Summary
Social rank can profoundly affect many aspects of mammalian reproduction and stress physiology, but little is known about how immune function is affected by rank and other socio-ecological factors in free-living animals.
In this study we examine the effects of sex, social rank, and reproductive status on immune function in long-lived carnivores that are routinely exposed to a plethora of pathogens, yet rarely show signs of disease.
Here we show that two types of immune defenses, complement-mediated bacterial killing capacity (BKC) and total IgM, are positively correlated with social rank in wild hyenas, but that a third type, total IgG, does not vary with rank.
Female spotted hyenas, which are socially dominant to males in this species, have higher BKC, and higher IgG and IgM concentrations, than do males.
Immune defenses are lower in lactating than pregnant females, suggesting the immune defenses may be energetically costly.
Serum cortisol and testosterone concentrations are not reliable predictors of basic immune defenses in wild female spotted hyenas.
These results suggest that immune defenses are costly and multiple socioecological variables are important determinants of basic immune defenses among wild hyenas. Effects of these variables should be accounted for when attempting to understand disease ecology and immune function.
Keywords: antibody, complement, ecoimmunology, hyena, immunology, lactation, social rank, sex, wild immunity
Introduction
Traditional immunology has made great strides in understanding immune function by performing experiments on animals whose diet, pathogen exposure and social interactions are all tightly controlled. However, the vertebrate immune system evolved in a complex, unpredictable, and resource-limited environment. Over the past few decades, a diverse group of scientists and health professionals have become increasingly interested in understanding immune function in an evolutionary ecological context. This has proven extremely difficult, at least in part due to the double-edge nature of immunity. For example, reduced investment in immune defenses can lead to pathogen-induced host morbidity or mortality, whereas overactive immune defenses can cause immunopathology or may divert energy from growth and reproduction (Svensson et al. 1998; Raberg, Graham & Read 2009). In light of the costs and benefits of immune defenses, quantification of immune defenses is most revealing when the samples are obtained from animals in their natural habitat (Brock et al. 2013). A major limitation to understanding immune function in the wild is the lack of long-term data sets that include measures of immune function and socio-ecological variables, which can shed light on within-group variation in immune function.
Social hierarchies in non-human animals, and socioeconomic status in humans, are associated with skewed resource distribution, variation in health, and varying levels of stress (reviewed in Sapolsky 2005; Cavigelli & Chaudhry 2012). High-ranking individuals often enjoy the highest priority of access to food resources, which are important for immune function in a wide range of taxa, including northern bobwhites (Colinus virginianu) (Lochmiller, Vestey & Jon 1993) tree lizards (Urosaurus ornatus) (French, Johnston & Moore 2007), zebra finches (Taeniopygia guttata) (Love et al. 2008), and tobacco hornworms (Manduca sexta) (Diamond & Kingsolver 2011). Here we investigate the effects of social rank and other socioecological variables on measures of immune function in wild spotted hyenas (Crocuta crocuta) (Fig. 1).
Fig. 1.
Spotted hyena (Crocuta crocuta) feeding on the carcass of a giraffe that has been dead for three days. Photo by Andrew S. Flies.
Spotted hyenas are known to survive exposure to pathogens such as rabies, canine distemper virus, and anthrax (East et al. 2001; Harrison et al. 2004; Lembo et al. 2011), making them a particularly interesting species in which to study immune function. To address the hypothesis that high-ranking individuals exhibit higher levels of immune defenses in nature, we used a 25-year data set to analyze relationships among social rank, sex, hormone concentrations, reproductive status, and immune function in wild hyenas. Hyena societies, or clans, may contain up to 130 individuals, and clans are rigidly structured by linear rank relationships that are stable over time (Frank 1986; Holekamp et al. 2015). Within each clan social rank is learned during the first several months of life, with the cubs assuming ranks immediately below those of their mothers, and thus dominance is not determined by size or fighting ability (Kruuk 1972; Holekamp & Smale 1993; Smale, Frank & Holekamp 1993; Smith 2010). Females remain in their natal clan for life, whereas males emigrate to a new clan after puberty (Smale, Frank & Holekamp 1993). Immigrant males assume the lowest rank in the clan, such that even juvenile natal hyenas of both sexes are dominant to immigrant adult males. Males move up in rank only when new males join the clan and assume lower ranks, but immigrant males never attain ranks higher than natal individuals.
Social rank determines priority of access to food at kills, so the highest-ranking hyenas enjoy the greatest quantity and quality of food from carcasses (Tilson & Hamilton 1984; Frank 1986; Engh et al. 2000; Smith et al. 2008). The highest-ranking females in a hyena clan start breeding at the youngest ages, experience the shortest interbirth intervals, wean their cubs at the youngest ages (Holekamp, Smale & Szykman 1996), and have the lowest circulating glucocorticoid levels when not lactating (Goymann et al. 2001).
Here we quantified three general measures of immune function in a wild spotted hyena population that has been intensively and continuously studied since 1988: in vitro serum bacterial killing capacity (BKC), total serum immunoglobulin G (IgG), and total serum immunoglobulin M (IgM). A bacterial killing assay provides a functional measure of the capacity of serum to kill bacteria, and serum BKC is mediated primarily by the complement system (Liebl & Martin 2009). Complement-mediated BKC is maintained in the absence of infection and functions as a preventative defense (Ricklin et al. 2010), but is briefly up-regulated upon pathogen recognition (Carroll 1998). IgG is an important acquired immune defense that can be produced in large quantities in response to infectious pathogens. IgG in all mammals studied to date consists of several subclasses (e.g. IgG1), which can have highly diverse antigenic specificity that is largely the result of individual immunological history; it remains unknown which IgG subclasses exist in spotted hyenas. IgG is particularly effective against pathogens that might be re-acquired later in life, and measurement of total serum IgG concentration represents a basic tool for evaluating infection status and abnormalities in immune function (Barnard, Behnke & Sewell 1996; Curno et al. 2009). IgM is important for early stage defense against pathogens during primary exposure, and for removal of dead host cells (Baumgarth, Tung & Herzenberg 2005). The important and diverse roles that complement and immunoglobulins play in host defense permit them to serve as reliable indicators of immune function in wild animals. Furthermore, non-cellular protein components of the immune system, such as immunoglobulins and complement, are accessible in peripheral blood, and they are more stable during handling and sample storage than are cellular components of the immune system (Mollnes, Garred & Bergseth 1988).
Our first goal in this study was to determine whether our three measures of immune function (i.e., BKC, IgM, and IgG) vary with sex in wild spotted hyenas. Higher levels of immune defenses in females than males have been observed in other mammals (reviewed in Libert, Dejager & Pinheiro 2010), and it is hypothesized that females can increase fitness by investing in immunity to extend their reproductive lifespan (Rolff 2002). Furthermore, because female spotted hyenas rank above immigrant males, if rank is important for immune function, then females should have higher levels of immune defenses than males. Second, we directly tested whether immune defenses vary with social rank among hyenas of both sexes. If immune function is affected by dietary quality in wild hyenas, then we expected to observe higher immunoglobulin concentrations and superior BKC in high- than low-ranking hyenas. Third, previous research suggests that energetic resources may be traded off between immune defenses and reproduction (French, Johnston & Moore 2007; Graham et al. 2010) or that resource acquisition may be lower during some stages of reproduction than others (Bashir-Tanoli & Tinsley 2014). Therefore we inquired whether immune defenses were reduced in females during lactation, which is the phase of mammalian reproduction known to incur the highest energetic costs (Gittleman & Thompson 1988). Finally, because certain steroid hormones have been found to have complex interactions with immune function (reviewed in Sapolsky 2005), we inquired whether immune measures varied with serum concentrations of cortisol and testosterone.
Materials and Methods
STUDY POPULATION
Behavioral observations of individually identifiable wild spotted hyenas were collected from 1988–2012 as part of a long-term study in the Maasai Mara National Reserve in Kenya; samples used in this study were collected between 1996–2009. Data documenting wins and losses as outcomes of agonistic interactions were extracted to generate rank matrices that were updated on an annual basis (Holekamp & Smale 1993; Smale, Frank & Holekamp 1993). To account for temporal variations in clan size and minimize the number of predictor variables tested, here we used relative rank instead of absolute rank. Relative rank for females was calculated using the following formula: (absolute rank - # of ranked females) / (maximum rank - # of ranked females), which assigned ranks from zero to one for lowest and highest ranked for each year, respectively; females and males are ranked separately. Our analyses of rank focus primarily on females because female ranks are highly stable over long periods of time, whereas male ranks change radically when males disperse from their natal clans.
Female hyenas were considered to be adults at 36 months or on the date they conceived their first litter, whichever came first (Swanson, Dworkin & Holekamp 2011). Briefly, age and birthdates (+/− 7 days) were assigned to cubs based on their appearance and behavior (i.e. pelage, size, motor coordination) at the time that we first observed them above ground at the den (Holekamp & Smale 1990; Holekamp, Smale & Szykman 1996). The age of immigrant males was estimated (+/− 4.9 month) using methods described in Van Horn, McElhinny and Holekamp (2003). Conception dates and reproductive states were calculated by subtracting the 110 day gestation period from parturition dates (Kruuk 1972). Two samples that were collected within nine days of parturition were removed from our data set due to major physiological and immunological changes, including decreased IgG and IgM levels and inhibition of complement activity associated with parturition (Smith et al. 1979; Bischof 1981; Klobasa et al. 1985; Markowska-Daniel, Pomorska-Mól & Pejsak 2010; Herr, Bostedt & Failing 2011); the two samples had BKCs that were nearly 4 standard deviations below the mean and had large influence on analyses involving bacterial killing capacity.
SAMPLE COLLECTION
Wild spotted hyenas were immobilized with tiletamine-zolazepam (6.5mg/kg Telazol; Fort Dodge Animal Health, Fort Dodge Iowa) in a plastic dart fired from an air rifle (Telinject Inc., Saugus, California) (Holekamp & Sisk 2003). All immobilizations and sample collection methods were approved by the MSU Institutional Animal Care and Use Committee (AUF # 07/08-099-00). Briefly, whole blood was collected from the jugular vein of anesthetized hyenas, allowed to clot at ambient temperature, centrifuged, and the sera were frozen in liquid nitrogen (−196°C). Samples were transported to MSU on dry ice and then stored at −80°C.
LABORATORY METHODS
BKC was determined using a method similar to that used by Flies et al. (2015). We assessed the effects of freezing, heating, and filtering serum samples on BKC, and a full description of the methods and validation of the bacterial killing assay used here is presented in the online Supporting Information (Figs S1 and S2). Total IgG and IgM were measured using a sandwich ELISA and reagents described by Flies et al. (2012). We used competitive binding ELISA kits for cortisol (Neogen #D402710) and testosterone (Neogen #D402510) according to the manufacturer’s instructions (See Supporting Information for additional methodological details).
STATISTICAL ANALYSIS
We used version 3.0.3 of the software package R (R Development Core Team 2014) to create linear models for assessing relationships between response and predictor variables. Because no single model is a perfect representation of nature, we used an approach that involved information theoretic multimodel inference rather than choosing a single best model (Burnham & Anderson 2002; Moore & Borer 2012). Using this approach we specified our predictor variables in the full model, and explored all possible subset models for each dependent variable (Moore & Borer 2012). The full set of candidate models for each response variable were ranked according to Akaike’s information criteria corrected for small sample size (AICc) (Burnham & Anderson 2002). Models that had a difference in AICc value of less than 2 (ΔAICc < 2) from the model with the lowest AICc value were considered to be equally acceptable (Burnham & Anderson 2002). We used the ‘MuMIn’ package in R to produce weighted averages of regression parameter coefficients, 95% confidence intervals, and p-values for each input variable in models with ΔAICc < 2, hereafter referred to as the “top models”. In cases where there was only one top model, we report the results directly from the linear model, rather than weighted averages.
As we had multiple samples from many individuals, we used linear mixed-effect models with individual identity as a random effect in our analyses of sex, female rank, and female reproductive status. All mixed models were run using the ‘lme4’ package in R (R Development Core Team 2014). We used linear models for analysis of immigrant male rank, as there was only one individual for which we had two samples; we used only the first sample from this individual for these analyses. For the analysis of sex, we used BKC, total IgG, and total IgM as the response variables, and sex, age, the number of months the serum samples were stored at −80°C, and minutes from darting to sample collection as predictor variables in the full models; this resulted in 20 candidate models derived from the full model for each response variable, inclusive of the null model and all possible combinations of one, two, three, or four predictor variables and an interaction between sex and age. In our analysis of reproductive status we included only females that were either pregnant or lactating. Adult female spotted hyenas in our study clans are nearly always either pregnant or lactating, and occasionally are both pregnant and lactating simultaneously. In our dataset we had three adult females of unknown reproductive status, and two adult females had not yet become reproductively active (age > 36 months but never pregnant), and thus were not included in our analysis. We tested for an interaction between rank and reproductive status and included serum cortisol and testosterone concentrations as covariates in our models of reproductive status, which resulted in 20 candidate models. Before performing linear regressions using the ‘stats’ package in R, all continuous variables were standardized by centering and dividing by the standard deviation to allow comparisons across tests and to reduce the effects of potentially collinear predictor variables (Gelman 2008; Schielzeth 2010; Grueber et al. 2011). See the online Supporting Information for details of model diagnostics and figure creation.
Results
SEX, AGE, AND IMMUNE DEFENSES
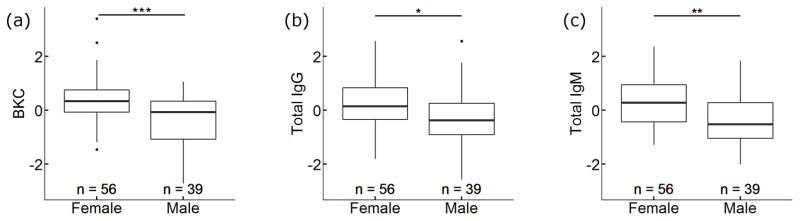
In our analysis of differences in immune function between females (n = 56 samples from 38 individuals) and males (n = 39 samples from 36 individuals) we found that serum from females had higher BKC (p < 0.001), total IgG (p = 0.030), and total IgM (p = 0.002) than did serum from males (Fig. 2). Age, latency to blood collection after darting, and years in frozen storage were not significant predictors of any of the immune measures tested here (Tables 1 and S1, Figs S3 and S4). Additionally, we had serum cortisol and testosterone concentrations for 53 adult hyenas (females: n = 37 samples from 29 individuals; males: n = 16 samples from 15 individuals). Analysis of this subset again showed that sex was a strong predictor of BKC (p = 0.022) and IgM (p = 0.015), but sex was not a significant predictor of IgG (p = 0.115) when cortisol and testosterone concentrations were included as covariates. Neither cortisol nor testosterone was significantly related to any of our immune measures (p > 0.176 in all cases), but there was a trend for an interaction between sex and cortisol in the IgG analysis (Tables S2 and S3).
Fig. 2.
Plots showing standardized comparisons of immune defenses in serum samples from female (n = 56 samples from 38 individuals) and male (n = 39 samples from 36 individuals) spotted hyenas. Females had significantly higher (a) BKC (p < 0.001), (b) IgG (p = 0. 030), and (c) total IgM (p = 0. 002) than males. Total IgG and total IgM were log transformed prior to standardization and regression analysis.
Table 1.
Results of AICc based multimodel weighted-average analysis of the relationship between sex and immune function between adult females (n = 56 samples, 38 individuals) and males (n = 39 samples, 36 individuals). Importance is the sum of Akaike weights over all models including the predictor variable.
| Response | Predictor | β | SE | Lower CI | Upper CI | p | Importance |
|---|---|---|---|---|---|---|---|
| BKC | Intercept | −0.05 | 0.10 | −0.25 | 0.15 | 0.606 | NA |
| Age | 0.15 | 0.10 | −0.04 | 0.35 | 0.125 | 0.74 | |
| Age X Sex | −0.17 | 0.10 | −0.36 | 0.02 | 0.074 | 0.45 | |
| Minutes | −0.13 | 0.09 | −0.31 | 0.06 | 0.175 | 0.42 | |
| Years | −0.13 | 0.09 | −0.32 | 0.06 | 0.180 | 0.40 | |
| Sex | 0.35 | 0.10 | 0.15 | 0.54 | < 0.001 | 1.00 | |
|
| |||||||
| IgG | Intercept | −0.04 | 0.10 | −0.24 | 0.16 | 0.714 | NA |
| Age | 0.14 | 0.11 | −0.07 | 0.35 | 0.177 | 0.44 | |
| Minutes | 0.07 | 0.10 | −0.13 | 0.26 | 0.502 | 0.09 | |
| Years | −0.16 | 0.10 | −0.36 | 0.03 | 0.106 | 0.63 | |
| Sex | 0.23 | 0.10 | 0.02 | 0.43 | 0.030 | 0.90 | |
|
| |||||||
| IgM | Intercept | −0.08 | 0.10 | −0.28 | 0.13 | 0.472 | NA |
| Years | −0.09 | 0.09 | −0.27 | 0.09 | 0.328 | 0.35 | |
| Sex | 0.32 | 0.10 | 0.11 | 0.52 | 0.002 | 1.00 | |
RANK, REPRODUCTIVE STATUS, AND IMMUNE DEFENSES
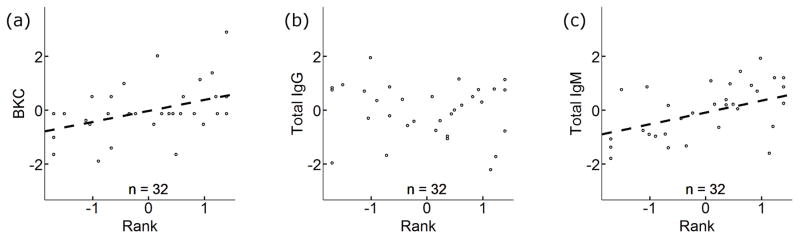
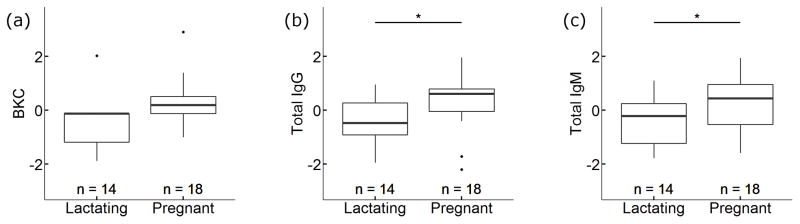
When we assessed the relationship between social rank, reproductive status, hormones, and immune function in adult females (n = 32 samples from 25 individuals), we found that serum BKC (p = 0.016) and total serum IgM (p = 0.021) were both positively correlated with social rank (Fig. 3). However, total serum IgG was unrelated to rank in adult females (p = 0.176) (Fig. 3). We found that total IgG and total IgM concentrations were significantly higher in 18 pregnant than in 14 lactating females (IgG: p = 0.019; IgM: p = 0.012) (Fig. 4). There was also a trend toward greater BKC in pregnant than lactating females (p = 0.093) (Fig. 4). We found no interaction effects between rank and reproductive status, and there were no strong correlations (p < 0.1) between our immune defense measures and the hormones we quantified (Tables 2 and S4). Although samples collected from females of unknown reproductive status (n = 3) and females that were neither lactating nor pregnant at the time of sample collection (n = 2) were not included in the analysis of reproductive status and rank, we performed an additional analysis of rank and immune defenses that included these samples, and again we found the rank was a strong predictor of BKC (p = 0.002) and IgM (p = 0.003), but was not correlated with IgG (p = 0.455) (Fig. S5, Tables S5 and S6). Age, cortisol, and testosterone were not correlated with immune defenses in these models (p > 0.1 in all cases), and we found no evidence of interaction effects between rank and age, rank and cortisol, or rank and testosterone. Additionally, we tested for an association between rank, age, and immune defenses in 16 immigrant males and found the male rank was inversely correlated with IgG (p = 0.01) (Fig. S6, Tables S7 and S8), but this relationship was largely driven by a single low-ranking male with a high IgG titer (p = 0.289 when the individual was removed from the analysis). We found no associations between male rank and IgM or BKC (Fig. S6, Tables S7 and S8).
Fig. 3.
Plots showing standardized relationships between immune defenses and social rank in adult female spotted hyenas (n = 32 samples, 25 individuals). Social rank was a positively correlated with (a) BKC (p = 0.016) and (c) total IgM (p = 0.021), but rank was not correlated with total (b) IgG (p = 0.176). Total IgG and total IgM were log transformed prior to standardization and regression analysis.
Fig. 4.
Plots showing standardized relationships between immune defenses and reproductive status in adult female spotted hyenas (n = 32 samples, 25 individuals). Lactating females had less (b) IgG (p = 0.019) and (c) total IgM (p = 0.012) than pregnant females; there was a trend for higher BKC in pregnant females than in lactating females (a) BKC (p = 0.093). Total IgG and total IgM were log transformed prior to standardization and regression analysis.
Table 2.
Results of AICc based multimodel weighted-average analysis of the relationship between reproductive status and immune function among adult females (n = 32 samples, 25 individuals). Importance is the sum of Akaike weights over all models including the predictor variable. Repro = reproductive status
| Response | Predictor | β | SE | Lower CI | Upper CI | p | Importance |
|---|---|---|---|---|---|---|---|
| BKC | Intercept | −0.02 | 0.15 | −0.33 | 0.30 | 0.912 | NA |
| Rank | 0.41 | 0.16 | 0.08 | 0.74 | 0.016 | 1.00 | |
| Repro | 0.27 | 0.16 | −0.05 | 0.59 | 0.093 | 0.52 | |
|
| |||||||
| IgG | Intercept | −0.08 | 0.18 | −0.45 | 0.28 | 0.658 | NA |
| Rank | −0.26 | 0.18 | −0.64 | 0.12 | 0.176 | 0.29 | |
| Repro | 0.39 | 0.16 | 0.06 | 0.72 | 0.019 | 1.00 | |
| Testosterone | −0.24 | 0.18 | −0.61 | 0.14 | 0.223 | 0.24 | |
|
| |||||||
| IgM | Intercept | −0.08 | 0.17 | −0.42 | 0.27 | 0.666 | NA |
| Cort | 0.18 | 0.13 | −0.08 | 0.44 | 0.168 | 0.39 | |
| Rank | 0.44 | 0.18 | 0.07 | 0.80 | 0.021 | 0.70 | |
| Repro | 0.35 | 0.14 | 0.08 | 0.63 | 0.012 | 0.70 | |
Discussion
SOCIAL RANK AS A PREDICTOR OF IMMUNE FUNCTION
It has been well-established that rank affects many aspects of the biology of spotted hyenas, including fitness (Holekamp, Smale & Szykman 1996; Swanson, Dworkin & Holekamp 2011), energy expenditure (Mills 1990; Boydston et al. 2003), and telomere length (Lewin et al. 2015), but prior to this study it was unknown whether rank affects their immune function. Here we show that rank is a strong predictor of immune defenses in wild spotted hyenas, but that neither age nor circulating hormone concentrations were significantly associated with any of the immune defenses that we quantified. In many species glucocorticoids and testosterone suppress aspects of immune function, but other cases have been documented in which one or both of these hormones augment immune function (Roberts, Buchanan & Evans 2004; Martin, Weil & Nelson 2006), so perhaps it is not surprising that we found no association between either testosterone or cortisol and our measures of immune function.
Prior research with both mammals and birds suggests that dominant individuals can experience faster recovery from wounds, infection, and disease than subordinates (Lindström 2004; Hawley et al. 2007; Archie, Altmann & Alberts 2012). However, rarely are the immunological mechanisms identified that mediate these health benefits. The positive correlations we observed here among rank, BKC, and IgM are consistent with the hypothesis that dominant hyenas maintain the highest levels of first line immune defenses, such as IgM and complement, operating to prevent disease and to permit fast recovery when infection does occur. In primates, early response to infection is a major determinant of disease outcome (Estes et al. 2008), and dietary restriction or malnourishment is often associated with reduced immune function, increased frequency of infection, and higher rates of chronic infection than those observed among individuals with nutrient-rich diets (Cunningham-Rundles, McNeeley & Moon 2005; Afacan, Fjell & Hancock 2012).
One of the primary mediators of the superior fitness enjoyed by high-ranking hyenas is their higher priority of access to food. Although we did not specifically quantify food intake in this study, previous studies have firmly established that high-ranking hyenas have better access to food resources in the spotted hyena societies studies here (Frank 1986; Smith et al. 2008), and we hypothesize that better access to food may have contributed to the higher levels of immune defenses found in high-ranking individuals. Energetic and nutritional resources are likely important for both complement and antibody production, as complement proteins and antibodies can each comprise more than ten percent of serum protein (Maes et al. 1995; Brock et al. 2013). A critical component of BKC, the complement protein C3, is produced by adipocytes and its production is significantly increased in response to dietary lipids (Maslowska et al. 1997). This is one potential mechanism through which nutritional status might mediate the rank-related variation in the immune measures reported here for hyenas.
Differences in pathogen exposure is another potential mediator of the relationship between rank and immune defenses observed here. In order to procure food, mates, and enhanced social position, high-ranking individuals in many species exhibit higher rates and intensities of aggressive behavior than do their low-ranking counterparts (Smith, Memenis & Holekamp 2006; Creel et al. 2012), and higher rates of aggression and social contact in turn may lead to higher stress, greater risk of injury, and increased pathogen exposure (Sands & Creel 2004; Rimbach et al. 2015). Conversely, low-ranking individuals may be the target of more aggression than high-ranking individuals, and thus could have higher stress, injury risk, and pathogen exposure. Previous research in hyenas and rodents suggests that higher pathogen exposure results in elevated IgG levels (Hooijkaas et al. 1984; Bos et al. 1989; Haury et al. 1997; Devalapalli et al. 2006; Flies et al. 2015), but that pathogen exposure is not correlated with serum BKC in hyenas (Flies et al. 2015). If rank is driving pathogen exposure then we would expect rank to be either positively or negatively correlated with IgG, but not correlated with BKC. We detected no evidence of an association between female rank and IgG, whereas we found a significant correlation between female rank and BKC, suggesting energetic resource allocation is more likely than pathogen exposure to mediate the relationship between rank and immune function observed here.
Immigrant males, on the other hand exhibited an inverse relationship between IgG and rank and no relationship between BKC and rank. However, the rank of immigrant males can change dramatically when males emigrate into a new clan in which they occupy the lowest rank in the social hierarchy and have poor access to food resources during competitive feeding events, so a correlative test between immigrant male rank and immune defenses is not well-suited to test the resource trade-off hypothesis. Additionally, the relationship between immigrant male rank and IgG was driven largely by the high IgG titer in one low-ranking individual. Finally, large fluctuations in total IgG may occur due to recent infections, and these may mask potential relationships between IgG and socioecological variables.
SEX AND REPRODUCTIVE STATUS AS PREDICTORS OF IMMUNE PERFORMANCE
Most studies of immune function in mammals have found that immune defenses are generally higher in females than in males (reviewed in Libert, Dejager & Pinheiro 2010), despite females being socially subordinate to males in most mammals. As female spotted hyenas outrank all immigrant males, it might be expected that this would lead to a sex-role reversal in immune defense levels. Interestingly, in our system the tendency still holds for females to exhibit higher immune defense levels than males, despite the sex-role-reversed patterns of body size, aggression and dominance seen in this species (Kruuk 1972; Swanson, Dworkin & Holekamp 2011). One possible explanation for this was proposed by Rolff (2002), who suggested that the higher immune defenses observed in females might function to increase reproductive longevity, whereas males are more likely to enhance fitness by increasing their mating rates than by investing in immunity.
Our observation that immune defense levels are lower in lactating than pregnant females is consistent with a hypothesis suggesting that energy resources are traded-off between immune defenses and the energy demands of reproduction (French, Johnston & Moore 2007; Graham et al. 2010). We found no association between cortisol or testosterone and immune function in reproductively active females, but it is possible that other hormones that we did not measure, such as oestrogen and progesterone, may affect the relationship between reproductive status and immunity. Testing immune defenses in females that were neither pregnant nor lactating would be a further test of the resource trade-off hypothesis, but as we sampled only two females that were neither pregnant nor lactating, we were unable to perform this test. East et al. (2015) recently found that Ancylostoma egg loads were higher in lactating that non-lactating female hyenas, and that individuals with high social status had lower Ancylostoma egg counts, which also suggests that immune defenses may be suppressed during lactation, but that high-ranking individuals may have more overall energetic resources and are thus buffered from immunological “trade-offs”. Although these data together support the hypothesis that energetic trade-offs may lead to reduced immune function during lactation, female mammals also transfer antibodies to offspring via colostrum and milk, and this transfer might also have contributed to the differences observed here between pregnant and lactating female immunoglobulin concentrations.
In summary, our data show that high-ranking hyenas have stronger immune defenses than their low-ranking conspecifics for two of the three immune parameters we tested. Furthermore, IgG and total IgM were both lower in lactating than pregnant females, and we also observed a trend toward lower BKC in lactating females. These data are consistent with the hypothesis that immune defenses are costly, and higher ranking individuals can allocate more resources to immune defenses. Our results are similar to those from previous studies showing that female mammals often have higher levels of immune defenses than do males; which, given the sex-role reversals observed in spotted hyenas, suggests that reproductive evolutionary forces as well as contemporary sociological forces both shape immune function. Taken together, our results suggest that a thorough understanding of socioecological variables may be needed before attempting to understand relationships between immunity and specific physiological measurements in gregarious animals.
Supplementary Material
Acknowledgments
We thank the Kenyan National Council for Science, Technology and Innovation for permission to conduct this research, and the Kenya Wildlife Service, Narok County Council and the Senior Warden of the Maasai Mara National Reserve for their assistance. We are deeply grateful to all those who contributed to the long-term data collection, particularly Kasaine Sankan. We wish to thank Eli Swanson, Julia Bell, Jean Tsao, and Barry Williams for helpful comments throughout this study. This work was supported by an NSF Graduate Research Fellowship, a Morris Animal Foundation Veterinary Student Scholars grant, a Sigma Xi Grant-in-Aid of Research, and a Grant-in-Aid of Research from the American Society of Mammalogists to A.S.F., by NSF grants IOS1121474 and DEB1353110 and N.I.H. grant 1R01GM105042 to K.E.H, and by grant W911NF-08-1-0310 from the Army Research Office to A.S.F. and K.E.H., and NIH NIAID grants K26RR023080 and U10 AI090872 to LSM. C.K.G. is president of Custom Monoclonal International, which supplied several of the antibodies used in this study.
Footnotes
Data Accessibility
Data are deposited in the Dryad Digital Repository doi:10.5061/dryad.934qn (Flies et al, 2016)
References
- Afacan NJ, Fjell CD, Hancock RE. A systems biology approach to nutritional immunology - focus on innate immunity. Molecular Aspects of Medicine. 2012;33:14–25. doi: 10.1016/j.mam.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Archie EA, Altmann J, Alberts SC. Social status predicts wound healing in wild baboons. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9017–9022. doi: 10.1073/pnas.1206391109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard CJ, Behnke JM, Sewell J. Social status and resistance to risease in house mice (Mus musculus): status-related modulation of hormonal responses in relation to immunity costs in different social and physical environments. Ethology. 1996;102:63–84. [Google Scholar]
- Bashir-Tanoli S, Tinsley MC. Immune response costs are associated with changes in resource acquisition and not resource reallocation. Functional Ecology. 2014;28:1011–1019. [Google Scholar]
- Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Seminars in Immunopathology. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- Bischof P. Pregnancy-associated plasma protein-A: An inhibitor of the complement system. Placenta. 1981;2:29–34. doi: 10.1016/s0143-4004(81)80037-9. [DOI] [PubMed] [Google Scholar]
- Bos NA, Kimura H, Meeuwsen CG, De Visser H, Hazenberg MP, Wostmann BS, Pleasants JR, Benner R, Marcus DM. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. European Journal of Immunology. 1989;19:2335–2339. doi: 10.1002/eji.1830191223. [DOI] [PubMed] [Google Scholar]
- Boydston EE, Kapheim KM, Szykman M, Holekamp KE. Individual variation in space use by female spotted hyenas. Journal of Mammalogy. 2003;84:1006–1018. [Google Scholar]
- Brock PM, Hall AJ, Goodman SJ, Cruz M, Acevedo-Whitehouse K. Immune activity, body condition and human-associated environmental impacts in a wild marine mammal. PloS one. 2013;8:e67132. doi: 10.1371/journal.pone.0067132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. Springer Verlag; 2002. [Google Scholar]
- Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annual Review of Immunology. 1998;16:545–568. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, Chaudhry HS. Social status, glucocorticoids, immune function, and health: Can animal studies help us understand human socioeconomic-status-related health disparities? Hormones and Behavior. 2012;62:295–313. doi: 10.1016/j.yhbeh.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Creel S, Dantzer B, Goymann W, Rubenstein DR. The ecology of stress: effects of the social environment. Functional Ecology. 2012:1–15. [Google Scholar]
- Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. The Journal of Allergy and Clinical Immunology. 2005;115:1119–1128. doi: 10.1016/j.jaci.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Curno O, Behnke JM, McElligott AG, Reader T, Barnard CJ. Mothers produce less aggressive sons with altered immunity when there is a threat of disease during pregnancy. Proceedings of the Royal Society B: Biological Sciences. 2009;276:1047–1054. doi: 10.1098/rspb.2008.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devalapalli AP, Lesher A, Shieh K, Solow JS, Everett ML, Edala AS, Whitt P, Long RR, Newton N, Parker W. Increased levels of IgE and autoreactive, polyreactive IgG in wild rodents: implications for the hygiene hypothesis. Scandinavian Journal of Immunology. 2006;64:125–136. doi: 10.1111/j.1365-3083.2006.01785.x. [DOI] [PubMed] [Google Scholar]
- Diamond SE, Kingsolver JG. Host plant quality, selection history and trade-offs shape the immune responses of Manduca sexta. Proceedings of the Royal Society B: Biological Sciences. 2011;278:289–297. doi: 10.1098/rspb.2010.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East M, Otto E, Helms J, Thierer D, Cable J, Hofer H. Does lactation lead to resource allocation trade-offs in the spotted hyaena? Behavioral Ecology and Sociobiology. 2015;69:805–814. [Google Scholar]
- East ML, Hofer H, Cox JH, Wulle U, Wiik H, Pitra C. Regular exposure to rabies virus and lack of symptomatic disease in Serengeti spotted hyenas. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:15026–15031. doi: 10.1073/pnas.261411898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engh AL, Esch K, Smale L, Holekamp KE. Mechanisms of maternal rank ‘inheritance’ in the spotted hyaena, Crocuta crocuta. Animal Behaviour. 2000;60:323–332. doi: 10.1006/anbe.2000.1502. [DOI] [PubMed] [Google Scholar]
- Estes JD, Gordon SN, Zeng M, Chahroudi AM, Dunham RM, Staprans SI, Reilly CS, Silvestri G, Haase AT. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. The Journal of Immunology. 2008;180:6798–6807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flies AS, Grant CK, Mansfield LS, Smith EJ, Weldele ML, Holekamp KE. Development of a hyena immunology toolbox. Veterinary Immunology and Immunopathology. 2012;145:110–119. doi: 10.1016/j.vetimm.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flies AS, Mansfield LS, Flies EJ, Grant CK, Holekamp KE. Socioecological predictors of immune defenses in wild spotted hyenas. Dryad Digital Repository. 2016 doi: 10.5061/dryad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flies AS, Mansfield LS, Grant CK, Weldele ML, Holekamp KE. Markedly elevated antibody responses in wild versus captive spotted hyenas show that environmental and ecological factors are important modulators of immunity. PloS one. 2015;10:e0137679. doi: 10.1371/journal.pone.0137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LG. Social organization of the spotted hyaena Crocuta crocuta. II. Dominance and reproduction. Animal Behaviour. 1986;34:1510–1527. [Google Scholar]
- French SS, Johnston GIH, Moore MC. Immune activity suppresses reproduction in food-limited female tree lizards Urosaurus ornatus. Functional Ecology. 2007;21:1115–1122. [Google Scholar]
- Gelman A. Scaling regression inputs by dividing by two standard deviations. Statistics in Medicine. 2008;27:2865–2873. doi: 10.1002/sim.3107. [DOI] [PubMed] [Google Scholar]
- Gittleman JL, Thompson SD. Energy allocation in mammalian reproduction. American Zoologist. 1988;28:863–875. [Google Scholar]
- Goymann W, East ML, Wachter B, Honer OP, Mostl E, Van’t Hof TJ, Hofer H. Social, state-dependent and environmental modulation of faecal corticosteroid levels in free-ranging female spotted hyenas. Proceedings of the Royal Society B: Biological Sciences. 2001;268:2453–2459. doi: 10.1098/rspb.2001.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Hayward AD, Watt KA, Pilkington JG, Pemberton JM, Nussey DH. Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science. 2010;330:662–665. doi: 10.1126/science.1194878. [DOI] [PubMed] [Google Scholar]
- Grueber CE, Nakagawa S, Laws RJ, Jamieson IG. Multimodel inference in ecology and evolution: challenges and solutions. Journal of Evolutionary Biology. 2011;24:699–711. doi: 10.1111/j.1420-9101.2010.02210.x. [DOI] [PubMed] [Google Scholar]
- Harrison TM, Mazet JK, Holekamp KE, Dubovi E, Engh AL, Nelson K, Van Horn RC, Munson L. Antibodies to canine and feline viruses in spotted hyenas (Crocuta crocuta) in the Masai Mara National Reserve. Journal of Wildlife Diseases. 2004;40:1–10. doi: 10.7589/0090-3558-40.1.1. [DOI] [PubMed] [Google Scholar]
- Haury M, Sundblad A, Grandien A, Barreau C, Coutinho A, Nobrega A. The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. European Journal of Immunology. 1997;27:1557–1563. doi: 10.1002/eji.1830270635. [DOI] [PubMed] [Google Scholar]
- Hawley DM, Jennelle CS, Sydenstricker KV, Dhondt AA. Pathogen resistance and immunocompetence covary with social status in house finches (Carpodacus mexicanus) Functional Ecology. 2007;21:520–527. [Google Scholar]
- Herr M, Bostedt H, Failing K. IgG and IgM levels in dairy cows during the periparturient period. Theriogenology. 2011;75:377–385. doi: 10.1016/j.theriogenology.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Holekamp KE, Dantzer B, Stricker G, Shaw Yoshida KC, Benson-Amram S. Brains, brawn and sociality: a hyaena’s tale. Animal Behaviour. 2015;103:237–248. doi: 10.1016/j.anbehav.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holekamp KE, Sisk CL. Effects of dispersal status on pituitary and gonadal function in the male spotted hyena. Hormones and Behavior. 2003;44:385–394. doi: 10.1016/j.yhbeh.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Holekamp KE, Smale L. Provisioning and food sharing by lactating spotted hyenas, Crocuta crocuta (Mammalia: Hyaenidae) Ethology. 1990;86:191–202. [Google Scholar]
- Holekamp KE, Smale L. Ontogeny of dominance in free-living spotted hyaenas: juvenile rank relations with other immature individuals. Animal Behaviour. 1993;46:451–466. [Google Scholar]
- Holekamp KE, Smale L, Szykman M. Rank and reproduction in the female spotted hyaena. Journal of Reproduction and Fertility. 1996;108:229–237. doi: 10.1530/jrf.0.1080229. [DOI] [PubMed] [Google Scholar]
- Hooijkaas H, Benner R, Pleasants JR, Wostmann BS. Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered “antigen-free” diet. European Journal of Immunology. 1984;14:1127–1130. doi: 10.1002/eji.1830141212. [DOI] [PubMed] [Google Scholar]
- Klobasa F, Habe F, Werhahn E, Butler JE. Changes in the concentrations of serum IgG, IgA and IgM of sows throughout the reproductive cycle. Veterinary Immunology and Immunopathology. 1985;10:341–353. doi: 10.1016/0165-2427(85)90023-6. [DOI] [PubMed] [Google Scholar]
- Kruuk H. The spotted hyena: a study of predation and social behavior. University of Chicago Press; Chicago: 1972. [Google Scholar]
- Lembo T, Hampson K, Auty H, Beesley CA, Bessell P, Packer C, Halliday J, Fyumagwa R, Hoare R, Ernest E, Mentzel C, Mlengeya T, Stamey K, Wilkins PP, Cleaveland S. Serologic surveillance of anthrax in the Serengeti ecosystem, Tanzania, 1996–2009. Emerging infectious diseases. 2011;17:387–394. doi: 10.3201/eid1703.101290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin N, Treidel LA, Holekamp KE, Place NJ, Haussmann MF. Socioecological variables predict telomere length in wild spotted hyenas. Biology Letters. 2015;11:20140991-1–4. doi: 10.1098/rsbl.2014.0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nature Reviews Immunology. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- Liebl AL, Martin LB. Simple quantification of blood and plasma antimicrobial capacity using spectrophotometry. Functional Ecology. 2009;23:1091–1096. [Google Scholar]
- Lindström KM. Social status in relation to sindbis virus infection clearance in greenfinches. Behavioral Ecology and Sociobiology. 2004;55:236–241. [Google Scholar]
- Lochmiller RL, Vestey MR, Jon CB. Relationship between protein nutritional status and immunocompetence in northern bobwhite chicks. The Auk. 1993;110:503–510. [Google Scholar]
- Love OP, Salvante KG, Dale J, Williams TD. Sex-specific variability in the immune system across life-history stages. The American Naturalist. 2008;172:E99–112. doi: 10.1086/589521. [DOI] [PubMed] [Google Scholar]
- Maes M, Wauters A, Neels H, Scharpé S, Van Gastel A, D’Hondt P, Peeters D, Cosyns P, Desnyder R. Total serum protein and serum protein fractions in depression: relationships to depressive symptoms and glucocorticoid activity. Journal of Affective Disorders. 1995;34:61–69. doi: 10.1016/0165-0327(94)00106-j. [DOI] [PubMed] [Google Scholar]
- Markowska-Daniel I, Pomorska-Mól M, Pejsak Z. Dynamic changes of immunoglobulin concentrations in pig colostrum and serum around parturition. Polish Journal of Veterinary Sciences. 2010;13:21–27. [PubMed] [Google Scholar]
- Martin LB, 2nd, Weil ZM, Nelson RJ. Refining approaches and diversifying directions in ecoimmunology. Integrative and Comparative Biology. 2006;46:1030–1039. doi: 10.1093/icb/icl039. [DOI] [PubMed] [Google Scholar]
- Maslowska M, Scantlebury T, Germinario R, Cianflone K. Acute in vitro production of acylation stimulating protein in differentiated human adipocytes. Journal of Lipid Research. 1997;38:1–11. [PubMed] [Google Scholar]
- Mills MGL. Kalahari Hyenas. Springer; 1990. Comparative foraging behaviour; pp. 71–129. [Google Scholar]
- Mollnes T, Garred P, Bergseth G. Effect of time, temperature and anticoagulants on in vitro complement activation: consequences for collection and preservation of samples to be examined for complement activation. Clinical and Experimental Immunology. 1988;73:484–488. [PMC free article] [PubMed] [Google Scholar]
- Moore SM, Borer ET. The influence of host diversity and composition on epidemiological patterns at multiple spatial scales. Ecology. 2012;93:1095–1105. doi: 10.1890/11-0086.1. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2014. [Google Scholar]
- Raberg L, Graham AL, Read AF. Decomposing health: tolerance and resistance to parasites in animals. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2009;364:37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nature Immunology. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbach R, Bisanzio D, Galvis N, Link A, Di Fiore A, Gillespie TR. Brown spider monkeys (Ateles hybridus): a model for differentiating the role of social networks and physical contact on parasite transmission dynamics. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2015;370 doi: 10.1098/rstb.2014.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts ML, Buchanan KL, Evans MR. Testing the immunocompetence handicap hypothesis: a review of the evidence. Animal Behaviour. 2004;68:227–239. [Google Scholar]
- Rolff J. Bateman’s principle and immunity. Proceedings of the Royal Society B: Biological Sciences. 2002;269:867–872. doi: 10.1098/rspb.2002.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands J, Creel S. Social dominance, aggression and faecal glucocorticoid levels in a wild population of wolves, Canis lupus. Animal Behaviour. 2004;67:387–396. [Google Scholar]
- Sapolsky RM. The Influence of Social Hierarchy on Primate Health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution. 2010;1:103–113. [Google Scholar]
- Smale L, Frank LG, Holekamp KE. Ontogeny of dominance in free-living spotted hyaenas: juvenile rank relations with adult females and immigrant males. Animal Behaviour. 1993;46:467–477. [Google Scholar]
- Smith JE. PhD. Michigan State University; 2010. Evolutionary and ecological forces shaping patterns of cooperation among spotted hyenas. 3417795. [Google Scholar]
- Smith JE, Kolowski JM, Graham KE, Dawes SE, Holekamp KE. Social and ecological determinants of fission–fusion dynamics in the spotted hyaena. Animal Behaviour. 2008;76:619–636. [Google Scholar]
- Smith JE, Memenis SK, Holekamp KE. Rank-related partner choice in the fission–fusion society of the spotted hyena (Crocuta crocuta) Behavioral Ecology and Sociobiology. 2006;61:753–765. [Google Scholar]
- Smith R, Bischof P, Hughes G, Klopper A. Studies on pregnancy-associated plasma protein A in the third trimester of pregnancy. BJOG: An International Journal of Obstetrics & Gynaecology. 1979;86:882–887. doi: 10.1111/j.1471-0528.1979.tb10716.x. [DOI] [PubMed] [Google Scholar]
- Svensson E, Råberg L, Koch C, Hasselquist D. Energetic stress, immunosuppression and the costs of an antibody response. Functional Ecology. 1998;12:912–919. [Google Scholar]
- Swanson EM, Dworkin I, Holekamp KE. Lifetime selection on a hypoallometric size trait in the spotted hyena. Proceedings of the Royal Society B: Biological Sciences. 2011;278:3277–3285. doi: 10.1098/rspb.2010.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilson RL, Hamilton WJ. Social dominance and feeding patterns of spotted hyaenas. Animal Behaviour. 1984;32:715–724. [Google Scholar]
- Van Horn RC, McElhinny TL, Holekamp KE. Age estimation and dispersal in the spotted hyena (Crocuta crocuta) Journal of Mammalogy. 2003;84:1019–1030. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.