Abstract
PURPOSE
We aimed to evaluate patterns of local tumor progression (LTP) after radiofrequency ablation (RF ablation) of colorectal cancer liver metastases (CRCLM) and to highlight the percentage of LTP not attributable to lesion size or RF ablation procedure-related factors (heat sink or insufficient ablation margin).
METHODS
CRCLM treated by RF ablation at a single tertiary care center from 2004–2012, with a minimum of six months of postprocedure follow-up, were included in this retrospective study. LTP morphology was classified as focal nodular (<90° of ablation margin), circumferential (>270°), or crescentic (90°–270°). Initial metastasis size, minimum ablation margin size, morphology of LTP, presence of a heat sink, and time to progression were recorded independently by two radiologists.
RESULTS
Thirty-two of 127 RF ablation treated metastases (25%) with a mean size of 23 mm (standard deviation 12 mm) exhibited LTP. Fifteen of 32 LTPs (47%) were classified as focal nodular, with seven having no procedure-related factor to explain recurrence. Ten of 32 LTPs (31%) were circumferential, with four having no procedure-related factor to explain recurrence. Seven of 32 LTPs (22%) were crescentic, with two having no procedure-related factor to explain recurrence. Of the 13 lesions without any obvious procedure-related reason for LTP, six (46%) were <3 cm in size.
CONCLUSION
Although LTP in RF ablation treated CRCLM can often be explained by procedure-related factors or size of the lesion, in this study up to six (5%) of the CRCLM we treated showed LTP without any reasonable cause.
Colorectal cancer is a common malignancy in North America. In both men and women, colorectal cancer is the third most commonly diagnosed cancer and the third leading cause of cancer death in the U.S. (1). One of the most common sites for metastatic colorectal cancer is the liver (1). When possible, surgical resection is considered the gold-standard treatment modality for colorectal cancer liver metastases (CRCLM), with neoadjuvant chemotherapy commonly used to downsize tumor burden prior to surgery, in addition to its use afterwards to reduce the risk of further local and metastatic disease (2). However, the majority of patients do not undergo surgery due to various comorbidities, extent of disease, or patient preference for locoregional therapy (3, 4). Of the currently available locoregional therapies, radiofrequency (RF) ablation is the most commonly used (5).
However, local tumor progression (LTP), defined by Goldberg et al. (6) as visualization of tumor within an area where complete tumor ablation was initially thought to have been achieved based on postablative cross-sectional imaging findings, can still occur. LTP rates following RF ablation have been quoted as ranging between 6% and 51% in the literature (7–9). This variation can be ascribed to multiple factors, such as differences in patient populations (some centers treat more advanced cases than other centers) and chemotherapy regimens (9, 10–14). Presence of a vessel, acting as a heat sink, in the vicinity of the ablated lesion can also affect the RF ablation procedure, and thus the incidence of LTP (15, 16). Finally, size of the lesion prior to RF ablation and the ablation margin following RF ablation have been found to be independent predictors of LTP, with lesions >3 cm and ablation margins <5 mm experiencing higher rates of recurrence (17, 18).
In this study, the incidence of LTP after RF ablation of CRCLM was compared with the initial size of the metastatic lesion, ablation margin size, and the presence of a heat sink. The aim of this retrospective study was to highlight the percentage of LTP without any attributable RF ablation procedure-related factors, such as presence of a heat sink or insufficient ablation margin, or lesion-related factors, such as size.
Methods
Institutional research ethics board approval was obtained for this retrospective study.
All RF ablations for CRCLM from 2004 through 2012 performed at a single tertiary care hospital with a minimum of six months of follow-up were included. CRCLM treated by RF ablation with <6 months of follow-up were excluded. Cases found to have obvious residual unablated tumor on the first follow-up imaging (rather than LTP, defined as a new abnormality in an area previously found to have a normal post-RF ablation appearance) were also excluded. Pre- or post-RF ablation treatment with chemotherapy was recorded for each patient.
Radiofrequency ablation
The included metastatic tumors underwent treatment by RF ablation with one of two systems (water cooled Cool-Tip RF ablation system; Covidien or multi-tined RF 3000 system; Boston Scientific). The ablations were performed either percutaneously under conscious sedation or intraoperatively with general anesthesia (usually during partial hepatectomy or during primary colon tumor resection). The decision to treat lesions percutaneously versus intraoperatively was made during multidisciplinary rounds that were attended by hepatobiliary and colorectal surgeons, oncologists, and radiologists.
During RF ablation, ultrasound guidance was used to visualize the lesion, which was subsequently ablated using the standard algorithm provided by the manufacturer of the given RF ablation system utilized during the procedure. The size of the probe used for each lesion ranged between 2–5 cm. The decision of which probe size to use was based on the size of the lesion to be treated, with the decision being made by the radiologist on the day of the procedure. If the lesion’s shape did not conform to the spherical ablation zone produced by the probe, or if the size of the lesion was sufficiently large so as to exceed the size of the ablation zone, the probe was repositioned and an additional ablation was performed at the time of the original treatment session to ensure adequate coverage of the lesion. In addition, to minimize the effect of a potential heat sink and ensure adequate ablation, in cases where a vessel >2 mm in diameter was noted to be closer than 4.5 mm to the lesion, a longer ablation was performed at the discretion of the radiologist (16). The probe track was ablated at the end of the procedure, both for percutaneous and intraoperative cases. Most patients that underwent percutaneous ablation were discharged home the same day as the procedure, with some occasionally having to remain in hospital overnight to receive treatment for a procedure-related complication such as pain management or lack of supervision at home.
Imaging
Imaging done prior to RF ablation consisted of multiphase computed tomography (CT) or magnetic resonance imaging (MRI) performed within two months of the ablation procedure. In addition, ultrasound consultation and sonographic imaging was performed prior to the scheduled RF ablation. This was carried out by one of five fellowship-trained radiologists that perform ablation so as to ensure feasibility of the procedure (i.e., adequate visualization, safe path to access the lesion, presence of heat sensitive structures nearby such as the colon, presence of a heat sink, adequate blood coagulation profile, and lack of important patient comorbidities/allergies). The RF ablation was performed within one week of the pre-RF ablation ultrasound consultation by one of the five subspecialized fellowship-trained radiologists.
Post-RF ablation imaging included either CT (16-slice or 64- slice VCT Lightspeed, GE Healthcare or 320-slice Aquilion ONE, Toshiba) or MRI (1.5 T Symphony Tim MRC37144, 1.5 T Symphony Maestro, or 3.0 T Magnetom Tim Trio 1, Siemens or Discovery MR750W 3.0 T, GE Healthcare) imaging modalities. Both CT and MRI imaging modalities were performed with pre- and post-dynamic intravenous contrast enhancement. MRI was the preferred modality for follow-up; however, due to various contraindications (e.g., the presence of a pacemaker or a comorbidity that precluded the patient from holding their breath), CT was performed in some cases.
In cases where MRI was utilized, standard MRI liver protocols were used. These varied slightly over the timeframe of the study; however, the key sequences of a liver MRI protocol included: T1-weighted images in and out of phase, balanced steady-state free precession, half-Fourier acquisition single-shot turbo spin echo, diffusion-weighted imaging with a B value that varied over the timeframe of the study, and pre- and post-gadolinium T1-weighted fat saturated imaging using arterial phase, portal venous phase, and 3–5 min delayed image acquisition. The follow-up cross-sectional studies were scheduled one, three, six, and 12 months after the RF ablation procedure, with additional imaging studies occurring yearly thereafter. A multiphasic pre- and post-contrast CT scan was performed on the same day as the RF ablation session if a suspected complication occurred during the procedure (i.e., the patient experienced some symptom out of the ordinary, such as extraordinary postprocedure pain).
All follow-up imaging and reports were reviewed independently by two fellowship-trained radiologists who specialize in abdominal imaging (one with six and the other with 16 years of radiology training experience). They were blinded to the results of the cross-sectional imaging reports. Axial and standard reformatted coronal images were reviewed in cases of local disease or tumor progression. The minimum distance from the CRCLM edge, when visible, to the edge of the RF ablation zone, as seen on contrast-enhanced images in the portal venous phase, was recorded. This distance was measured using the standard measurement tool available on a PACS workstation (McKesson Radiology; McKesson Corp.). Pre-RF ablation images were reviewed first, followed by the first post-RF ablation images. These were then carefully compared side-by-side with images where the first appearance of local disease progression could be identified.
Data collection
The classification system used for the morphologic pattern of local disease progression was based on the pattern of LTP around the circumference of the initial lesion before RF ablation, and was adapted from that of King and Breen (19). In particular, the patterns of LTP were classified as either focal nodular (<90° of the circumference), crescentic (between 90° and 270° of the circumference), or circumferential (>270° of the circumference) around the ablation margin.
The size of the metastatic lesion before RF ablation, minimum distance between the edge of the tumor and the edge of the RF ablation zone, morphology of LTP, time to progression, and suspected cause of LTP were all recorded on a standardized sheet, independently by the two radiologists. Further, the presence of potential heat sinks, including the portal and hepatic veins, was also recorded by the evaluating radiologist. Disagreements in measurements (occurring in 5 of 127 cases; 4%) were resolved by consensus.
The ablation procedure was deemed a technical success if a minimum ablation margin of 5 mm was achieved along all margins of the tumor on follow-up imaging. LTP was defined as new abnormal tumor tissue, which was not present on initial or follow-up post-RF ablation imaging, and not related to any procedure-related technical factors.
Most lesions were not biopsied at the time of identified local progression of disease, although the imaging characteristics were the same as the preprocedure tumor. All tumors treated with RF ablation were previously discussed at weekly multidisciplinary case conference. These conferences were attended by radiologists, oncologist, hepatobiliary surgeons, and pathologists to determine the best course of action for each patient.
Statistical analysis
The RF ablation terms and reporting outcomes used in this manuscript were adapted from the reporting standards of Ahmed et al. (20). Statistical analysis was performed using a computer spreadsheet program (Microsoft Excel; Microsoft Corp.). Descriptive data were summarized as number and percentage or mean±standard deviation.
Results
In total, 80 consecutive patients were treated with ultrasound-guided RF ablation over the study period. These procedures included both percutaneous and intraoperative cases. Seven patients were excluded, having <6 months of follow-up. Further, two patients with obvious residual untreated tumor on initial follow-up were excluded as well. Thus, 71 patients with 127 CRCLM lesions treated with RF ablation met the inclusion criteria (50 males and 21 females with a mean age of 68.3±10.2 years). Thirty-four patients (27%) underwent intraoperative RF ablation, while the remainder underwent percutaneous treatment. Patient characteristics are recorded in Table 1.
Table 1.
Patient characteristics
| All patients | Patients having lesions with LTP | Patients having lesions without LTP | |
|---|---|---|---|
| Patientsa | 71 (100) | 24 (31) | 53 (75) |
| Age (years)b | 68.3±10.2 (49.6–85.9) | 69.1±10.6 (51.6–85.7) | 68.4±10.3 (49.6–85.7) |
| Male | 50 (70) | 17 (71) | 38 (72) |
| Received pre-RF ablation chemotherapy | 54 (76) | 17 (71) | 41 (77) |
| Received post-RF ablation chemotherapy | 48 (68) | 18 (75) | 35 (67) |
| Follow-up (months) | 23.7±10.8 (7.0–54.3) | 23.7±10.4 (10.8–54.3) | 23.7±10.9 (7.0–52.0) |
| Deceased at last follow-up | 20 (28) | 8 (33) | 15 (28) |
Data are presented as n (%) or mean±standard deviation (range).
LTP, local tumor progression; RF, radiofrequency.
Of the patients with multiple metastases, six had some metastases with local tumor progression and other metastases without local tumor progression.
Age at the time of the radiofrequency ablation procedure.
The two most common sites for metastatic lesions were segments 7 and 8 of the liver, accounting for 34 and 32 incidents of metastases, respectively. In addition, 73 of 127 metastases (57%) were located in the periphery of the liver, as opposed to the parenchyma. Thirty-two of 127 RF ablation treated CRCLM lesions (25%) in 24 of 71 patients (34%) showed local tumor progression 1.9–50.4 months post-RF ablation (mean, 11.8±9.9 months). The mean index size of the lesions that exhibited LTP was 23±12 mm. Twenty-one of the 32 incidents of LTP (66%) were peripherally located in the liver, with the most incidents of LTP occurring in segments 7 and 8 (12 and 9 incidents of LTP, respectively). The localization and characteristics of the treated lesions are recorded in Tables 2 and 3, respectively.
Table 2.
Localization of liver metastases
| Liver segment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4a | 4b | 5 | 6 | 7 | 8 | |
| All metastases (n=156)a | 2 (1) | 10 (6) | 8 (5) | 22 (14) | 11 (7) | 17 (11) | 20 (13) | 34 (22) | 32 (21%) |
| Metastases with LTP (% of segment lesions) | 1 (50) | 3 (30) | 1 (13) | 5 (23) | 3 (27) | 3 (18) | 5 (25) | 12 (35) | 9 (28%) |
| Metastases without LTP (% of segment lesions) | 1 (50) | 7 (70) | 7 (87) | 17 (77) | 8 (73) | 14 (82) | 15 (75) | 22 (65s) | 23 (72%) |
Data are presented as n (%).
LTP, local tumor progression.
Of the 127 metastatic lesions, 29 were situated in two liver segments.
Table 3.
Characteristics of radiofrequency ablation treated metastases
| All treated metastases | Treated metastases with LTP | Treated metastases without LTP | |
|---|---|---|---|
| Lesions | 127 (100) | 32 (25) | 95 (75) |
| Peripheral liver metastases | 73 (57) | 21 (66) | 52 (55) |
| Lesion size (mm)a | 23±12 (3–71) | 32±14 (11–71) | 20±10 (3–50) |
| Lesion follow-up (months) | 23.7±10.8 (7.0–54.3) | 23.7±10.4 (10.8–54.3) | 23.7±10.9 (7.0–52.0) |
| Local recurrence time (months) | - | 11.8±9.9 (1.9–50.4) | - |
| Intraoperative ablation | 34 (27) | 4 (13) | 30 (32) |
Data are presented as n (%) or mean±standard deviation (range).
LTP, local tumor progression.
Size as determined at the time of the radiofrequency ablation procedure.
The morphologic patterns of LTP in patients and CRCLM lesions, as well as the hypothesized causes of recurrence, are described in Table 4. In particular, nineteen of the 32 lesions that experienced LTP (59%) occurred as a result of procedure-related factors. In the remaining 13 of 32 lesions that experienced LTP (41%; mean size, 35±14 mm), no obvious procedure-related reason for LTP could be ascertained. In this group of 13 lesions, six were <3 cm in size, four lesions measured between 3–4 cm, and the remaining three were >4 cm in diameter.
Table 4.
Local tumor progression morphology and hypothesized cause
| Focal (<90°) | Crescentic (90°–270°) | Circumferential (>270°) | Total | |
|---|---|---|---|---|
| Patients with LTP (total no. of patients=71) | 11 (46) | 5 (21) | 8 (33) | 24 (34) |
| Lesions with LTP, n (%) (total no. of lesions=127) | 15 (47) | 7 (22) | 10 (31) | 32 (25) |
|
| ||||
| Hypothesized cause of LTP | ||||
| Aggressive disease (drug resistance and metastatic relapse) | 0 (0) | 3 (43) | 0 (0) | 3 (9) |
| <5 mm ablation margin | 5 (33) | 2 (29) | 4 (40) | 11 (34) |
| Probe malposition resulting in <5 mm ablation margin | 0 (0) | 0 (0) | 1 (10) | 1 (3) |
| >5 mm ablation margin, but next to a heat sink | 3 (20) | 0 (0) | 1 (10) | 4 (13) |
| >5 mm ablation margin, no heat sink, but >3 cm in size | 5 (33) | 0 (0) | 2 (20) | 7 (22) |
| No identifiable procedure-related or lesion-related cause | 2 (13) | 2 (29) | 2 (20) | 6 (19) |
Data are presented as n (%).
LTP, local tumor progression.
Of the 24 patients who experienced LTP, 17 patients (71%) accounting for a total of 22 lesions (69%) received pre-RF ablation chemotherapy. Eighteen patients (75%) who experienced LTP, accounting for 26 lesions (81%), received post-RF ablation chemotherapy. Chemotherapy consisted of a variety of regimens including: 5-FU, FOLFIRI (5-FU, folinic acid and irinotecan based therapy), FOLFOX (5-FU, folinic acid and oxaliplatin based therapy), capecitabine (Xeloda), cetuximab (Erbitux), panitumumab (Vectibix), bevacizumab (Avastin), and raltitrexed (Tomudex). The particular regimen used varied over the period of the study due to changes in chemotherapy protocol.
Twelve of the 127 RF ablation treated CRCLM lesions (9%) required intraprocedural repositioning of the ablation probe in order to ensure that the entire lesion was ablated. Eight of these 12 lesions (67%) experienced LTP.
Seventy-three of the 127 RF ablation treated CRCLM lesions (57%) were peripherally located in the liver. In such cases, the radiologist performing the ablation had to take into account nearby heat sensitive structures (e.g., small and large bowel) so as not to damage them. Only one case of intra-RF ablation necessitated the use of hydrodissection of a segment of contiguous bowel that was next to a peripherally located CRCLM. There were no complications associated with this case and no local progression of disease was identified on any subsequent imaging.
None of the patients had any significant complications related to their RF ablation procedure.
Discussion
It has been recommended in the literature that a minimum ablation margin of 5 mm around each RF ablation treated CRCLM lesion be achieved in order to ensure adequate local control of disease (17). Issues related to the ablation procedure itself can result in an insufficient ablation margin. Such issues include: poor sonographic visibility due to chemotherapy-induced fatty liver, a poor acoustic window for adequate placement of the probe, inadequate probe size in relation to the size of the treated lesion, and operator positioning of the ablation probe. CT-guidance, with or without the use of ultrasound-guidance, may help with some of the above issues, but was not part of the ablation protocol used in this study.
However, there are other factors that may contribute to the ultimate success or failure of the RF ablation procedure to control local progression of disease. For example, flow in nearby vessels may act as a heat sink, diminishing the ability of the ablation probe to achieve a temperature of 60°C in the area of interest (the temperature needed to achieve adequate thermal necrosis) (6). This heat sink effect may vary depending on the size of the vessel, the size of the tumor being treated, and the distance of the tumor with respect to the diameter of the vessel in question (6, 16).
In this study, 12 of 32 lesions (38%) that resulted in local progression of disease had an insufficient ablation margin (<5 mm). A minimum ablation margin of >5 mm was achieved in four of 32 lesions (13%), but the lesion was situated next to a heat sink; likely resulting in LTP. The number of the lesions that were situated next to a heat sink was statistically higher for the group that experienced LTP (P < 0.05). Despite this, in 13 of 32 CRCLM lesions in this study (41%), no procedure-related factor could be found to account for LTP.
Size of the initial lesion has also been shown to be a predictive factor for local tumor progression, with lesions >3 cm in size having recurrence rates up to seven times higher than those <3 cm in size (18). Although this may be a factor in some cases, other biological factors should still be considered, rather than simply an arbitrary size cutoff. In our group of 13 lesions without obvious procedure-related factors identified for LTP, six of 13 lesions (46%) were <3 cm in size, accounting for six of 127 (5%) of all treated lesions, still lending support for the need to identify other factors for these recurrences.
With no procedure-related or lesion-related factors identified for causing LTP, other potential causes should be investigated, including hypotheses of inflammatory patterns following ablations in terms of cytokines levels rising in the blood stream (21–23). Hyperemia around the area of RF ablation occurs as a result of reparative processes in adjacent viable tissues (4). Post-RF ablation, the blood vessels in the partly damaged surrounding tissues become leaky and, in conjunction with hyperemia incited by the thermal damage, may present a suitable location for new micrometastases (21, 22). Thus, LTP after a RF ablation procedure that was a technical success has been postulated to be either due to microscopic residual disease or reseeding of circulating malignant cells in a patient who has metastatic colorectal cancer (21, 22). A similar hypothesis has been posited for primary hepatocellular carcinoma lesions insufficiently treated with RF ablation (23, 24).
This study is not without limitations. The sample size was small and this is a single center retrospective study. For this study population, we only included patients with CRCLM and did not compare these results to primary liver tumors, such as hepatocellular carcinoma, which may have different pathophysiology with respect to local progression of disease. This could be an area of future study and comparison.
In the vast majority of cases, particularly on MRI (including post-gadolinium images and T2-weighted images), the location of the original CRCLM could be seen as having a slightly different signal intensity (or density in the case of CT imaging) from the ablated area. Thus, the radiologists measured the distance from the original tumor margin to the shortest margin at the outer edge of the ablation zone on both axial and coronal images. However, due to intra-lesional hemorrhage, which can occur as a result of RF ablation, it is possible that the margins of the primary tumor, compared with the ablation area, could have been over- or underestimated. In two of 32 lesions (6%) exhibiting LTP, due to the hypodensity and lack of enhancement of the tumor pre- and post-contrast enhancement (both before and after RF ablation), the distance between the edge of the original CRCLM and ablation margin could not be reliably identified.
Further, although it rarely completely disappears, over time, the treated area of the primary tumor can involute and decrease in size. Thus, the imaging at one month may have allowed a slight interval for reduction in the original treated tumor size. This could have led to an inaccurate measurement of the ablation margin. However, the magnitude of such inaccuracy is difficult to predict.
In cases where intraprocedural repositioning of the ablation probe was needed (12 of 127, or 9%, of lesions in this study), although care was taken to ensure adequate coverage, small areas could potentially have been missed. Thus, residual unablated tumor may have been falsely interpreted as LTP. In this study eight of 12 lesions (67%) that required intraprocedural ablation probe repositioning experienced LTP. However, post-RF ablation imaging was performed after each case to ensure at least a 5 mm ablation margin and adequate ablation coverage of the lesion itself.
In this small retrospective study the effect of preprocedural and postablative chemotherapies, as well as the various regimes used, could have had variable effects on LTP. However, these effects cannot be measured nor the degree of their effect determined.
DNA analysis to assess for any genetic changes associated with the tumor (i.e., if a second primary had developed or if there was seeding from a different metastasis) could have possibly been of benefit.
In conclusion, although local disease progression in RF ablation treated CRCLM could be due to procedure-related or lesion-related factors, in cases where no such factors can be found, consideration should be given to other possibilities in order to allow radiologists to provide optimal care for this patient population. In our study, up to 5% of the CRCLM we treated showed LTP without any reasonable cause. Further studies including larger, prospective multicenter trials as well as multivariate analyses are warranted to further elucidate the underlying mechanisms resulting in LTP.
Main points.
Heat sink, probe size with respect to tumor size and probe positioning can be potential causes for initial technical failure and local tumor progression in RF ablation.
Meticulous technique is important when performing radiofrequency ablation for colorectal tumor liver metastases; factors such as large size and location of the tumor can have an impact on local tumor progression.
There are cases in which no procedure-related or lesion-related factors can be identified as a cause for local tumor progression. In up to 5% of treated cases no procedure-related or lesion-related factors were identified as a cause for local tumor progression. Thus, other potential causes should be considered, such as cytokine release and other local biological changes.
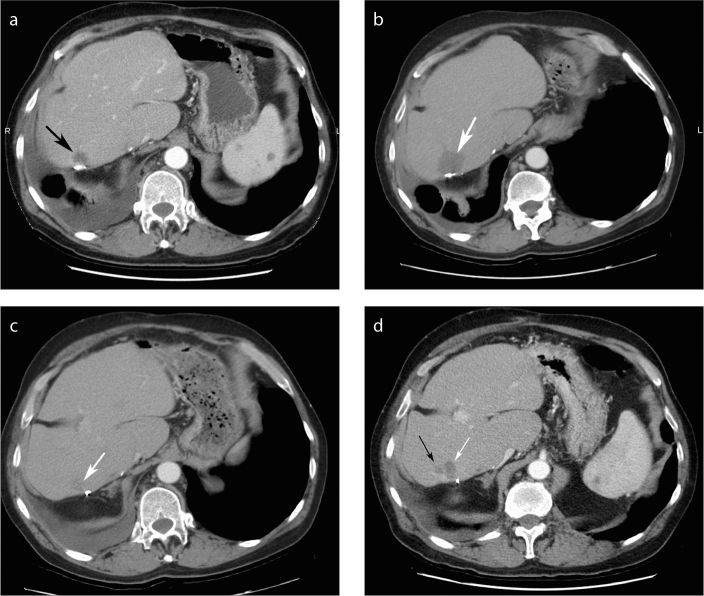
Figure 1.
a–d. Example of focal nodular local progression of disease in a 65-year-old man with remote right hepatectomy for colorectal cancer liver metastases (CRCLM). Contrast-enhanced CT image (a) shows development of local progression of disease along the suture line two years after surgery (black arrow). One month post-RF ablation (b), the area of ablation appears larger that the original area, making it difficult to identify the initial lesion on CT within the area of the ablation bed (white arrow). The lower edge of the tumor on the same initial post-RF ablation imaging appears smooth (c, white arrow). Six months later a subtle focal area of new nodularity is identified along the inferior margin (d, black arrow) next to the previously treated CRCLM (white arrow) that was retreated with RF ablation. This demonstrates the challenges of interpreting LTP on post-RF ablation CT imaging.
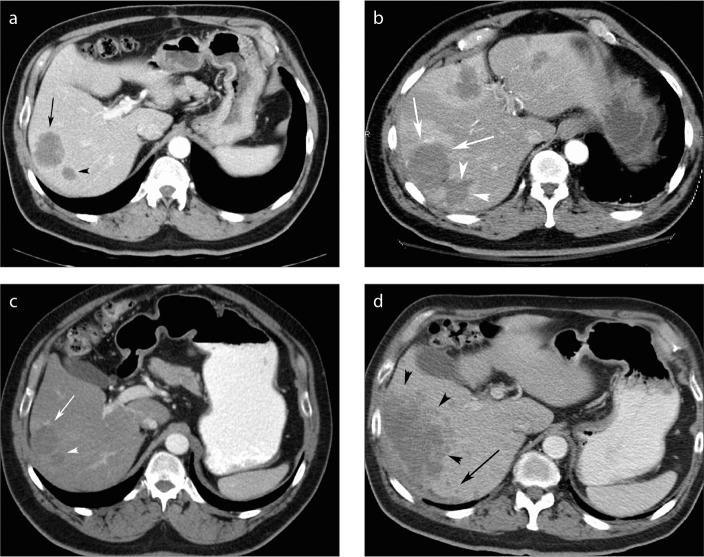
Figure 2.
a–d. Example of circumferential local progression of disease in a 67-year-old man on chemotherapy for CRCLM. Axial contrast-enhanced CT six weeks pre-RF ablation (a) demonstrates two lesions next to each other in the right lobe of the liver (black arrow and black arrowhead). CT performed one month post-RF ablation (b) demonstrates primary technical success with ablation margins measuring >5mm compared with the original tumors (white arrows and arrowheads). Two extra areas of ablation were performed in this patient since two new hypodense lesions were identified at the time of the RF ablation in segments 4 and 2/3 of the liver (not shown). Three months post-RF ablation (c), the ablated areas had reduced in size and were similar in size to the original tumors (white arrow and arrowhead). The patient’s condition deteriorated and a large area of local progression of disease was noted around the entire margin of the previously treated lesion (d, black arrowheads). In addition, new subcentimeter hypodense satellite lesions (black arrow) can be seen. The aggressiveness of the patient’s underlying malignant disease may have contributed to the development of local progression of disease and satellite lesions.
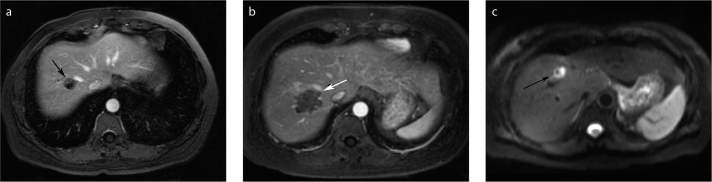
Figure 3.
a–c. Example of a heat sink from the right hepatic vein in a 51-year-old woman with CRCLM. MRI performed pre-RF ablation during the portal venous phase of the gadolinium-enhanced axial image (a) reveals a 1.8 cm lesion (black arrow) adjacent to the right hepatic vein. MRI performed one-month post-RF ablation (b) shows that RF ablation was a technical success. On post-gadolinium imaging there is ablation right up to the hepatic vein (white arrow). Diffusion-weighted imaging performed two years later (c) shows an area of abnormal high signal intensity (black arrow) with corresponding abnormal enhancement post-gadolinium.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
Funding Information
This project is funded by a GE sponsored CHAR grant.
References
- 1.American Cancer Society. Colorectal cancer facts & figures 2011–2013. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 2.Cauchy F, Faivre S, Belghiti J. Surgical results after downstaging of initially marginal or non-resectable liver metastases. Dig Dis. 2012;30:2–14. doi: 10.1159/000342048. http://dx.doi.org/10.1159/000342048. [DOI] [PubMed] [Google Scholar]
- 3.King A, Breen D. Understanding the current status of image-guided ablation for metastatic colorectal disease. Abdom Imaging. 2013;38:1234–1244. doi: 10.1007/s00261-013-0020-x. http://dx.doi.org/10.1007/s00261-013-0020-x. [DOI] [PubMed] [Google Scholar]
- 4.Adam R, Vinet E. Regional treatment of metastasis: surgery of colorectal liver metastases. Ann Oncol. 2004;15:iv103–106. doi: 10.1093/annonc/mdh912. [DOI] [PubMed] [Google Scholar]
- 5.Evans J. Ablative and catheter-delivered therapies for colorectal liver metastases (CRLM) Eur J Surg Oncol. 2007;33(Suppl 2):S64–75. doi: 10.1016/j.ejso.2007.09.027. http://dx.doi.org/10.1016/j.ejso.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg N, Grassi C, Cardella J, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S377–390. doi: 10.1016/j.jvir.2009.04.011. http://dx.doi.org/10.1016/j.jvir.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Bageacu S, Kaczmarek D, Lacroix M, Dubois J, Forest J, Porcheron J. Cryosurgery for resectable and unresectable hepatic metastases from colorectal cancer. Eur J Surg Oncol. 2007;33:590–596. doi: 10.1016/j.ejso.2007.01.003. http://dx.doi.org/10.1016/j.ejso.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Sofocleous CT, Petre EN, Gonen M, et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J Vasc Interv Radiol. 2011;22:755–761. doi: 10.1016/j.jvir.2011.01.451. http://dx.doi.org/10.1016/j.jvir.2011.01.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol. 2005;23:1358–1364. doi: 10.1200/JCO.2005.12.039. http://dx.doi.org/10.1200/JCO.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 10.Gillams AR, Lees WR. Radio-frequency ablation of colorectal liver metastases in 167 patients. Eur Radiol. 2004;14:2261–2267. doi: 10.1007/s00330-004-2416-z. http://dx.doi.org/10.1007/s00330-004-2416-z. [DOI] [PubMed] [Google Scholar]
- 11.Gleisner AL, Choti MA, Assumpcao L, Nathan H, Schulick RD, Pawlik TM. Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg. 2008;143:1204–1212. doi: 10.1001/archsurg.143.12.1204. http://dx.doi.org/10.1001/archsurg.143.12.1204. [DOI] [PubMed] [Google Scholar]
- 12.Cui Y, Li H, Wu Q, et al. Treatment of colorectal cancer with unresectable synchronous liver-only metastases with combined therapeutic modalities. J Gastrointest Surg. 2011;15:285–293. doi: 10.1007/s11605-010-1357-x. http://dx.doi.org/10.1007/s11605-010-1357-x. [DOI] [PubMed] [Google Scholar]
- 13.Elias D, Baton O, Sideris L, et al. Hepatectomy plus intraoperative radiofrequency ablation and chemotherapy to treat technically unresectable multiple colorectal liver metastases. J Surg Oncol. 2005;90:36–42. doi: 10.1002/jso.20237. http://dx.doi.org/10.1002/jso.20237. [DOI] [PubMed] [Google Scholar]
- 14.Evrard S, Ménétrier-Caux C, Biota C, et al. Cytokines pattern after surgical radiofrequency ablation of liver colorectal metastases. Gastroenterol Clin Biol. 2007;31:141–145. doi: 10.1016/s0399-8320(07)89344-4. http://dx.doi.org/10.1016/S0399-8320(07)89344-4. [DOI] [PubMed] [Google Scholar]
- 15.Lu DS, Raman SS, Limanond P, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267–1274. doi: 10.1097/01.rvi.0000092666.72261.6b. http://dx.doi.org/10.1097/01.RVI.0000092666.72261.6B. [DOI] [PubMed] [Google Scholar]
- 16.Zorbas G, Samaras T. A study of the sink effect by blood vessels in radiofrequency ablation. Comput Biol Med. 2015;57:182–186. doi: 10.1016/j.compbiomed.2014.12.014. http://dx.doi.org/10.1016/j.compbiomed.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175. doi: 10.1007/s00270-012-0377-1. http://dx.doi.org/10.1007/s00270-012-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanis E, Nordlinger B, Mauer M, et al. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer. Eur J Cancer. 2014;50:912–919. doi: 10.1016/j.ejca.2013.12.008. http://dx.doi.org/10.1016/j.ejca.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 19.King A, Breen D. Understanding the current status of image-guided ablation for metastatic colorectal disease. Abdom Imaging. 2013;38:1234–1244. doi: 10.1007/s00261-013-0020-x. http://dx.doi.org/10.1007/s00261-013-0020-x. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—A 10-year update. Radiology. 2014;273:241–260. doi: 10.1148/radiol.14132958. http://dx.doi.org/10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frich L, Bjørnland K, Pettersen S, Clausen OP, Gladhaug IP. Increased activity of matrix metalloproteinase 2 and 9 after hepatic radiofrequency ablation. J Surg Res. 2006;135:297–304. doi: 10.1016/j.jss.2006.05.010. http://dx.doi.org/10.1016/j.jss.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Nikfarjam M, Muralidharan V, Christophi C. Altered growth patterns of colorectal liver metastases after thermal ablation. Surgery. 2006;139:73–81. doi: 10.1016/j.surg.2005.07.030. http://dx.doi.org/10.1016/j.surg.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 23.Kong J, Kong L, Kong J, et al. After insufficient radiofrequency ablation, tumor-associated endothelial cells exhibit enhanced angiogenesis and promote invasiveness of residual hepatocellular carcinoma. J Transl Med. 2012;10:230. doi: 10.1186/1479-5876-10-230. http://dx.doi.org/10.1186/1479-5876-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang N, Wang L, Chai ZT, et al. incomplete radiofrequency ablation enhances invasiveness and metastasis of residual cancer of hepatocellular carcinoma cell HCCLM3 via activating β-Catenin signaling. PLoS One. 2014;9:e115949. doi: 10.1371/journal.pone.0115949. http://dx.doi.org/10.1371/journal.pone.0115949. [DOI] [PMC free article] [PubMed] [Google Scholar]