Abstract
Background
The autosomal recessive hyper IgE syndrome (AR-HIES) due to Dedicator Of Cytokinesis 8 (DOCK8) deficiency shares clinical features with the autosomal dominant HIES (AD-HIES) due to Signal Transducer and Activator of Transcription 3 (STAT3) mutations, including recurrent infections and mucocutaneous candidiasis, suggestive of Th17 cell dysfunction. The mechanisms underlying this phenotypic overlap are unclear.
Objective
We sought to elucidate common mechanisms operative in the different forms of HIES.
Experimental Design
We analyzed the differentiation of CD4+ T helper (Th) cell subsets in control and DOCK8-deficient subjects. We also examined the role of DOCK8 in regulating STAT3 activation in T cells.
Methods
Th cell differentiation was analyzed by ELISA, flow cytometry and real time PCR measurements of cytokines and Th cell transcription factors. The interaction of DOCK8 and STAT3 signaling pathways was examined by flow cytometry, immunofluorescence, co-immunoprecipitation and gene expression analysis.
Results
There was a profound block in the differentiation of DOCK8-deficient naïve CD4+ T cells into Th17 cells. A missense mutation that disrupts DOCK8 guanine exchange factor (GEF) activity while sparing protein expression also impaired Th17 cell differentiation. DOCK8 constitutively associated with STAT3 independent of GEF activity, while it regulated STAT3 phosphorylation in a GEF activity-dependent manner. DOCK8 also promoted STAT3 translocation to the nucleus and induction of STAT3-dependent gene expression.
Conclusion
DOCK8 interacts with STAT3, regulates its activation and the outcome of STAT3-dependent Th17 differentiation. These findings may explain the phenotypic overlap between DOCK8 deficiency and AD-HIES.
Keywords: Cdc42, DOCK8, Guanine Nucleotide Exchange Factor, Hyper IgE Syndrome, Mucocutaneous Candidiasis, SOCS3, STAT3, Th17
Introduction
The autosomal recessive form of the hyper IgE syndrome (AR-HIES) is a combined primary immunodeficiency disease (PID) characterized by susceptibility to viral and bacterial infections, mucocutaneous candidiasis, atopic dermatitis and food allergy, virus-induced malignancies and autoimmunity 1–4. Most subjects with this disorder suffer from loss-of-function lesions in DOCK8. The latter encodes Dedicator Of Cytokinesis 8 (DOCK8), a guanine nucleotide exchange factor (GEF) member of the DOCK180 superfamily of guanine-nucleotide exchange factors 5. DOCK8 coordinates the actin cytoskeleton response to mitogenic and chemokine signals through the reversible activation of small G proteins, most notably cell division cycle 42 (Cdc42) 6–9. Patients with DOCK8 deficiency carry deletions, splice junction mutations, premature stop codons and very rarely missense mutations in DOCK8, leading to absent or trace amounts of expressed DOCK8 protein 2, 3, 10–16. Somatic reversions of some mutations may lead to partial expression of DOCK8 protein in some cell lineages but not others 13.
Several features of DOCK8 deficiency overlap with those of the autosomal dominant form of HIES (AD-HIES) due to loss of function mutations in the gene encoding Signal Transducer and Activator of Transcription 3 (STAT3) 1, 17–20. Subjects in both disorders exhibit high serum IgE, eczema, recurrent staphylococcal skin abscesses, frequent upper and lower respiratory tract infections, candidiasis, and hypereosinophilia. In particular, subjects with AD-HIES manifest a profound block in Th17 differentiation, reflecting the requirement for STAT3 in this process 21–24. Those with AR-HIES were also shown to exhibit defective Th17 differentiation18. However, the subjects studied were very few in number and included both DOCK8 deficient and sufficient individuals, making it difficult to definitively attribute Th17 deficiency to the absence of DOCK8. Furthermore, the status of STAT3 activation in DOCK8 deficiency remains poorly defined. In this study, we have sought to specifically examine the impact of DOCK8 on STAT3 activation and STAT3-dependent Th17 cell differentiation. Our studies identify a novel function for DOCK8 in amplifying STAT3 activation in GEF-dependent manner.
MATERIAL AND METHODS
Subjects
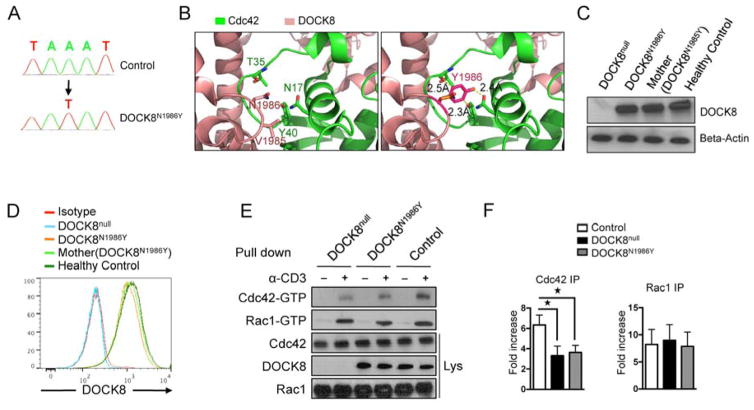
A child with functional DOCK8 deficiency (Pt1; Table E1 in the Online Repository) originally presented at 4 years of age with a history of recurrent upper and lower respiratory tract infections, mucocutaneous candidiasis, recurrent oral herpes infection and hepatomegaly. She had elevated serum IgE and decreased IgM concentrations, peripheral blood eosinophilia, and CD4 T cell lymphopenia (Table E1 in the Online Repository). Her liver enzymes were mildly increased (70–100 U/L), but her liver biopsy was diagnostic of cirrhosis. Sanger sequencing analysis of DOCK8 revealed a missense mutation in exon 45 (c.5956 A>T), resulting in a N1986Y substitution in DOCK8 (see Results Section). She was given a diagnosis of functional DOCK8 deficiency, and started on anti-bacterial and anti-fungal prophylaxis and monthly intravenous immunoglobulin infusions. On follow-up, her liver and spleen were found enlarged secondary to portal hypertension, and endoscopic evaluation revealed portal hypertensive gastropathy and esophageal varicoses. She successfully underwent bone morrow transplantation at the age of 7 years, with full engraftment. However, she passed away 4 months after her transplant because of decompensated liver disease and encephalopathy triggered by infection.
Sixteen other patients with DOCK8-deficiency (1–9 year old, 6 males/10 females, followed up over 6 months-6 years) were included in the study. Diagnosis was established by clinical history, corroborated by flow cytometric analysis of DOCK8 expression in lymphocytes and confirmed by mutational analysis of DOCK8 (Table E1 in the Online Repository). Patient recruitment and the studies reported herein were approved by Institutional Review Board at the Boston Children’s Hospital and by the local ethics committees at the institutions of the respective referring physicians. Written informed consent was obtained from participating families. Healthy parents of subjects or healthy control were included as control.
Protein Modeling
The N1986Y mutation was generated from the crystal structure of DOCK8 7 in Coot 25 using the same side chain torsion angle in residue N1986. The molecular representation was displayed in Pymol 26.
STAT3 phospho-flow assay
Cytokine (IL-6 or IL-21) induced STAT3 phosphorylation was evaluated by flow cytometry 18. Briefly, T cell blasts were expanded from PBMCs by treatment with CD2/CD3/CD28 mAbs for 5 days in the presence of IL-2 (100ng/ml). T cell blasts were treated with different concentrations of the indicated cytokines and time intervals. The cells were then fixed in 4% paraformaldehyde, permeabilized by treatment with 90% methanol, stained with anti-CD3, -CD4 (Biolegend), and -pSTAT3 (Y705) mAbs (BD bioscience) and evaluated for STAT3 phosphorylation by FACS FORTESSA cell analyzer (BD bioscience) 18.
pSTAT3 imaging
Subcellular localization of pSTAT3 was determined by confocal microscopy as described.14 Briefly, T cell blast were spun down over coverslips coated with poly-D-lysine (50 μg/ml; Sigma) and either sham coated (PBS) or coated with anti-CD3 mAb (2 μg/ml in PBS overnight) and incubated for 30 min at 37 °C in the absence or presence of added IL-6 (20ng/ml). The cover slips were then washed with PBS, and the cells were fixed with 4% PFA, permeabilized with 0.2% saponin, and blocked with 4% BSA. They were stained with anti-CD4 mAb, anti pSTAT3 mAb and Prolong Gold Antifade Reagent with DAPI (Invitrogen) and evaluated by confocal microscopy 14.
Rac 1 and cdc42 pull down assays
Rac1 and cdc42 pull down assays were carried out using Pierce Thermo Scientific pull down assay kits. Briefly, T cells from control, DOCK8N1986Y and DOCK8null patients were either left untreated or were treated with 1 μg/m anti-CD3 mAb for 30 min. Cell lysates were derived and precipitated with a chimeric protein composed of glutathione S-transferase fused with the GTPase-binding domain of p21-activated kinase (GST1-PAK1). The precipitates and aliquots of the total cell extracts were subjected to immunoblot analysis using anti-Rac1 or anti Cdc42 mAbs 14, 27.
Transient transfection assays
cDNA encoding Flag-tagged human DOCK8 isoform 1 and hemagglutinin (HA)-tagged human STAT3 isoform 1 were obtained from Origene and GeneCopoeia, respectively. Mutagenesis of the respective cDNA clone was carried out using QuikChange II and Q5 site directed mutagenesis kits (Agilent Technologies and New England Biolabs, respectively). Plasmids were transfected into Jurkat or HEK293 cells by electroporation. At 36h post transfection, the cells were lysed with buffer containing 0.75% NP-40. Immunoprecipitation and immunoblotting was carried out as described in the Results section.
Autoantibody array
Plasma aliquots from patient and control subjects were analyzed using microarrays spotted with 84 autoantigens (University of Texas Southwestern Medical Center, Genomic and Microarray Core Facility), as described 28. Data was normalized to healthy controls. A value of 1 (white) is equal to the control average + 1 standard deviation (SD). A value of >1 (red) or <1 (blue) is more or less than 1 SD above or below the healthy control mean, respectively.
Immunoprecipitation
Cellular lysates were derived and precleared by incubation with Protein G Dynabeads (Life Technologies), followed by immunoprecipitation with Protein G Dynabeads and the indicated antibodies. MOPC21 mAb was used as an IgG isotype control in immunoprecipitation studies. Immunoprecipitates were resolved by SDS-PAGE, transferred to PVDF membranes and immunoblotted with the indicated antibodies as previously described 3.
Statistical analysis
Significance was calculated by using both parametric and nonparametric methods (One and two way ANOVA, Student’s T test and Man Whitney-U test). A p value of <0.05 was considered significant.
Other Methods
Information on additional real time PCR analysis, flow cytometry and intracellular staining reagents, antibodies, ELISA, Th cell differentiation, immunoblotting and chromatin immunoprecipitation (ChIP) assays is provided in the Methods section in this article’s Online Repository at www.jacionline.org.
RESULTS
DOCK8 deficiency profoundly impairs Th17 Cell differentiation
Our previous studies demonstrated defective differentiation of AR-HIES T cells into Th17 cells, but the genotype of those patients was undetermined 18. To determine the impact of DOCK8 deficiency on Th17 cell differentiation, we examined the capacity of naïve CD4+ T cells isolated from patients with complete DOCK8 deficiency (DOCK8null) and control subjects to differentiate in vitro into Th17 cells. DOCK8-sufficient naïve CD4+ T cells effectively differentiated into Th17 cells when stimulated with anti-CD2/CD3/CD28 mAbs in the presence of pro-Th17 polarizing cytokines. In contrast, DOCK8null naïve CD4+ T cells failed to differentiate into Th17 cells, as revealed by profoundly decreased RORC transcripts, encoding the Th17 lineage-specifying transcription factor RORγt, as well as IL17 transcripts and IL-17 protein in patient as compared to control T cells (Figure 1A–C)18, 29. Ineffective Th17 cell differentiation was not corrected by using a combination of phorbol myristate acetate (PMA) and Ionomycin to bypass surface receptor activation, indicating that the impact of DOCK8 on Th17 differentiation extended downstream of T cell receptor stimulation.
Figure 1.
DOCK8 deficiency impairs Th17 cell differentiation. A–C. Expression of RORC and IL17A transcripts (A, B), and IL-17A (C) by naïve CD4+ T cells from control and DOCK8-deficient subjects differentiated under polarizing Th17 cell conditions then either left unstimulated (medium) or stimulated with anti(α)-CD2/CD3/C28 mAbs or PMA+ Ionomycin (Io). D–F. Expression of TBX21 and IFNG transcripts (D, E), and IFN-γ (F) in naïve T cells differentiated under Th1 cell polarizing conditions and stimulated as in A–C. G–L. Expression of GATA3 and IL4 transcripts (G, H), IL-4 and IL-5 (I, J), IL9 transcripts (K) and IL-9 (L) in naïve CD4+ T cells differentiated under Th2 cell polarizing conditions and stimulated as in A–C. Results are representative of at least two independent experiments. *p<0.05, **p<0.01 and ****p<0.0001 by one way ANOVA and post test analysis.
To determine whether the defect in Th17 differentiation reflected a generalized abnormality in Th cell differentiation, naïve CD4+ T cells of patient and control subjects were analyzed for Th1, Th2 and Th9 differentiation in the presence of T cell mitogens and the respective polarizing cytokines. Results revealed that TBX21 transcripts, encoding the master Th1 cell differentiation factor T-bet, IFNG transcripts and IFN-γ protein were similarly expressed in Th1 differentiated DOCK8-deficient T cells as compared to controls (Figure 1D–F). While expression of transcripts encoding the master Th2 cytokine GATA3 was similar in Th2 differentiated patient and control T cells, there was a trend towards increased IL4 transcripts as well as increased IL-4 and IL-5 expression by DOCK8-deficient, Th2 differentiated T cells. In contrast, Th9 differentiation was similar in DOCK8-deficient and -sufficient T cells (Figure 1G–L).
A missense mutation in DOCK8 GEF catalytic center recapitulates the phenotype of DOCK8 deficiency and Th17 differentiation defect
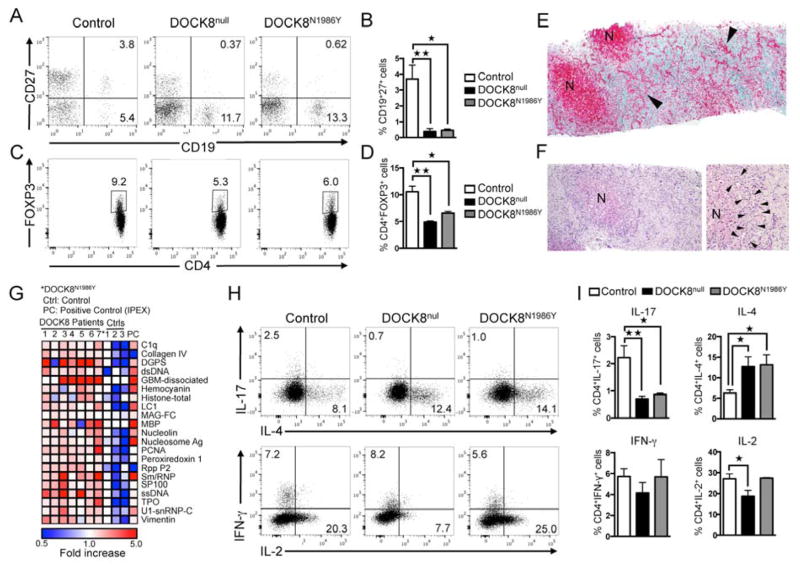
As indicated above, the overwhelming majority of patients with DOCK8 deficiency carry deletions, splice junction mutations or nonsense mutations in DOCK8 that severely compromise or abolish protein expression 11. Uniquely, one subject (P1) out of 61 patients whom we have identified with verified DOCK8 mutations carried a missense mutation (c.5956 A>T), resulting in a N1986Y amino acid substitution in the GEF catalytic loop, immediately next to the invariant V1985 nucleotide sensor (Figure 2A) 30, 31. The mutant tyrosine residue was determined to be deleterious by POLYPHEN2 (score 1.0) and SIFT (score 0.05) analyses 32, 33. The human N1986Y mutation was modeled onto the mouse DOCK8 structure in complex with Cdc42 7. The Y1986 side chain was predicted by protein modeling onto the mouse DOCK8 structure in complex with Cdc42 to create steric clash with Cdc42 at several residues including residue T35 at the Switch 1 region, N17 and Y40 (Figure 2B) 7. Expression of the mutant protein was preserved, as revealed by immunoblotting and flow cytometric analysis (Figure 2C, D) 34. However, Cdc42 activation was defective in DOCK8N1986Y-expressing T cells, while Rac1 activation was normal (Figure 2E, F). The N1986Y mutation recapitulated the clinical and immunological features of DOCK8 deficiency including eczema, persistent viral infections and recurrent sinopulmonary infections, failure of memory B cell differentiation, and regulatory T cell deficiency (Figure 3A–D) 12, 14. The patient also suffered from a well-established biliary-type cirrhosis. The pathology was consistent with advanced primary sclerosing cholangitis with no identified microsporidia or cryptosporidia organisms, similar to what has been previously described for some DOCK8-deficient patients and suggestive of an autoimmune process (Figure 3E, F) 4. Consistent with autoimmunity, the patient demonstrated evidence of active auto-antibody production, as previously described for other patients with DOCK8 deficiency (Figure 3G) 12. Importantly, flow cytometric analysis revealed decreased IL-17+ T cells in PBMCs of patient as compared to control subjects, indicating a requirement for DOCK8 GEF activity in Th17 cell differentiation (Figure 3H–I).
Figure 2.
DOCK8N1986Y mutation disrupts GEF catalytic activity and impairs Th17 cell response. A. Sanger sequencing flurograms showing genomic DNA mutation in patient P1 as compared to a healthy control subject (Ctrl). B. Ribbon diagram, produced using PyMOL, of the DOCK8 DHR2-Cdc42 interface, showing the effects of the N1986Y substitution. C, D. Immunoblot (C) and flow cytometric analysis (D) of DOCK8 expression in T cell blasts of a DOCK8-deficient subject (DOCKnull), patient with DOCK8N1986Y, her mother, and a healthy control subject. E. GTP-bound Cdc42 and Rac1 pull-down assay carried out on control, DOCK8null and DOCK8N1986Y T cells at baseline and following activation with aCD3 mAb. F, G. Densitometric analysis of Rac1 and CDC42 precipitates (n=3/group). *p<0.05, by one way ANOVA and post test analysis.
Figure 3.
DOCK8N1986Y mutation recapitulates the phenotype of DOCK8 deficiency and impairs Th17 cell responses. A, B. Flow cytometric analysis of CD19 and CD27 expression (A) and percentage of CD27+CD19+ memory B cells (B) in PBMC of controls, DOCK8null subjects and the DOCK8N1986Y patient. C, D. Flow cytometric analysis (C) and frequencies (D) of CD4+Foxp3+ cells in PBMC of control and DOCK8null subjects and those of the DOCK8N1986Y patient (two separate observations), gated on CD4+ cells. E. Liver histology of DOCK8N1986Y patient, showing bile duct proliferation (arrowheads) and nodular regeneration (N); collagen is stained green, Trichrome stain. F. Left regenerative nodul (N) with irregular outlines due to cholangiolar damage and hypertrophy H&E stain. Right. Cholangioles, along the limiting plate (between arrowheads) show inflammation with scattered lymphocytes and neutrophils, H&E stain. G. Heat map display of autoantibody reactivity against self-proteins in 6 DOCK8 deficient patients (#1–6), DOCK8N1986Y patient (#7*), controls (Ctrls), and a positive control (PC, patient with FOXP3 deficiency). H, I. Flow cytometric analysis (H) and frequencies (I) of IL17, IL-4, IFNγ, and IL-2 expression in peripheral blood CD4+ T cells of controls, DOCK8null subjects and DOCK8N1986Y patient stimulated ex-vivo with PMA+Io then stained for respective cytokine. Results are representative of at least two-three independent experiments. *p<0.05, **p<0.01 by one way ANOVA and post test analysis.
DOCK8 deficiency results in defective STAT3 phosphorylation and nuclear translocation
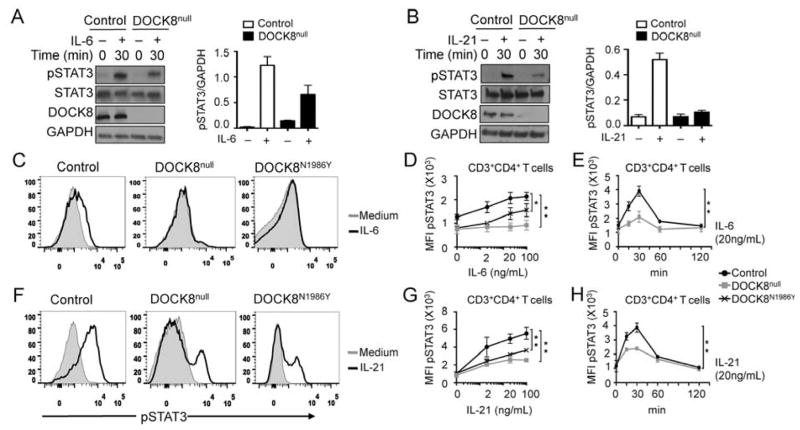
Th17 cell differentiation is orchestrated by STAT3 activating cytokines, including IL-6 and IL-21, acting in synergy with TCR/CD3 signals and TGFβ 35, 36. Both antigen and cytokine receptor signaling are associated with STAT3 phosphorylation, and the two pathways synergistically induce STAT3 activation and translocation to the nucleus. To determine whether DOCK8 deficiency in T cells affected the activation of STAT3 by Th17 polarizing cytokines, we examined STAT3 phosphorylation at the regulatory Y705 residue (pSTAT3) in T cells following IL-6 or IL-21 treatment. Patients with DOCK8 deficiency exhibited a profound defect in pSTAT3 phosphorylation in response to stimulation with both cytokines as revealed by immunoblotting (Figure 4A–B). The role of DOCK8 GEF activity on STAT3 activation was further deduced from studies on pSTAT3 induction in primary DOCK8null and DOCK8N1986Y T cells stimulated with IL-6 or IL-21, as determined by flow cytometry. Results showed that DOCK8null and DOCK8N1986Y CD4+ T cells both exhibited a profound decrease in pSTAT3 induction in response to both IL-6 and IL-21 in a cytokine concentration and treatment time-dependent manner as compared to control CD4+ T cells (Figure 4C–H). Expression of IL-6 receptor alpha chain (IL-6Ra), IL-6R signal transducer (gp130) and IL-21Ra chain was normal, indicating that the defect in STAT3 phosphorylation was not due to deficiency of cytokine-binding and signal transducing receptor subunits (data not shown).
Figure 4.
Defective STAT3 activation in primary DOCK8 mutant T cells. A and B, left panels: Immunoblot analysis of pY705-STAT3 present in lysates of control and DOCK8null T cells at baseline and following treatment for 30 min with IL-6 (A) or IL-21 (B). Densitometric quantitation of pSTAT3 in the respective lanes of Fig 4, A and Fig 4, B, normalized for total GAPDH content in the lysates. Results are shown as means of two experiments. Error bars represent S.E.M. C–H. Flow cytometric analysis and mean fluorescence intensity (MFI) quantitation of pY705-STAT3 in control, DOCK8null and DOCK8N1986Y T cells treated with increasing concentrations of IL-6 (C, D) or IL21 (F, G) for 30 min or 20 ng/ml of IL-6 or IL-21 for the indicated time periods (E, H). Results are representative of at least two-three independent experiments (n=2/group for A, B and 3/group for D, E and G, H). *P < .05 and **P < .01, repeated-measures 2-way ANOVA.
The role of DOCK8 in amplifying cytokine-induced pSTAT3 formation was confirmed using Jurkat human leukemic T cells, which lack DOCK8 expression. Transfection of Jurkat cells with a construct encoding DOCK8, but not empty construct, resulted in marked increase in pSTAT3 formation in response to IL-21 treatment in association with DOCK8 expression (Figure 5A, B). We further compared the activity of DOCK8N1986Y to wild-type DOCK8 protein (DOCK8WT) using the Jurkat cell reconstitution system. Results showed that whereas DOCK8WT enabled cytokine-induced pSTAT3 formation, DOCK8N1986Y did not (Figure 5C, D). These results established that DOCK8 acts to amplify STAT3 phosphorylation in response to different activating cytokines in a GEF activity-dependent manner.
Figure 5.

DOCK8 amplifies STAT3 activation in Jurkat T cells by a GEF-dependent mechanism. A. Immunblot analysis of pY705-STAT3 in lysates of Jurkat cells transfected with empty vector or one encoding DOCK8WT and either left untreated or treated with IL-21. B. Densitometric quantitation of pSTAT3 in the respective lanes of A, normalized for total STAT3 protein content (n=2 experiments /group). C. Immunblot analysis of pY705-STAT3 in lysates of Jurkat cells transfected with empty vector or ones encoding Flag DOCK8WT or DOCK8N1986Y then left untreated or treated with IL-6 or IL-21. D. Densitometric quantitation of pSTAT3 in the respective lanes of C, normalized for total GAPDH protein content.
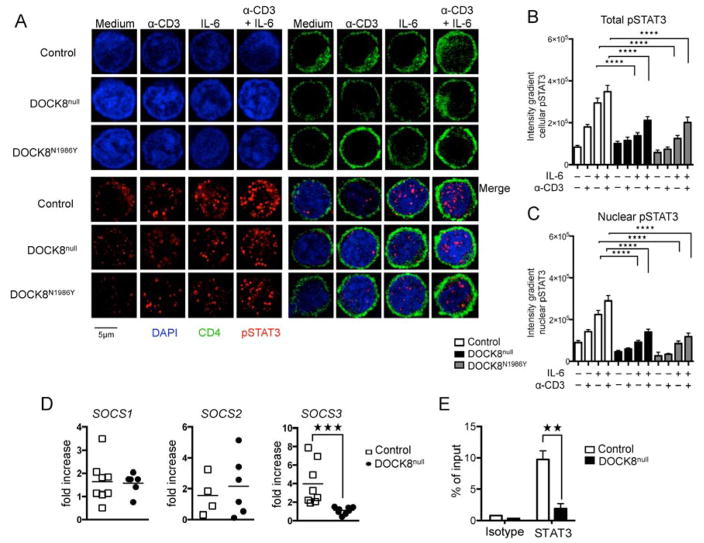
The defect in cytokine-induced STAT3 activation by IL-6 in DOCK8-deficienct T cells was associated with decreased presence of pSTAT3 into the nucleus, as detected by immunofluorescence analysis using anti-pSTAT3 antibody. Stimulation of control T cells with anti-CD3 mAb, and pronouncedly more so with IL-6, resulted in the induction of pSTAT3 and its translocation to the nucleus, and the combination of the two stimuli was additive (Figure 6A–C). In contrast, pSTAT3 induction and nuclear translocation by these two stimuli, alone or in combination, was profoundly decreased in DOCK8 deficient T cells. DOCK8N1986Y T cells also demonstrated defective pSTAT3 induction and nuclear translocation, consistent with a requirement for intact GEF activity for STAT3 activation (Figure 6A–C).
Figure 6.
DOCK8 deficiency impairs pSTAT3 translocation to the nucleus and induction of STAT3-dependent gene expression. A. DOCK8 deficiency impairs activation-induced pSTAT3 translocation to the nucleus. Cells were treated with anti-CD3 mAb, IL-6 or both and stained for nuclear DNA (DAPI) and pSTAT3 and examined by confocal microscopy for total cellular versus nuclear pSTAT3. N=30–50 cells/group; Scale bar: 5 μm. B, C. Quantitation of total cellular versus nuclear pSTAT3 staining. D. RT- PCR analysis of SOCS1, 2 and 3 transcript expression, expressed as fold increase over baseline, in T cell blasts of DOCK8-deficient versus control subjects treated with IL-6 (100 ng/ml; 30 min). E. ChIP analysis of STAT3 binding at the SOCS3 promoter in patient and control T cell blasts either untreated or stimulated with IL-6 (n=5/group). Results are representative of at least two independent experiments. **p<0.01, ***p<0.001 and ****p<0.0001 by one and two way ANOVA with post test analysis.
To examine the consequences of decreased STAT3 activation in DOCK8 mutant cells on gene expression, we analyzed the capacity of IL-6 treatment to induce expression of transcripts encoding Suppressor of Cytokine Signaling 3 (SOCS3), which is dependent on STAT3 activation 37. Results revealed that SOCS3 transcripts were markedly decreased in DOCK8-deficient T cells. In contrast, transcripts encoding SOCS1 and SOCS2, whose expression is not regulated by STAT3 signaling, were unaffected (Figure 6D). Furthermore, Chromatin immunoprecipitation (ChIP) assays revealed decreased binding of pSTAT3 to the SOCS3 promoter in DOCK8-deficient T cells treated with IL-6, consistent with the decreased SOCS3 transcription in these cells (Figure 6E).
DOCK8 associates with STAT3 in a nucleotide sensor-independent manner
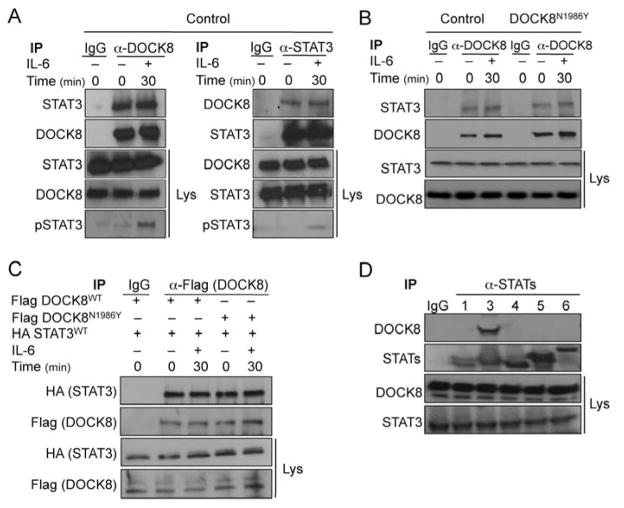
To determine whether DOCK8 and STAT3 associate in a complex, we examined their co-immunoprecipitation with antibodies to the respective protein. Immunoprecipitation of DOCK8 from lysates of control T cells with an anti-DOCK8 Ab resulted in the co-immunoprecipitation of STAT3, as detected by immunoblotting with an anti-STAT3 Ab (Figure 7A, left panel). Treatment with IL-6 resulted in no significant changes in the amounts of STAT3 protein co-precipitating with DOCK8 despite the increase in pSTAT3, indicating that the interaction is independent of Y705-STAT3 phosphorylation (Figure 7A, left panel). In reciprocal experiments, immunoprecipitation with anti-STAT3 antibodies resulted in co-immunoprecipitation of DOCK8, as detected by immunoblotting with an anti-DOCK8 Ab (Figure 7A, right panel).
Figure 7.
DOCK8 associates with STAT3 independent of GEF activity. A. DOCK8 and STAT3 are constitutively associated in primary T cells. DOCK8 (left) and STAT3 (right) immunprecipitates derived from T cell lysates were blotted with anti-STAT3 or anti-DOCK8 Abs, respectively. The immunoblots were reprobed with anti-STAT3 (left) or DOCK8 (right). Lysates were also probed for STAT3, DOCK8 and pSTAT3. B. DOCK8N1986Y associates with STAT3 in primary T cells. DOCK8 immunprecipitates were derived from control and DOCK8N1986Y T cell lysates. The immunoprecipitates and total cellular lysates were probed for STAT3 and DOCK8 by immunblotting. C. Flag-tagged DOCK8WT and DOCK8N1986Y proteins expressed in Jurkat human leukemic T cells co-immunoprecipitate with HA-tagged STAT3. Cells were transfected with indicated constructs and anti-Flag immunoprecipitated derived and probed with the indicated Abs. D. DOCK8 specifically associates with STAT3 but not other STAT proteins. STAT proteins were immunoprecipitated with the respective STAT protein Ab and the immunoprecipitates probed with anti-STAT3 mAb. Results are representative of at least three independent experiments.
We also examined the impact of the DOCK8N1986Y mutation on the association of DOCK8 with STAT3 using patient T cell blasts. Results revealed that STAT3 also co-immunoprecipitated with DOCK8N1986Y (Figure 7B). To confirm the association of DOCK8 with STAT3, we transfected Jurkat human leukemic T cells with constructs encoding Flag-tagged DOCK8WT and DOCKN1986Y and HA-tagged STAT3. Immunoprecipitation studies with an anti-Flag mAb revealed that a HA-reactive 100 KDa protein, consistent with recombinant STAT3, associated equally well with both Flag-tagged DOCK8WT and -DOCKN1986Y proteins. Together, these results confirmed that STAT3 and DOCK8 associate independent of DOCK8 GEF activity (Figure 7C).
To determine whether other STAT proteins interact with DOCK8, we carried out immunoprecipitation studies on T cells blasts using Abs specific for STAT1, -3, -4, -5 and -6. Results revealed that whereas DOCK8 co-immunoprecipitated with STAT3, it failed to do so with other STAT proteins, indicating that DOCK8 specifically interacted with STAT3 (Figure 7D).
DOCK8 directly interacts with STAT3 independent of Y705-STAT3 phosphorylation
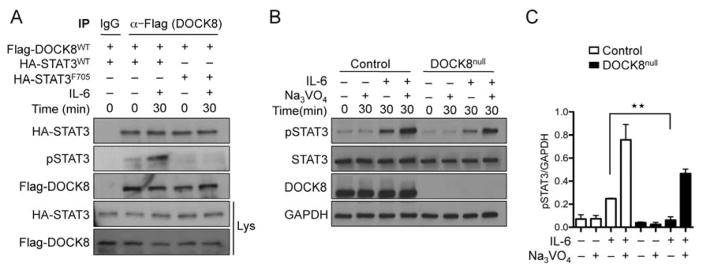
Y705 phosphorylation regulates the transcriptional activity of STAT338, 39. To determine if Y705 phosphorylation was important for the association of STAT3 with DOCK8, we generated cDNA encoding a HA-tagged STAT3 mutant in which the Y705 residue was substituted with phenylalanine (F705). Jurkat cells were transfected with cDNA encoding Flag-tagged DOCK8 together with HA-tagged wild-type (WT) STAT3 protein (HA-STAT3WT) or HA-STAT3F705. Co-precipitation experiments revealed that both HA-STAT3WT and HA-STAT3F705 were recovered in equal amounts upon precipitation of recombinant DOCK8 with anti-Flag mAb (Figure 8A). pSTAT3 was detected in immunoprecipitates of WT but not HA-STAT3F705 transfected cells, consistent with the STAT3F705 mutation acting as a dominant negative fashion similar to what is seen in patients with hyper IgE syndrome 18. These results indicated that the co-association of STAT3 and DOCK8 was independent of the phosphorylation status of STAT3.
Figure 8.
DOCK8 and STAT3 directly associate independent of STAT3-Y705 phosphorylation status. A. DOCK8 co-immunoprecipitates with STAT3F705. Immunoprecipitates were derived from Jurkat cells expressing Flag-DOCK8 and HA-STAT3WT or HA-STAT3F705 proteins using anti-Flag mAbs, and probed with anti-HA, anti-Flag and anti-p705-STAT3 mAb. The lysates were probed with anti-Flag and anti-HA mAbs. B. Rescue of defective STAT3 phosphorylation in DOCK8 deficient cells with Na3VO4 treatment. Primary T cell blasts from control and DOCK8-deficient subjects were either left untreated or treated with Na3VO4 for 30 min then stimulated with IL-6 as indicated. Cellular lysates were immunoblotted with pSTAT3, STAT3, DOCK8 and GAPDH mAbs, as indicated. C. Densitometric quantitation of pSTAT3 formation in experiments carried out as in B (n=3). Results are representative of at least three independent experiments. **p<0.01 by one and two way ANOVA with post test analysis.
STAT3 phosphorylation at Y705 is regulated by tyrosine phosphatases 40, 41. To determine if decreased pSTAT3 formation due to DOCK8 deficiency or DOCK8N1986Y mutation reflected enhanced dephosphorylation of STAT3, we analyzed pY705-STAT3 formation in DOCK8-deficient T cells treated with IL-6 in the absence of presence of the phosphotyrosine phosphatase inhibitor sodium orthovanadate (Na3VO4). Whereas treatment with Na3VO4 on its own induced no discernible increase in pY705-STAT3, it potentiated the IL-6-induced pY705-STAT3 formation in both WT and DOCK8-deficient T cells, with the latter reaching levels close to those observed in WT T cells (Figure 8B, C). These results suggest that DOCK8-STAT3 association may protect the pY705 residue of the latter from dephosphorylation.
Discussion
Our results showed that DOCK8 deficient patients have a profound defect in Th17 differentiation related to decreased STAT3 phosphorylation, translocation to the nucleus and transcriptional activity. DOCK8 was found to constitutively and specifically associate with STAT3 but not other STAT proteins, and to enable activation-induced STAT3 translocation to the nucleus. Furthermore, DOCK8 GEF activity was necessary for optimal STAT3 phosphorylation and Th17 differentiation but not STAT3-DOCK8 interaction. These findings establish a mechanistic basis for the phenotypic convergence of the autosomal dominant and recessive forms of HIES centered on DOCK8 regulation of STAT3.
DOCK8 interacts with the small GTPase Cdc42 through the DHR-2 domain and mediates its activation by stimulating the GTP-GDP exchange reaction 7. DOCK8 deficiency did not affect Rac1 activation, which is compromised in DOCK2 deficiency 27. Importantly, a mutation in the DOCK8 nucleotide sensor loop, DOCK8N1986Y, which disrupted the GEF activity, recapitulated the phenotype of DOCK8 deficiency including impaired STAT3 Y705 phosphorylation and Th17 cell deficiency, indicating an essential role for DOCK8 GEF function in STAT3 activation and disease pathogenesis.
While the promotion of STAT3 phosphorylation by DOCK8 was dependent on the latter’s GEF activity, the physical association of the two proteins was not, evidenced by normal association of STAT3 with DOCK8N1986Y. The association was also independent of STAT3 Y705 phosphorylation status. Nevertheless, DOCK8N1986Y mutation abolished the enhancement of STAT3 phosphorylation induced by DOCK8, indicating a requirement for the GEF activity for this effect. Cdc42 activates downstream kinases, including activated kinase (ACK1), that have been implicated in STAT3 activation, suggesting a role for such kinase(s) in mediating DOCK8-dependent upregulation of STAT3 phosphorylation 42.
Previous studies have shown that DOCK8 links TLR9-MyD88 signaling in B cells to STAT3 activation via a Src-Syk kinase cascade, whereas cytokine induced STAT3 phosphorylation appeared normal 14. DOCK8 has also been implicated in the generation of RORγt+ type 3 innate lymphoid cells (ILC3), and in promoting IL-23-dependent STAT3 activation 43. The failure in earlier studies to detect marked differences in cytokine-induced STAT3 phosphorylation in DOCK8-deficient lymphocytes probably reflects the low concentrations and delayed time courses of cytokine treatment in those studies, which masked the function of DOCK8 in amplifying STAT3 phosphorylation that is more readily detected at higher concentrations and earlier time points (Figure 4E and H) 14. Our present studies expand on these observations in several ways. First, we have demonstrated that DOCK8 amplifies cytokine-dependent STAT3 activation in a GEF activity-dependent manner. We have also demonstrated that DOCK8 and STAT3 physically associate as a signaling module independent of the former’s GEF activity, thus allowing more focused channeling of STAT3 activation via DOCK8. Finally, DOCK8 promotes STAT3 nuclear translocation, an event that may contribute to sustained STAT3 activation in the nuclear compartment.
While both STAT3 mutations in AD-HIES and DOCK8 deficiency impair STAT3 activation and consequently Th17 cell differentiation, the two gene defects differ in their impact. AD-HIES is associated with STAT3-dependent extra-hematopoietic manifestations that are either lacking or uncommon in DOCK8 deficiency 44. DOCK8 function as a signal amplifier of STAT3 activation is particularly relevant in immune cells, where DOCK8 is highly expressed, but not in other tissues, where alternative, compensatory mechanisms for amplifying STAT3 activation may be operative 45. Other phenotypes of DOCK8 deficiency not shared with AD-HIES, such as autoimmunity and susceptibility to viral infections, may also reflect distinct functions of DOCK8 beyond STAT3 activation, such as its regulation of the actin cytoskeleton, chemotaxis and antiviral cytokine production 7, 12, 16, 46.
In conclusion, our studies identify an essential and specific function for DOCK8 in mediating STAT3 activation and nuclear translocation. DOCK8-STAT3 interaction illustrates a novel mechanism for the regulation of STAT signaling by DOCK proteins. Putative interactions between different members of the DOCK and STAT family proteins may play an important role in the regulation of STAT signaling, including the differentiation of Th cell subsets.
Supplementary Material
Key Messages.
DOCK8 Regulates Th17 differentiation by promoting STAT3 phosphorylation and translocation to the nucleus.
DOCK8 constitutively associates with STAT3 in a GEF activity-independent manner, but amplifies STAT3 activation in a GEF activity-dependent manner.
Th17 deficiency may contribute to the heightened susceptibility of DOCK8-deficient subjects to candida and bacterial infections
Acknowledgments
This manuscript is dedicated to the memory of Professor Dr. Işil Berat Barlan (1958–2015). This work was supported by the National Institutes of Health (5R01AI065617), to T.A.C. and a grant from the Scientific and Technological Research Council of Turkey (1059B191300622) to S.K. We thank Drs. Michel Massad and Luigi Notarangelo for critical review of the manuscript.
Abbreviations
- Cdc42
Cell division cycle 42
- DOCK8
Dedicator Of Cytokinesis 8
- GEF
Guanine nucleotide exchange factor
- PBMC
Peripheral blood mononuclear cells
- Rac1
Ras-related C3 botulinum toxin substrate 1
- SOCS1-3
Suppressor of cytokine signaling 1-3
- STAT
Signal Transducer and Activator of Transcription
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Renner ED, Puck JM, Holland SM, Schmitt M, Weiss M, Frosch M, et al. Autosomal recessive hyperimmunoglobulin E syndrome: a distinct disease entity. J Pediatr. 2004;144:93–9. doi: 10.1016/S0022-3476(03)00449-9. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046–55. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124:1289–302. e4. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aydin SE, Kilic SS, Aytekin C, Kumar A, Porras O, Kainulainen L, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options - a review of 136 patients. J Clin Immunol. 2015;35:189–98. doi: 10.1007/s10875-014-0126-0. [DOI] [PubMed] [Google Scholar]
- 5.Cote JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 2007;17:383–93. doi: 10.1016/j.tcb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGhee SA, Chatila TA. DOCK8 immune deficiency as a model for primary cytoskeletal dysfunction. Dis Markers. 2010;29:151–6. doi: 10.3233/DMA-2010-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood. 2012;119:4451–61. doi: 10.1182/blood-2012-01-407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizesko MC, Banerjee PP, Monaco-Shawver L, Mace EM, Bernal WE, Sawalle-Belohradsky J, et al. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2013;131:840–8. doi: 10.1016/j.jaci.2012.12.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ham H, Guerrier S, Kim J, Schoon RA, Anderson EL, Hamann MJ, et al. Dedicator of cytokinesis 8 interacts with talin and Wiskott-Aldrich syndrome protein to regulate NK cell cytotoxicity. J Immunol. 2013;190:3661–9. doi: 10.4049/jimmunol.1202792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Herz W, Ragupathy R, Massaad MJ, Al-Attiyah R, Nanda A, Engelhardt KR, et al. Clinical, immunologic and genetic profiles of DOCK8-deficient patients in Kuwait. Clin Immunol. 2012;143:266–72. doi: 10.1016/j.clim.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelhardt KR, Gertz ME, Keles S, Schaffer AA, Sigmund EC, Glocker C, et al. The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2015;136:402–12. doi: 10.1016/j.jaci.2014.12.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen E, Morbach H, Ullas S, Bannock JM, Massad C, Menard L, et al. Dedicator of cytokinesis 8-deficient patients have a breakdown in peripheral B-cell tolerance and defective regulatory T cells. J Allergy Clin Immunol. 2014;134:1365–74. doi: 10.1016/j.jaci.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jing H, Zhang Q, Zhang Y, Hill BJ, Dove CG, Gelfand EW, et al. Somatic reversion in dedicator of cytokinesis 8 immunodeficiency modulates disease phenotype. J Allergy Clin Immunol. 2014;133:1667–75. doi: 10.1016/j.jaci.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jabara HH, McDonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A, et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol. 2012;13:612–20. doi: 10.1038/ni.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Zahrani D, Raddadi A, Massaad M, Keles S, Jabara HH, Chatila TA, et al. Successful interferon-alpha 2b therapy for unremitting warts in a patient with DOCK8 deficiency. Clin Immunol. 2014;153:104–8. doi: 10.1016/j.clim.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keles S, Jabara HH, Reisli I, McDonald DR, Barlan I, Hanna-Wakim R, et al. Plasmacytoid dendritic cell depletion in DOCK8 deficiency: rescue of severe herpetic infections with IFN-alpha 2b therapy. J Allergy Clin Immunol. 2014;133:1753–5. e3. doi: 10.1016/j.jaci.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, et al. Hyper-IgE syndrome with recurrent infections--an autosomal dominant multisystem disorder. N Engl J Med. 1999;340:692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- 18.Al Khatib S, Keles S, Garcia-Lloret M, Karakoc-Aydiner E, Reisli I, Artac H, et al. Defects along the T(H)17 differentiation pathway underlie genetically distinct forms of the hyper IgE syndrome. J Allergy Clin Immunol. 2009;124:342–8. 8 e1–5. doi: 10.1016/j.jaci.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 20.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–62. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 21.de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–50. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–7. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–6. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renner ED, Rylaarsdam S, Anover-Sombke S, Rack AL, Reichenbach J, Carey JC, et al. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol. 2008;122:181–7. doi: 10.1016/j.jaci.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 26.Delano WL. The PyMol Molecular Graphics System. 2002 Available at http://www.pymol.org.
- 27.Dobbs K, Dominguez Conde C, Zhang SY, Parolini S, Audry M, Chou J, et al. Inherited DOCK2 Deficiency in Patients with Early-Onset Invasive Infections. N Engl J Med. 2015;372:2409–22. doi: 10.1056/NEJMoa1413462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li QZ, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR, et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol. 2007;147:60–70. doi: 10.1111/j.1365-2249.2006.03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Primary immunodeficiency diseases. Report of an IUIS Scientific Committee. International Union of Immunological Societies. Clin Exp Immunol. 1999;118(Suppl 1):1–28. doi: 10.1046/j.1365-2249.1999.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Zhang Z, Roe SM, Marshall CJ, Barford D. Activation of Rho GTPases by DOCK exchange factors is mediated by a nucleotide sensor. Science. 2009;325:1398–402. doi: 10.1126/science.1174468. [DOI] [PubMed] [Google Scholar]
- 31.Kulkarni K, Yang J, Zhang Z, Barford D. Multiple factors confer specific Cdc42 and Rac protein activation by dedicator of cytokinesis (DOCK) nucleotide exchange factors. J Biol Chem. 2011;286:25341–51. doi: 10.1074/jbc.M111.236455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng PC, Henikoff S. Accounting for human polymorphisms predicted to affect protein function. Genome Res. 2002;12:436–46. doi: 10.1101/gr.212802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pai SY, de Boer H, Massaad MJ, Chatila TA, Keles S, Jabara HH, et al. Flow cytometry diagnosis of dedicator of cytokinesis 8 (DOCK8) deficiency. J Allergy Clin Immunol. 2014;134:221–3. doi: 10.1016/j.jaci.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 36.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Badgwell DB, Bevers JJ, 3rd, Schlessinger K, Murray PJ, Levy DE, et al. IL-6 signaling via the STAT3/SOCS3 pathway: functional analysis of the conserved STAT3 N-domain. Mol Cell Biochem. 2006;288:179–89. doi: 10.1007/s11010-006-9137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–8. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 39.Sasse J, Hemmann U, Schwartz C, Schniertshauer U, Heesel B, Landgraf C, et al. Mutational analysis of acute-phase response factor/Stat3 activation and dimerization. Mol Cell Biol. 1997;17:4677–86. doi: 10.1128/mcb.17.8.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ten Hoeve J, de Jesus Ibarra-Sanchez M, Fu Y, Zhu W, Tremblay M, David M, et al. Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol Cell Biol. 2002;22:5662–8. doi: 10.1128/MCB.22.16.5662-5668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Guo A, Yu J, Possemato A, Chen Y, Zheng W, et al. Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T. Proc Natl Acad Sci U S A. 2007;104:4060–4. doi: 10.1073/pnas.0611665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Debidda M, Wang L, Zang H, Poli V, Zheng Y. A role of STAT3 in Rho GTPase-regulated cell migration and proliferation. J Biol Chem. 2005;280:17275–85. doi: 10.1074/jbc.M413187200. [DOI] [PubMed] [Google Scholar]
- 43.Singh AK, Eken A, Fry M, Bettelli E, Oukka M. DOCK8 regulates protective immunity by controlling the function and survival of RORgammat+ ILCs. Nat Commun. 2014;5:4603. doi: 10.1038/ncomms5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woellner C, Gertz EM, Schaffer AA, Lagos M, Perro M, Glocker EO, et al. Mutations in STAT3 and diagnostic guidelines for hyper-IgE syndrome. J Allergy Clin Immunol. 2010;125:424–32. e8. doi: 10.1016/j.jaci.2009.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su HC, Jing H, Zhang Q. DOCK8 deficiency. Ann N Y Acad Sci. 2011;1246:26–33. doi: 10.1111/j.1749-6632.2011.06295.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Q, Dove CG, Hor JL, Murdock HM, Strauss-Albee DM, Garcia JA, et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med. 2014;211:2549–66. doi: 10.1084/jem.20141307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.