Abstract
Introduction
In 2014, the NRG Oncology Group initiated the first NCI-sponsored, phase I clinical trial of SBRT for the treatment of multiple metastases in multiple organ sites (XXXX; NCTXXX). The primary endpoint is to test the safety of SBRT for the treatment of multiple lesions (2–4) in several anatomic sites in a multi-institutional setting. Because of the technical challenges inherent to treating multiple lesions as their spatial separation decreases, we present the technical requirements for NRG-XXXX and the rationale for their selection.
Methods and Materials
Patients with controlled primary tumors of breast, non-small cell lung, or prostate are eligible if they have 2–4 metastases distributed among 7 extracranial anatomic locations throughout the body. Prescription and organ-at-risk (OAR) doses were determined by expert consensus. Credentialing requirements include 1) irradiation of the IROC phantom with SBRT, 2) submitting IGRT case studies, and 3) planning the benchmark. Guidelines for navigating challenging planning cases including assessing composite dose are discussed.
Results
Dosimetric planning to multiple lesions receiving differing doses (45–50Gy) and fractionation (3–5) while irradiating the same organs-at-risk is discussed, particularly for metastases in close proximity (≤5cm). The benchmark case was selected to demonstrate the planning trade-offs required to satisfy protocol requirements for two nearby lesions. Examples of passing benchmark plans exhibited a large variability in plan conformity.
Discussion
NRG-XXXX was developed using expert consensus on multiple issues from the dose fractionation regimen to the minimum IGRT guidelines. Credentialing was tied to the task rather than the anatomic site in order to reduce its burden. Every effort was made to include a variety of delivery methods to reflect current SBRT technology. While some simplifications were adopted, the successful completion of this trial will inform future designs of both national and institutional would allow immediate clinical adoption of SBRT trials for oligometastases.
Keywords: Oligometastases, SBRT, clinical trial, NRG
Background
Oligometastases defines the proposed clinical state between locoregionally confined cancer and widespread metastases (1). Cancer patients with few metastases limited in number and distribution could potentially be cured if all visible tumors were ablated with radiotherapy or surgery. With the increasing availability of the technological advances enabling SBRT (2), the use of ablative radiotherapy to treat oligometastases is increasing (3). To date, several single arm trials using ablative radiotherapy to treat oligometastases have been conducted (4–8). In these studies, including trials allowing up to 6 metastases, the median number of treated metastases was 2 (9, 10). Furthermore, in a recently reported international survey, while most respondents were comfortable treating ≤ 3 metastases, even more would not treat >3 metastases in the same treatment course (3). Therefore, most practicing radiation oncologists do not have experience delivering ablative radiotherapy to multiple targets in the same patient during the same treatment course.
To determine tolerable doses for multiple metastases treated with ablative radiotherapy, in 2014 NRG Oncology initiated the first NCI-sponsored, phase I trial of stereotactic body radiotherapy (SBRT) for the treatment of 3–4 metastases, or 2 metastases within 5 cm, in multiple organs (XXXX; NCTXXX). The goal of NRG XXXX is to robustly determine the safety of validated phase II single organ SBRT doses when delivered simultaneously in the same treatment course to multiple targets potentially in multiple organs.
Given that available evidence suggests that most practitioners do not have experience with the delivery of ablative radiotherapy in these clinical scenarios, extensive care was taken during protocol development to ensure that these techniques would be applied carefully and appropriately. This included compounding available dose volume metrics for multi-organ SBRT, developing a benchmark study for treatment planning education, developing minimum technical standards necessary to implement accurate dose delivery for multi-site, multi-organ SBRT, as well as providing rapid pre-treatment review of plans prior to delivery. Herein we summarize these technical tools developed to assist centers implementing multi-target ablative radiotherapy for multiple metastases.
Rationale for Technical Requirements
As this is the first NTCN SBRT trial intending to treat patients to multiple anatomic sites, exhaustive technical challenges were discussed and addressed during protocol development. Some of the items that had to be reconciled among the treatment sites included motion management, image-guidance (IGRT), composite dose calculation, organ-at-risk (OAR) dose constraints (considering variable time courses and fractionation schedules), and positioning accuracy of multiple lesions that may be treated on separate days. These technical requirements are often intertwined. Consider the example of a patient with a thoracic spinal metastasis and two lung metastases, one located centrally while the other is located in the posterior aspect of the lung near the spinal metastasis. A single computed tomography (CT) scan must be used to calculate the true composite dose to the spinal cord. If the lung lesions require motion management, this would affect the CT scan acquisition (e.g., breath hold) which in turn would affect the spinal metastasis treatment. In this clinical scenario, the spinal metastasis would be treated in the breath hold position, which is not standardly used for spine, to match the treatment of the lung metastases and enable calculation of the dose contributed to the spinal cord from the aggregate treatment. Moreover, the organ-at-risk (OAR) dose limits from the 3 fractionation regimen must be used since the central lung and spinal metastases are treated in 3 fractions. Because the distribution of all possible combinations of metastatic locations is unknown, the full range of technical challenges could not be tabulated. However, the majority were incorporated into the protocol based on past experience of SBRT for oligometastases by expert radiation oncologists.
Considerations on Selection of Appropriate SBRT Doses
Reported phase II SBRT studies have typically described patients receiving ablative radiotherapy to a single organ (11, 12). As such, each target organ has developed a literature of dose volume metrics for acceptable treatment plans and expected toxicity. However, NRG-XXXX is investigating the safety of treating 2–4 metastases distributed among 7 extracranial anatomic locations throughout the body (Table 1). To adapt these differing dose-volume metrics for multi-organ stereotactic ablative radiotherapy (MOSART), we adopted the straightforward approach of assigning each metastasis to a location as listed in Table 1 based on the potential for normal tissue toxicity. We also encouraged sites to consider the potential for normal tissue toxicity when assigning location. For example, a rib metastasis would by definition be assigned to the osseous location. However, if it were located in the thorax near the lung, it should be assigned to the peripheral lung location because the toxicity of greatest concern is from pulmonary effects. On the other hand, if it were adjacent to the liver, it should be assigned to the liver location.
Table 1.
Initial and decreased doses if dose-limiting toxicities are encountered for the 7 anatomic sites of metastases eligible for treatment on NRG-XXX (data partially appears in NRG-XXX: Table XXX).
| Prescription Doses | |||
|---|---|---|---|
| Metastatic Locations | Initial Starting Dose | BED per USC* | Decreased DLT Dose |
| Lung - Peripheral | 45 Gy (3 fractions) | 96 Gy | 42 Gy (3 fractions) |
| Lung - Central | 50 Gy (5 fractions) | 99 Gy | 47.5 Gy (3 fractions) |
| Mediastinal/Cervical lymph node | 50 Gy (5 fractions) | 99 Gy | 47.5 Gy (3 fractions) |
| Liver | 45 Gy (3 fractions) | 96 Gy | 42 Gy (3 fractions) |
| Spinal/Paraspinal | 30 Gy (3 fractions) | 60 Gy | 27 Gy (3 fractions) |
| Osseous | 30 Gy (3 fractions) | 60 Gy | 27 Gy (3 fractions) |
| Abdominal-pelvic metastases (lymph node/adrenal gland | 45 Gy (3 fractions) | 96 Gy | 42 Gy (3 fractions) |
Biological Effective Dose per the Universal Survival Curve, a measure of relative dose potency (13), assuming alpha = 0.33Gy−1, beta = 0.0385Gy−1, alpha/beta = 8.6, D0 = 1.25Gy
Initial SBRT doses for metastases in each anatomic location are based on single organ SBRT phase II trials (e.g., RTOG 0631, RTOG 0438) and, where unavailable, retrospective data (2) and/or expert consensus. Although fractionation schedules vary from either three or five fractions, most of the selected doses in Table 1 are equipotent per the universal survival formalism (13) except for tumors in the spinal/paraspinal and osseous categories where proximity to critical structures (e.g., the spinal cord) has obvious dose potency limitations. Of note, different metastases in a given patient may be assigned to different locations and be treated with different SBRT doses. If dose-limiting toxicities (DLT) are encountered, the prescription doses (which vary in fractionation regimens) among the various anatomic sites will be de-escalated as shown in Table 1.
SBRT Simulation and Target Delineation
Many challenges are faced when simulating patients for multi-organ ablative radiotherapy. While immobilization is critical to ensure reproducible set-up and minimal intra-fraction motion, there is no gold standard and patient immobilization should always prioritize patient comfort for otherwise longer than typical treatments. The CT scan must include all metastases intended for SBRT in order to allow for tabulation of composite dose to the OAR. Occasionally, more than one treatment position is required (e.g., arms above the head to treat two lung metastases but arms to the side to treat a third metastasis in the humerus). While intravenous contrast during simulation is encouraged to aid in target delineation, it is only required for liver metastases. Motion management is required for treatment planning when tumor excursion exceeds 1 cm to limit normal organ volumes being irradiated. Motion management must be maintained for all organs irradiated in a given treatment plan in order to ensure accuracy of composite dose calculations.
Treatment Planning Challenges for MOSART
Challenges also arise when planning with different ablative dose fractionation schemes in the same patient. In particular, there is no standard guidance on how to assess the contributions to a single organ-at-risk from different metastases treated with different fractionation regimens. Also, we acknowledge that clinicians must always balance planning target volume (PTV) coverage against OAR limits to select treatments that they believe will provide the best possible chance of tumor control without OAR risk. In line with this strategy, explicit planning priorities were developed for NRG-XXXX (see Figure 1) and can be summarized as:
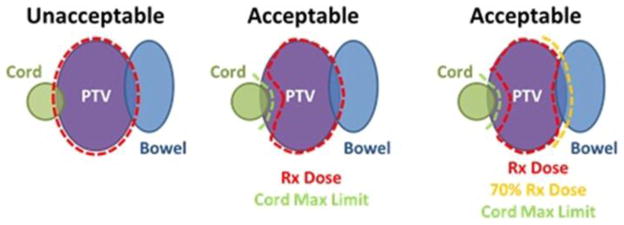
Figure 1.
Depictions of protocol acceptable and unacceptable doses to spinal cord and a second serial organ (bowel) that both overlap with a PTV for a patient intended to be treated on NRG-XXX.
Spinal dose constraints, as assessed on the composite dose map, must always be met.
SBRT dose compactness must be maintained, with doses > 100% encompassed within the PTV.
PTV coverage may fall to 70% of the prescription dose in order to meet non-spinal OAR constraints.
Given that multiple metastases will be irradiated in the same treatment course, we mandated that composite doses be used to assess dose to OARs. However, we also mandated that the coverage and conformity for each individual PTV must be sufficient using the individual plan for each target. It was important to ensure that the dose coverage for each PTV does not rely on the scatter contribution from a nearby metastasis’ treatment which may be delivered on a different day. If the treatment is delivered on the same day, this consideration may not apply. Additionally, in cases when a single plan (e.g., VMAT) is used to simultaneously treat two nearby metastases, the dose contribution cannot be de-convolved for each PTV separately and therefore dose will be assessed on a single plan. Thus, a conservative approach to document composite dose to OAR was adopted whereby if a given organ has > 1 Gy dose contribution from both a three and five fractionation plan, then the three fraction OAR dose constraints is to be used.
Benchmark Credentialing
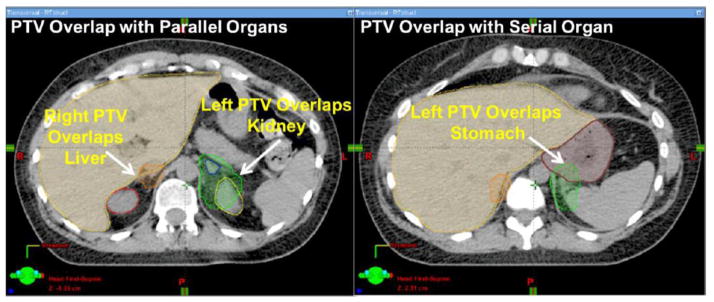
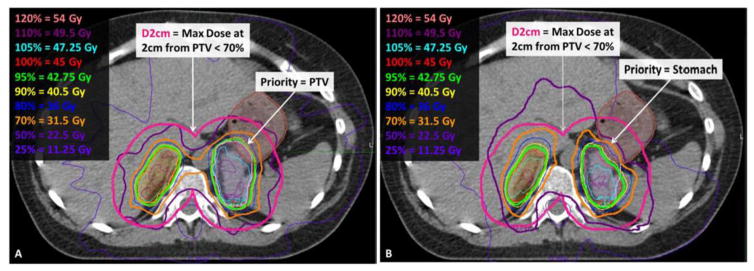
To aid centers participating in this protocol as well as others adopting multi-target multi-organ SBRT, we developed a benchmark case to serve as a planning tool to familiarize participating institutions with the specific planning goals of the protocol prior to enrolling/planning their first patient. Feedback regarding plan quality can be given to an institution without the pressure of a patient urgently waiting to begin treatment. Figure 2 depicts the benchmark case for NRG-XXXX, consisting of bilateral adrenal metastases separated by < 5 cm of tissue. The right PTV overlaps with liver while the left PTV overlaps with the left kidney and 4cc of the stomach. This challenging case was selected to provide a real-world example highlights the difficulty of treating two nearby lesions with separate plans, thereby encouraging institutions to evaluate whether they wish to credential for irradiation of two lesions with a single plan and isocenter. In this case, the treating physician and team must balance various planning priorities outlined in NRG-XXXX, allowing institutions to discover that there are a variety of methods by which to develop an acceptable plan, as shown in Figure 3. Physicians must choose whether to prioritize target coverage or limit dose to OAR. For example, the PTV may be fully covered with the prescription dose as in Figure 3.A (95% isodose shown in green) as long as the PTV volume that overlaps the stomach, liver, or kidneys does not exceed 105% of the prescription dose (cyan isodose). Alternatively, coverage of the PTV that overlaps with stomach may be reduced to 70% (orange isodose) of the prescription dose (Figure 3.B). Specific guidelines for planning the benchmark and examples of cases that did or did not pass have been posted on IROC Houston’s website (http://irochouston.mdanderson.org) under “Credentialing.”
Figure 2.
Axial CT views of the benchmark depict PTVs of bilateral adrenal metastases (in green and orange colorwash) overlapping with both parallel (liver, left kidney) and serial (stomach) OAR.
Figure 3.
The same axial CT slice depicts that the benchmark can be planned by prioritizing the PTV coverage (lilac colorwash) where it overlaps with the stomach (brown colorwash) as shown in 3.A or by prioritizing the stomach sparing as shown in 3.B.
SBRT Treatment Planning and Evaluation
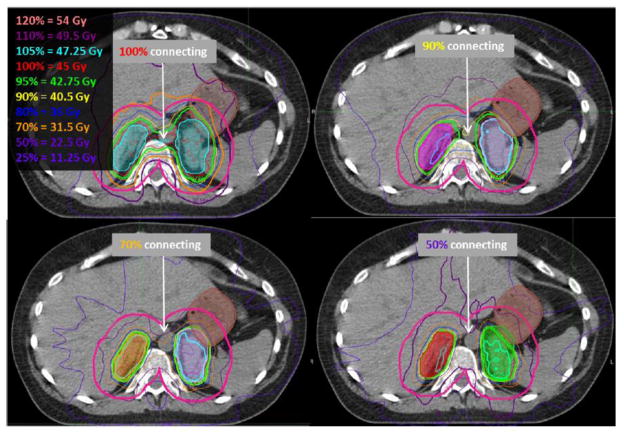
Previous lung SBRT protocols (e.g., RTOG 0236, RTOG 0813) provided general guidelines for dose fall-off, e.g., dose at 2cm away from the target (D2cm) and the 50% volume as a ratio of the PTV volume (R50%). Similar guidelines do not exist for non-lung SBRT. However, as they were developed for targets within lung tissue, it is expected that comparable or superior dose gradients are achievable in higher density organs throughout the rest of the body. While these guidelines should be followed as closely as possible, the R50% and D2cm are expected to grow as the distance between any two metastases decreases. This effect could be exacerbated when an OAR is located in between the metastases (e.g., spinal cord in the benchmark case in Figure 2). However, given that these criteria help to generate compact dose distributions, similar guidelines were incorporated into NRG-XXXX (see Table X-X in NRG-XXXX). While NRG-XXXX allows for planning with IMRT or VMAT, these techniques are required to meet the dose compactness criteria (i.e., ratio of prescription dose to PTV volume < 1.2–1.5) that are typical of 3DCRT SBRT plans. Note that without careful selection of the optimization criteria, it is possible to create a non-compact plan using IMRT/VMAT and thus the use of these planning techniques should only be used when 3DCRT cannot achieve the desired plan. Figure 4 depicts benchmark plans developed with VMAT from different institutions in which the conformity between the two PTVs varies dramatically. On the other hand, IMRT/VMAT plans enable fine control of dose within the PTV which becomes important when trying to limit the dose in the PTV volumes that overlap normal tissue. Thus, given the lack of a priori testing of these criteria, we did not consider violating these dose fall-off criteria an unacceptable deviation. In fact, we anticipate that the collection of these data during NRG-XXXX will allow the generation of future recommendations for the R50% and D2cm for the treatment of nearby multiple metastases with SBRT.
Figure 4.
Benchmark plans from four different institutions developed with VMAT depict varying degrees of conformity, as shown by the isodose lines connecting between the two PTVs. The magenta line depicts the D2cm structure, which represents the 3D distance of 2cm away from the PTVs.
Target coverage is assessed as the dose delivered to 95% of the PTV and compared to the minimum coverage requirements in the tables found in NRG-XXXX for initial starting doses (Table X-X) and decreased DLT doses (Table X-X). Any dose greater than prescribed (i.e., > 100%) must be confined to the PTV, especially because a portion of the PTV volume contains normal tissue. By definition, SBRT does not limit the highest dose within the PTV in order to ensure a rapid dose fall-off away from the target. In fact, high doses within the target are considered acceptable and are thought to potentially provide a tumoricidal advantage (2). If the PTV overlaps with an OAR, it is permissible to reduce the minimum point dose within the PTV to no less than 70% of the prescription volume (Figure 1). Every effort should be made to maintain adequate GTV coverage. Note that a treating physician can choose to use the lower end of the variation acceptable prescription doses in order to de-escalate dose if OAR limits are near tolerance.
OAR constraints are listed in NRG-XXXX for three (NRG-XXXX Table X-X) and five (NRG-XXXX Table X-X) fractionation treatments, and separated into serial and parallel organ groups. When available, these OAR limits were drawn from past RTOG protocols and published single institution trials. Because these OAR constraints are consensus-based and not evidence-based, approved plans may exceed them in this phase I trial. To be prudent, spinal cord limits must never be exceeded. Every effort should be made to meet OAR constraints unless there is direct overlap with PTV (Figure 1).
Image-Guidance and Radiation Dose Delivery Credentialing for MOSART
Ensuring that SBRT can be delivered safely and accurately to any target in the body is a challenging endeavor, even more so when targeting multiple lesions in the same treatment course. Therefore, it was important to establish a mechanism where the targeting and delivery of SBRT could be validated for each institution prior to patient treatment. Therefore we adapted credentialing from previous RTOG SBRT protocols for a single anatomic site but generalized it to the functional task being tested rather than the anatomic site being treated. We determined that this would avoid an onerous credentialing burden for each anatomic site being treated (for both the institutions and reviewers) as well as efficiently focus on necessary tasks. We identified that the important tasks for accurate and safe delivery include verification of the accuracy of: 1) irradiation of multiple targets with isocenters that are not centered within each target 2) irradiation with or without motion management, 3) IGRT for targets in soft-tissue versus bony anatomy.
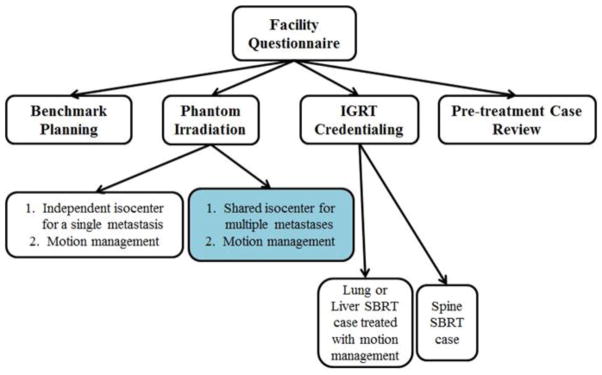
Figure 5 summarizes the various credentialing steps required for NRG-XXXX. Irradiation of a phantom provided by the Imaging and Radiation Oncology Core (IROC) utilizing the same motion management intended for patients on the protocol is required. Once an institution demonstrates that they can irradiate a moving target in a lung phantom with their motion management strategy of choice, it is assumed that they can do so to a liver target. Because the protocol allows for a variety of planning techniques (e.g., 3DCRT, IMRT, VMAT) the phantom irradiation could be accomplished with any of these methods. If an institution successfully irradiates the SBRT phantom with 3DCRT but has previously demonstrated their ability to deliver an IMRT plan to a non-SBRT phantom, this would enable them to credential for both SBRT and IMRT. Institutions are “grandfathered” so that they do not have to repeatedly demonstrate their capability to accurately deliver dose, even allowing interchangeability among the SBRT phantoms irradiated (i.e., liver phantom instead of the lung phantom).
Figure 5.
The pre-enrollment and pre-treatment steps required for NRG-XXX credentialing. The blue-shaded bubble demonstrates the additional credentialing step unique to this SBRT protocol
To address the uniqueness of NRG-XXXX in treating multiple targets, an additional phantom credentialing step was added. If an institution plans to irradiate targets separated by more than 10 cm with a single isocenter for patients enrolled onto this protocol, the protocol requires credentialing by irradiating the lung/spine or liver phantom, each with two targets. However, the protocol cautions against the use of a single treatment isocenter for lesions separated by > 10 cm as this may compromise targeting accuracy (NRG-XXXX: Section XXX). The dosimetric impact of small rotational positioning errors on multiple targets treated with a single isocenter has been documented for stereotactic treatments in the body (14) and brain (15). Even if these rotational errors could be corrected using IGRT and 6-degree-of-freedom treatment tables, relative changes in alignment between multiple targets due to organ motion could not be easily remedied. Note that by design, Cyberknife, is defined as a non-isocentric technique and phantom irradiation automatically credentials the institution for the multi-isocenter delivery technique. Finally, to limit the credentialing burden, institutions were urged to combine as many of their techniques as possible into a single irradiation.
Credentialing of image-guidance was separated into two distinct tasks: verification of target positioning for treatment of soft-tissue targets (e.g., lung/liver) and of targets within bony anatomy (e.g., spine/osseous). As guidelines for these tasks did not exist during NRG XXXX protocol development, we polled experts and developed consensus recommendations shown here in Table 2. To summarize, verification of targets within soft tissue requires 3D volumetric imaging because osseous structures visible on 2D imaging are not good surrogates for these targets. Targets that track bony anatomy can be verified by 2D IGRT. The only exception is when the soft-tissue targets have implanted fiducials that are visible on 2D imaging enabling the target to be directly tracked without the need of a surrogate. To ensure that institutions could integrate IGRT techniques with motion management techniques being used, credentialing cases were required to be submitted from patients treated with a motion management technique that matches the one intended for use in patients enrolled onto NRG-XXXX.
Table 2.
Minimum IGRT required for each of the 7 anatomic sites (appears as Table XXX in NRG-XXX).
| Metastatic Location | Minimum IGRT requirement | |
|---|---|---|
| No Fiducials | With Fiducials** | |
| Lung – Peripheral+ | Volumetric (3D) | Orthogonal kV (2D) |
| Lung – Central+ | Volumetric (3D) | Orthogonal kV (2D) |
| Mediastinal/Cervical LN | Volumetric (3D) | N/A |
| Liver+ | Volumetric (3D) | Orthogonal kV (2D) |
| Spinal | Orthogonal kV (2D) | Orthogonal kV (2D) |
| Osseous* | Orthogonal kV (2D) | N/A |
| Abdominal-pelvic+ | Volumetric (3D) | Orthogonal kV (2D) |
NOTE: When osseous/rib metastases are classified into another metastatic location, follow the IGRT guidelines for that site.
NOTE: When a metastasis contains an implanted fiducial that is clearly visible on kV orthogonal or volumetric imaging, either method can be used.
NOTE: Registration using a soft tissue surrogate for the tumor is recommended for lung, liver and abdominal-pelvic metastases for both 3D and 2D IGRT datasets.
Take-Home Points
In order to ensure safe and accurate delivery of ablative radiotherapy to multiple targets in multiple organ sites, credentialing and technical requirements were developed for NRG-XXXX to ensure minimum technical competency without regard to the anatomic site (i.e., to test function independent of anatomic site). To acknowledge the technical difficulty of treating targets with high doses with nearby or overlapping OARs, prioritization of prescription doses and OAR constraints were interleaved. Certain challenging scenarios were highlighted in the protocol as requiring additional credentialing steps such as: treating multiple targets with a single isocenter and the importance of different IGRT workflows for soft-tissue targets compared to those than track boney anatomy.
Conclusions
NRG-XXXX is the first NCI-supported national protocol to study the safety and feasibility of the treatment of multiple metastases at various anatomic locations. Technical challenges spanning treatment planning and radiation delivery were addressed in developing a generalized SBRT protocol for treatment to any anatomic organ. While the results of this protocol will provide invaluable data on technical and planning guidelines that will guide the development of future oligometastases trials, protocol development necessitated the generation of expert consensus guidance on multiple issues from the dose fractionation regimen to the minimum IGRT guidelines inclusive of a variety of delivery methods in order to test the current state of clinically available SBRT technology. These guidelines can be adopted by institutions starting MOSART programs. The successful completion of this trial will inform future designs of both national and institutional SBRT trials for oligometastases. Future technological (i.e., deformable registration algorithms) and biological (i.e., accurate BED modelling) advances could be incorporated as they become available.
Acknowledgments
This project was supported by grants U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC), U24CA180803 (IROC) from the National Cancer Institute (NCI).
We thank the following individuals for providing their expert opinion during protocol development: Indrin Chetty, Ph.D., Laura Dawson, M.D., Timothy Solberg, Ph.D., Fang-Fang Yin, Ph.D.
Footnotes
Conflicts of Interest Notification: Ms. Winter reports grants from NCI Grant - subcontract to ACR, during the conduct of the study. Dr. Robinson reports grants and personal fees from Varian, personal fees from Radialogica, personal fees from ViewRay, grants from Elekta, personal fees from DFINE, outside the submitted work. Dr. Timmerman reports grants from Varian Medical systems, grants from Accuray, Inc., grants from Elekta Oncology, outside the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol Off J Am Soc ClinOncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 2.XXX.
- 3.Lewis SL, Porceddu S, Nakamura N, et al. Definitive Stereotactic Body Radiotherapy (SBRT) for Extracranial Oligometastases: An International Survey of >1000 Radiation Oncologists. Am J Clin Oncol. 2015 doi: 10.1097/COC.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 4.Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol StockhSwed. 1995;34:861–870. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 5.Ryu SI, Chang SD, Kim DH, et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49:838–846. doi: 10.1097/00006123-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 6.XXX.
- 7.XXX
- 8.Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol Off J Am Soc ClinOncol. 2014;32:3824–3830. doi: 10.1200/JCO.2014.56.7412. [DOI] [PubMed] [Google Scholar]
- 9.XXX
- 10.XXX.
- 11.Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol StockhSwed. 2006;45:823–830. doi: 10.1080/02841860600904854. [DOI] [PubMed] [Google Scholar]
- 12.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol Off J Am Soc ClinOncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 13.Park C, Papiez L, Zhang S, et al. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol BiolPhys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Shiu A, Wang C, et al. Dosimetric effect of translational and rotational errors for patients undergoing image-guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol BiolPhys. 2008;71:1261–1271. doi: 10.1016/j.ijrobp.2008.02.074. [DOI] [PubMed] [Google Scholar]
- 15.Roper J, Chanyavanich V, Betzel G, et al. Single-Isocenter Multiple-Target Stereotactic Radiosurgery: Risk of Compromised Coverage. Int J Radiat Oncol BiolPhys. 2015;93:540–546. doi: 10.1016/j.ijrobp.2015.07.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]