Abstract
Reproductive division of labour is a hallmark of eusociality, but disentangling the underlying proximate mechanisms can be challenging. In bumblebees, workers isolated from the queen can activate their ovaries and lay haploid, male eggs. We investigated if volatile, contact, visual or behavioural cues produced by the queen or brood mediate reproductive dominance in Bombus impatiens. Exposure to queen-produced volatiles, brood-produced volatiles and direct contact with pupae did not reduce worker ovary activation; only direct contact with the queen could reduce ovary activation. We evaluated behaviour, physiology and gene expression patterns in workers that were reared in chambers with all stages of brood and a free queen, caged queen (where workers could contact the queen, but the queen was unable to initiate interactions) or no queen. Workers housed with a caged queen or no queen fully activated their ovaries, whereas ovary activation in workers housed with a free queen was completely inhibited. The caged queen marginally reduced worker aggression and expression of an aggression-associated gene relative to queenless workers. Thus, queen-initiated behavioural interactions appear necessary to establish reproductive dominance. Queen-produced chemical cues may function secondarily in a context-specific manner to augment behavioural cues, as reliable or honest signal.
Keywords: chemical communication, pheromone, behaviour, altruism, eusocial, division of labour
1. Background
In colonies of eusocial insects, one or a small number of females (queens) dominate the reproduction of the group, whereas the other females (workers) remain (mostly) sterile [1]. Proximate mechanisms that regulate this reproductive dominance include aggression and pheromones [2–4]. It has been hypothesized that aggression originally mediated this conflict in the ancestral state, and the subsequent evolution of queen pheromones allowed for the development of larger, more harmonious social groups [5]. Although it has not been extensively studied, there is some evidence for the transition from aggression to pheromone signalling in social insects [6–9].
In primitively eusocial insects, such as bumblebees, it has been suggested that reproductive division of labour is regulated by a combination of chemical and behavioural cues [10,11]. In Bombus terrestris bumblebees, queens dominate reproduction early in the colony cycle, but near the end of the colony cycle workers activate their ovaries and compete with the queen and each other over the production of haploid males (reviewed in [11]). Furthermore, under queenless conditions, workers form a dominance hierarchy [12] where one (or a few) workers exhibit the majority of the aggressive behaviours, have the highest ovarian activation levels [13] and can inhibit reproduction in subordinate workers [14]. The mandibular gland was initially thought to produce a putative pheromone [15,16], but subsequent studies demonstrated that extracts of queen mandibular glands had no inhibitory effect on biosynthesis of the gonadotropin juvenile hormone in workers [17]. Full body queen extracts did decrease juvenile hormone biosynthesis in workers after three days of exposure but did not impact worker aggression, and ovary activation/egg-laying were not assessed [17]. Furthermore, worker reproduction was not inhibited if workers were separated from the queen by a double mesh screen, and thus volatile chemical cues do not function in this system [18]. Non-volatile compounds on the cuticle of queens and fertile workers were suggested to operate as a reliable fertility signal of their presence and reproductive status [19], and chemical cues from the nest wax affected worker reproduction [20].
Recent studies have suggested that the cuticular hydrocarbon pentacosane (C25) may play a role in inhibiting worker reproduction in B. terrestris, because workers in queenless colonies exposed to pentacosane exhibited significantly higher rates of ‘oocyte resorption’ [21] and had significantly fewer developing oocytes in their ovaries than workers in queenless colonies exposed to solvent controls [22]. However, exposure of queenless Bombus impatiens worker groups to pentacosane did not significantly impact ovary activation rates or terminal oocyte sizes after 10 days of exposure [23]. Furthermore, in this study [23], ‘oocyte resorption’ rates were positively correlated with the duration of egg-laying, suggesting this is not an appropriate parameter to quantify reproductive inhibition. Thus, current evidence suggests that queen-produced chemicals alone may only slightly reduce or delay worker reproduction in bumblebees overall. However, pheromone blends are often quite complex and exposure to single chemicals or extracts may not be sufficient to elicit an appropriate behaviour or physiological response.
Previous studies have suggested that B. impatiens, a North American bumblebee species, exhibits less aggression than B. terrestris in late-stage colonies and in queenless worker groups [24,25]. Comparisons of our behavioural analyses of these two species also support this hypothesis, though without a common garden experiment it is difficult to know if variation in behaviours are due to species-specific differences or differences in environment or observers (see electronic supplementary material, figure S1). Additionally, while ovary activation rates in small queenless worker groups appear to be similar in both species [23,26,27], rates in full-sized late-stage colonies are higher in B. terrestris (50–64%, see [27,28]) than in B. impatiens (9–11%, see [23,29]). Overall, these studies suggest that B. impatiens may rely more heavily on chemical communication to regulate the reproductive division of labour, making comparative studies between B. impatiens and B. terrestris an especially interesting system in which to investigate the transition from aggression to pheromone signalling.
Here, we investigated the role of different social cues in inhibiting worker reproduction in B. impatiens. First, we evaluated the effect of volatile and contact cues from the queen and pupae/brood on worker ovary activation and egg production (experiment 1). These studies demonstrated that direct contact with the queen was necessary to inhibit worker ovary activation. Next, we examined what aspect of ‘direct contact’ was most relevant: the ability of the workers to visually inspect the queen, the ability of the workers to contact the queen with their antennae (and therefore be exposed to possible contact pheromones), or the ability of the queen to freely behaviourally interact with the workers (experiment 2). For this, we reared the groups of B. impatiens workers with (i) an uncaged, free queen (ii) a caged queen and (iii) no queen. We examined the effects of these treatments on worker attraction to the queen, aggression among workers, ovary activation and gene expression patterns associated with reproductive dominance.
2. Methods
2.1. General bumblebee rearing
Colonies of B. impatiens were obtained from Koppert Biological Systems (Howell Michigan, USA). Colonies were approximately two weeks old (based on first worker emergence), with less than 30 workers each and all stages of brood. Colonies were maintained in nest-boxes at a constant temperature of 28–30°C and 40–50% humidity, and supplied ad libitum with a sugar solution and honeybee-collected pollen. Callow workers (less than 24 h) of approximately the same size were collected from queenright colonies and randomly assigned to different treatments. Callow workers were used, because there is no impact from combining workers from different colonies (they have not yet established colony-specific nest-mate recognition cues), thereby allowing us to increase the sample sizes [8,27]. For experiment 2, all workers were individually tagged to monitor their behaviours. All workers were flash frozen on dry ice after 10 days of treatment and stored at −80°C until dissection.
2.2. Assessment of ovary activation and egg-laying
Ovaries were dissected under a stereomicroscope in double distilled water, and the largest terminal oocyte from three ovarioles (two from one ovary and one from the second ovary) was measured with an eyepiece micrometer. The mean of these oocytes for each bee was used as an index of ovary activation [8,13,23]. Newly laid eggs were counted immediately after workers were collected in queenless/caged queen groups, but not in queenright/‘free’ queen worker groups, because it would be impossible to differentiate worker-laid eggs from queen-laid eggs in these groups.
2.3. Worker–worker aggression
Discrete aggressive behaviours were recorded in 10 min intervals at approximately three time points (07.00, 11.00 and 15.00) on days 3 and 4 post-group establishment. Observations were performed on days 3 and 4, because aggression in B. terrestris workers was found previously to be highest on these days [13]. The behaviours were (i) attacking, where one bee physically contacts another bee aggressively, this may result in biting, pushing, dragging, struggling or attempted stinging; (ii) darting, where one bee makes a sudden and directed movement towards another bee without making actual contact; and (iii) humming, characterized by short bursts of wing vibrations directed at another bee. Behavioural information was collected for each individually tagged worker.
2.4. Expression levels of vitellogenin and Krüppel homologue 1
From each group of workers in experiment 2, trial 1, we selected the most behaviourally dominant worker based on the highest total number of aggressive behaviours she performed over the 60 min of observation time and examined gene expression patterns in the heads of these workers. We homogenized the heads of individual bees and extracted RNA (see electronic supplementary material). We identified the B. impatiens homologues for two candidate genes, vitellogenin (vg) and Krüppel-homologue 1 (Kr-h1), which have previously been shown to vary with reproductive dominance in B. terrestris [30,31], and two previously validated housekeeping genes [31,32], arginine kinase and phospholipase A2, using the NCBI database. Accession numbers, primer sequences and primer efficiencies are presented in the electronic supplementary material, table S1. Details on primer design, RNA extraction, cDNA synthesis and qRT-PCR can be found in the electronic supplementary material.
2.5. Experiment 1: effects of volatile chemicals and contact cues from queen and pupae/brood
We evaluated the oocyte size and egg-laying in workers exposed to queen's volatiles from either reduced (three workers and a queen) or full-sized colonies. All workers at the onset of the experiment were callow (less than 24 h), kept in sets of three, randomly selected from full-sized colonies, and were sampled at the age of 10 days.
A ‘reduced colony’ was created by placing a mated, laying queen from a full-sized colony (n = 8, each queen was taken from a different colony, each queen was only used once) into an airtight mason jar (16 oz, wide mouth) with three to five pupae from her colony, along with three callow workers. Reduced colonies were then attached to airtight jars containing three additional callow workers via PVC tubing (VWR, USA). Air was drawn from the callow groups at 200 µl s−1 using a laboratory air pump (Gast Manufacturing, Benton Harbor, MI) creating an air flow from the reduced colonies into these groups (figure 1). We employed a double tube system to prevent the buildup of pressure in the jars. As controls, groups of callow workers were exposed to the volatiles from three queenless workers and pupae from the same colony used to generate the ‘reduced colony’. Worker oocyte size was thus measured in 30 groups (workers in reduced colony: n = 8 groups; workers exposed to volatiles from reduced colony: n = 8 groups; workers with pupae: n = 7 groups; workers exposed to volatiles from workers with pupae: n = 7 groups). As noted above, the number of eggs laid was measured in queenless groups.
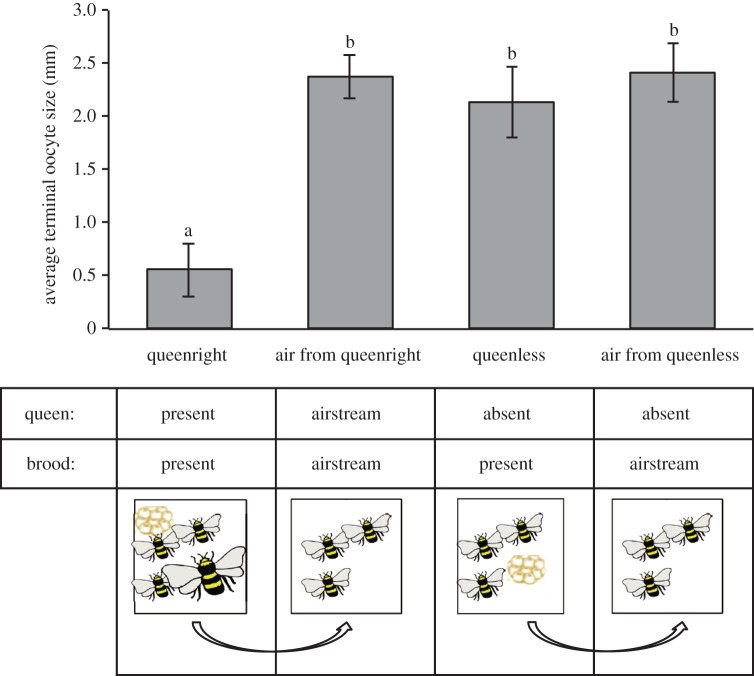
Figure 1.
The effect of volatile and contact cues from the queen and brood (pupae) on worker oocyte size. Worker oocytes in reduced colonies (workers + queen + pupae) are significantly smaller than worker oocytes from all other groups, and there are no significant differences observed among the other groups. Thus, only contact with the queen significantly reduces oocyte activation. Note that oocytes are considered ‘ready to lay’ at 2–3 mm [11]. Statistical analyses were performed using a mixed model (F3,20 = 22.5, p < 0.0001 followed by a post hoc Tukey test; different letters denote significant differences (p < 0.05). Data are presented as means ± standard errors.
Workers were exposed to full colonies, using the same experimental design as above. Treatment groups containing three callow workers received volatiles from full colonies (queen, more than 30 workers and brood in different stages) housed in brood boxes provided by Koppert (n = 12 groups). Control groups received volatiles from an empty brood box (n = 12 groups).
2.6. Experiment 2: evaluating the effects of visual, contact and behavioural cues from queens
We evaluated the oocyte size, behaviour and gene expression of workers exposed to visual, contact and behavioural cues from the queen. Groups of three callow workers were placed in wooden rearing chambers (21 × 21 × 12 cm) with brood at all stages of development. Workers were either grouped with an uncaged laying queen (free queen), a caged laying queen or left queenless (see below for information on replicates). The queen was caged over the brood and a pollen ball. The mesh cage was small enough that the workers could contact the queen through the mesh easily from all sides, regardless of the position of the queen. Workers were observed to regularly contact the queen through the mesh: we measured worker contact events with the queen during 10 min observation periods every day, and found workers contact the queen an average of 4.38 ± 0.09 times over a 10 min observation period during days 2, 3 and 4 post-worker introduction (data not shown).
We repeated this experiment twice with small modifications. For trial 1, the control queenless worker groups were supplied with an empty cage. In trial 2, to better determine the effect that a moving object within a cage may have, workers were housed with one caged worker. All workers at the onset of the experiment were callows (less than 24 h), randomly selected from full-sized colonies (trials 1: four colonies; trials 2: six colonies), and were sampled at the age of 10 days. Individual workers were also paint-marked to track individuals during behavioural observations.
Worker ovary activation and worker–worker aggression were measured in 19 groups for trial 1 (queenless workers: n = 7; caged queen: n = 6; uncaged queen: n = 6) and 19 groups for trial 2 (queenless workers: n = 7; caged queen: n = 6; uncaged queen: n = 6). Egg number was evaluated only in trial 2. Variation in gene expression levels associated with treatment was evaluated only in trial 1 (queenless workers: n = 6; caged queen: n = 6; uncaged queen: n = 6).
2.7. Statistical analyses
The data for all of the experiments are available in the electronic supplementary material, table S2. All statistical analyses were performed using JMP Pro v. 12.1. The oocyte size data were analysed using a mixed model with ‘treatment’ as fixed effect and ‘cage’ as random factor. We also used ‘brood identity’ as random factor but this was not significant (p = 0.93, 0.89 for experiment 1 and 2, respectively) and thus was not included in the model. ‘Queen identity’ also did not affect oocyte size (examined using a mixed model with ‘queen identity’ as fixed effect and ‘cage’ as random factor). Multiple comparisons between treatments were performed using a Tukey post hoc test. Prior to the analysis, data were log-transformed.
The effect of treatment on egg-laying was examined using a generalized linear mixed model (GLMM) with a Poisson distribution and log as link function (with overdispersion). ‘Queen and brood identity’ were included as random factors. Aggression was analysed using the same GLMM including ‘queen identity’ and ‘cage’ as random factors, with ‘treatment’ and ‘trial’ and the interaction between them as fixed effects. Pairwise comparisons were used in order to compare aggression in the different treatments. Gene expression data were first log-transformed and then analysed using a mixed model with ‘treatment’ as a fixed effect and ‘cage’ as a random factor, followed by a Tukey post hoc test. ‘Queen identity’ did not affect gene expression data and thus was not included in the model (examined using a mixed model with ‘queen identity’ as fixed effect and ‘cage’ as a random factor, p = 0.44 and 0.68 for vg and kr-h1, respectively).
Power for the oocyte size and egg count data was computed using R for two-sided tests with no multiple testing adjustment. Results are summarized in the electronic supplementary material, table S3. For oocyte size in experiment 1, power was computed using simulation. For oocyte size in experiment 2, power was based on the ANOVA of log(response). In studies of queen pheromone in honeybees, exposure to a synthetic blend resulted in 50% reduction in the rates of ovary activation [33], whereas studies in a stingless bee species found a greater than 50% decrease in ovary activation rates in workers exposed to a queen extract [34]. For oocyte size, the power to detect a 50% reduction or increase in size was 100% for experiment 1 and 96.2% in experiment 2. For a more modest 25% reduction, power was 60.5% and 43.3%, respectively, but the combined power over the two independent experiments is 77.8%. The power for the egg count data was 86.1% and 10.4%, respectively, for a 50% reduction or increase in count, giving a combined power over the two experiments of 87.5%.
3. Results
3.1. Experiment 1: effects of volatile chemicals and contact cues from queen and pupae/brood
There was a significant effect of treatment on worker oocyte size in reduced colonies (F3,20 = 22.5, p < 0.0001, figure 1). Workers within the reduced colony (which had direct contact with the queen) had significantly smaller terminal oocytes (0.56 ± 0.25 mm, p < 0.001 for all post hoc comparisons) than workers exposed to air from reduced colonies (2.37 ± 0.21 mm), workers in queenless groups containing pupae (2.41 ± 0.27 mm) or workers exposed to the air from these groups (2.14 ± 0.33 mm). Thus, in all groups where workers had no direct contact with the queen, worker ovaries were fully activated (p > 0.8, ‘ready to lay eggs’ are more than 2 mm), whereas the ovaries of workers that had direct contact with the queen were essentially inactive. Although there was a trend for worker groups exposed to air from reduced colonies to produce fewer eggs (16.4 ± 3.0 eggs, n = 7) compared with worker groups exposed to air from workers and pupae (21.6 ± 2.1 eggs, n = 8), egg production was highly variable and this difference was not significant (χ2 = 1.74, d.f. = 1, p = 0.19).
Next, we repeated the same experiment using three worker groups exposed to volatiles from full colonies (contained a queen and all stages of brood). Terminal oocyte size in workers exposed to volatiles from full colonies did not differ significantly from that of the control group, which consisted of workers exposed to volatiles from an empty rearing chamber, and workers from both treatments had fully activated ovaries (terminal oocyte size of 2.41 ± 0.57 mm versus 2.63 ± 0.087 mm, F1,2.6 = 0.42, p = 0.57). Differences in total eggs laid from these two groups were also not statistically significant (workers exposed to volatiles from full colonies: 18.3 ± 2.3 eggs; workers exposed to volatiles from an empty cage: 22.6 ± 3.3 eggs, χ2 = 1.20, d.f. = 1, p = 0.27).
3.2. Experiment 2: evaluating the effects of visual, contact and behavioural cues from queens
Worker oocyte size was examined in three treatment groups: (i) three workers grouped with a free, uncaged queen; (ii) three workers grouped with a caged queen; and (iii) three queenless control workers (exposed to an empty cage in trial 1 and to a caged worker in trial 2). All groups contained brood at all stages of development. Worker terminal oocyte size was significantly impacted by treatment (F2,32 = 8.45, p = 0.001), but not by trial (F1,32 = 1.68, p = 0.2) or their interactions (F2,32 = 0.15, p = 0.85; figure 2a). Workers housed with free queens had significantly smaller oocytes than workers housed with caged queens or no queens (p < 0.012), and there was no difference in oocyte size of workers housed with caged queens or queenless controls (p = 0.59). Only workers that had contact with a freely behaving queen had inactive ovaries, whereas workers under queenless conditions or housed with a caged queen had mature, ready-to-lay eggs (terminal oocyte size more than 2 mm) and fully activated ovaries. Note that, because all treatments contained brood at all stages of development, contact with brood was not sufficient to prevent workers from fully activating their ovaries.
Figure 2.
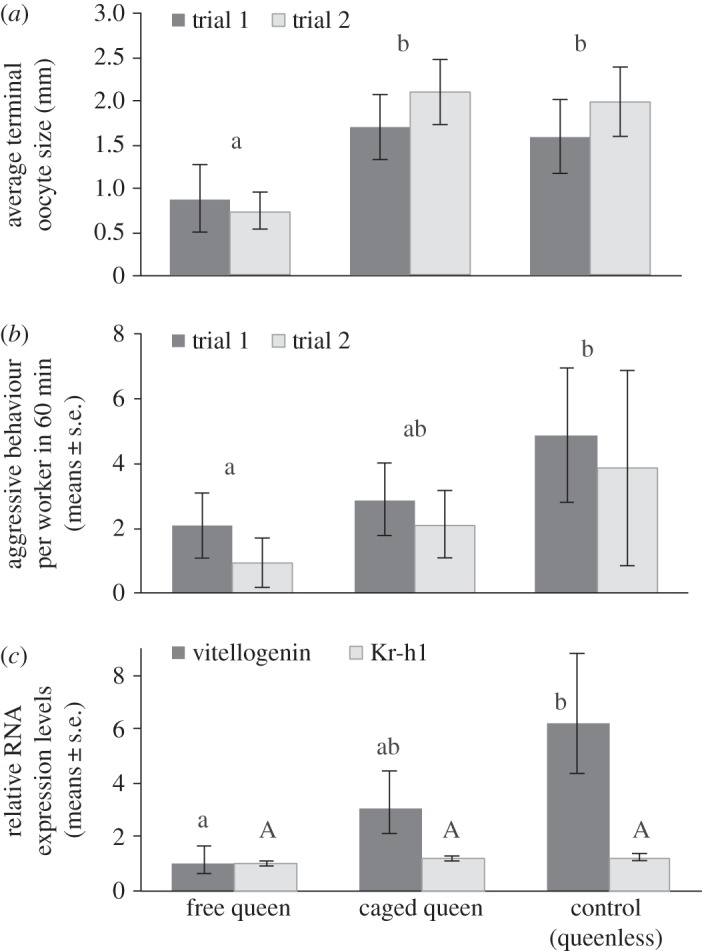
Evaluating the effects of visual, contact and behavioural cues from queens on workers. (a) There is a significant effect of treatment on worker oocyte size in both trials. Worker ovary activation is higher in the queenless and caged queen groups relative to the free queen groups, and there is no significant different between queenless workers and workers housed with the caged queen. Different letters denote significant differences (p < 0.05). Data are presented as means ± standard errors. (b) Aggressive behaviours are significantly higher in queenless worker groups compared with free queen groups, and caged queen groups are intermediate. Additive total of attacking, humming and darting are presented as means ± s.e. Different letters denote significant differences (p < 0.05). (c) Expression of vitellogenin is significantly higher in the heads of behaviourally dominant workers from the queenless controls compared with behaviourally dominant workers in the free queen groups, whereas workers from caged queen groups are intermediate. There is no effect of treatment on Kr-h1 expression. Different letters denote significant differences (p < 0.05); data are presented as means ± s.e.
There were no significant differences in egg production (measured in trial 2) between workers housed with a caged queen or queenless controls, though again there was a trend for fewer eggs laid by workers housed with a caged queen (queenless control: 10 ± 2.90 eggs, caged queen: 5.67 ± 2.95 eggs, χ2 = 1.05, d.f. = 1, p = 0.31).
Next, we evaluated worker–worker aggression among these groups. There were significant differences among the treatment groups (χ2 = 9.87, d.f. = 2, p = 0.007, figure 2b), but there was no trial effect (χ2 = 1.92, d.f. = 1, p = 0.17) or interaction (χ2 = 0.47, d.f. = 2, p = 0.79). Aggressive behaviours were significantly higher in queenless worker groups compared with the free queen groups, whereas caged queen groups were intermediate (multiple comparisons: queenless versus free: p = 0.002; queenless versus caged queen, p = 0.07, caged versus free, p = 0.19). Aggression between queens and workers was never observed.
In B. terrestris, expression levels of Krüppel homologue 1 and vitellogenin are linked to aggression and dominance. Expression of Kr-h1 is downregulated in workers in the presence of a queen or a dominant worker [30], and expression of vitellogenin is upregulated in aggressive and aggressed workers compared with passive workers [31]. Thus, Kr-h1 and vg are both sensitive to social context, making them appropriate candidate genes to investigate the social cues mediating the reproductive division of labour in bumblebees.
Relative mRNA expression levels of vg and Kr-h1 were analysed for the behaviourally dominant worker (identified based on aggression score) in each cage in each treatment group in trial 1 only. For vg, there was a significant effect of treatment (F2,15.5 = 4.37, p = 0.031, figure 2c), with expression levels significantly higher in queenless worker groups than free queen groups (p = 0.027), whereas caged queen groups were intermediate. There was no significant effect of treatment on Kr-h1 expression levels (F2,15.5 = 1.39, p = 0.28, figure 2c).
4. Discussion
Our results demonstrate that reproductive dominance of the B. impatiens queen is mediated by behavioural interactions with the queen, which possibly may be reinforced by visual, volatile or contact chemical cues which may serve to place the queen's behaviour in the correct colony context. Interaction with a freely behaving queen is necessary to inhibit ovary activation over a 10 day period: workers exposed to all other treatments had fully activated ovaries with mature, ‘ready-to-lay’ eggs. It is important to note that though our experiments used small groups of three workers, the presence of a freely interacting queen fully inhibited worker ovary activation in these groups, as it would in a full, pre-competition phase colony, and thus the mechanisms needed to reduce worker ovary activation were functional in these small group settings. Workers exposed to a caged queen—and her associated visual, volatile, and contact chemical cues—showed a non-significant trend (relative to queenless workers) of reduction in both aggression at 3–4 days post-group establishment and vitellogenin expression levels at 10 days post-group establishment. Furthermore, there was non-significant trend for egg production to be lower in workers exposed to queen volatiles and caged queens relative to queenless controls. Overall, these results suggest that chemical cues from the queen may result in a slight delay in the onset of worker reproduction, but are not sufficient to inhibit it (unlike a freely behaving queen) or even significantly delay it, even over a relatively short timescale of 10 days.
Studies in B. terrestris have also suggested that contact with a freely behaving queen is necessary to establish the queen's reproductive dominance in this species, though this can be modulated by colony environment (e.g. in post-competition colonies, workers will activate their ovaries, regardless of their contact with the queen). In B. terrestris, queen chemical extracts did not impact aggression [17] and exposure to the hydrocarbon pentacosane (which is found at higher levels in queens than workers, but is still found in workers) did not inhibit worker ovary activation [21], though it was associated with reduced numbers of visible oocytes in the ovaries of exposed workers [22]. Thus, in both B. impatiens and B. terrestris, queen-produced chemical cues seem to only marginally modulate the timing of worker dominance hierarchy establishment and/or worker egg-laying behaviour when compared with the effects of a freely behaving queen. A previous study in B. terrestris [18] used a queen excluder to limit the queen's movement in a confined compartment within the nest while at the same time presenting the workers (which could freely move between compartments) with the opportunity to evade the queen and reproduce. Although worker-laid eggs in the queen-excluded compartment occurred earlier than in the queenright compartment, it was not dramatically earlier, and much later compared with queenless workers. Furthermore, the amount of time the workers spent in the queenright versus queen-excluded compartment was similar and irrespective of ovary activation. These results suggest that the queen's behavioural and chemical cues do not actively and/or negatively inhibit worker ovary activation (if so, the workers would counteract these signals simply by avoiding the queen) but instead the cues allow the workers to adjust the timing of their ovary activation, so they initiate reproduction at the time when it is most beneficial to them. Thus, if any chemical cues produced by the queen operate in bumblebees to regulate worker reproduction, then they are likely to be context-dependent, and to provide workers with information about the queen presence and fecundity rather than to elicit a primer or releaser physiological response in workers. Overall, it suggests that these chemical cues in bumblebees are serving as reliable or honest signals (depending on whether their production is costly) of queen presence and fecundity rather than a pheromonal queen ‘control’ mechanism (see [35] for further discussion). Intriguingly, it may be that chemical signals in the colony serve not to inhibit ovary activation but rather to stimulate it and coordinate its timing with the colony cycle. Indeed, a recent study found that wax scent enables workers to time their reproduction by providing essential information concerning the social condition of the colony: queenright workers exposed to wax from the competition phase were more aggressive and more likely to compete over reproduction than queenright workers without wax or with wax from the early social phase [20].
Previous studies in B. impatiens found that aggression and oviposition were less likely to co-occur in workers housed with brood and workers housed without brood tended to be more aggressive and have higher ovarian development, suggesting the presence of a brood pheromone that inhibited worker reproduction [26,27]. In our study, we showed that volatiles from full-sized colonies (which included both queen and brood of all ages), the presence of pupae alone (experiment 1) or physical contact with brood of all ages (experiment 2) did not prevent workers from fully activating their ovaries. The differences between the studies could stem from the differences in the age of the examined workers (12 day old at the onset of the experiment in Sibbald & Plowright [27] and 1 day old in this study) and the aspect of reproduction that was tested (ovarian activation and egg-laying/aggression). Older workers are likely to have activated their ovaries prior to the onset of the experiment (Sibbald and Plowright kept their workers isolated for 12 days prior to pairing them for the experiment) and to modulate their egg-laying in the presence of brood. In accordance with this explanation, studies in B. terrestris did not find any effect of brood on the time for workers to initiate their first egg-laying [18] and brood presence did not alter behaviour or worker physiology in queenright groups consisting of 6 day old workers compared with empty-queenright control groups [20].
Interestingly, though vitellogenin is typically described as a ‘yolk protein’, in our studies vitellogenin expression closely tracked aggression, but not ovary activation. Vitellogenin RNA levels were intermediate in workers housed with a caged queen compared with workers housed with a free queen or no queen, despite the fact that these workers had fully activated ovaries. Previous studies have also found similar results in B. terrestris workers [31]: vitellogenin RNA levels were not associated with worker task or juvenile hormone titres (but see [36]), and were only partially associated with age, queen presence, caste and ovary activation. However, in B. terrestris workers, vitellogenin RNA levels were increased in worker bees experiencing high levels of aggression [31]. These results suggest that vitellogenin RNA levels in both B. terrestris and B. impatiens bumblebees are primarily associated with social aggression, though vitellogenin, as a protein, may still play a role in ovary activation. Note that in honeybees (Apis mellifera), vitellogenin RNA levels are strongly correlated with worker division of labour and juvenile hormone titres [37], though again there is little correlation with queen or worker reproductive state [38], indicating that vitellogenin's functions in social insects are likely much broader than a yolk protein. Kr-h1 expression levels did not differ significantly between groups, despite the significant differences in ovarian activation and aggression, which is in contrast to studies in B. terrestris, where Kr-h1 expression is reduced in the presence of the queen or dominant workers and positively correlated with juvenile hormone levels [30,36]. However, previous studies evaluated expression in brain and fat body, while we used whole heads, and thus further analysis is necessary to determine if these differences are due to species-level effects or tissue differences.
The factors that inhibit reproduction in workers exposed to a freely behaving B. impatiens queen remain to be determined. Aggressive interactions were not observed between workers and queens in our studies, but it may be that more subtle behaviours play a role or low-frequency aggression is sufficient to maintain dominance. It is likely that multiple signals (chemical, visual, behavioural) are used by workers to assess the presence of a fertile queen, with behaviour playing a major role. If the workers do indeed adjust their reproduction according to the cues they obtain from the queen or nest environment, then it will be interesting to determine if there is variation in their likelihood to activate their ovaries and what causes it—in honeybees, for example, worker reproductive potential is negatively correlated with cooperative interactions with the queen [39,40].
Thus, though there is a reduction in worker ovary activation rates in late-stage colonies (see [23,27–29]) and a reduction in aggression during the establishment of reproductive dominance hierarchies (see electronic supplementary material, figure S1 and [24,25]) in B. impatiens relative to B. terrestris, our results provide no indication that these species-level differences are due to an increased reliance on chemical signalling in B. impatiens, and thus some other factors must underpin the observed species-level differences. Indeed, in both species, the presence of an active, live queen that freely interacts with workers appears to be critical for fully inhibiting worker reproduction. Inhibition of worker reproduction appears to be driven primarily by behavioural interactions with the queen, though chemical signals may play a minor role in a context-dependent manner (e.g. they may augment to impact of the behavioural signals obtained from the queen, serve to marginally delay the onset of worker reproduction in the absence of these behavioural signals or allow workers to assess the stage of the colony cycle). However, there are considerable differences in the chemical profiles of sterile and reproductive queens and workers in B. terrestris [19,20,31,41] and B. impatiens (A.H. 2016, unpublished data), and recent studies demonstrated that chemical cues in the wax of post-competition colonies can actually trigger worker reproduction [20]. Thus, there is substantial chemical complexity in the bumblebee colonies, and it remains to be determined if and how these chemicals function in the social organization of bumblebee colonies.
Across species, the resolution of reproductive conflicts in social Hymenoptera appears to require an interplay between behavioural (e.g. aggression) and chemical signalling. It is generally accepted that in the more primitive clades or in species where colonies have small worker populations, aggression is the primary means for maintaining reproductive skew, but in more advanced species—particularly in species where colonies have a large number of workers—pheromonal signalling has replaced aggression. Although the shift to pheromonal regulation seems adaptive in that it replaces costly behaviour, it is also prone to cheating because the totipotent females have the same genome. Consequently, in most social Hymenoptera, workers can not only activate their ovaries, but also concomitantly start to produce the typical reproductively associated chemical signals or pheromones. Thus, it is maladaptive to totally abandon aggressive behaviour in order to maintain reproductive skew. An example of the tight interplay between aggression and pheromone signalling is demonstrated in the ponerine ant Dinoponera quadriceps [42–44]. The gamergate first gains its reproductive dominance by aggression, and after establishing reproductive dominance, these females are typified by producing elevated amounts of 9-hentriacotene. However, this pheromone seems insufficient, because, when challenged, the alpha ant not only aggresses the challenger but also marks her with another pheromone bouquet, emanating from Dufour's gland, which provokes other workers to aggress the challenger. Ants of the genus Diacamma possess a pair of thoracic appendages called gemma that produce a pheromone signalling reproductive dominance. Challengers in some of these species are neutralized a priori, because upon emergence they are aggressed by nest-mates that mutilate and remove their gemma [45,46]. This physical aggression effectively sterilizes the emerging worker and prevents it from becoming a challenger. Even in societies where the conflict was seemingly evolutionarily resolved (e.g. honeybees, see [47,48]), there is still interplay between pheromone production and aggressive behaviour. When rendered hopelessly queenless, workers start to activate ovaries and concomitantly produce the queen pheromone in their mandibular glands. These workers are recognized by rival workers that aggressively compete with them over reproductive dominance [49]. Therefore, it appears that in the race for reproductive dominance, aggression and chemical signalling are closely intertwined, and their relative importance seems to be idiosyncratic to the species.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Nate McCartney (Tumlinson Laboratory) for assistance in designing the equipment used in experiment 1. We also thank members of the Hefetz and Grozinger laboratories for helpful discussions and critical reading of earlier drafts of the manuscript.
Ethics
As insects, bumblebees are not subject to IUCAC approval. However, experiments were carefully designed to minimize any stress experienced by the bumblebees during the course of this study.
Data accessibility
All the datasets (oocyte size, egg number, behaviour, gene expression) used in this manuscript are presented in the electronic supplementary material, table S2. The data have also been deposited in Dryad (doi:10.5061/dryad.3p0b5) [50].
Authors' contributions
M.P. and E.A. carried out the experimental work and analysed the data. N.A. provided support for the statistical analyses. All authors participated in the conception and design of the experiment and drafting the manuscript. All authors gave final approval for publication.
Competing interests
The authors do not have any competing interests.
Funding
M. P. was supported by a Bunton-Waller Graduate Award from Penn State University. E.A. was supported by a Vaadia-BARD Postdoctoral Fellowship (FI-49113). This research was supported by funding from the United States–Israel Binational Science Foundation (grant no. 2013104) to C.M.G., N.A. and A.H.
References
- 1.Wilson E. 1971. The insect societies. Cambridge, MA: Belknap. [Google Scholar]
- 2.Ratnieks FLW, Foster KR, Wenseleers T. 2006. Conflict resolution in insect societies. Annu. Rev. Entomol. 51, 581–608. (doi:10.1146/annurev.ento.51.110104.151003) [DOI] [PubMed] [Google Scholar]
- 3.Fletcher D, Ross K. 1985. Regulation of reproduction in eusocial Hymenoptera. Annu. Rev. Entomol. 30, 319–343. (doi:10.1146/annurev.en.30.010185.001535) [Google Scholar]
- 4.Le Conte Y, Hefetz A. 2008. Primer pheromones in social Hymenoptera. Annu. Rev. Entomol. 53, 523–542. (doi:10.1146/annurev.ento.52.110405.091434) [DOI] [PubMed] [Google Scholar]
- 5.Kocher SD, Grozinger CM. 2011. Cooperation, conflict, and the evolution of queen pheromones. J. Chem. Ecol. 37, 1263–1275. (doi:10.1007/s10886-011-0036-z) [DOI] [PubMed] [Google Scholar]
- 6.Cuvillier-Hot V, Lenoir A, Crewe R, Malosse C, Peeters C. 2004. Fertility signalling and reproductive skew in queenless ants. Anim. Behav. 68, 1209–1219. (doi:10.1016/j.anbehav.2003.11.026) [Google Scholar]
- 7.Monnin T, Peeters C. 1999. Dominance hierarchy and reproductive conflicts among subordinates in a monogynous queenless ant. Behav. Ecol. 10, 323–332. (doi:10.1093/beheco/10.3.323) [Google Scholar]
- 8.Amsalem E, Hefetz A. 2010. The appeasement effect of sterility signaling in dominance contests among Bombus terrestris workers. Behav. Ecol. Sociobiol. 64, 1685–1694. (doi:10.1007/s00265-010-0982-4) [Google Scholar]
- 9.Toth AL, Tooker JF, Radhakrishnan S, Minard R, Henshaw MT, Grozinger CM. 2014. Shared genes related to aggression, rather than chemical communication, are associated with reproductive dominance in paper wasps (Polistes metricus). BMC Genomics 15, 75 (doi:10.1186/1471-2164-15-75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michener C. 1974. The social behavior of the bees: a comparitive study. Cambridge, MA: Harvard University Press. [Google Scholar]
- 11.Amsalem E, Grozinger C, Padilla M, Hefetz A. 2015. The physiological and genomic bases of bumble bee social behaviour. Adv. Insect Physiol. 48, 37–93. (doi:10.1016/bs.aiip.2015.01.001) [Google Scholar]
- 12.Doorn A, Van, Heringa J. 1986. The ontogeny of a dominance hierarchy in colonies of the bumblebee Bombus terestris (Hymenoptera, Apidae). Insect. Soc. 33, 3–25. (doi:10.1007/BF02224031) [Google Scholar]
- 13.Amsalem E, Hefetz A. 2011. The effect of group size on the interplay between dominance and reproduction in Bombus terrestris. PLoS ONE 6, e18238 (doi:10.1371/journal.pone.0018238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloch G, Hefetz A. 1999. Regulation of reproduction by dominant workers in bumblebee (Bombus terrestris) queenright colonies. Behav. Ecol. Sociobiol. 45, 125–135. (doi:10.1007/s002650050546) [Google Scholar]
- 15.van Honk C, Velthuis H, Röseler P, Malotaux M. 1980. The mandibular glands of Bombus terrestris queens as a source of queen pheromones. Entomol. Exp. Appl. 28, 191–198. (doi:10.1111/j.1570-7458.1980.tb03004.x) [Google Scholar]
- 16.Röseler P, Röseler I, van Honk C. 1981. Evidence for inhibition of corpora allata activity in workers of Bombus terrestris by a pheromone from the queen's mandibular glands. Experientia 37, 348–351. (doi:10.1007/BF01959856) [Google Scholar]
- 17.Bloch G, Hefetz A. 1999. Reevaluation of the role of mandibular glands in regulation of reproduction in bumblebee colonies. J. Chem. Ecol. 25, 881–896. (doi:10.1023/A:1020805103379) [Google Scholar]
- 18.Alaux C, Jaisson P, Hefetz A. 2004. Queen influence on worker reproduction in bumblebees (Bombus terrestris) colonies. Insect. Soc. 51, 287–293. (doi:10.1007/s00040-004-0741-5) [Google Scholar]
- 19.Sramkova A, Schulz C, Twele R, Francke W, Ayasse M. 2008. Fertility signals in the bumblebee Bombus terrestris (Hymenoptera: Apidae). Naturwissenschaften 95, 515–522. (doi:10.1007/s00114-008-0353-4) [DOI] [PubMed] [Google Scholar]
- 20.Rottler-Hoermann A-M, Schulz S, Ayasse M. 2016. Nest wax triggers worker reproduction in the bumblebee Bombus terrestris. R. Soc. open sci. 3, 150599 (doi:10.1098/rsos.150599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Oystaeyen A, et al. 2014. Conserved class of queen pheromones stops social insect workers from reproducing. Science 343, 287–290. (doi:10.1126/science.1244899) [DOI] [PubMed] [Google Scholar]
- 22.Holman L. 2014. Bumblebee size polymorphism and worker response to queen pheromone. PeerJ 2, e604 (doi:10.7717/peerj.604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amsalem E, Orlova M, Grozinger C. 2015. A conserved class of queen pheromones? Re-evaluating the evidence in bumblebees (Bombus impatiens). Proc. R. Soc. B 282, 20151800 (doi:10.1098/rspb.2015.1800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cnaani J, Schmid-Hempel R, Schmidt J. 2002. Colony development, larval development and worker reproduction in Bombus impatiens Cresson. Insect. Soc. 49, 164–170. (doi:10.1007/s00040-002-8297-8) [Google Scholar]
- 25.Cnaani J, Wong A, Thomson J. 2007. Effect of group size on ovarian development in bumblebee workers (Hymenoptera: Apidae: Bombus). Entomol. Gen. 29, 305–314. (doi:10.1127/entom.gen/29/2007/305) [Google Scholar]
- 26.Sibbald ED, Plowright CMS. 2012. On the relationship between aggression and reproduction in pairs of orphaned worker bumblebees (Bombus impatiens). Insect. Soc. 60, 23–30. (doi:10.1007/s00040-012-0261-7) [Google Scholar]
- 27.Sibbald ED, Plowright CMS. 2014. Social interactions and their connection to aggression and ovarian development in orphaned worker bumblebees (Bombus impatiens). Behav. Process. 103, 150–155 . (doi:10.1016/j.beproc.2013.11.012) [DOI] [PubMed] [Google Scholar]
- 28.Duchateau M, Velthuis H. 1989. Ovarian development and egg laying in workers of Bombus terrestris. Entomol. Exp. Appl. 51, 199–213. (doi:10.1111/j.1570-7458.1989.tb01231.x) [Google Scholar]
- 29.Alaux C, Savarit F, Jaisson P, Hefetz A. 2004. Does the queen win it all? Queen–worker conflict over male production in the bumblebee, Bombus terrestris. Naturwissenschaften 91, 400–403. (doi:10.1007/s00114-004-0547-3) [DOI] [PubMed] [Google Scholar]
- 30.Shpigler H, Patch H, Cohen M, Fan Y, Grozinger C, Bloch G. 2010. The transcription factor Krüppel homolog 1 is linked to hormone mediated social organization in bees. BMC Evol. Biol. 10, 120 (doi:10.1186/1471-2148-10-120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amsalem E, Malka O, Grozinger C, Hefetz A. 2014. Exploring the role of juvenile hormone and vitellogenin in reproduction and social behavior in bumble bees. BMC Evol. Biol. 14, 45 (doi:10.1186/1471-2148-14-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hornáková D, Matousková P, Kindl J, Valterová I, Pichová I. 2010. Selection of reference genes for real-time polymerase chain reaction analysis in tissues from Bombus terrestris and Bombus lucorum of different ages. Anal. Biochem. 397, 118–120. (doi:10.1016/j.ab.2009.09.019) [DOI] [PubMed] [Google Scholar]
- 33.Hoover SER, Keeling CI, Winston ML, Slessor KN. 2003. The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften 90, 477–480. (doi:10.1007/s00114-003-0462-z) [DOI] [PubMed] [Google Scholar]
- 34.Nunes TM, et al. 2014. Queen signals in a stingless bee: suppression of worker ovary activation and spatial distribution of active compounds. Sci. Rep. 4, 7449 (doi:10.1038/srep07449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller L, Nonacs P. 1993. The role of queen pheromones in social insects: queen control or queen signal? Anim. Behav. 45, 787–794. (doi:10.1006/anbe.1993.1092) [Google Scholar]
- 36.Shpigler H, Amsalem E, Huang ZY, Cohen M, Siegel AJ, Hefetz A, Bloch G. 2014. Gonadotropic and physiological functions of juvenile hormone in bumblebee (Bombus terrestris) workers. PLoS ONE 9, e100650 (doi:10.1371/journal.pone.0100650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page RE, Rueppell O, Amdam GV. 2012. Genetics of reproduction and regulation of honeybee (Apis mellifera L.) social behavior. Annu. Rev. Genet. 46, 97–119. (doi:10.1146/annurev-genet-110711-155610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grozinger C, Fan Y, Hoover S, Winston M. 2007. Genome-wide analysis reveals differences in brain gene expression patterns associated with caste and reproductive status in honey bees (Apis mellifera). Mol. Ecol. 16, 4837–4848. (doi:10.1111/j.1365-294X.2007.03545.x) [DOI] [PubMed] [Google Scholar]
- 39.Kocher SD, Ayroles JF, Stone EA, Grozinger CM. 2010. Individual variation in pheromone response correlates with reproductive traits and brain gene expression in worker honey bees. PLoS ONE 5, e9116 (doi:10.1371/journal.pone.0009116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galbraith DA, Wang Y, Amdam GV, Page RE, Grozinger CM. 2015. Reproductive physiology mediates honey bee (Apis mellifera) worker responses to social cues. Behav. Ecol. Sociobiol. 69, 1511–1518. (doi:10.1007/s00265-015-1963-4) [Google Scholar]
- 41.Amsalem E, Twele R, Francke W, Hefetz A. 2009. Reproductive competition in the bumble-bee Bombus terrestris: do workers advertise sterility? Proc. R. Soc. B 276, 1295–1304. (doi:10.1098/rspb.2008.1688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monnin T, Ratnieks FL. 2001. Policing in queenless ponerine ants. Behav. Ecol. Sociobiol. 50, 97–108. (doi:10.1007/s002650100351) [Google Scholar]
- 43.Monnin T, Ratnieks FLW, Jones GR, Beard R. 2002. Pretender punishment induced by chemical signalling in a queenless ant. Nature 419, 61–65. (doi:10.1038/nature00932) [DOI] [PubMed] [Google Scholar]
- 44.Peeters C, Monnin T, Malosse C. 1999. Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc. R. Soc. Lond. B 266, 1323–1327. (doi:10.1098/rspb.1999.0782) [Google Scholar]
- 45.Billen J, Peeters C. 1991. Fine-structure of the gemma gland in the ant Diacamma australe (Hymenoptera, Formicidae). Belgian J. Zool. 121, 203–210. [Google Scholar]
- 46.Gronenberg W, Peeters C. 1993. Central projections of the sensory hairs on the gemma of the ant Diacamma: substrate for behavioural modulation? Cell Tissue Res. 273, 401–415. (doi:10.1007/BF00333695) [Google Scholar]
- 47.Woyciechowski M, Łomnicki A. 1987. Multiple mating of queens and the sterility of workers among eusocial hymenoptera. J. Theor. Biol. 128, 317–327. (doi:10.1016/S0022-5193(87)80074-7) [Google Scholar]
- 48.Ratnieks F, Visscher P. 1989. Worker policing in the honeybee. Nature 342, 796–797. (doi:10.1038/342796a0) [Google Scholar]
- 49.Malka O, Shnieor S, Katzav-Gozansky T, Hefetz A. 2008. Aggressive reproductive competition among hopelessly queenless honeybee workers triggered by pheromone signaling. Naturwissenschaften 95, 553–559. (doi:10.1007/s00114-008-0358-z) [DOI] [PubMed] [Google Scholar]
- 50.Padilla M, Amsalem E, Altman N, Hefetz A, Grozinger CM. 2016. Data from: Chemical communication is not sufficient to explain reproductive inhibition in the bumblebee Bombus impatiens. Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.3p0b5) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the datasets (oocyte size, egg number, behaviour, gene expression) used in this manuscript are presented in the electronic supplementary material, table S2. The data have also been deposited in Dryad (doi:10.5061/dryad.3p0b5) [50].