 2011;22:127–47. doi: 10.1684/ecn.2011.0288. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Wang Y, Liang L, et al. IL-37 mediates the antitumor activity in renal cell carcinoma. Med Oncol. 2015;32:250. doi: 10.1007/s12032-015-0695-7. [DOI] [PubMed] [Google Scholar]
- 13.Henry CJ, Casas-Selves M, Kim J, et al. Aging-associated inflammation promotes selection for adaptive oncogenic events in B cell progenitors. J Clin Invest. 2015;125:4666–80. doi: 10.1172/JCI83024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni M, Yang ZW, Li DJ, et al. A potential role of alpha-7 nicotinic acetylcholine receptor in cardiac angiogenesis in a pressure-overload rat model. J Pharmacol Sci. 2010;114:311–19. doi: 10.1254/jphs.09335fp. [DOI] [PubMed] [Google Scholar]
- 15.Vacca A, Ria R, Reale A, Ribatti D. Angiogenesis in multiple myeloma. Chem Immunol Allergy. 2014;99:180–96. doi: 10.1159/000353312. [DOI] [PubMed] [Google Scholar]
- 16.Ramanujan S, Koenig GC, Padera TP, et al. Local imbalance of proangiogenic and antiangiogenic factors: A potential mechanism of focal necrosis and dormancy in tumors. Cancer Res. 2000;60:1442–48. [PubMed] [Google Scholar]
- 17.Belloni D, Marcatti M, Ponzoni M, et al. Angiopoietin-2 in bone marrow milieu promotes multiple myeloma-associated angiogenesis. Exp Cell Res. 2015;330:1–12. doi: 10.1016/j.yexcr.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Nold MF, Nold-Petry CA, Zepp JA, et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–22. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao JJ, Pan QZ, Pan K, et al. Interleukin-37 mediates the antitumor activity in hepatocellular carcinoma: Role for CD57+ NK cells. Sci Rep. 2014;4:5177. doi: 10.1038/srep05177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berardi S, Ria R, Reale A, et al. Multiple myeloma macrophages: Pivotal players in the tumor microenvironment. J Oncol. 2013;2013:183602. doi: 10.1155/2013/183602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teng X, Hu Z, Wei X, et al. IL-37 ameliorates the inflammatory process in psoriasis by suppressing proinflammatory cytokine production. J Immunol. 2014;192:1815–23. doi: 10.4049/jimmunol.1300047. [DOI] [PubMed] [Google Scholar]
- 22.Vacca A, Ribatti D, Presta M, et al. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood. 1999;93:3064–73. [PubMed] [Google Scholar]