Abstract
BACKGROUND
In patients with myocardial infarction (MI), leaflet tethering by displaced papillary muscles induces mitral regurgitation (MR), which doubles mortality. Mitral valves (MVs) are larger in such patients but fibrosis sets in counterproductively. The investigators previously reported that experimental tethering alone increases mitral valve area in association with endothelial-to-mesenchymal transition.
OBJECTIVES
This study explored the clinically relevant situation of tethering and MI, testing the hypothesis that ischemic milieu modifies MV adaptation.
METHODS
Twenty-three adult sheep were examined. Under cardiopulmonary bypass, the PM tips in 6 sheep were retracted apically to replicate tethering, short of producing MR (tethered-alone). PM retraction was combined with apical MI created by coronary ligation in another 6 sheep (tethered + MI), and left ventricular (LV) remodeling was limited by external constraint in 5 additional sheep (LV constraint). Six sham-operated sheep were controls. Diastolic MV surface area was quantified by 3-dimensional echocardiography at baseline and after 58 ± 5 days, followed by histopathology and flow cytometry of excised leaflets.
RESULTS
Tethered + MI leaflets were markedly thicker than tethered-alone valves and sham controls. Leaflet area also increased significantly. EMT, detected as α-smooth muscle actin-positive endothelial cells, significantly exceeded that in tethered-alone and control valves. Transforming growth factor-β, matrix metalloproteinase expression, and cellular proliferation were markedly increased. Uniquely, tethering + MI showed endothelial activation with vascular adhesion molecule expression, neovascularization, and cells positive for CD45, considered a hematopoietic cell marker. Tethered + MI findings were comparable with external ventricular constraint.
CONCLUSIONS
MI altered leaflet adaptation, including a profibrotic increase in valvular cell activation, CD45-positive cells, and matrix turnover. Understanding cellular and molecular mechanisms underlying leaflet adaptation and fibrosis could yield new therapeutic opportunities for reducing ischemic MR.
Keywords: echocardiography, endothelial-to-mesenchymal transition, inflammation, mitral regurgitation, papillary muscle
A common complication of ischemic heart disease, mitral regurgitation (MR) doubles mortality and increases heart failure (HF) after myocardial infarction (MI) (1). Left ventricular (LV) remodeling causes papillary muscle (PM) displacement that tethers the mitral valve (MV) leaflets and restricts closure (Figures 1A and 1D) (2,3).
FIGURE 1. MV Tethering and MI.
Normal mitral valve (MV) closure with convex leaflets towards the left atrium (LA) (A) versus a tethered MV after papillary muscle (PM) retraction with concave leaflets (D). Echocardiographic views of a sheep heart at baseline (B) and 2 months after PM tethering and apical myocardial infarction (MI) (E). Limited apical MI results in apical left ventricular (LV) remodeling (solid white line) (B vs. E). Tethering of the MV leaflets relative to the annulus and tenting volume are increased (B vs. E; C vs. F, where darker colors indicate increased tenting). PM tethering and apical MI promote leaflet adaptation with increased MV surface area over time (C vs. F). Ao = aorta.
As the LV remodels, the surface area of the stretched MV increases adaptively to reduce MR (4–6). Ischemic MR remains common, however, indicating inadequate leaflet compensation (4,5). Valves excised at the late HF stage are stiff and fibrotic (7–9), further impairing closure by reducing systolic leaflet expansion and the flexibility needed to bend and seal effectively, as shown by finite-element analysis (10). Intrinsic MV changes are therefore important for the full pathogenesis of ischemic MR and its persistence following annuloplasty (11–13).
Valve adaptation can be affected not only by mechanical stretch but also by the ischemic milieu with its known cytokine release (14–16) and by MR turbulence (17,18). We developed a large-animal model to vary these factors independently, following MV area noninvasively by 3-dimensional echocardiography (3DE; Figures 1B, 1C, 1E, and 1F) (4,5,19).
Tethering alone without infarction, induced surgically by PM traction short of producing MR, increased MV area and thickness over 2 months (19). The normally quiescent adult valve endothelium (20) showed reactivated endothelial-to-mesenchymal transition (EMT), an early developmental process (21–23).
How the tethered valve is affected by ischemia, with release of inflammatory cytokines and transforming growth factor-beta (TGF-β) (14–16,24–27), is unknown. We therefore tested the hypothesis that MI alters adaptation of tethered MVs. A limited apical MI was used to avoid direct PM displacement by inferior MI (Figure 1D) (28). MV area changes over 2 months were explored by excised-leaflet molecular histopathology and flow-cytometric analysis.
METHODS
Data from 23 adult Dorsett hybrid sheep (weight >45 kg) were compared: six tethered + MI; six tethered + MI with LV constraint; six tethered-alone; and six sham-operated controls. (The last 2 groups, previously reported, now have extensive additional analyses [19]).
The tethered + MI group required controlled leaflet tethering without MR or leaflet disruption, achieved after left thoracotomy and pericardial cradle construction under cardiopulmonary bypass. The left atrium (LA) was opened and suture loops inserted via the MV orifice into the exposed PM tips (both medial PM heads), buttressed by Teflon felt pledgets, and exteriorized to the epicardium overlying the PMs, pulled through a Dacron anchoring patch, in a model developed by J. Luis Guerrero. Retracting these sutures parallel to the PM axis pulled the PM tips and leaflets apically (19). The heart was restarted and a limited apical infarct produced by distal left anterior descending coronary artery ligation, avoiding directly increased PM tethering from inferior wall bulging (28). Final suture length was then adjusted in the beating heart under echocardiographic guidance just short of producing MR, the sutures knotted against the anchoring patch, and the chest closed.
To limit post-infarction LV remodeling and its potential effects on leaflet tethering and MV adaptation, the tethered + MI with LV constraint group required suturing a flexible prolene surgical mesh (Ethicon) circumferentially from base to apex around both ventricles after creation of the same MV tethering and MI model (3). This allowed us to explore MV adaptation in an ischemic milieu with limited post-infarction LV dilation.
All sheep underwent cardiopulmonary bypass, with no MI created in the tethered-alone group, and no surgical intervention in the controls after MV and PM exposure.
Animals were cared for 58 ± 5 days and euthanized after left thoracotomy with echocardiography to confirm absence of MR and reconstruct MV surface area. These studies conformed to National Institutes of Health animal care guidelines and had institutional animal care approval.
IMAGING AND ANALYSIS
Echocardiographic data were collected using epicardial high-frequency (3.5 to 5 MHz) 2D-3D echo probes (S5, X3) with an iE33 scanner (Philips, Andover, Massachusetts). 3D volumetric data sets were electrocardiogram-gated from 4 to 7 consecutive heartbeats. Full datasets were acquired in standardized planes at baseline and before euthanasia. MR absence or grading by long-axis-view vena contracta and successful MV leaflet tethering were assessed in the beating heart just after PM tethering and MI creation and again prior to euthanasia. 3D LV volumes were calculated from 6 equiangular rotated apical views. 3D tenting volume was measured between the closed leaflet atrial surface and the annular least-squares plane (Figures 1C and 1F) (3).
Total MV leaflet area was measured at full diastolic opening to factor out superimposed passive systolic stretch. (Total leaflet area cannot be measured precisely in systole since the coapted portions cannot be optimally resolved.) Leaflet area was measured in a blinded fashion using validated custom Omni4D software (MD Handschumacher, Boston, MA) (4, 29),
The LA was opened and the LV wall dissected from the antero-lateral commissure under irrigation of pre-cooled sterile phosphate-buffered saline. Both leaflets including chordae were divided for histopathology (frozen in optimal cutting temperature compound at −80° C) and cell isolation and flow-cytometry (transported in pre-cooled physiologic collecting medium).
Microscopy was used to measure leaflet thickness in the 10 thickest areas across the leaflet mid-portion and anterior and posterior strut chordal thickness.
STATISTICS
One-way analysis of variance with post hoc contrast analysis and Tukey-Kramer test were used when appropriate to compare multiple groups of interest. Paired Student t test compared baseline and euthanasia results within animals. Data are summarized as mean ± SD for continuous variables and median with percentiles when appropriate. Statistical significance was set at p < 0.05 (2-sided).
RESULTS
The tethered + MI group sheep all had apical MI with decreased LV ejection fraction (LVEF) at euthanasia (58 ± 3 to 31 ± 4%; p < 0.001) and PM retraction with tented mitral leaflets (baseline vs. euthanasia, Figure 1B vs. 1E; early systolic tenting volume: 1.76 ± 0.52 increasing to 3.32 ± 0.95 cm3 before euthanasia; p = 0.017; late systolic tenting volume: 0.85 ± 0.19 increasing to 2.03 ± 0.69 cm3 before euthanasia; p = 0.009). Diastolic and systolic mitral annular area increased from baseline to euthanasia; MR remained trace after model creation and before euthanasia (vena contracta: 0.12 ± 0.1 vs. 0.13 ± 0.1 cm; p = 0.771) (Table 1).
TABLE 1.
Echocardiographic Baseline and Euthanasia Measures: Tethered + MI Model
| Baseline | Euthanasia | p Value | |
|---|---|---|---|
| LVEDV, ml | 57 ± 7 | 82 ± 17 | 0.023 |
| LVESV, ml | 24 ± 4 | 56 ± 13 | 0.002 |
| LVEF, % | 58 ± 3 | 31 ± 4 | <0.001 |
| Posteromedial PM to lateral MA trigone (systole), mm | 32 ± 4 | 40 ± 3 | <0.001 |
| Anterolateral PM to medial MA trigone (systole), mm | 29 ± 3 | 33 ± 2 | 0.010 |
| Anterolateral to posteromedial PM distance (systole), mm | 21 ± 4 | 26 ± 4 | 0.019 |
| MV leaflet area, cm2 | 13.9 ± 1.8 | 17.8 ± 2.3 | <0.001 |
| Leaflet length A2, mm | 15.4 ± 1.8 | 18.6 ± 2.3 | 0.003 |
| Leaflet length P2, mm | 13.4 ± 1.8 | 16.4 ± 1.6 | 0.010 |
| Annular area (diastole), cm2 | 8.66 ± 1.44 | 11.49 ± 1.11 | 0.002 |
| Annular area (systole), cm2 | 7.98 ± 1.17 | 10.12 ± 1.28 | 0.006 |
| Early systolic tenting volume, cm3 | 1.76 ± 0.52 | 3.32 ± 0.95 | 0.017 |
| Late systolic tenting volume, cm3 | 0.85 ± 0.19 | 2.03 ± 0.69 | 0.009 |
Values are mean ± SD.
A2/P2 = anterior/posterior mitral valve leaflet, middle scallop; LVEDV = left ventricular enddiastolic volume; LVESV = left ventricular end-systolic volume; LVEF = left ventricular ejection fraction; MA = mitral annulus; MV = mitral valve; PM = papillary muscle.
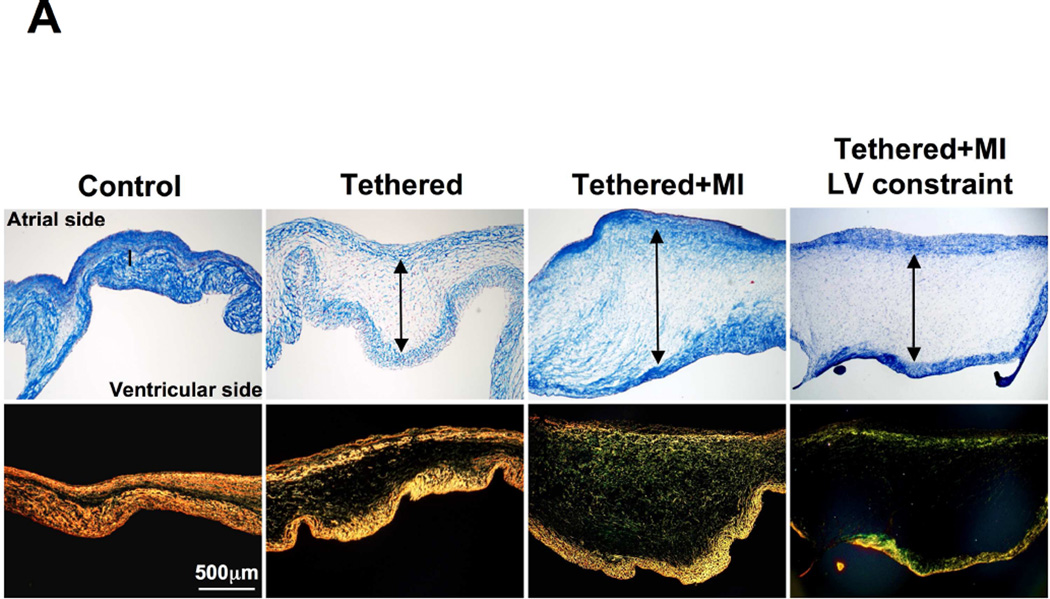
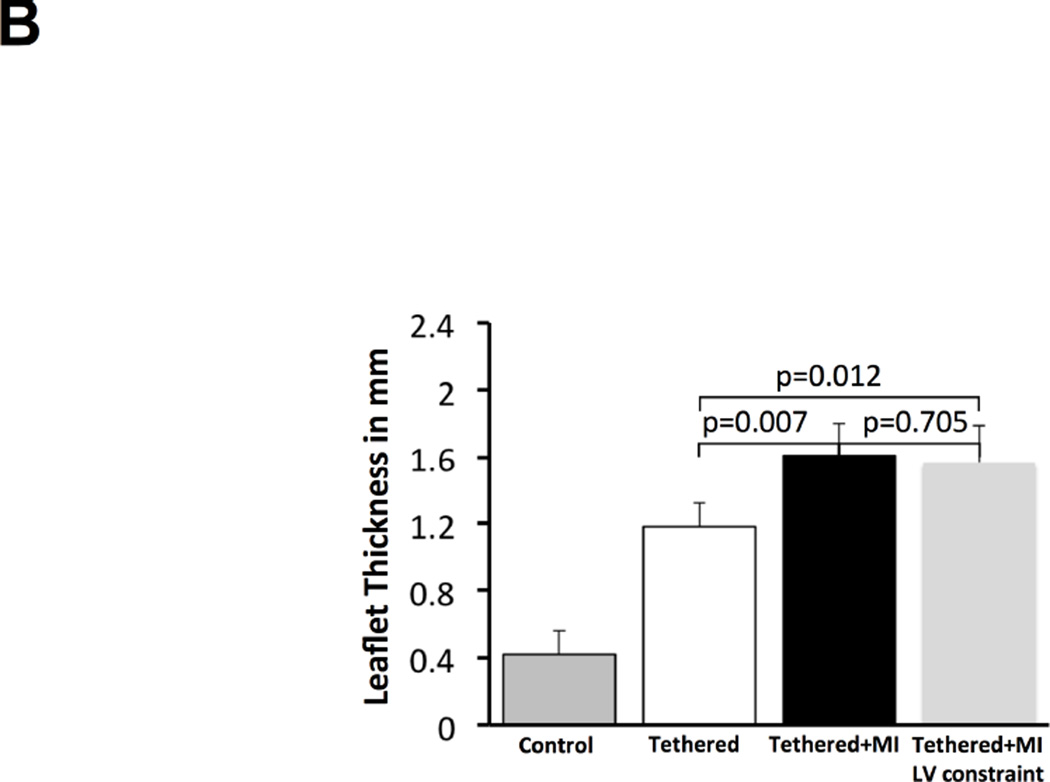
In tethered + MI sheep, total leaflet area consistently increased by 3.9 ± 0.9 cm2 (28 ± 6%) from 13.9 ± 1.8cm2 to 17.8 ± 2.3 cm2 over 2 months (p < 0.001), as did leaflet length (Table 1). The leaflet area increase was greater than in animals with tethering-alone or sham surgery (28% ± 6% vs. 17% ± 10% vs. 1% ± 4%, respectively; p < 0.001). Tethered + MI MVs were thicker than tethered-alone MVs and 3.8 times thicker than controls (1.61 ± 0.19 vs. 1.18 ± 0.14 vs. 0.42 ± 14mm, p <0.001) (Table 2, Figure 2A). Increased thickness and disrupted matrix were apparent in adjacent sections stained with Masson and picrosirius red (Figures 2A and 2B). Increased leaflet size and opacity, consistent with thickening, was visible at euthanasia (Figures 2C and 2D). In the tethered + MI sheep, although diastolic annular area increased from baseline to euthanasia by an average of 34%, there was no significant change in the ratio of leaflet/annular area as a measure of adaptation (1.61 ± 0.07 vs. 1.54 ± 0.09; p = 0.331) – comparable to the ratio in patients with tethered versus normal leaflets (5).
TABLE 2.
Echocardiographic, Histological, and Flow Cytometry Results
| Control | Tethered-alone | Tethered + MI | Tethered + MI LV constraint |
|
|---|---|---|---|---|
| MV leaflet area increase, % | 1 ± 4 | 17 ± 10* | 28 ± 6‡ | 18 ± 4 |
| MV leaflet length increase, % | 0.5 ± 2.9 | 10.9 ± 6.8* | 22.3 ± 7.5‡ | 10 ± 7.7 |
| MV leaflet thickness, mm | 0.42 ± 0.14 | 1.18 ± 0.14*† | 1.61 ± 0.19 | 1.57 ± 0.22 |
| VECs coexpressing | 7 ± 4 | 40 ± 19* | 62 ± 16 | 52 ± 12 |
| α-SMA, % | 6 (4 – 11) | 39 (21 – 59) | 61 (51 – 73) | 53 (44 – 57) |
| CD45-positive cells/HPF | 3 ± 1 | 2 ± 3*† | 25 ± 4 | 22 ± 3 |
| 3 (2 – 4) | 2 (0 – 5) | 26 (22 – 29) | 20 (19 – 24) | |
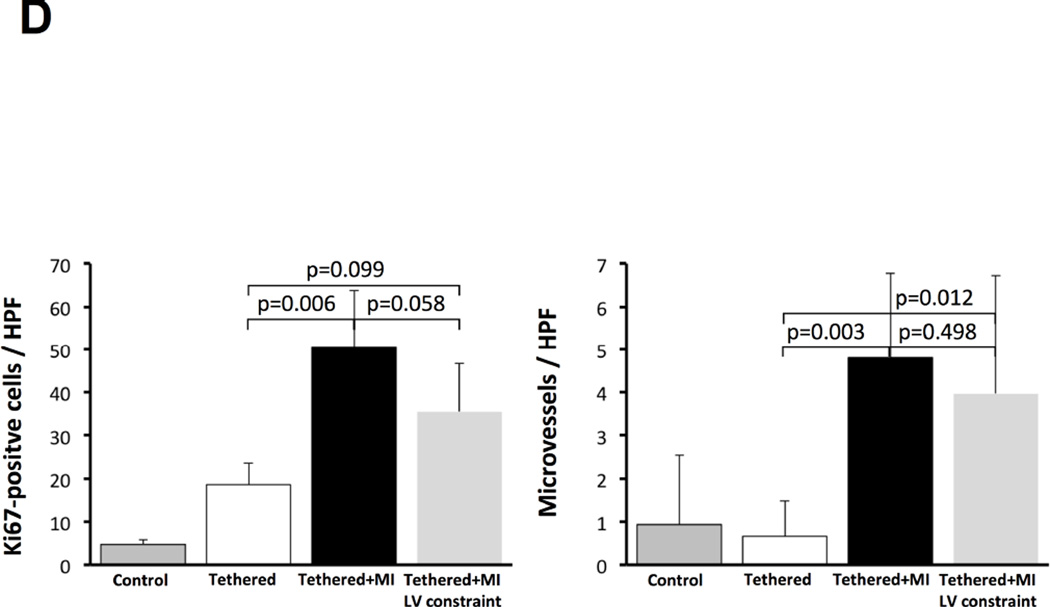
| Ki67-positive cells/HPF | 5 ± 1 | 19 ± 5* | 50 ± 13 | 35 ± 11 |
| 5 (4 – 5) | 19 (15 – 22) | 51 (42 – 55) | 31 (29 – 45) | |
| Microvessels/HPF | 1 ± 2 | 1 ± 1*† | 5 ± 2 | 4 ± 3 |
| 0 (0 – 3) | 1 (0 – 1) | 5 (5 – 6) | 4 (3 – 6) |
Values are mean ± SD or median (interquartile range).
p < 0.05 tethered only vs. tethered + MI;
p < 0.05 tethered only vs. tethered + MI LV constraint;
p < 0.05 tethered + MI vs. tethered + MI LV constraint.
α-SMA = alpha-smooth muscle actin; HPF = high-powered field; MI = myocardial infarction; VEC = valvular endothelial cell; other abbreviations as in Table 1.
FIGURE 2. Mitral Leaflet Thickness and Surface Area.
(A) Leaflet tethering alone and with MI increase leaflet thickness via expansion of spongiosa layer, even when controlled for LV volume by constraint in tethered + MI sheep (Masson, above; Picrosirius red, below; arrows: spongiosa). (B) Quantitative comparison of leaflet thickness in all groups. (C) Tethered + MI MV leaflets show increased area and opacity consistent with increased thickness (units in cm). (D) Quantitative comparison of leaflet area increase in all groups. Abbreviations as in Figure 1.
CELLULAR EXPRESSION, RECRUITMENT, AND PROLIFERATION
Normal endothelial cells express CD31 but not α-smooth muscle actin (α-SMA) (Figure 3A). Tethering only, as previously reported, induces α-SMA expression in the endothelium on the MV’s atrial side, indicating endothelial activation and EMT, with mild interstitial penetration of α-SMA+ cells (Figure 3A) (19). In contrast, tethered + MI sheep showed exuberant α-SMA expression along the MV’s atrial side, extending deep into the interstitium (Figure 3A [right-hand panels]). Expression of the endothelial cell-surface marker CD31 was diminished with tethering + MI, further indicating EMT with loss of cell-cell contacts, allowing interstitial migration (Figure 3A [third lower panel]). Weak CD31 staining could be seen in isolated cells within the interstitium (Figure 3A [inset]), consistent with penetration of cells undergoing EMT. Flow cytometry of cells digested from the leaflets similarly showed an increased percent of endothelial cells (CD31+) co-expressing α-SMA in the tethered + MI, more than tethered-alone MVs and controls (62 ± 16% vs. 40 ± 19% vs. 7 ± 4%; p < 0.001; Figure 3B, Online Figure 1, Table 2).
FIGURE 3. MV Endothelial-to-mesenchymal Transformation.
(A) Staining for interstitial marker α-smooth muscle actin (α-SMA) along the CD31+ endothelium shows increasing extension of α-SMA+ cells into the interstitium (asterisks) for the study groups with tethered MVs; occasional CD31+ staining was seen in the interstitium. (B) Quantitative analysis of endothelial cells (EC) detected by flow cytometry that are also α-SMA+, indicating endothelial-to-mesenchymal transformation (EMT), and expressed as percentage of total CD31+ cells. Other abbreviations as in Figure 1.
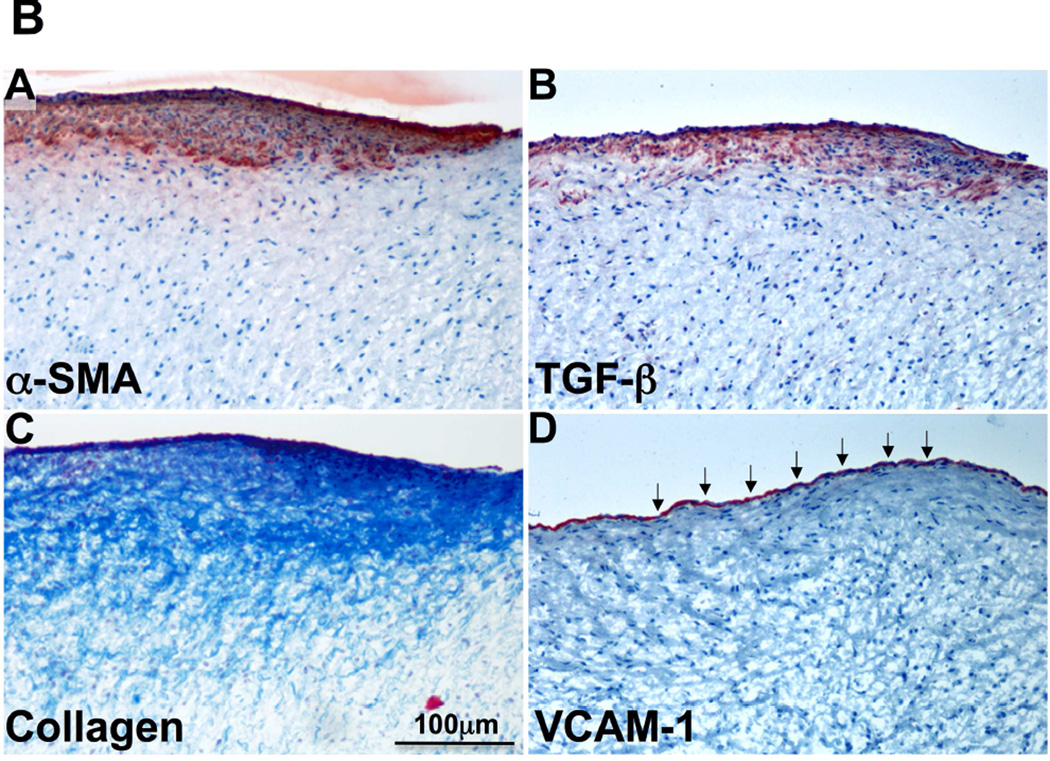
Vascular cell adhesion molecule-1 (VCAM-1), a leukocyte adhesion molecule marking endothelial activation, was consistently expressed in the endothelium of tethered + MI valves (Figures 4A [upper panels] and 4B, red staining absent in tethered-alone MVs). TGF-β expression, not detected in normal adult valves and only mildly evident in tethered-alone MVs, was prominent on the atrial side of tethered + MI MVs extending interstitially (Figures 4A [lower panels] and 4B). Figure 4B shows adjacent sections of a tethered + MI valve stained for α-SMA, TGF-β, collagen accumulation and VCAM-1; all but VCAM-1 show endothelial and sub-endothelial co-localization.
FIGURE 4. VCAM-1 and TGF-β Staining.
(A) Endothelial vascular cell adhesion molecule-1 (VCAM-1) staining (red, upper panel) only in the tethered + MI and tethered + MI LV constraint valves. Increased interstitial transforming growth factor-beta (TGF-β) staining in tethered + MI and tethered + MI LV constraint valves versus tethered-alone valves (lower panels). (B) Adjacent sections of tethered + MI MV showing co-localization of α-SMA (A), TGF-β (B), fibrosis (collagen accumulation) (C, Masson) and endothelial cell activation (VCAM-1, arrows, D).
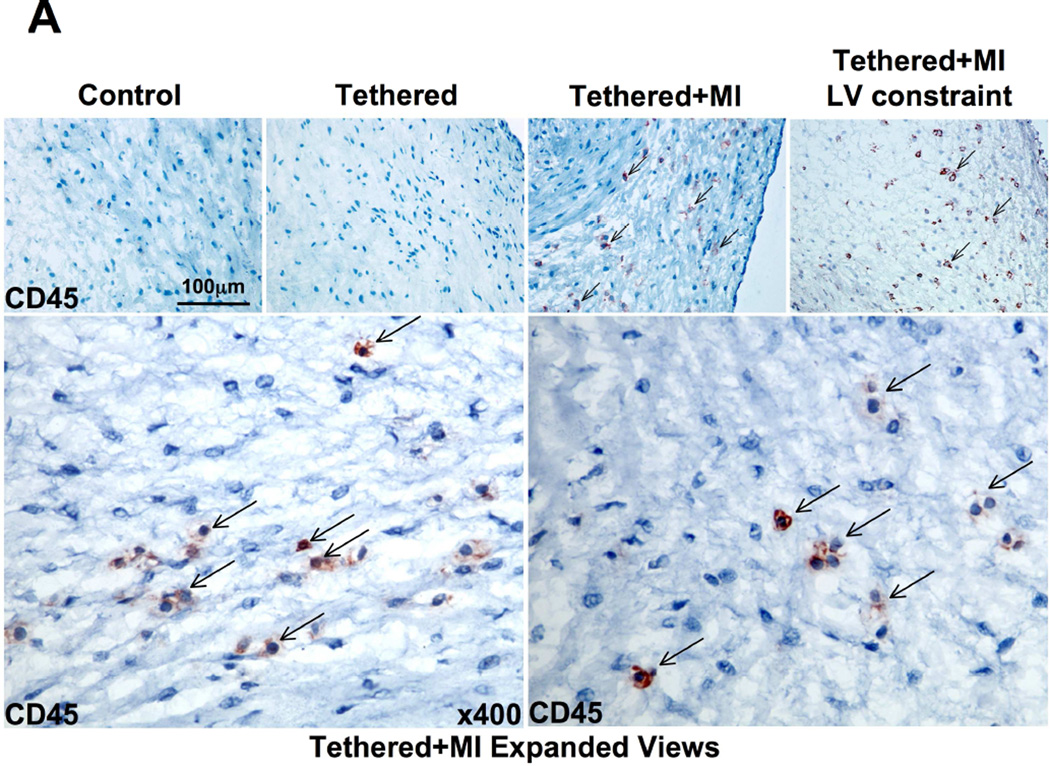
Increased VCAM-1 along the endothelium could increase cellular recruitment into the valve. We therefore evaluated MV sections for cells expressing the hematopoietic marker CD45. Tethered + MI MVs contained significantly more CD45-positive cells, uncommon in tethered-alone MVs and controls (Figures 5A and 5B). Cell proliferation detected by Ki67 immunostaining was also strongly increased in both endothelial and interstitial cells of tethered + MI MVs (Figures 5C and 5D). CD45 and Ki67-positive cells were predominantly in the atrialis and spongiosa.
FIGURE 5. MV CD45+ Cells, Cell Proliferation, and Microvessels.
(A) CD45+ cells (arrows) in tethered + MI and tethered + MI LV constraint valves are shown (bottom panels: high magnification of representative fields) and analyzed (B). (C) Increased cellular proliferation (Ki67, upper panels, asterisks) and microvessels (lower panels) are greatest in tethered + MI and tethered + MI LV constraint valves, also seen by quantitative analysis (D). HPF = high-powered field.
INGROWTH AND REMODELING
Normal cardiac valves are largely avascular. Neovascularization is common in degenerative disease and potentially facilitates inflammation and cell recruitment. Tethered + MI valves showed more microvessels in their mid-portions than tethered-alone and control MVs (Figures 5C and 5D).
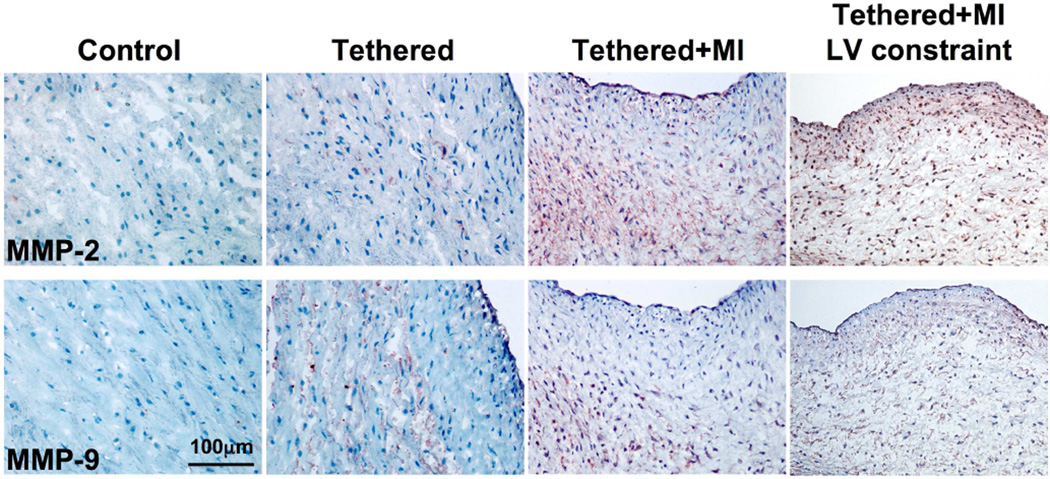
Overexpression of matrix-degrading enzymes by activated myofibroblasts occurs in human myxomatous degeneration (30). The present study demonstrated abundant expression of proteolytic matrix metalloproteinases (MMP)-2 and MMP-9, particularly in the spongiosa (Figure 6), suggesting excessive collagen degradation/remodeling in tethered + MI MVs. Picrosirius red collagen staining (Figure 2A [lower panels]) confirmed these results, demonstrating poorly organized collagen in the enlarged spongiosa, also evident by Masson staining (Figure 2A [upper panels]). Online Figure 2 shows heterogeneous areas of increased and reduced subatrial collagen (compare the focal collagen deposition in Figure 4B [lower left]). Online Figure 3 indicates subatrial elastin loss in tethered + MI valves.
FIGURE 6. MV MMP Staining.
Matrix metalloproteinase (MMP-2 and MMP-9) staining is greatest in tethered + MI and tethered + MI LV constraint valves.
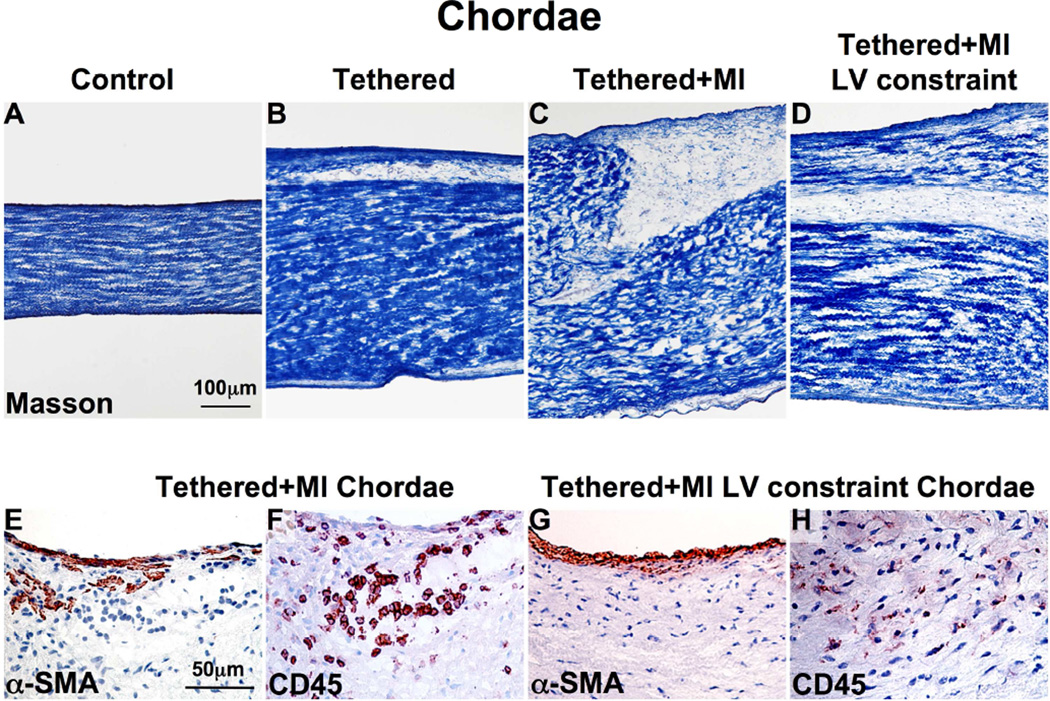
Anterior and posterior strut chordae in the tethered + MI sheep were thicker compared to tethered-alone and sham surgery animals (2.2 ± 1 vs. 1.2 ± 0.1 vs. 0.9 ± 0.1 mm, p < 0.05) (Figures 7A to 7C). Figures 7D and 7E show significant α-SMA expression and CD45-positive cells in tethered + MI chordae.
FIGURE 7. Chordal Changes.
(A–D) Chordal thickness is greatest in the tethered + MI and tethered + MI LV constraint sheep. α-SMA+ (E and G) and CD45+ (F and H) cells are seen near the chordal endothelium and within the interstitium in tethered + MI and tethered + MI LV constraint chords, suggesting chordal EMT. Abbreviations as in Figures 1 and 3.
LV CONSTRAINT
3D echocardiographic analyses (Online Appendix) showed that tethered + MI sheep had increased LV volumes and tethering distances at euthanasia compared with tethered-alone sheep. To explore whether the MV changes also occur without such increases in volume we studied 5 additional tethered + MI sheep in which LV volumes were limited by external constraint. With this constraint, LV volumes and tethering distances at euthanasia were comparable to those with tethering alone and significantly less than in tethered + MI sheep without constraint (Online Appendix and Figure 4). Despite this reduction in LV expansion and tethering, MV changes were qualitatively and quantitatively comparable to those in tethered + MI sheep without constraint, including consistently and prominently increased leaflet thickness (Figures 2A and 2B), EMT with extensive subendothelial α-SMA staining (Figures 3A and 3B), TGF-β and endothelial VCAM-1 staining (Figure 4A), CD45-positive cells (Figures 5A and 5B), Ki67-positive cells (Figures 5C and 5D), microvasculature ingrowth (Figures 5C and 5D) and MMP-2/MMP-9 (Figure 6). Leaflet area increased less in the constrained tethered + MI sheep, consistent with the smaller LV (Figure 2D), and potentially corresponding to the mildly but not significantly lower percentage of endothelial cells undergoing EMT and Ki67+ cells (Figures 3B and 5D). Chordal thickness, EMT, and CD45-positive cells were also comparable (Figure 7).
DISCUSSION
This study goes beyond prior in vivo observations that mechanical MV tethering reactivates EMT, an embryonic growth process, increasing both leaflet area and thickness (19,21–23). Stretch induces valve expression of TGF-β (31), an EMT promoter (32). TGF-β was expressed but not strongly with tethering alone, suggesting that mild TGF-β induction may suffice to promote leaflet growth, or that other signals may also activate EMT. These findings indicate that adult valves, normally quiescent (20), can respond to stress and altered ventricular geometry (19,20,23), as seen with their enlargement in the remodeled ventricles of patients with aortic insufficiency (AI) (33).
When apical MI is added to mechanical tethering, EMT and TGF-β are markedly augmented, with prominently increased cellular proliferation and leaflet and chordal thickness. Additional changes, previously unrecognized post-MI, occur that are not evident with tethering alone: endothelial activation, indicated by VCAM-1 expression; the presence of CD45-positive cells; neovascularization; and ECM remodeling with increased MMP expression (Central Illustration).
CENTRAL ILLUSTRATION. Ischemia alters mitral valve adaptation: Cellular and molecular events in the tethered mitral valve with MI compared with tethered valve alone, and impact on valve function.
(A) Compared to tethered-alone leaflets, the endothelium of tethered + myocardial infarction (MI) mitral valves undergoes excessive endothelial-to-mesenchymal cell transition (EMT) and is vascular cell adhesion molecule-1 (VCAM-1)-positive, indicating endothelial activation; the interstitium shows abundant CD45-positive cells and microvessels that may act as additional ports of cellular entry. Extracellular matrix remodeling is markedly upregulated in tethered + MI leaflets (increased TGF-β, MMPs), along with a hyperproliferative increase in Ki67-positive cells. (B) Leaflet area increase has the potential to adapt to post-MI left ventricular (LV) remodeling and tethering and prevent mitral regurgitation (MR); inadequate area increase, with potential retraction and thickening of the tethered leaflets and chordae by induced α-smooth muscle actin+ myofibroblasts, can augment MR following MI. MMP = matrix metalloproteinase; TGF-β = transforming growth factor-beta; VIC = valvular interstitial cell.
As expected, tethering is mildly augmented by MI-induced LV remodeling, and this may contribute to changes in the MV. However, MI-induced valve changes persist when external constraint limits LV remodeling and reduces tethering distances. Further, tethered-alone and tethered + MI valves are different not only in degree of EMT, thickness, and TGF-β expression, but also in nature of changes, with endothelial VCAM-1 activation, accumulation of CD45-positive cells, and neovascularization. The post-MI effects on MV adaptation therefore do not appear to depend solely on LV remodeling, although it seems reasonable that they may increase with LV remodeling-induced tethering.
Understanding the nature and mechanisms of post-MI MV changes can potentially provide insights into clinically important questions: Why is leaflet adaptation frequently inadequate to prevent MR post-MI, unlike adequate adaptation in AI (4,5,33,34)? Why does deleterious leaflet stiffening occur, impairing coaptation (8,10)? Why do current therapies have limitations? And what therapies might help (11–13)? Several possible mechanisms influencing MV remodeling and growth active post-MI are suggested by our observations.
For example, TGF-β, pro-fibrotic in many organs, is overexpressed in the tethered + MI valves, and seen in areas of increased collagen deposition (Figures 4A and 4B). While TGF-β is a key stimulus for EMT and growth in the developing valve (21–23), it could drive valve fibrosis by activating valvular interstitial cells to become contractile α-SMA-positive myofibroblasts that secrete, compact, and remodel the extracellular matrix (24–28,35,36). The fibrogenic effects of TGF-β might be amplified by its activation of angiotensin type-1 (AT1) receptors (26) that in turn would increase TGF-β expression.
TGF-β as a compensatory response to stretch may therefore become counterproductive post-MI (30), causing excessive EMT as a substrate for valve fibrosis, stiffening, and regurgitation (8–10,35). TGF-β may further lead to myofibroblast-induced matrix contraction (25) that can limit the ability of the valve to increase its area, possibly explaning the relative deficiency in valve area post-MI despite increased ventricular size (Central Illustration). TGF-β can also drive the increased chordal EMT and fibrotic thickening seen in the tethered + MI valves (Figure 7), further restricting leaflet closure and increasing regurgitation.
TGF-β may originate from the stretched valve itself; TGF-β is also markedly increased in ischemic myocardium and released into the cardiac extracellular fluid (24). Conceivably, post- MI neurohumoral activation of pro-fibrotic angiotensin II and aldosterone may promote fibrosis, synergistically with TGF-β.
Activation of the endothelial leukocyte adhesion molecule VCAM-1, seen only in the tethered + MI valves (Figures 4A and 4B), suggests the presence of inflammatory cytokines (37). Myocardial ischemia and infarction strongly induce release of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 from infarct zones and, later in ventricular remodeling, from noninfarcted myocardium (14–16). Endothelial activation by these cytokines recruits circulating cells into the infarct (37). Cytokines promote ECM remodeling with MMP activation, interstitial fibrosis, and collagen deposition in infarcted and remote zones (38). Infarct-induced cytokines may likewise explain the endothelial activation (VCAM-1 expression) seen in tethered + MI valves, contributing to their matrix remodeling and abundant MMP expression (Figure 6).
An unexpected finding was the prominence of cells positive for CD45, considered a pan-hematopoietic cell marker, in the tethered + MI leaflets and chordae (Figures 5A, 5B, and Figure 7). While these cells require further characterization, one hypothesis consistent with other findings is that they are fibrocytes, circulating bone marrow-derived cells (BMCs) that enter sites of injury and inflammation and adopt an α-SMA-positive myofibroblast phenotype (38–41). Fibrocytes, beneficial in normal wound healing and compaction, produce stiffened, sclerotic tissue in pulmonary fibrosis, asthma, and fibrosing renal and cutaneous diseases (39–41). They are also recruited to ischemic myocardium, where their inhibition reportedly reduces cardiac remodeling (42).
An equilibrium normally exists between circulating BMCs and the cardiac valves (43); this may be altered by activating cell adhesion molecules (37), as in the tethered + MI valves. Marrow-derived cell recruitment is a ubiquitous response to diverse vascular and valvular injuries. Tanaka et al. have shown that marrow-derived cells recruited to the aortic valve display a mesenchymal phenotype in hypercholesterolemic mice (44).
At least a subset of the observed CD45+ cells may therefore represent fibrocytes recruited into the stressed post-MI valve, influenced by VCAM-1 activation and TGF-β (38–40,45), which promotes their differentiation to myofibroblasts (49). Activated fibrocyte-derived myofibroblasts secrete additional TGF-β (39–41), pro-inflammatory cytokines, MMPs, and ECM components, reinforcing post-MI valve changes.
CLINICAL AND THERAPEUTIC IMPLICATIONS
The observations in this study suggest post-MI transition from adaptive MV enlargement to maladaptive limitation of enlargement and counterproductive stiffening (15,26). TGF-β overexpression, exuberant EMT as substrate for fibrosis, and endothelial activation with circulating cell recruitment in the fibrocyte hypothesis can promote fibrosis and matrix compaction (Central Illustration).
Understanding these mechanisms has potential therapeutic implications, with the long-range goal of augmenting MV area and preserving leaflet flexibility to reduce ischemic MR. TGF-β pathways can be inhibited, for example, using AT1 receptor blockers as TGF-β antagonists to limit tissue growth in a Marfan mouse model (46). Pharmacological modification may require a specific time window to permit early EMT but prevent later fibrosis (24).
FUTURE DIRECTIONS
This study compared changes when MI was superimposed on mechanical tethering without MR. Further work will be needed to explore the effects of MI alone, MR turbulence, and TGF-β blockade. MR alone increases MV collagen deposition, MMPs, and circulating TNF-α (18,47), but downregulates myocardial noncollagen ECM components and TGF-β (48); MR combined with tethering can be tested in inferior MI. Inferior MI increases valve area, but insufficiently to prevent MR (5). Whether CD45+ cells are beneficial or adverse needs to be explored (43,49), as do the roles of VCAM-1 and TGF-β in cell recruitment.
To determine why cellular changes (EMT, VCAM-1) mainly affect the atrial side of the MV requires testing biological differences between atrial and ventricular endothelium, compounding the higher atrialis radius of curvature and stress. Longitudinal studies are needed to explore clinically important questions of how the MV changes over time, why leaflet adaptation is frequently inadequate to prevent MR, and how this can be improved.
CONCLUSIONS
MV adaptation to mechanical stretch created by PM tethering with concomitant MI is different in nature and extent than with PM tethering alone, including exuberant EMT, cellular proliferation, TGF-β overexpression, endothelial activation, accumulation of CD45-positive cells, neovascularization, and matrix remodeling. Potential explanations based on synergistic stretchand MI-induced pathways involve TGF-β, pro-inflammatory cytokines, and recruited fibrocytes. Understanding these mechanisms could lead to new therapeutic opportunities to increase leaflet area and flexibility and preserve coaptation.
Supplementary Material
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE
A tethered MV has the potential for compensatory leaflet adaptation. In patients with volume overload from chronic aortic insufficiency, MV growth matches ventricular dilation and MR is rare. With comparable ventricular dilation after MI, however, MV growth is often insufficient and the leaflets become thick and fibrosed; MR is frequent, and heart failure and mortality risks are doubled.
TRANSLATIONAL OUTLOOK
Future research should seek to identify the cellular pathways through which post-MI tethering promotes MV leaflet and chordal growth so that therapeutic strategies can be developed based on these adaptive mechanisms.
Acknowledgments
The biostatistical work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Funding Sources: This work was supported in part by grant 07CVD04 from the Fondation Leducq, Paris, France, for the Transatlantic MITRAL Network of Excellence and by the NHLBI of the National Institutes of Health under award number R01HL109506 to R.A.L., E.A. and J.B. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support was from grant K24 HL67434 from the National Institutes of Health, an American Society of Echocardiography Career Development Award, and an Erwin-Schrödinger Stipend (FWF Austrian Science Fund).
ABBREVIATIONS AND ACRONYMS
- α-SMA
α-smooth muscle actin
- ECM
extracellular matrix
- EMT
endothelial-to-mesenchymal transition
- MMP
matrix metalloproteinase
- MR
mitral regurgitation
- MV
mitral valve
- PM
papillary muscle
- TGF-β
transforming growth factor-beta
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
REFERENCES
- 1.Grigioni F, Enriquez-Sarano M, Zehr KJ, et al. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 2.Kono T, Sabbah HN, Rosman H, et al. Left ventricular shape is the primary determinant of functional mitral regurgitation in heart failure. J Am Coll Cardiol. 1992;20:1594–1598. doi: 10.1016/0735-1097(92)90455-v. [DOI] [PubMed] [Google Scholar]
- 3.Otsuji Y, Handschumacher MD, Schwammenthal E, et al. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997;96:1999–2008. doi: 10.1161/01.cir.96.6.1999. [DOI] [PubMed] [Google Scholar]
- 4.Chaput M, Handschumacher MD, Tournoux F, et al. Mitral leaflet adaptation to ventricular remodeling: occurrence and adequacy in patients with functional mitral regurgitation. Circulation. 2008;118:845–852. doi: 10.1161/CIRCULATIONAHA.107.749440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaput M, Handschumacher MD, Guerrero JL, et al. Mitral leaflet adaptation to ventricular remodeling: prospective changes in a model of ischemic mitral regurgitation. Circulation. 2009;120:S99–S103. doi: 10.1161/CIRCULATIONAHA.109.844019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rausch MK, Tibayan FA, Miller DC, Kuhl E. Evidence of adaptive mitral leaflet growth. J Mech Behav Biomed Mater. 2012;15:208–217. doi: 10.1016/j.jmbbm.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quick DW, Kunzelman KS, Kneebone JM, Cochran RP. Collagen synthesis is upregulated in mitral valves subjected to altered stress. Asaio J. 1997;43:181–186. [PubMed] [Google Scholar]
- 8.Grande-Allen KJ, Barber JE, Klatka KM, et al. Mitral valve stiffening in end-stage heart failure: evidence of an organic contribution to functional mitral regurgitation. J Thorac Cardiovasc Surg. 2005;130:783–790. doi: 10.1016/j.jtcvs.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Grande-Allen KJ, Borowski AG, Troughton RW, et al. Apparently normal mitral valves in patients with heart failure demonstrate biochemical and structural derangements: an extracellular matrix and echocardiographic study. J Am Coll Cardiol. 2005;45:54–61. doi: 10.1016/j.jacc.2004.06.079. [DOI] [PubMed] [Google Scholar]
- 10.Kunzelman KS, Quick DW, Cochran RP. Altered collagen concentration in mitral valve leaflets: biochemical and finite element analysis. Ann Thorac Surg. 1998;66:S198–S205. doi: 10.1016/s0003-4975(98)01106-0. [DOI] [PubMed] [Google Scholar]
- 11.Acker MA, Parides MK, Perrault LP, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. New Engl J Med. 2014;370:23–32. doi: 10.1056/NEJMoa1312808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung J, Papakostas L, Tahta SA, et al. Mechanism of recurrent ischemic mitral regurgitation after annuloplasty: continued LV remodeling as a moving target. Circulation. 2004;110:II85–II90. doi: 10.1161/01.CIR.0000138192.65015.45. [DOI] [PubMed] [Google Scholar]
- 13.McGee EC, Gillinov AM, Blackstone EH, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2004;128:916–924. doi: 10.1016/j.jtcvs.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 14.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 15.Mann DL. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu Rev Physiol. 2003;65:81–101. doi: 10.1146/annurev.physiol.65.092101.142249. [DOI] [PubMed] [Google Scholar]
- 16.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 17.Fornes P, Heudes D, Fuzellier JF, et al. Correlation between clinical and histologic patterns of degenerative mitral valve insufficiency: a histomorphometric study of 130 excised segments. Cardiovasc Pathol. 1999;8:81–92. doi: 10.1016/s1054-8807(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 18.Stephens EH, Nguyen TC, Itoh A, et al. The effects of mitral regurgitation alone are sufficient for leaflet remodeling. Circulation. 2008;118:S243–S249. doi: 10.1161/CIRCULATIONAHA.107.757526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dal-Bianco JP, Aikawa E, Bischoff J, et al. Active adaptation of the tethered mitral valve: insights into a compensatory mechanism for functional mitral regurgitation. Circulation. 2009;120:334–342. doi: 10.1161/CIRCULATIONAHA.108.846782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aikawa E, Whittaker P, Farber M, et al. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105:408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markwald RR, Norris RA, Moreno-Rodriguez R, Levine RA. Developmental basis of adult cardiovascular diseases: valvular heart diseases. Ann N Y Acad Sci. 2010;1188:177–183. doi: 10.1111/j.1749-6632.2009.05098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker GA, Masters KS, Shah DN, et al. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res. 2004;95:253–260. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- 26.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 27.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 28.Gorman JH, 3rd, Gorman RC, Plappert T, et al. Infarct size and location determine development of mitral regurgitation in the sheep model. J Thorac Cardiovasc Surg. 1998;115:615–622. doi: 10.1016/S0022-5223(98)70326-5. [DOI] [PubMed] [Google Scholar]
- 29.Beaudoin J, Thai WE, Wai B, et al. Assessment of mitral valve adaptation with gated cardiac computed tomography: validation with three-dimensional echocardiography and mechanistic insight to functional mitral regurgitation. Circ Cardiovasc Imaging. 2013;6:784–789. doi: 10.1161/CIRCIMAGING.113.000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabkin E, Aikawa M, Stone JR, et al. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 31.Ku CH, Johnson PH, Batten P, et al. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res. 2006;71:548–556. doi: 10.1016/j.cardiores.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- 33.Beaudoin J, Handschumacher MD, Zeng X, et al. Mitral valve enlargement in chronic aortic regurgitation as a compensatory mechanism to prevent functional mitral regurgitation in the dilated left ventricle. J Am Coll Cardiol. 2013;61:1809–1816. doi: 10.1016/j.jacc.2013.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito K, Okura H, Watanabe N, et al. Influence of chronic tethering of the mitral valve on mitral leaflet size and coaptation in functional mitral regurgitation. JACC Cardiovasc Imaging. 2012;5:337–345. doi: 10.1016/j.jcmg.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 36.Liu AC, Gotlieb AI. Transforming growth factor-beta regulates in vitro heart valve repair by activated valve interstitial cells. Am J Pathol. 2008;173:1275–1285. doi: 10.2353/ajpath.2008.080365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kakio T, Matsumori A, Ono K, Ito H, Matsushima K, Sasayama S. Roles and relationship of macrophages and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the ischemic and reperfused rat heart. Lab Invest. 2000;80:1127–1136. doi: 10.1038/labinvest.3780119. [DOI] [PubMed] [Google Scholar]
- 38.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herzog EL, Bucala R. Fibrocytes in health and disease. Exp Hematol. 2010;38:548–556. doi: 10.1016/j.exphem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keeley EC, Mehrad B, Strieter RM. Fibrocytes: bringing new insights into mechanisms of inflammation and fibrosis. Int J Biochem Cell Biol. 2010;42:535–542. doi: 10.1016/j.biocel.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong KM, Belperio JA, Keane MP, et al. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:22910–22920. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- 42.Haudek SB, Xia Y, Huebener P, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Visconti RP, Ebihara Y, LaRue AC, et al. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res. 2006;98:690–696. doi: 10.1161/01.RES.0000207384.81818.d4. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka K, Sata M, Fukuda D, et al. Age-associated aortic stenosis in apolipoprotein E-deficient mice. J Am Coll Cardiol. 2005;46:134–141. doi: 10.1016/j.jacc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 45.Wahl SM, Hunt DA, Wakefield LM, et al. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987;84:5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng CM, Cheng A, Myers LA, et al. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oral H, Sivasubramanian N, Dyke DB, et al. Myocardial proinflammatory cytokine expression and left ventricular remodeling in patients with chronic mitral regurgitation. Circulation. 2003;107:831–837. doi: 10.1161/01.cir.0000049745.38594.6d. [DOI] [PubMed] [Google Scholar]
- 48.Zheng J, Chen Y, Pat B, et al. Microarray identifies extensive downregulation of noncollagen extracellular matrix and profibrotic growth factor genes in chronic isolated mitral regurgitation in the dog. Circulation. 2009;119:2086–2095. doi: 10.1161/CIRCULATIONAHA.108.826230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deb A, Wang SH, Skelding K, et al. Bone marrow-derived myofibroblasts are present in adult human heart valves. J Heart Valve Dis. 2005;14:674–678. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.