Abstract
Sciatica may result from pathologies affecting the nerve both in its intraspinal and extraspinal course. In daily routine, the vast majority of cases are caused by herniation of the lumbar discs compressing the neural roots. Extraspinal causes of sciatic pain are usually underestimated and the imaging study may be completed after reporting the lumbar MRIs. However, early diagnosis of the exact etiology of sciatica is paramount for both relieving the symptoms and preventing any additional neurologic injury. In this pictorial assay, some relatively rare causes of sciatic neuralgia along the route of the sciatic nerve after leaving the sacral foramen will be displayed.
In daily clinical practice, it is not rare to encounter patients with neurogenic claudication emanating from the abnormalities of the lumbosacral spine, mostly secondary to disc diseases, compression osteophytes, or spinal canal stenosis (1). Another subgroup with radicular pain of sciatic nerve (SN) have nothing to do with lumbosacral spinal etiologies but may be affected by pathologies beyond the sacral foramen, along the nerve path before, through, and after the sciatic foramen.
This study will review the anatomy of the SN after the sacral foramen through deep gluteal space on magnetic resonance imaging (MRI), with pathologies either belonging to the nerve itself or tissues in its vicinity causing sciatic neuralgia in this route.
Anatomical landmarks
The SN is formed by the L4–S3 nerve roots leaving the sacral foramen, it lies antero-inferiorly to the sacroiliac joint and the piriformis muscle through the greater sciatic foramen (2). After the ischial spine, in the lesser sciatic foramen, the nerve extends between obturatorius internus muscle anteriorly and gluteus muscle posteriorly. At the level of the ischial tuberosity medially, the nerve can be identified in the subgluteal space, where the anterior border is formed by quadratus femoris and the posterior border by gluteus maximus muscles (3). Down to the posterior aspect of the thigh, SN travels between the biceps femoris and the adductor magnus (Fig. 1). Right above the knee level, it branches off into the common peroneal and tibial nerves.
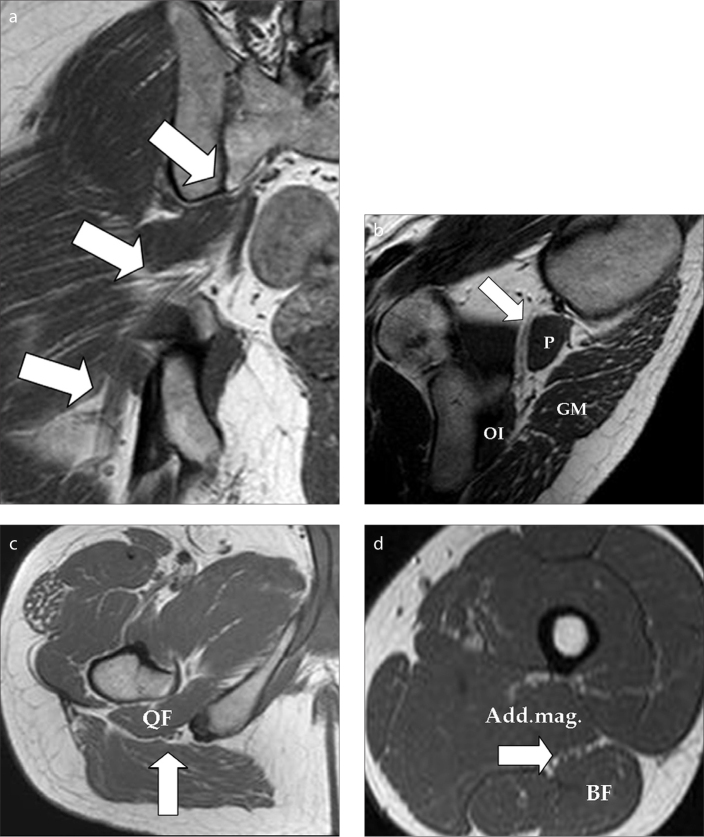
Figure 1.
a–d. Coronal T1-weighted image (a) shows the right-sided sciatic nerve (SN) leaving the sacral foramen and continuing inferolaterally through the pelvis and the sciatic foramina (arrows). Oblique-sagittal T1-weighted image (b) at the level of the greater and lesser sciatic foramina with piriformis (P) muscle the landmark of the greater sciatic foramina. The lesser sciatic foramen contains the obturator internus muscle (OI) anteriorly and gluteus maximus (GM) posteriorly. Note the extension of the SN anteriorly to piriformis muscle down to lesser sciatic foramen (arrow). Axial T1-weighted image (c) at the level of the infragluteal region shows the ischial tuberosity and the greater trochanter of the femur. The SN (arrow) extends between the quadratus femoris (QF) muscle and gluteus maximus muscle in this image. Axial T1-weighted image (d) at the mid level of the femur. The SN (arrow) lies in between the adductor magnus and biceps femoris (BF) muscles.
Clinical findings
Other than pain radiating from the lower back down to the leg and foot, patients may experience either deep gluteal syndrome findings or secondary findings of denervation in the territory of the SN. The most common symptoms of deep gluteal syndrome include: hip or buttock pain; tenderness in the gluteal and retro-trochanteric region; sciatica-like pain, often unilateral but sometimes bilateral, exacerbated with rotation of the hip in flexion and knee extension; intolerance of sitting more than approximately half an hour; and lumbago (3). Biceps femoris, semitendinosus, semimembranosus, ischiocondylar part of adductor magnus, and all muscles below the knee can display denervation findings, as these muscles are supplied by SN.
MRI technique
Magnetic resonance neurography protocols prescribed for the SN both on 1.5 T and 3.0 T systems have been reported before (1). Magnetic resonance neurography relies on high-resolution (2–3 mm section thickness) T1-weighted spin echo (SE)/T1-weighted fluid-attenuated inversion recovery images and T2-weighted fat-saturated SE/spectral-attenuated inversion recovery (SPAIR)/short-tau inversion recovery (STIR) images in multiple planes. While STIR works best at 1.5 T, SPAIR with varying flip angles is preferred in 3.0 T systems, owing to a higher signal-to-noise ratio compared with STIR images (4). High-resolution MRI techniques are of utmost help in demonstrating the fibrous bands around the SN, which may be the cause of diminished sciatic mobility during hip and knee movements with the precipitating cause of sciatic neuropathy (3). Three-dimensional sequences with the addition of STIR have been routinely used in clinical practice for many years.
As stated before, on fluid sensitive sequences, increased nerve signal intensity approaching to that of neighborhood vessels may indicate neuropathy. In addition, enlargement of the nerve and abnormal fascicules are further indicators for neuropathy. Denervation changes such as edema, fatty infiltration, and atrophy of the related muscles may accompany sciatic neuralgia and may even be considered the first imaging proofs of disease. However, absence of these secondary findings cannot totally exclude possible SN pathology.
Pathologies proximal to the sciatic foramen
Beyond the sacral foramen in its pelvic extension anterior to the sacroiliac joints, SN may be affected by any pelvic soft tissue and bone tumors (Fig. 2); hematoma (Fig. 3); abscess formation mainly in the setting of sacroiliitis, either secondary to nonspecific agents or specific infections such as brucellosis or tuberculosis (Fig. 4); or inflammation and osteoarthritic changes of the sacroiliac joints, either through compression, invasion, or encroachment of the nerve with these disease processes.
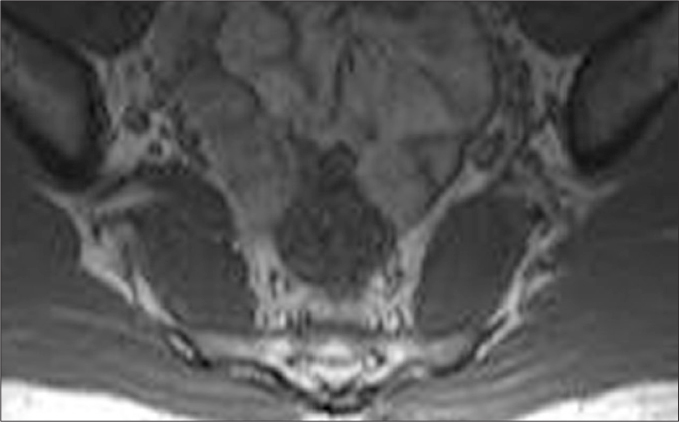
Figure 2.
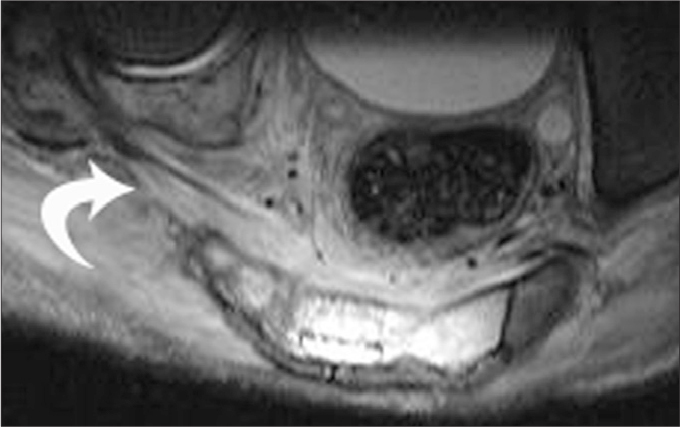
A 22-year-old male with lymphoma involving the sacrum and the right pelvic ring. The SN, in its extension in the pelvis through the sciatic foramen, is hard to identify in the soft tissue component/edema of the tumor as a separate structure at its expected location (arrow).
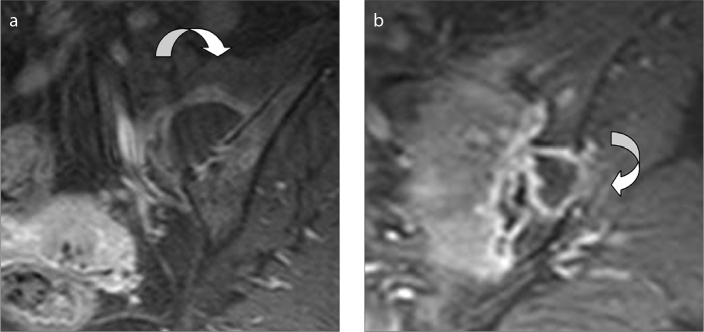
Figure 3.
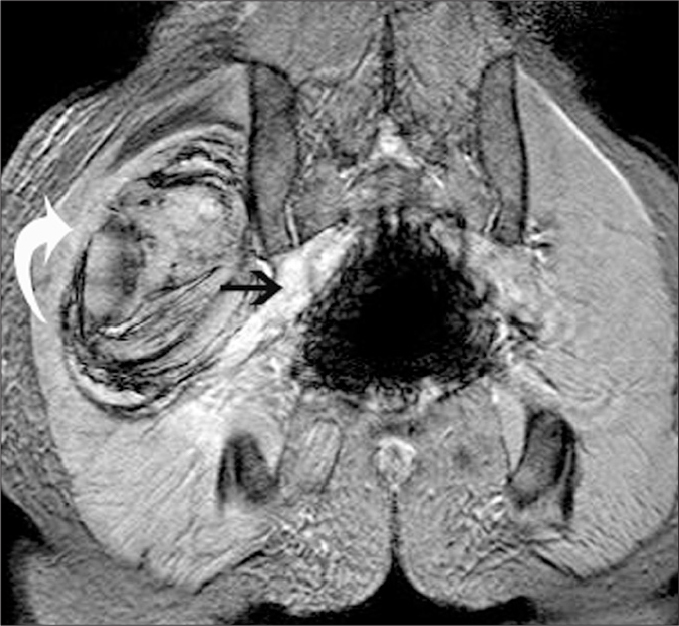
A 20-year-old male with acute myeloid leukemia, who developed a huge hematoma on the right gluteal region after a bone marrow biopsy. On coronal gradient recalled echo (GRE) T2-weighted image on the coronal plane, the hematoma (curved arrow) is seen in the close vicinity of the edematous, thickened SN (straight arrow).
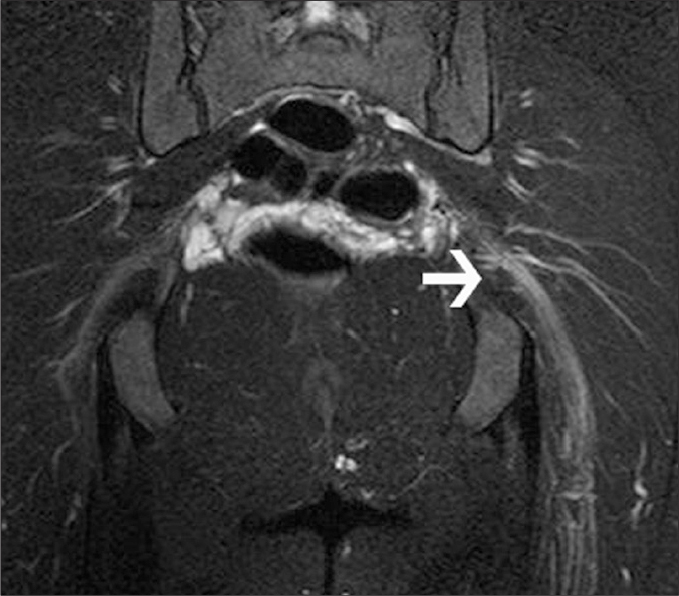
Figure 4.
a, b. A 51-year-old female with a history of epidural analgesia. Oblique-coronal fat-saturated T1-weighted contrast-enhanced (intravenous gadolinium-based agent) images show septic sacroiliitis on her left sacroiliac joint with multiple abscess formations (arrows) extending to presacral region (a) and posteriorly to gluteal muscles (b). The abscesses lying adjacent to the SN adds to her severe lower back pain. Bacterial
Piriformis syndrome
Piriformis syndrome is another cause of pain radiating from the sacrum through the gluteal area and down to the posterior aspect of the thigh, caused by an abnormal condition of the piriformis muscle. The possible causative factors for this syndrome are traumatic injury and reflex spasm of the muscle, which results in edema and contractures with subsequent compression and entrapment of the SN (5). Hypertrophy of the muscle, dynamic entrapment of the nerve by the muscle, and an anomalous course of the SN due to anatomical variations are the other etiologies for piriformis syndrome (3). As an adjunct to clinical diagnosis, MRI plays a role in revealing an enlarged or anomalous muscle with changes in the SN (5) (Fig. 5).
Figure 5.
A 32-year-old male referred to MRI for right hip pain. Unenhanced axial T1-weighted image shows an accessory piriform muscle; piriformis syndrome was potentially caused by nerve compression during prolonged sitting, walking, and running.
Pathologies through the sciatic foramen
Trauma neuropathy
Pilates is a technique that aims to improve the core strength and flexibility through six basic principles of balance, concentration, control, precision, breathing, and flow. It is reported that when practiced correctly, some Pilates exercises can relieve sciatic pain (6). Although it is unlikely that Pilates may cause sciatica, certain exercises might exacerbate a preexisting sciatic condition (7). The female case displayed in Fig. 6 presented with pain on her left buttock, and had a history of Pilates exercises she had been performing on her own. The SN shows hyperintensity in a short segment, which is found compatible with neuropraxia with the given history. Neuropraxia is the lowest degree of damage of the myelin sheath surrounding the axon causing transient functional loss, with an excellent prognosis. On MRI, neuropraxia is seen as mild thickening of the nerve with hyperintensity on fluid sensitive sequences.
Figure 6.
A 38-year-old woman with pain on her left gluteal region extending down to the thigh, with a history of Pilates exercises. Duration of exercise, workout routine, and preexisting etiology for sciatica were not clear on her reports. On fat-saturated T2-weighted coronal image, the SN displays hyperintensity in a short segment in the greater sciatic foramen (arrow), which was found to be compatible with neuropraxia according to the given history.
Postinjection sciatic neuropathy
Postinjection sciatic neuropathy is a disorder caused by an improper technique of injection or the introduction of a medicinal agent into the gluteal region. Either a direct puncture of the SN or an adjacent blood vessel by an injection needle or compression of the nerve by a hematoma can be the cause of the injury. The spectrum of neurologic presentation ranges from minor transient pain to severe sensory disturbance and even motor loss. Early recognition of the nerve injection injury and appropriate management via drug treatment aiming pain relief, physiotherapy, and surgical exploration are needed to prevent neurologic deficits. On T2-weighted images hyperintense intraneural lesion, entirely or partially involving nerve fascicles, is reported as the main imaging criterion (Fig. 7) (8).
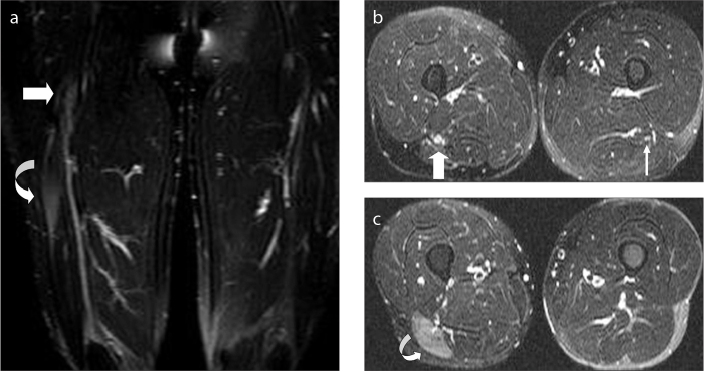
Figure 7.
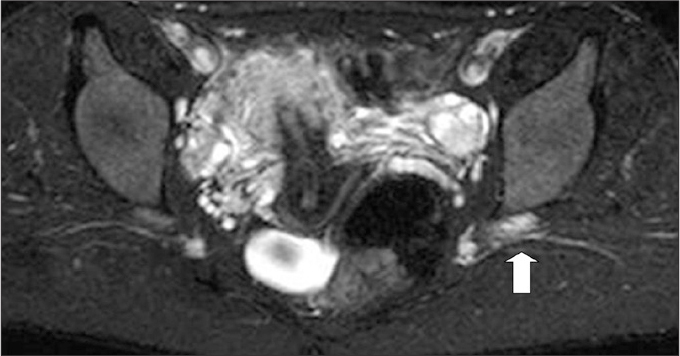
A 26-year-old female with a history of intramuscular nonsteroidal antiinflammatory drug injection to her left dorso-gluteal region in a primary healthcare unit six months ago, for her back pain. During the injection she documented severe pain and numbness on her left leg. On admission to MRI, she was using a brace for her foot drop. On axial fat-saturated T2-weighted image through the greater sciatic foramen, SN segment is hyperintense (arrow) compared with the right side.
Pathologies distal to the sciatic foramen
Paradoxic hypertrophy
Paradoxic SN hypertrophy may occur after a lower limb amputation performed for both malignant and nonmalignant conditions. Hypertrophy is reported to be greatest near the transection site extending proximally. It is termed paradoxical because usually transection of the limb is followed by atrophy of the nerve instead of hypertrophy. The etiology remains unclear; however, some propose that it may be secondary to dysregulated axonal neurofilament transport (9). Differentiating this benign process from a residual or locally recurrent tumor may prevent unnecessary biopsies (Fig. 8) (9).
Figure 8.
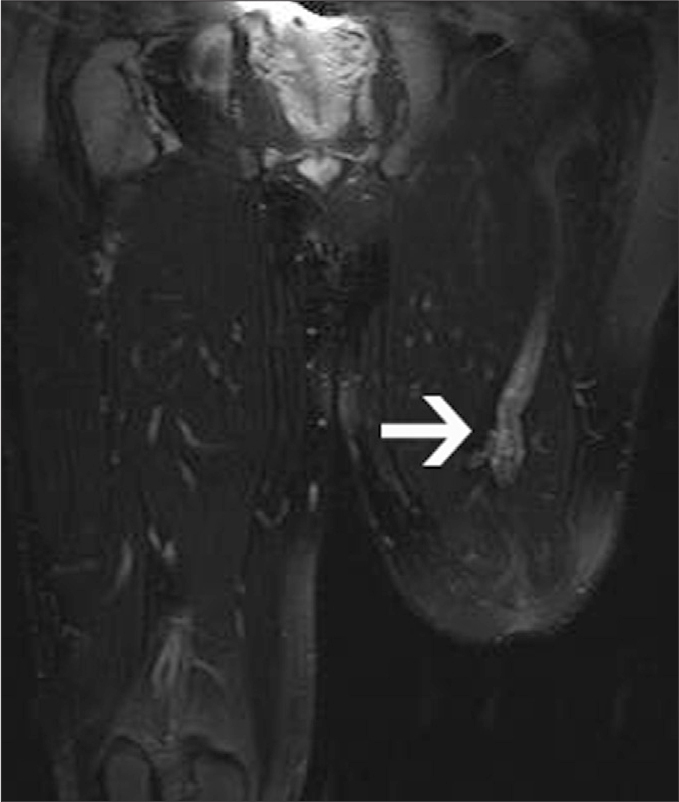
A 33-year-old man with a left above-knee amputation referred to MRI for the evaluation of his stump pain, which has been hindering him from using his prostheses. Coronal fat-saturated T2-weighted image of the lower extremities shows prominent enlargement of the SN, which is more prominent at the end of the nerve as detected by higher signal intensity, compatible with paradoxic hypertrophy (arrow).
Posttraumatic neuroma
Neuroma is a non-neoplastic proliferation at the end of an injured nerve, usually 1–12 months following the amputation. Two types of postamputation neuromas are encountered: terminal neuroma (end-bulb), which originates at the end of the injured nerve, represents a normal healing pattern of the nerve and is often asymptomatic; while, spindle neuroma (neuroma-in continuity) is localized within the nerve, away from the injured nerve ending. While the first pathology occurs in a completely transected nerve, which is not in apposition with the distal nerve, the latter displays a fusiform thickening of the nerve due to injury or chronic friction of the intact nerve (Fig. 9) (10). Patients cannot always point the pain when there is neuroma, making it difficult to distinguish from phantom limb pain. Soft tissue mass and pain generated by percussion on stump are among the possible clinical findings. Pain relief after injection of lidocaine to the painful area may help confirm the diagnosis.
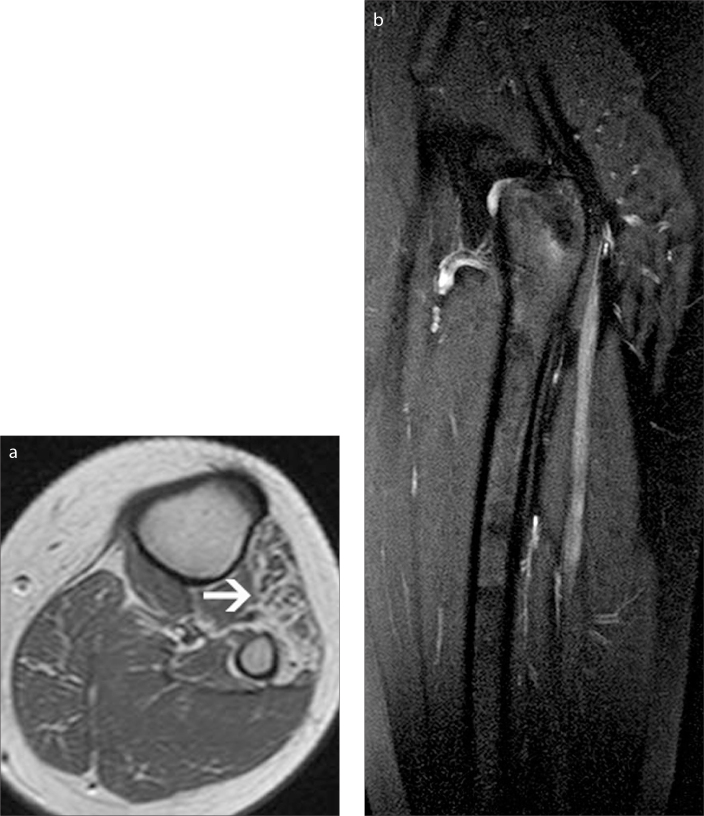
Figure 9.
a–c. A 26-year-old male with a history of sharp cut wound to his right thigh, who presented with pain and plantar flexion weakness. Coronal (a) and axial (b, c) fat-saturated T2-weighted images of both thighs show fusiform enlargement of the SN (thick arrows) with nerve continuity about the level of the previous trauma (a, b). The axial plane on panel (b) corresponds to the level where the fusiform enlargement of the nerve is shown on panel (a). Compare the thickened right-sided SN with the normal left side (thin arrow) (b). Denervation edema (curved arrow) in the long head of the biceps femoris muscle (a, c) is seen with diffuse hyperintensity in the muscle.
Localized hypertrophy
Localized hypertrophic neuropathy of the SN in children is a rare condition, presenting with entrapment neuropathy in the territory muscles of the SN (11). The findings can be summarized as i) segmental, focal enlargement of the SN; ii) increased hyperintensity of the effected nerve segment on T2-weighted images; iii) “salt and pepper” appearance of the segment indicating the preservation of fascicular configuration; and iv) possible enhancement after intravenous gadolinium administration (Fig. 10) (11). Pathology of the swollen nerve segment reveals onion-bulb like formations of perineural cells giving the nomenclature of localized hypertrophic neuropathy. This condition should be among the differential diagnoses for several etiologies of monomelic amyotrophy.
Figure 10.
a, b. An 11-year-old boy with a complaint of weakness of the ankle dorsiflexion and evertion, which yielded a peroneal nerve compromise. Axial T1-weighted image (a) of the knee shows fatty atrophy indicating chronic denervation of the lateral compartment muscles (arrow). As the common peroneal nerve was extending uneventfully, MRI of the SN was recommended. On sagittal fat-saturated T2-weighted image (b), the SN is thickened with preservation of the fascicles seen as tiny hypointense dots on axial fat saturated T2-weighted images (not shown). Findings are compatible with localized hypertrophy of the SN in this case.
Entrapment secondary to vascular prominence
Vascular lesions such as venous angioma, arteriovenous malformation, venous malformations in the setting of Klippel-Trenaunay syndrome, and capillary hemangioma in the vicinity of the SN may be the cause of sciatica (12). In the case presented in Fig. 11, the dilated vascular segment may cause pressure over SN, particularly in certain body positions.
Figure 11.
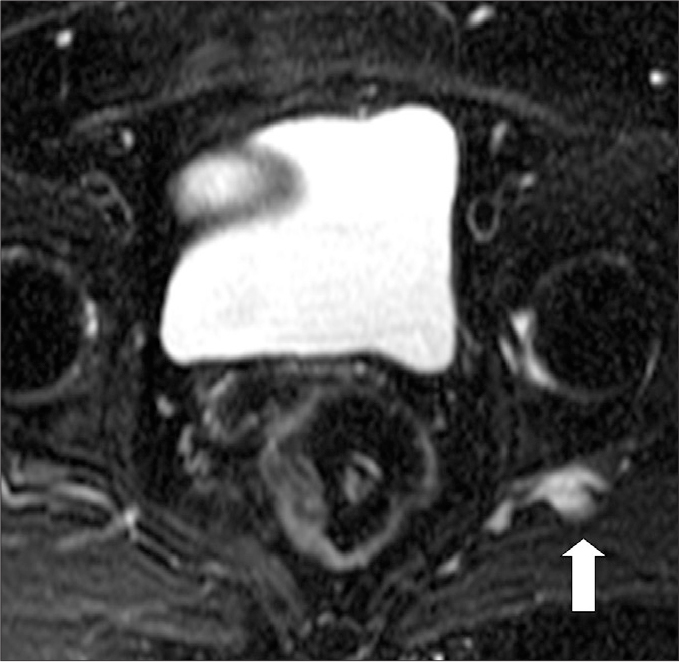
A 64-year-old female with left-sided buttock pain. Axial fat-saturated T2-weighted image shows an enlarged vessel (arrow) at the posterior aspect of the SN, which may be exerting pressure over the nerve.
Bony trauma
Although the frequently associated posterior luxation of the femoral head plays a more significant role in the SN injuries, acetabular fractures may also cause damage to the nerve. SN injury in the setting of acetabular fractures may result from i) initial direct damage; ii) during reconstructive surgery with intraoperative positioning and the usage of several instruments; or iii) as a late complication of surgery including wear debris, implant migration, hematoma, scarring, and heterotopic ossification. Patients with hip injuries and acetabular fractures should be evaluated for possible SN injuries. On MRI, muscular enlargement, edema, or scarring around the SN can be seen as a representation of the nerve injury (Fig. 12) (13).
Figure 12.
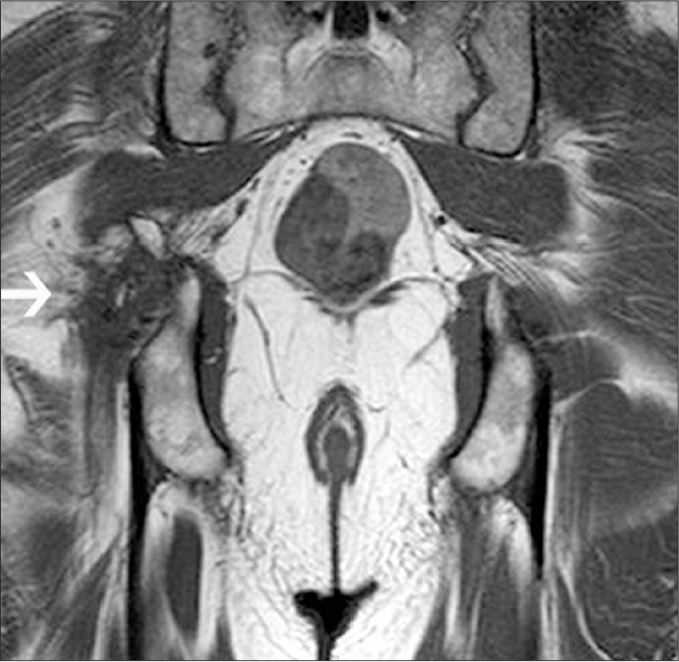
Coronal T1-weighted image shows trauma to the SN and possibly fibrosis around the nerve (arrow) in the chronic stage caused by acetabular fracture in a male. Compare the findings with the left-sided normal SN.
Benign peripheral nerve sheath tumor
Schwannoma of the SN may originate at any level of the nerve from pelvis to thigh. These rare tumors of SN arise from the sheath of the nerve. Since schwannomas do not penetrate the nerve fascicles, their enucleation is possible, preserving nerve continuity. On the other hand, neurofibromas deeply affect the nerve and thus require complete resection. Differential diagnosis between schwannoma and neurofibroma on MRI may help the surgeon to foresee the extension of his operation and its consequences. Classically, the nerve lies eccentric to the mass in schwannoma, but central or obliterated by the mass in neurofibroma (14). The “target sign”, low signal intensity centrally and high signal intensity peripherally at T2-weighted images, is more frequent in neurofibromas, although it may be seen in schwannomas as well. While schwannoma has a true capsule composed of epineurium, neurofibromas are rarely encapsulated (Fig. 13) (14).
Figure 13.
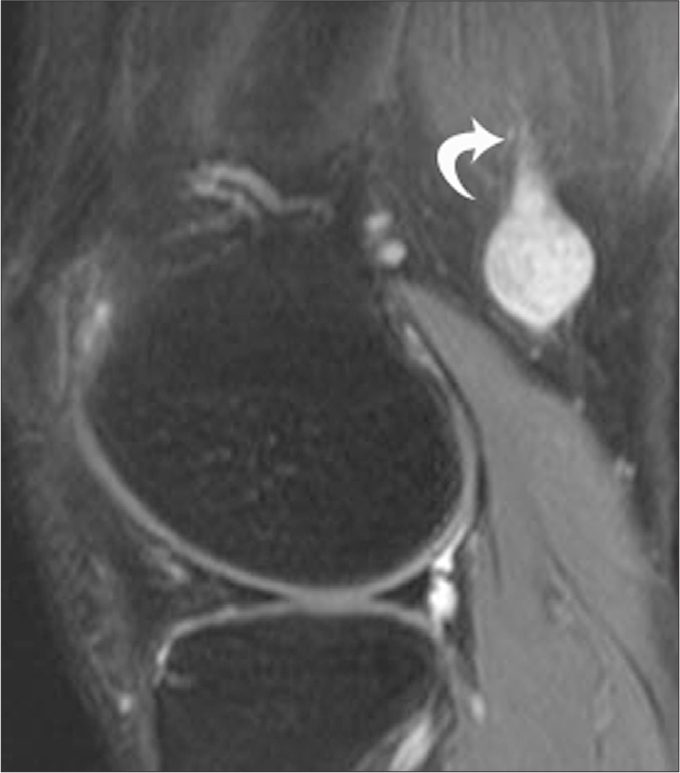
A 47-year-old male with schwannoma. Sagital fat saturated T2-weighted image shows a well-defined hyperintense mass in the posterior supracondylar region of the knee, with the SN entering the mass (curved arrow).
Malignant peripheral nerve sheath tumor
Malignant peripheral nerve sheath tumor (MPNST) is uncommon and can be associated with neurofibromatosis type I. A comparison of 41 MPNST and 20 neurofibroma cases revealed four significant features useful for distinguishing between MPNST and neurofibromas: i) increased largest dimension of the mass; ii) peripheral enhancement; iii) perilesional edema-like zone; and iv) intratumoral cystic lesion (Fig. 14) (15).
Figure 14.
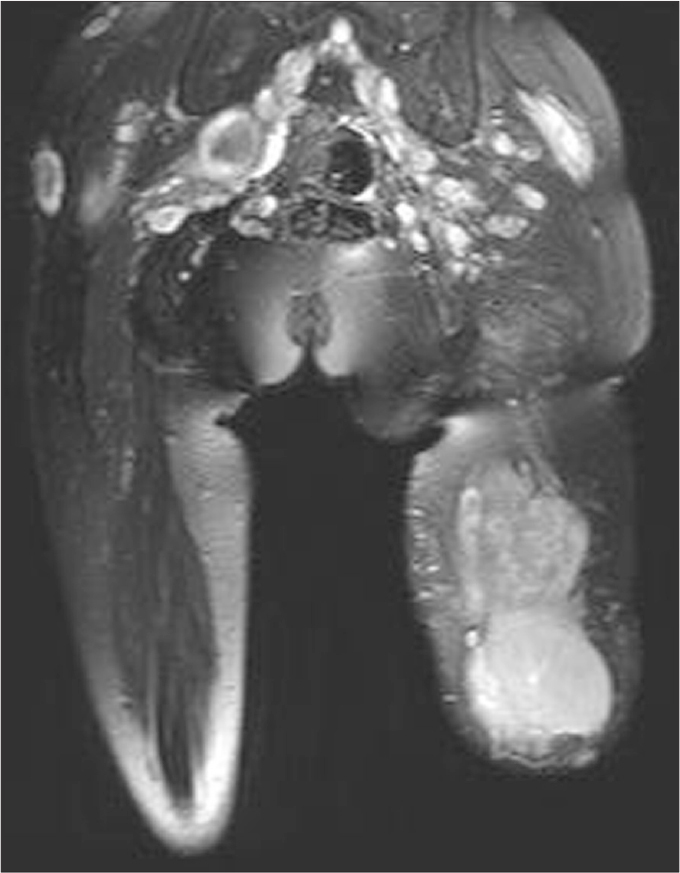
Giant malignant nerve sheath tumor of the SN on the left amputated lower extremity of a 28-year-old female patient with neurofibromatosis type 1. Multiple neurofibromas are evident with their grape-like, saturated T2-weighted image.
Conclusion
Patients with SN pathologies may refer to MRI either with secondary findings of denervation in the related muscles or radicular pain in the territory of the nerve. If the MRI of the lumbosacral spine is not satisfactory to explain the present clinical setting, a dedicated SN MRI examination may be needed. On these images, SN should be tracked at those certain anatomical landmarks, and any change in signal intensity, thickness, fascicular arrangement should be evaluated. The presence of any adjacent bone and soft tissue pathologies should be investigated for possible adverse effect on the SN.
Main points.
Sciatic nerve (SN) pathologies may present with pain radiating from lower back down to the leg and foot, deep gluteal syndrome clinics, or secondary findings of denervation in the territory of the nerve.
SN pathologies can be recognized either by the changes of the nerve itself such as increased signal intensity on fluid sensitive sequences, enlargement, and abnormal fasciculations, or by denervation changes in the related muscles such as edema, fatty infiltration, and atrophy.
Distal to the sacral foramen, anatomical landmarks in the course of the SN such as the greater sciatic foramen (antero-inferiorly the sacroiliac joint and the piriformis muscle), the lesser sciatic foramen (obturatorius internus muscle anteriorly and gluteus muscle posteriorly), and the subgluteal space (quadratus femoris anteriorly and gluteus maximus muscle posteriorly) may enhance the understanding of different pathologies of the nerve.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Moore KR, Tsuruda JS, Dailey AT. The value of MR neurography for evaluating extraspinal neuropathic leg pain: a pictorial essay. Am J Neuroradiol AJNR. 2001;22:786–794. [PMC free article] [PubMed] [Google Scholar]
- 2.Ergun T, Lakadamyalı H. CT and MRI in the evaluation of extraspinal sciatica. Br J Radiol. 2010;83:791–803. doi: 10.1259/bjr/76002141. http://dx.doi.org/10.1259/bjr/76002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernando MF, Cerezal L, Carro P, Abascal F, Canga A. Deep gluteal syndrome: anatomy, imaging, and management of sciatic nerve entrapments in the subgluteal space. Skeletal Radiol. 2015;44:919–934. doi: 10.1007/s00256-015-2124-6. http://dx.doi.org/10.1007/s00256-015-2124-6. [DOI] [PubMed] [Google Scholar]
- 4.Thawait SK, Chaudhry V, Thawait GK, et al. High-resolution MR neurography of diffuse peripheral nerve lesions. AJNR Am J Neuroradiol. 2011;32:1365–1372. doi: 10.3174/ajnr.A2257. http://dx.doi.org/10.3174/ajnr.A2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee EY, Margherita AJ, Gierada DS, Narra VR. MRI of piriformis syndrome. AJR Am J Roentgenol. 2004;183:63–64. doi: 10.2214/ajr.183.1.1830063. http://dx.doi.org/10.2214/ajr.183.1.1830063. [DOI] [PubMed] [Google Scholar]
- 6.Caceres MF. Rehabilitation of the sciatic nervein Pilates. Austin, Tx: Aug 17, 2014. pp. 1–11. [Google Scholar]
- 7.Mercer L. Is Pilates good for sciatica? Available at: http://livewell.jillianmichaels.com/pilates-good-sciatica-4723.html.
- 8.Pham M, Wessig C, Brinkhoff J, Reiners K, Stoll G, Bendszus M. MR neuroragraphy of sciatic nerveinjection injury. J Neurol. 2011;258:1120–1125. doi: 10.1007/s00415-010-5895-7. http://dx.doi.org/10.1007/s00415-010-5895-7. [DOI] [PubMed] [Google Scholar]
- 9.Ma M, Guillerman RP. Paradoxic hypertrophy of the sciatic nerve. Pediatr Radiol. 2010;40:S177. doi: 10.1007/s00247-010-1867-4. http://dx.doi.org/10.1007/s00247-010-1867-4. [DOI] [PubMed] [Google Scholar]
- 10.Narayanan S, Sarawagi R. Teaching NeuroImages: Posttraumatic neuroma-in-continuity of the right tibial nerve. Neurology. 2014;82:e198–e199. doi: 10.1212/WNL.0000000000000472. http://dx.doi.org/10.1212/WNL.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 11.Roux A, Tréquier C, Bruneau B, et al. Localized hypertrophic neuropathy of the sciatic nervein children: MRI findings. Pediatr Radiol. 2012;42:952–958. doi: 10.1007/s00247-012-2418-y. http://dx.doi.org/10.1007/s00247-012-2418-y. [DOI] [PubMed] [Google Scholar]
- 12.Gompel JJV, Griessenauer CJ, Scheithauer BW, Amrami KK, Spinner RJ. Vascular malformations, rare causes of sciatic neuropathy: a case series. Neurosurgery. 2010;67:1133–1142. doi: 10.1227/NEU.0b013e3181ecc84e. http://dx.doi.org/10.1227/NEU.0b013e3181ecc84e. [DOI] [PubMed] [Google Scholar]
- 13.Issack PS, Helfet DL. Sciatic nerve injury associated with acetabular fractures. HSS J. 2009;5:12–18. doi: 10.1007/s11420-008-9099-y. http://dx.doi.org/10.1007/s11420-008-9099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaman FD, Kransdorf MJ, Menke DM. Schwannoma: radiologic-pathologic correlation. Radiographics. 2004;24:1477–1481. doi: 10.1148/rg.245045001. http://dx.doi.org/10.1148/rg.245045001. [DOI] [PubMed] [Google Scholar]
- 15.Wasa J, Nishida Y, Tsukushi S, et al. MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas. AJR Am J Roentgenol. 2010;194:1568–1574. doi: 10.2214/AJR.09.2724. http://dx.doi.org/10.2214/AJR.09.2724. [DOI] [PubMed] [Google Scholar]