Abstract
Enteric fever affects more than 25 million people annually and results from systemic infection with Salmonella enterica serovar Typhi or Paratyphi pathovars A, B or C1. We conducted a genome-wide association study of 432 individuals with blood culture–confirmed enteric fever and 2,011 controls from Vietnam. We observed strong association at rs7765379 (odds ratio (OR) for the minor allele = 0.18, P = 4.5 × 10−10), a marker mapping to the HLA class II region, in proximity to HLA-DQB1 and HLA-DRB1. We replicated this association in 595 enteric fever cases and 386 controls from Nepal and also in a second independent collection of 151 cases and 668 controls from Vietnam. Imputation-based fine-mapping across the extended MHC region showed that the classical HLA-DRB1* 04:05 allele (OR = 0.14, P = 2.60 × 10−11) could entirely explain the association at rs7765379, thus implicating HLA-DRB1 as a major contributor to resistance against enteric fever, presumably through antigen presentation.
Enteric (typhoid) fever remains a considerable public health problem worldwide. It has been estimated that there are 26.9 million new enteric fever infections per year globally, with 200,000 deaths1,2. Individuals are normally infected with the causative agents (S. enterica Typhi (hereafter, S. Typhi) and S. enterica Paratyphi (hereafter, S. Paratyphi) pathovars) after the consumption of fecally contaminated food or water. Although industrialization, improvements in sanitation and the provision of clean water have effectively mitigated the disease burden in many countries3,4, enteric fever continues to be endemic in many lower-income countries. Protective vaccines against S. Typhi exist, but they have limited efficacy and are not suitable for young children (who represent the most at-risk group). Consequently, these vaccines are not widely deployed in the populations with the greatest need. Notably, there is currently no licensed vaccine against enteric fever caused by S. Paratyphi pathovars, potentially constituting a huge problem as the incidence of S. Paratyphi A infection is increasing in many countries across Asia5,6.
The interface between the human host and the pathogen likely has a critical role in determining outcome during an enteric fever infection. The genetic variability of the most common agent of enteric fever (S. Typhi) together with its virulence mechanisms and epidemiology have been investigated in detail7,8, whereas little is known about the human host determinants influencing susceptibility to enteric fever. There are a number of very rare genetic diseases, for example, mediated by mutations in the interleukin (IL)-12 or interferon (IFN)-γ pathways, that result in hypersusceptibility to non-typhoidal Salmonella and other intracellular bacteria9, but the corresponding mutations have not, as yet, been associated with enteric fever susceptibility. Candidate gene studies10–13 have been compromised by limited sample size and consequent lack of power to identify robust associations14.
To further evaluate the role of host genetics in susceptibility to enteric fever, we performed a genome-wide association study (GWAS) on 432 individuals with blood culture–confirmed S. Typhi infection and 2,011 controls from Vietnam. These same cases and controls also underwent a second round of genotyping using the Illumina HumanExome BeadChip15 (Online Methods). An overall association analysis across all SNP markers tested showed little evidence of global test statistic inflation (λGC = 1.05; Supplementary Fig. 1), suggesting that cryptic population stratification between enteric fever cases and controls was minimal. This finding was indeed supported by principal-components analysis of all subjects (Supplementary Figs. 2 and 3, Supplementary Tables 1 and 2, and Supplementary Note). The strongest evidence for association mapped to SNP rs7765379 at the class II human leukocyte antigen (HLA) region near the HLA-DQB1 and HLA-DRB1 genes (HLA-DRB1 mRNA, NM_002124; HLA-DQB1 mRNA, NM_002123). The minor allele of rs7765379 was under-represented in enteric fever cases (frequency = 1.04%) with respect to the control subjects (frequency = 5.5%; OR = 0.18, P = 4.5 × 10−10; Table 1). We also identified a second SNP marker exceeding genome-wide significance at rs6841458, which mapped near GUCY1A3 on chromosome 4 (OR = 1.55, P = 3.3 × 10−8). No other genomic region exhibited significant evidence of association (P > 5 × 10−7; Supplementary Figs. 4 and 5).
Table 1.
Association results for SNP rs7765379 in the class II HLA region with enteric fever in Vietnamese and Nepalese subjects
| SNP (gene) | Reference allele |
Effect allele |
Genotype counta in cases (freq.) |
Genotype counta in controls (freq.) |
P valueb | OR | Collection |
|---|---|---|---|---|---|---|---|
| rs7765379 (HLA-DRB1–HLA- DQB1) |
A | C | 0/9/423 (0.0104) | 6/208/1,797 (0.055) | 4.5 × 10−10 (2.53 × 10−8) | 0.18 | Discovery (Vietnam)c |
| A | C | 0/16/574d (0.0136) | 3/24/359 (0.039) | 0.00070 (0.00062) | 0.34 | Replication (Nepal)e | |
| A | C | 0/3/148 (0.010) | 1/69/598 (0.053) | 0.00013 (0.00098) | 0.18 | Replication (Vietnam)f | |
| 2.29 × 10−13 | 0.22 (0.15–0.34) | Meta-analysis |
Genotype counts in cases and controls are given in the following format: homozygotes for the minor (effect) allele/heterozygous/wild type. The frequency of each allele is shown in parentheses.
P values were derived from the likelihood ratio test using logistic regression (P values in parentheses were derived from the trend test).
Discovery with 432 enteric fever cases and 2,011 controls.
A total of 590 of 595 cases were successfully genotyped.
Replicaiton with 595 enteric fever cases and 386 controls.
Replicaiton with 151 enteric fever cases and 668 controls.
We attempted to replicate the associations observed at rs7765379 in the major histocompatibility complex (MHC) region and the GUCY1A3 variant rs6841458 in an independent collection of 595 enteric fever cases and 386 geographically matched controls from Nepal (Online Methods). We observed significant evidence of replication for rs7765379: the minor allele was again under-represented in the cases (frequency = 1.36%) in comparison to the controls (frequency = 3.9%; OR = 0.34, P = 7.0 × 10−4), a finding consistent with the discovery results (Table 1). However, the association with the GUCY1A3 rs6841458 marker on chromosome 4 (OR = 0.97, P = 0.71) did not replicate (Supplementary Table 3). As the Nepal replication collection comprised groups with diverse ancestry for cases and controls, which could confound the association analysis, we reanalyzed the association between rs7765379 and enteric fever with stratification for self-reported ancestry using previously described methods16. The association observed remained robust and consistent across all ancestry groups where the marker was polymorphic (Supplementary Table 4). We also did not observe any difference in the magnitude of the association when the analysis was stratified for S. Typhi or S. Paratyphi A (Online Methods and Supplementary Table 5).
To provide additional confidence in the observed association at rs7765379, we performed further replication in samples originating from 151 individuals with enteric fever and 668 controls independently enrolled in Vietnam. We again observed significant association at rs7765379 (OR = 0.18, P = 1.3 × 10−4; Table 1). Meta-analysis of the data from the discovery and both replication collections showed a genome-wide significant association at rs7765379: the minor allele was associated with 4.55-fold increased resistance to enteric fever (ORmeta = 0.22, Pmeta = 2.29 × 10−13; Table 1).
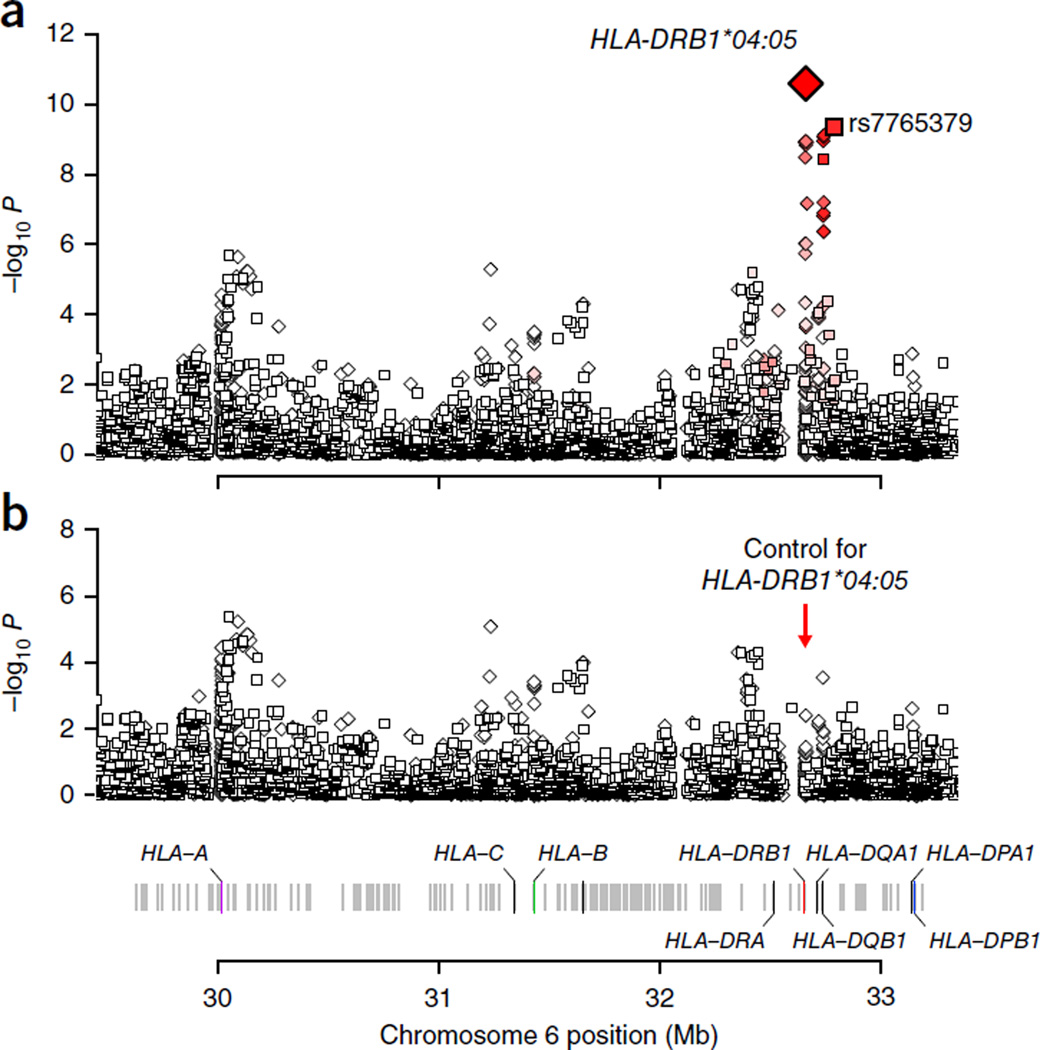
We next explored the possibility that the association at rs7765379 might be driven by functional variation within the classical HLA genes, which might not have been directly captured by the GWAS. To this end, we performed statistical imputation of classical HLA alleles and their corresponding amino acid polymorphisms using a combined reference panel of East Asian and European individuals with previously described methodologies17–19 (Online Methods). We observed the strongest association with the classical HLA-DRB1*04:05 allele (OR = 0.14, P = 2.60 × 10−11; Fig. 1), which was unsurprisingly in tight linkage disequilibrium (LD) with rs7765379 (r2 = 0.83). The association at this four-digit allele was more significant than any associations at SNPs or amino acid polymorphisms (Supplementary Table 6). We also performed omnibus tests for association at each amino acid position encoded by eight HLA genes to examine whether multiallelic polymorphism at any amino acid position could explain the association better20. None of the omnibus test P values were more significant than the P value for HLA-DRB1*04:05. After we adjusted for the effect of HLA-DRB1*04:05, no other variant remained significant (P > 1 × 10−6)21, including rs7765379 (adjusted P = 0.62; Supplementary Table 6). In contrast, after we adjusted for the effect of rs7765379, there was still a nominally significant association for the HLA-DRB1*04:05 allele (adjusted P = 0.016), making it more likely that the HLA-DRB1*04:05 allele is a driving factor for this observed HLA association signal. To verify the accuracy of our imputation, we performed HLA typing for 140 individuals from the Vietnam GWAS discovery collection. The allelic concordance between the typed and imputed alleles for HLA-DRB1*04:05 was 99.3%, thus reflecting the robustness of our imputation. Taking the directly typed HLA alleles as the ‘gold standard’, the sensitivity of the imputation was measured at 100% while the specificity of the imputation was 98.8% (refs. 17,22). Although our imputation accuracy was very high, we note that imputation errors normally result in loss of statistical power23 and thus reduce the degree of statistical significance observed.
Figure 1.
Association results for enteric fever within the broad HLA region. (a) We observed the most significant association at HLA-DRB1*04:05 (OR = 0.14, P = 2.6 × 10−11). Squares denote directly genotyped SNPs, and diamonds denote imputed markers. HLA-DRB1*04:05 was in tight LD with the most significant directly genotyped SNP, rs7765379 (r2 = 0.83). (b) When conditioning the analysis on HLA-DRB1*04:05, we no longer observed any convincing evidence of association (P > 4 × 10−6).
The Salmonellae are a genus of facultative intracellular pathogens with the ability to survive and replicate in an array of phagocytic and non-phagocytic cells by residing in a specialized cellular compartment, named the Salmonella-containing vacuole (SCV)24,25. The intracellular survival and replication of Salmonella is critical for virulence and essential for systemic disease26. Salmonella inject bacterial-derived effector proteins into host cells, such as macrophages, to influence host cell activation and promote bacterial survival. Additionally, Salmonella use dendritic cells for dissemination and restrict the ability of these cells to process antigens and present peptides27. Enteric fever is the most severe manifestation of Salmonella infection in humans. It is fatal in 10–25% of patients without antimicrobial treatment, and surviving patients have longer fever clearance times and more severe clinical complications than those that received antimicrobial therapy28. This variability in disease severity and outcome is presumably dependent on external and environmental factors, such as exposure and the virulence of the pathogen, and host factors, such as variation in innate and adaptive immune responses during exposure and acute infection. The intimate and streamlined relationship between the human host and the invading Salmonella, in which the bacteria attempt to establish infection in the face of an immune response from the host29, represents a fine balance in terms of pathogenicity.
HLA-DRB1 encodes the β chain of HLA-DR and belongs to the HLA class II molecules, which present antigen to CD4+ T lymphocytes. Variation in HLA-DR might affect the capacity of the HLA class II molecules to present antigen, which might in turn result in a differential immune response. Here we show that HLA-DRB1*04:05 is strongly protective against enteric fever. Presence of the HLA-DRB1* 04:05 allele might tip the poise of pathogenicity between the invading pathogen and host immune response toward the human host, resulting in as much as fivefold greater protection from enteric fever. The minor allele of rs7765379 that we observed to confer resistance to enteric fever has previously been shown to strongly associate with increased susceptibility to Crohn’s disease and rheumatoid arthritis30,31. This observation is consistent with the possible counterbalancing effects between determinants of infectious disease susceptibility and potential autoimmunity in the host32,33 and perhaps more so with genetic determinants exerting as strong an effect as HLA molecules. The unequivocal detection of positive signals of evolutionary selection in the HLA region can be complicated by the presence of balancing selection occurring at this locus34, so further efforts will be needed to explore the contribution of these opposing forces in depth.
Thus far, there have been few published GWAS of infectious disease susceptibility in comparison to other genetic traits and non-communicable diseases14,17,35–40. In published studies of infectious disease susceptibility, the HLA region has been positively identified as encoding host factors for HIV control (but not for susceptibility to HIV infection itself)29, leprosy, visceral leishmaniasis and chronic hepatitis B virus infection. Conversely, there was no signal of association implicating the HLA region for other infectious diseases such as meningococcal sepsis, dengue, tuberculosis and malaria. Before the introduction of technological advances permitting GWAS, candidate gene studies interrogating the involvement of the broad MHC region found variable evidence of associations in individuals with enteric fever at the class II and III regions without having the statistical power to be conclusive11–13. Our previous study on 111 enteric fever cases and 77 controls in Vietnam showed evidence of association implicating HLA-DRB1*04 in resistance against enteric fever (P = 0.02), but this finding was not robust to correction for multiple testing11. We were able to confirm this association with HLA-DRB1*04 in our current Vietnamese GWAS discovery collection (OR = 0.33, P = 1.5 × 10−9; Supplementary Table 6). This association is specifically driven by HLA-DRB1*04:05 (OR = 0.14, P = 2.6 × 10−11; Supplementary Table 6), as performing the association analysis conditioning for the allele dosage of HLA-DRB1*04:05 extinguished all evidence of association seen at HLA-DRB1*04 (Pconditioned = 0.056).
To our knowledge, this study is the first large-scale, unbiased effort to investigate host genetic factors associated with enteric fever, providing strong evidence for a role for HLA-DRB1*04:05 as a protective factor against enteric fever. Our data document one of the more substantial effect sizes (averaging nearly ~5-fold greater resistance against disease for the minor allele) reported thus far for any genetic locus determining human susceptibility to an infectious disease. Further attention should now be focused on determining the precise nature of HLA class II variation in determining susceptibility to enteric fever and other invasive bacterial pathogens, thus offering the potential to contribute to improvements in the rational design of vaccines for enteric fever and other serious bacterial infections.
ONLINE METHODS
Enteric fever cases and population controls
DNA samples from individuals with enteric fever (Vietnam, n = 432 for the GWAS discovery stage and n = 151 for replication; Nepal, n = 595 for replication) were collected as part of larger epidemiological or clinical studies. Individuals with enteric fever were defined as children or adults with clinical signs and symptoms of enteric fever with culture-confirmed S. Typhi or S. Paratyphi A in their blood or bone marrow. In Vietnam, >99% of cases were infected with S. Typhi, whereas, in Nepal, 68% (402/595) of cases were colonized with S. Typhi and 32% (188/595) were colonized with S. Paratyphi A. Blood samples for research were collected at the time of study enrollment, and demographic and clinical information was recorded into case report forms for the duration of the hospital stay or study. The clinical studies in Vietnam were performed between 1992 and 2002 at the Hospital for Tropical Diseases in Ho Chi Minh City, the Dong Nai Pediatric Centre in Dong Nai Province and the Dong Thap Provincial Hospital in Dong Thap Province, Vietnam, and have been described previously44–48. In Nepal, the clinical studies were performed between 2005 and 2014 at Patan Hospital, Kathmandu, and have been described previously49–51.
The population control individuals from Vietnam and Kathmandu were cord blood controls. In Vietnam, cord blood control samples (n = 2,011 for the GWAS discovery stage and n = 668 for the replication stage) were collected from babies born between 2003 and 2012 at Hung Vuong Obstetric Hospital in Ho Chi Minh City and Dong Thap Hospital in Dong Thap province. In Nepal, cord blood samples (n = 386) were collected at Patan Hospital, Kathmandu, between 2008 and 2012. All cases and controls were unrelated, and the majority of Vietnamese individuals were of Vietnamese Kinh ancestry (>98%), as assessed by questionnaire. There was more ancestry diversity within the Nepalese cases and controls, where individuals self-reported as 1 of 15 different ancestry groups. Genomic DNA was extracted from blood samples using the Qiagen blood midi or maxi kits (Qiagen) or the Nucleon BACC genomic DNA extraction kits (GE Healthcare). The treating physician was responsible for obtaining informed consent from the patients or their parent or guardian for clinical study participation and from the baby’s mother for population controls. Informed consent was obtained from all subjects involved in this research. Ethical approvals were granted by the scientific and ethical committees at the Hospital for Tropical Diseases, the Dong Thap Provincial Hospital and the Health Services of Dong Thap Province in Vietnam. The clinical trials in Nepal were approved by the Nepal Health Research Council, Kathmandu. Protocols were also approved by the Oxford Tropical Research Ethics Committee, Oxford University, UK.
Genotyping
Genome-wide genotyping for the Vietnamese enteric fever cases and cord blood controls was performed using the Illumina OmniExpress BeadChip and Illumina 660W BeadChip, which have been very well described40. These samples also underwent exome-wide genotyping using the Illumina Human Exome BeadChip, which assays for 247,870 SNP markers enriched in the coding exome, following the manufacturer’s specifications. Replication genotyping for the independently enrolled cases and controls from Nepal and Vietnam was performed with TaqMan allelic discrimination assays (Applied Biosystems) using routine laboratory techniques described elsewhere40.
Statistical analysis
Stringent quality control filters were used to remove poorly performing samples and SNP markers in both the discovery (GWAS and exome chip) and replication (de novo genotyping) phases. SNPs with a call rate of <95%, non-polymorphic SNP markers and those showing significant deviation from Hardy-Weinberg equilibrium (P value for deviation of <1 × 10−6) were removed from further statistical analysis. For the exome chip that contained nonsynonymous variants, SNPs that were not monomorphic (corresponding to at least one heterozygous carrier individual) were included for downstream analysis. For the GWAS chip, SNPs with a MAF of >1% were included for downstream analysis. A total of 709,725 markers passed quality control filters in this manner (642,445 from the GWAS chip and 67,280 from the exome chip).
Similarly stringent quality control criteria were also applied on a per-sample basis, and samples with an overall genotyping success rate of <95% were removed from further analysis. The remaining samples were then subjected to biological relationship verification using the principle of variability in allele sharing according to the degree of relationship. Identity-by-state (IBS) information was derived using the PLINK software package52. For those pairs of individuals who showed evidence of relatedness reflecting pairs of first-degree relatives (for example, a parent and offspring, full siblings or monozygotic twins), we removed the sample with the lower call rate before performing principal-component analysis. Principal-component analysis was undertaken to account for spurious associations resulting from ancestral differences for individual SNPs, and principal-component plots were generated using the R statistical program. For the discovery stage, all enteric fever cases had perfectly matched controls, as visualized spatially on principal-component analysis (Supplementary Fig. 2), using previously reported criteria53.
For both the discovery and replication stages, analysis of association contrasting SNP genotypes between enteric fever cases and controls was carried out using logistic regression, with adjustment for the first ten principal components of genetic stratification. This test models for a trend-per-copy effect of the minor allele on disease risk. The association P value was obtained by a log-likelihood ratio test comparing the likelihood of the null model against the likelihood of the fitted model. We verified non-departure from the additive genetic model by additionally performing the conventional trend test. Genotyping cluster plots for genetic markers surpassing genome-wide significance at the discovery stage were visually checked and verified to be of good quality (Supplementary Figs. 6 and 7).
Genome-wide imputation was performed using IMPUTE2 software (see URLs) with the 1000 Genomes Project reference panel54.
HLA imputation and association analysis
We imputed classical HLA alleles and corresponding amino acid polymorphisms using a combined Asian and European reference panel with high-density SNP genotypes and 4-digit classical HLA allele genotypes (530 Asian individuals and 5,225 European individuals) using SNP2HLA software (see URLs), as described elsewhere18,19,22. We extracted SNP genotypes located in the broad MHC region (chr. 6: 29–34 Mb) to impute 92 two-digit classical alleles, 152 four-digit classical alleles and 763 amino acid polymorphisms of the 8 class I and class II HLA genes (HLA-A, HLA-B, HLA-C, HLA-DRB1, HLA-DQA1, HLA-DQB1, HLA-DPA1 and HLA-DPB1).
For the association analysis, we used a logistic regression model assuming additive effects of allele dosages on the log-odds scale. We defined HLA variants to include biallelic SNPs in the MHC region, two-digit and four-digit classical HLA alleles, biallelic amino acid polymorphisms and multiallelic amino acid positions. To account for potential population substructure, we included the top ten principal components as covariates. For HLA variants with m alleles (m = 2 for biallelic variants and m > 2 for multiallelic variants), we included m − 1 alleles as independent variables in the regression model, excluding the most frequent allele as a reference. This resulted in the following logistic regression model:
where β0 is the logistic regression intercept, β1,j is the additive effects of the dosage of allele j for the variant xj, L is the number of principal components enrolled in the analysis, yl is the lth principal component and β2,l is the effect of yl. An omnibus P value was obtained by a log-likelihood ratio test comparing the likelihood of the null model against the likelihood of the fitted model. We further assessed the significance of the improvement in fit by calculating the deviance (= −2 times the log likelihood), which follows a χ2 distribution with m − 1 degree(s) of freedom.
Statistical power calculation for the replication stage
Statistical power calculations were performed using previously described approaches for complex disease genetic studies55. To observe a reasonably convincing replication (for example, at P < 0.001), our approach comprising a first-stage replication using 595 enteric fever cases and 386 controls from Nepal and a second-stage replication using 151 enteric fever cases and 668 controls from Vietnam would be able to reliably detect (>90% power) modest genetic effects (OR > 1.5–1.6) at MAFs in excess of 20%. For strong genetic effects with OR > 3, the replication stages were sufficiently powered to detect these even at MAFs as low as 5% (Supplementary Table 7). Thus, for potential true positive associations to achieve genome-wide significance in the combined discovery and replication data sets, we brought forward sentinel SNPs showing P < 5 × 10−7 from the discovery stage for testing in the replication collections56.
Supplementary Material
Acknowledgments
This work was supported by the Wellcome Trust, UK as part of their Major Overseas Program in Viet Nam (089276/Z/09/Z) and by the Biomedical Research Council, Agency for Science, Technology and Research, Singapore. S.B. is a Sir Henry Dale Fellow, jointly funded by the Wellcome Trust and the Royal Society (100087/Z/12/Z). P.I.W.d.B. acknowledges support from the Netherlands Organization for Scientific Research (Vernieuwingsimpuls VIDI Award NWO project number 016.126.354).
Footnotes
URLs. R statistical software, http://www.r-project.org/; IMPUTE2, http://mathgen.stats.ox.ac.uk/impute/impute_v2.html; SNP2HLA, https://www.broadinstitute.org/mpg/snp2hla/.
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
AUTHOR CONTRIBUTIONS
C.C.K. and S.J.D. are the principal investigators for the study who conceived and obtained funding for the project. C.C.K. organized and supervised the GWAS and replication genotyping pipeline, devised the overall analysis plan and wrote the first draft of the manuscript with input from S.J.D. S.J.D. established the enteric fever cohorts for the discovery and replication stages of this genetics study by working with J.J.F., T.T.H., N.P.H.L., N.T.H., T.T.B.T., C.M.P., N.T.C., H.V., L.T.P., M.N.L., N.T.V.T. and P.V.V. in Vietnam and with B.B., S.K., S.D., A.A., A.K., O.S., C.D. and S.B. in Nepal to coordinate the collection of clinical samples and phenotype data. K.L.A. and C.P.S. coordinated and collected the Vietnamese control cohorts. T.D., D.N.H. and T.A. contributed data from Vietnamese replication cohorts with other diseases. Z.L., K.S.S. and J.N.F. performed genotyping and DNA quality checks on all samples. C.C.K., Y.Y.T., P.I.W.d.B., B.H., Y.O., M.L.H. and S.R. analyzed the data and performed imputation. All authors critically reviewed manuscript revisions and contributed intellectual input to the final submission.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J. Glob. Health. 2012;2:010401. doi: 10.7189/jogh.02.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull. World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 3.Cvjetanović B, Grab B, Uemura K. Epidemiological model of typhoid fever and its use in the planning and evaluation of antityphoid immunization and sanitation programmes. Bull. World Health Organ. 1971;45:53–75. [PMC free article] [PubMed] [Google Scholar]
- 4.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid fever. Clin. Infect. Dis. 2010;50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGregor AC, Waddington CS, Pollard AJ. Prospects for prevention of Salmonella infection in children through vaccination. Curr. Opin. Infect. Dis. 2013;26:254–262. doi: 10.1097/QCO.0b013e32835fb829. [DOI] [PubMed] [Google Scholar]
- 6.Karki S, Shakya P, Cheng AC, Dumre SP, Leder K. Trends of etiology and drug resistance in enteric fever in the last two decades in Nepal: a systematic review and meta-analysis. Clin. Infect. Dis. 2013;57:e167–e176. doi: 10.1093/cid/cit563. [DOI] [PubMed] [Google Scholar]
- 7.Kingsley RA, et al. Genome and transcriptome adaptation accompanying emergence of the definitive type 2 host-restricted Salmonella enterica serovar Typhimurium pathovar. MBio. 2013;4:e00565–13. doi: 10.1128/mBio.00565-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong VK, et al. Characterization of the yehUT two-component regulatory system of Salmonella enterica serovar Typhi and Typhimurium. PLoS ONE. 2013;8:e84567. doi: 10.1371/journal.pone.0084567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jong R, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor–deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 10.Dunstan SJ, et al. Typhoid fever and genetic polymorphisms at the natural resistance–associated macrophage protein 1. J. Infect. Dis. 2001;183:1156–1160. doi: 10.1086/319289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunstan SJ, et al. Genes of the class II and class III major histocompatibility complex are associated with typhoid fever in Vietnam. J. Infect. Dis. 2001;183:261–268. doi: 10.1086/317940. [DOI] [PubMed] [Google Scholar]
- 12.Dunstan SJ, et al. A TNF region haplotype offers protection from typhoid fever in Vietnamese patients. Hum. Genet. 2007;122:51–61. doi: 10.1007/s00439-007-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dharmana E, et al. HLA-DRB1*12 is associated with protection against complicated typhoid fever, independent of tumour necrosis factor α. Eur. J. Immunogenet. 2002;29:297–300. doi: 10.1046/j.1365-2370.2002.00318.x. [DOI] [PubMed] [Google Scholar]
- 14.Fakiola M, et al. Common variants in the HLA-DRB1–HLA-DQA1 HLA class II region are associated with susceptibility to visceral leishmaniasis. Nat. Genet. 2013;45:208–213. doi: 10.1038/ng.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huyghe JR, et al. Exome array analysis identifies new loci and low-frequency variants influencing insulin processing and secretion. Nat. Genet. 2013;45:197–201. doi: 10.1038/ng.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helgason A, et al. Refining the impact of TCF7L2 gene variants on type 2 diabetes and adaptive evolution. Nat. Genet. 2007;39:218–225. doi: 10.1038/ng1960. [DOI] [PubMed] [Google Scholar]
- 17.Pereyra F, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia X, et al. Imputing amino acid polymorphisms in human leukocyte antigens. PLoS ONE. 2013;8:e64683. doi: 10.1371/journal.pone.0064683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillai NE, et al. Predicting HLA alleles from high-resolution SNP data in three Southeast Asian populations. Hum. Mol. Genet. 2014;23:4443–4451. doi: 10.1093/hmg/ddu149. [DOI] [PubMed] [Google Scholar]
- 20.Raychaudhuri S, et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat. Genet. 2012;44:291–296. doi: 10.1038/ng.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gockel I, et al. Common variants in the HLA-DQ region confer susceptibility to idiopathic achalasia. Nat. Genet. 2014;46:901–904. doi: 10.1038/ng.3029. [DOI] [PubMed] [Google Scholar]
- 22.Okada Y, et al. Risk for ACPA-positive rheumatoid arthritis is driven by shared HLA amino acid polymorphisms in Asian and European populations. Hum. Mol. Genet. 2014 Jul 28; doi: 10.1093/hmg/ddu387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang L, Wang C, Rosenberg NA. The relationship between imputation error and statistical power in genetic association studies in diverse populations. Am. J. Hum. Genet. 2009;85:692–698. doi: 10.1016/j.ajhg.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jantsch J, Chikkaballi D, Hensel M. Cellular aspects of immunity to intracellular Salmonella enterica. Immunol. Rev. 2011;240:185–195. doi: 10.1111/j.1600-065X.2010.00981.x. [DOI] [PubMed] [Google Scholar]
- 25.Malik-Kale P, et al. Salmonella—at home in the host cell. Front. Microbiol. 2011;2:125. doi: 10.3389/fmicb.2011.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 27.Cheminay C, Mohlenbrink A, Hensel M. Intracellular Salmonella inhibit antigen presentation by dendritic cells. J. Immunol. 2005;174:2892–2899. doi: 10.4049/jimmunol.174.5.2892. [DOI] [PubMed] [Google Scholar]
- 28.van den Bergh ET, Gasem MH, Keuter M, Dolmans MV. Outcome in three groups of patients with typhoid fever in Indonesia between 1948 and 1990. Trop. Med. Int. Health. 1999;4:211–215. doi: 10.1046/j.1365-3156.1999.43374.x. [DOI] [PubMed] [Google Scholar]
- 29.Khor CC, Hibberd ML. Host-pathogen interactions revealed by human genome-wide surveys. Trends Genet. 2012;28:233–243. doi: 10.1016/j.tig.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Freudenberg J, et al. Genome-wide association study of rheumatoid arthritis in Koreans: population-specific loci as well as overlap with European susceptibility loci. Arthritis Rheum. 2011;63:884–893. doi: 10.1002/art.30235. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki K, et al. A genome-wide association study identifies 2 susceptibility loci for Crohn’s disease in a Japanese population. Gastroenterology. 2013;144:781–788. doi: 10.1053/j.gastro.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Sfriso P, et al. Infections and autoimmunity: the multifaceted relationship. J. Leukoc. Biol. 2010;87:385–395. doi: 10.1189/jlb.0709517. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson EK, Kwiatkowski DP, Sabeti PC. Natural selection and infectious disease in human populations. Nat. Rev. Genet. 2014;15:379–393. doi: 10.1038/nrg3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Bakker PI, et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat. Genet. 2006;38:1166–1172. doi: 10.1038/ng1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Bakker PI, Telenti A. Infectious diseases not immune to genome-wide association. Nat. Genet. 2010;42:731–732. doi: 10.1038/ng0910-731. [DOI] [PubMed] [Google Scholar]
- 36.Davila S, et al. Genome-wide association study identifies variants in the CFH region associated with host susceptibility to meningococcal disease. Nat. Genet. 2010;42:772–776. doi: 10.1038/ng.640. [DOI] [PubMed] [Google Scholar]
- 37.Thye T, et al. Common variants at 11p13 are associated with susceptibility to tuberculosis. Nat. Genet. 2012;44:257–259. doi: 10.1038/ng.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thye T, et al. Genome-wide association analyses identifies a susceptibility locus for tuberculosis on chromosome 18q11.2. Nat. Genet. 2010;42:739–741. doi: 10.1038/ng.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fellay J, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khor CC, et al. Genome-wide association study identifies susceptibility loci for dengue shock syndrome at MICB and PLCE1. Nat. Genet. 2011;43:1139–1141. doi: 10.1038/ng.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dean M, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 43.Modiano D, et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature. 2001;414:305–308. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- 44.Cao XT, et al. A comparative study of ofloxacin and cefixime for treatment of typhoid fever in children. The Dong Nai Pediatric Center Typhoid Study Group. Pediatr. Infect. Dis. J. 1999;18:245–248. doi: 10.1097/00006454-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Chinh NT, et al. A randomized controlled comparison of azithromycin and ofloxacin for treatment of multidrug-resistant or nalidixic acid–resistant enteric fever. Antimicrob. Agents Chemother. 2000;44:1855–1859. doi: 10.1128/aac.44.7.1855-1859.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luxemburger C, et al. Risk factors for typhoid fever in the Mekong delta, southern Viet Nam: a case-control study. Trans. R. Soc. Trop. Med. Hyg. 2001;95:19–23. doi: 10.1016/s0035-9203(01)90318-9. [DOI] [PubMed] [Google Scholar]
- 47.Vinh H, et al. Double blind comparison of ibuprofen and paracetamol for adjunctive treatment of uncomplicated typhoid fever. Pediatr. Infect. Dis. J. 2004;23:226–230. doi: 10.1097/01.inf.0000114905.87426.c2. [DOI] [PubMed] [Google Scholar]
- 48.House D, et al. Cytokine release by lipopolysaccharide-stimulated whole blood from patients with typhoid fever. J. Infect. Dis. 2002;186:240–245. doi: 10.1086/341298. [DOI] [PubMed] [Google Scholar]
- 49.Arjyal A, et al. Gatifloxacin versus chloramphenicol for uncomplicated enteric fever: an open-label, randomised, controlled trial. Lancet Infect. Dis. 2011;11:445–454. doi: 10.1016/S1473-3099(11)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandit A, et al. An open randomized comparison of gatifloxacin versus cefixime for the treatment of uncomplicated enteric fever. PLoS ONE. 2007;2:e542. doi: 10.1371/journal.pone.0000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koirala S, et al. Gatifloxacin versus ofloxacin for the treatment of uncomplicated enteric fever in Nepal: an open-label, randomized, controlled trial. PLoS Negl. Trop. Dis. 2013;7:e2523. doi: 10.1371/journal.pntd.0002523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tall AR. CETP inhibitors to increase HDL cholesterol levels. N. Engl. J. Med. 2007;356:1364–1366. doi: 10.1056/NEJMe078029. [DOI] [PubMed] [Google Scholar]
- 54.1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- 56.Purdue MP, et al. Genome-wide association study of renal cell carcinoma identifies two susceptibility loci on 2p21 and 11q13.3. Nat. Genet. 2011;43:60–65. doi: 10.1038/ng.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.