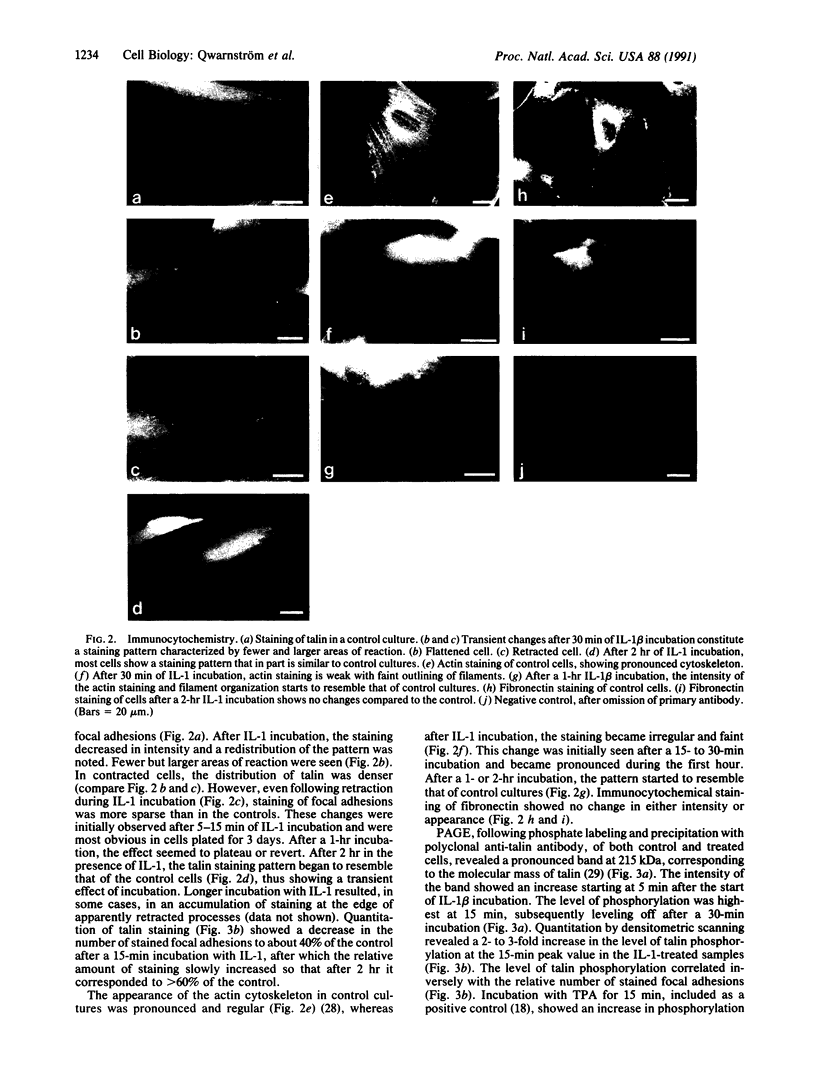
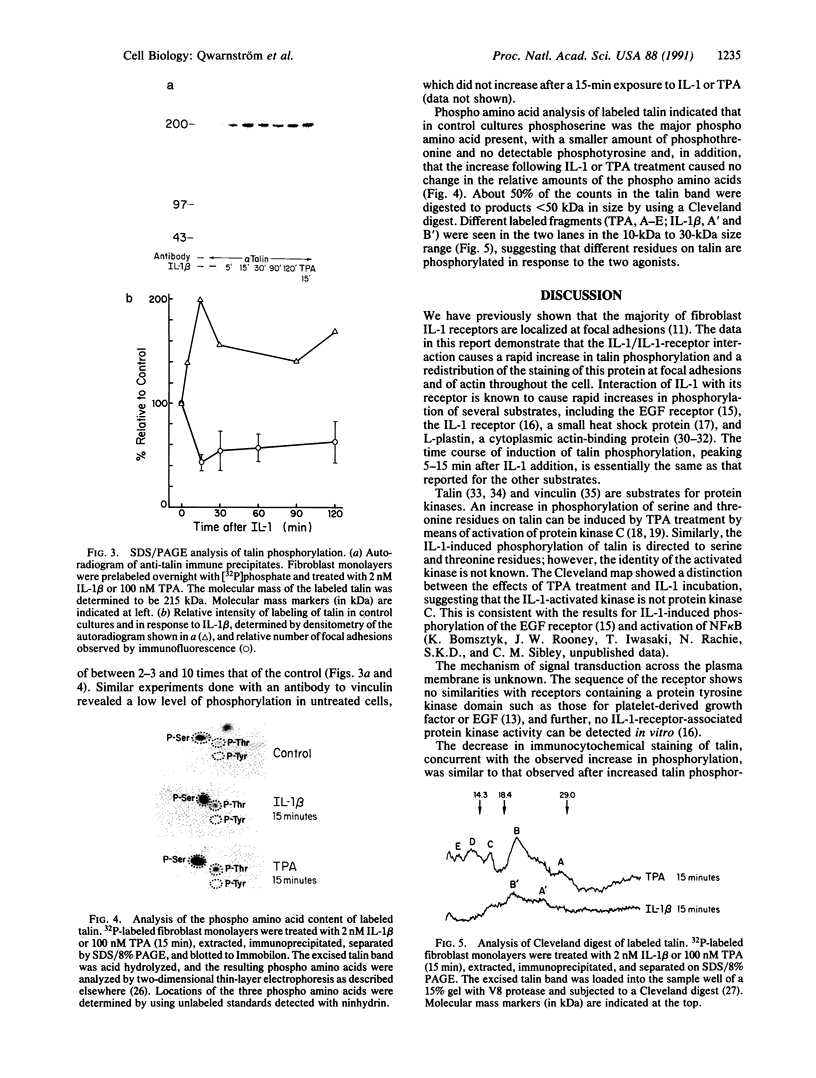
Abstract
The majority of interleukin 1 (IL-1) receptors in human fibroblasts has been shown to be localized at focal adhesions. This study describes rapid alterations caused by IL-1 beta/IL-1-receptor interaction at these sites. Fibroblast monolayers, incubated with IL-1 beta and prepared for electron microscopy, showed successive loss of cell-substratum contact and fewer and less-pronounced processes. Immunocytochemistry revealed loss and redistribution of the talin staining initially observed after 5-15 min of IL-1 beta incubation. Similarly, the cytoskeleton showed a decrease in staining and a disorganization starting from 15 to 30 min after IL-1 addition, whereas extracellular fibronectin appeared largely unaffected. Prelabeling with [32P]phosphate showed a 2- to 3-fold increase in the level of talin phosphorylation, peaking at 15 min. Phospho amino acid analyses revealed a higher level of serine and threonine phosphorylation. The data suggest that the action of IL-1 beta on fibroblasts may be partially mediated by direct phosphorylation of talin via activation of a protein serine/threonine kinase, leading to changes in transmembrane linkage proteins and the cytoskeleton. Such alterations at focal adhesions may provide a mechanism by which IL-1 can rapidly modulate cell-matrix interactions during inflammation and wound healing.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badley R. A., Woods A., Carruthers L., Rees D. A. Cytoskeleton changes in fibroblast adhesion and detachment. J Cell Sci. 1980 Jun;43:379–390. doi: 10.1242/jcs.43.1.379. [DOI] [PubMed] [Google Scholar]
- Beckerle M. C., Burridge K., DeMartino G. N., Croall D. E. Colocalization of calcium-dependent protease II and one of its substrates at sites of cell adhesion. Cell. 1987 Nov 20;51(4):569–577. doi: 10.1016/0092-8674(87)90126-7. [DOI] [PubMed] [Google Scholar]
- Beckerle M. C. The adhesion plaque protein, talin, is phosphorylated in vivo in chicken embryo fibroblasts exposed to a tumor-promoting phorbol ester. Cell Regul. 1990 Jan;1(2):227–236. doi: 10.1091/mbc.1.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird T. A., Saklatvala J. IL-1 and TNF transmodulate epidermal growth factor receptors by a protein kinase C-independent mechanism. J Immunol. 1989 Jan 1;142(1):126–133. [PubMed] [Google Scholar]
- Brown P. J., Juliano R. L. Selective inhibition of fibronectin-mediated cell adhesion by monoclonal antibodies to a cell-surface glycoprotein. Science. 1985 Jun 21;228(4706):1448–1451. doi: 10.1126/science.4012302. [DOI] [PubMed] [Google Scholar]
- Burridge K. Are stress fibres contractile? Nature. 1981 Dec 24;294(5843):691–692. doi: 10.1038/294691a0. [DOI] [PubMed] [Google Scholar]
- Burridge K., Connell L. A new protein of adhesion plaques and ruffling membranes. J Cell Biol. 1983 Aug;97(2):359–367. doi: 10.1083/jcb.97.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Burridge K., Feramisco J. R. Microinjection and localization of a 130K protein in living fibroblasts: a relationship to actin and fibronectin. Cell. 1980 Mar;19(3):587–595. doi: 10.1016/s0092-8674(80)80035-3. [DOI] [PubMed] [Google Scholar]
- Chen J. M., Chen W. T. Fibronectin-degrading proteases from the membranes of transformed cells. Cell. 1987 Jan 30;48(2):193–203. doi: 10.1016/0092-8674(87)90423-5. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Chen J. M., Parsons S. J., Parsons J. T. Local degradation of fibronectin at sites of expression of the transforming gene product pp60src. Nature. 1985 Jul 11;316(6024):156–158. doi: 10.1038/316156a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Danilov Y. N., Juliano R. L. Phorbol ester modulation of integrin-mediated cell adhesion: a postreceptor event. J Cell Biol. 1989 May;108(5):1925–1933. doi: 10.1083/jcb.108.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue J. E., Martin G. S. Phosphorylation of talin at tyrosine in Rous sarcoma virus-transformed cells. Mol Cell Biol. 1987 Jan;7(1):371–378. doi: 10.1128/mcb.7.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower S. K., Kronheim S. R., March C. J., Conlon P. J., Hopp T. P., Gillis S., Urdal D. L. Detection and characterization of high affinity plasma membrane receptors for human interleukin 1. J Exp Med. 1985 Aug 1;162(2):501–515. doi: 10.1084/jem.162.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallis B., Prickett K. S., Jackson J., Slack J., Schooley K., Sims J. E., Dower S. K. IL-1 induces rapid phosphorylation of the IL-1 receptor. J Immunol. 1989 Nov 15;143(10):3235–3240. [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979 Sep;18(1):193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Hirst R., Horwitz A., Buck C., Rohrschneider L. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Kaur P., Saklatvala J. Interleukin 1 and tumour necrosis factor increase phosphorylation of fibroblast proteins. FEBS Lett. 1988 Dec 5;241(1-2):6–10. doi: 10.1016/0014-5793(88)81019-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Aebersold R. H., Kent S. B., Varma M., Leavitt J. Molecular cloning and characterization of plastin, a human leukocyte protein expressed in transformed human fibroblasts. Mol Cell Biol. 1988 Nov;8(11):4659–4668. doi: 10.1128/mcb.8.11.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Matsushima K., Shiroo M., Kung H. F., Copeland T. D. Purification and characterization of a cytosolic 65-kilodalton phosphoprotein in human leukocytes whose phosphorylation is augmented by stimulation with interleukin 1. Biochemistry. 1988 May 17;27(10):3765–3770. doi: 10.1021/bi00410a037. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Kilian P. L., Lewis J. C., Paganelli K. A., Chizzonite R. A. The interleukin 1 receptor. Dynamics of interleukin 1 binding and internalization in T cells and fibroblasts. J Immunol. 1987 May 1;138(9):2906–2912. [PubMed] [Google Scholar]
- Pasquale E. B., Maher P. A., Singer S. J. Talin is phosphorylated on tyrosine in chicken embryo fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5507–5511. doi: 10.1073/pnas.83.15.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Lachman L. B., Mainardi C. L., Kang A. H. Interleukin 1 stimulation of collagenase production by cultured fibroblasts. J Exp Med. 1983 Feb 1;157(2):801–806. doi: 10.1084/jem.157.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Raghow R., Stricklin G. P., Poppleton H., Seyer J. M., Kang A. H. Modulation of fibroblast functions by interleukin 1: increased steady-state accumulation of type I procollagen messenger RNAs and stimulation of other functions but not chemotaxis by human recombinant interleukin 1 alpha and beta. J Cell Biol. 1988 Feb;106(2):311–318. doi: 10.1083/jcb.106.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qwarnstrom E. E., Page R. C., Gillis S., Dower S. K. Binding, internalization, and intracellular localization of interleukin-1 beta in human diploid fibroblasts. J Biol Chem. 1988 Jun 15;263(17):8261–8269. [PubMed] [Google Scholar]
- Qwarnström E. E., MacFarlane S. A., Page R. C. Effects of interleukin-1 on fibroblast extracellular matrix, using a 3-dimensional culture system. J Cell Physiol. 1989 Jun;139(3):501–508. doi: 10.1002/jcp.1041390308. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin in cell adhesion and invasion. Cancer Metastasis Rev. 1984;3(1):43–51. doi: 10.1007/BF00047692. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Sims J. E., Acres R. B., Grubin C. E., McMahan C. J., Wignall J. M., March C. J., Dower S. K. Cloning the interleukin 1 receptor from human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8946–8950. doi: 10.1073/pnas.86.22.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J. E., March C. J., Cosman D., Widmer M. B., MacDonald H. R., McMahan C. J., Grubin C. E., Wignall J. M., Jackson J. L., Call S. M. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science. 1988 Jul 29;241(4865):585–589. doi: 10.1126/science.2969618. [DOI] [PubMed] [Google Scholar]
- Turner C. E., Pavalko F. M., Burridge K. The role of phosphorylation and limited proteolytic cleavage of talin and vinculin in the disruption of focal adhesion integrity. J Biol Chem. 1989 Jul 15;264(20):11938–11944. [PubMed] [Google Scholar]
- Werb Z., Hembry R. M., Murphy G., Aggeler J. Commitment to expression of the metalloendopeptidases, collagenase and stromelysin: relationship of inducing events to changes in cytoskeletal architecture. J Cell Biol. 1986 Mar;102(3):697–702. doi: 10.1083/jcb.102.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- de Arruda M. V., Watson S., Lin C. S., Leavitt J., Matsudaira P. Fimbrin is a homologue of the cytoplasmic phosphoprotein plastin and has domains homologous with calmodulin and actin gelation proteins. J Cell Biol. 1990 Sep;111(3):1069–1079. doi: 10.1083/jcb.111.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]