Abstract
Although a number of studies have reported that children of depressed, compared to nondepressed, parents exhibit biased attention to sad facial stimuli, the direction of this bias remains unclear; some studies find evidence of preferential attention towards sad faces whereas others find evidence of attention avoidance. In the current study, we used event-related potentials (ERPs) to assess children’s attention to emotional stimuli using a spatial cueing task. We found that children of depressed mothers generally exhibited less attention to sad facial stimuli than children of nondepressed mothers. Across all indices of attention bias (N2pc and SPCN time locked to face onset, P3b time locked to probe onset, reaction times to probes), children of mothers with a history of major depressive disorder (MDD) during the child’s life exhibited less attention to sad faces than children of never depressed mothers. For two of these indices (SPCN and reaction times), the attention biases for the offspring of depressed mothers was not specific to sadness and was observed for all emotional expressions. Group differences in the ERP indices was maintained when controlling for the influence of mothers’ and children’s current symptoms of depression and anxiety, mothers’ history of anxiety disorders, and children’s history of MDD and anxiety disorders, suggesting that the results are specific to mothers’ history of MDD.
Keywords: maternal depression, intergenerational transmission, attention bias, ERP
Scientific Summary
Although there is growing evidence that children of depressed mothers exhibit attentional biases for sad faces, the direction of this bias is unclear. The current study examined ERP indices of biased attention within the context of a spatial cueing task and showed that children of depressed, compared to never depressed, mothers exhibit attentional avoidance of sad faces.
Decades of research have documented the increased risk of depression in children of depressed mothers (for reviews, see Goodman, 2007; Gotlib & Colich, 2014). Yet, the specific mechanisms that place these children at risk remain poorly understood. Theorists (e.g., Goodman, 2007; Goodman & Gotlib, 1999) have proposed that information-processing biases featured in cognitive models of depression may represent a final common pathway of risk for various genetic and environmental influences on the intergenerational transmission of depression. According to cognitive models of depression (e.g., Clark, Beck, & Alford, 1999; Disner, Beevers, Haigh, & Beck, 2011), individuals’ characteristic ways of attending to, interpreting, and remembering stimuli may contribute to the development and maintenance of depression. According to these models, depressed and at-risk individuals should exhibit preferential attention toward depression-relevant stimuli (e.g., sad faces). This attentional bias is hypothesized to be specific to depression-relevant stimuli rather than other types of stimuli (e.g., threat-relevant). This attentional bias is also hypothesized to be manifest as an increase in sustained attention toward, and/or difficulty disengaging attention from, depression-relevant stimuli (e.g., sad faces), rather than a bias in initial orienting of attention. These hypotheses have been supported through various studies of adults and adolescents using both reaction time and eye tracking measures of attentional bias (see Armstrong & Olatunji, 2012; Hankin, Gibb, Abela, & Flory, 2010; Joormann & Arditte, 2014).
To date, however, only three studies have examined attentional biases in school-aged children of depressed mothers. Each has found evidence of attentional biases specifically for sad facial expressions in children of depressed, compared to never depressed, mothers. However, the direction of this bias differed across the studies. Specifically, whereas two of the studies (Joormann, Talbot, & Gotlib, 2007; Kujawa et al., 2011) observed preferential attention toward sad faces in children of mothers with a history of major depressive disorder (MDD), the other study (Gibb, Benas, Grassia, & McGeary, 2009) found evidence of attentional avoidance of sad facial stimuli. Although the former findings are consistent with the adult literature, the latter finding is more consistent with what is observed in the infant literature in which there is clear evidence that infants of depressed mothers spend less time looking at their mothers’ faces than do infants of nondepressed mothers (Boyd, Zayas, & McKee, 2006; Diego et al., 2004; Field, 1984, 1995). Indeed, this attentional avoidance of sad faces has been proposed as an emotion regulation strategy among children of depressed mothers (Bistricky, Ingram, & Atchley, 2011; Termine & Izard, 1988), consistent with current emotion regulation models of attentional allocation (Gross, 2014), though it does not appear sufficient for preventing these children from becoming depressed themselves (Gibb et al., 2009; Harrison & Gibb, 2015).
There are a number of potential reasons for these mixed findings across studies. One difference across studies is that a mood induction was used in the two studies that found evidence of preferential attention toward sad faces in children of depressed mothers (Joormann et al., 2007; Kujawa et al., 2011), but not in the studies that found evidence of attentional avoidance (Boyd et al., 2006; Field, 1995; Gibb et al., 2009). This said, there is evidence that higher levels of depression in children are associated with greater attentional avoidance of sad faces (Gibb et al., 2009; Harrison & Gibb, 2015), suggesting that the presence of a sad mood may not account for differences across studies. A second possibility is that the effects are moderated by child sex, with preferential attention to sad faces being observed in daughters depressed mothers (Joormann et al., 2007; Kujawa et al., 2011) but not sons (Gibb et al., 2009; Kujawa et al., 2011). Finally, a third possibility is methodological: the differences in findings across studies may due, in part, to the method used to assess attentional biases.
Specifically, each of the three studies described above (Gibb et al., 2009; Joormann et al., 2007; Kujawa et al., 2011) used a modified dot probe task (MacLeod, Mathews, & Tata, 1986) in which attentional biases are inferred from reaction times to the appearance of a probe. In this task, pairs of facial expressions containing one emotional (sad, happy, or angry) and one neutral photograph from the same actor are presented briefly on a computer screen. After the faces disappear, a probe appears in the location of one of the faces. Preferential attention for emotional faces (e.g., sad versus neutral) is inferred when participants’ reaction times to the probe are faster when the probe appears in the location of the emotional face than in the location of the neutral face. This reasoning is predicated on the assumption that reaction times to the probe will be faster if one’s attention is already allocated to that side of the computer screen. This paradigm has been used successfully in countless studies in cognitive and clinical psychology (for a review, see Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007). However, recent research has raised questions about the reliability of reaction time indices of attentional bias (see Gibb, McGeary, & Beevers, 2016). Specifically, reaction time indices of attentional bias exhibit poor split-half and retest reliability (Brown et al., 2014; Kappenman, Farrens, Luck, & Proudfit, 2014; Kappenman, MacNamara, & Proudfit, 2015; Price et al., 2015; Schmukle, 2005; Staugaard, 2009; Waechter et al., 2014), which can increase risk for Type I and Type II errors. Another limitation of this approach is that, at best, it provides only a snap shot of attentional processes – where attention was allocated at the precise moment the probe appeared (e.g., 1000ms after stimulus onset). However, attention is a multi-faceted construct that can be decomposed into a number of distinct processes including involuntary capture of attention by a salient stimulus, voluntary shift of attention toward a stimulus, disengagement of attention, and inhibition of return to a recently-attended location.
To address these limitations, we used event related potentials (ERPs) to index attentional bias in a spatial cueing task (Posner, 1980). We chose this task rather the dot probe because depression is hypothesized to be specifically associated with biases in sustained attention and difficulty disengaging attention (Disner et al., 2011; Gotlib & Joormann, 2010; Joormann & Arditte, 2014) and Posner’s task captures initial orienting versus disengagement of attention from a cued location. Because this task uses only a single cue (e.g., emotional face) presented on each trial, researchers have suggested that it may provide a better assessment of attentional disengagement than the dot probe task in which two stimuli are presented simultaneously (Fox, Russo, Bowles, & Dutton, 2001; Rossignol, Philippot, Bissot, Rigoulot, & Campanella, 2012). For the Posner spatial cueing task, an emotional face (cue) is presented on either the left or right side of a computer screen for a given amount of time (e.g., 1000 ms). After the face disappears, a probe (target) appears on either the same side of the screen or on the opposite side of the screen. In this paradigm, the face is considered to be a valid predictor of the location of the probe if the probe appears on the same side of the screen as the face and an invalid predictor if the probe appears on the opposite side of the screen as the face. This design allowed us to examine ERP components time-locked to the onset of the face or to the probe.
To assess initial allocation of attention to the presentation of faces, we focused on the N2pc, which is an early emerging ERP component (~125–225 ms after stimulus onset) consisting of a larger negativity over occipito-parietal sites in the hemisphere contralateral to the attended stimulus (Luck, 2012; Woodman & Luck, 1999). To assess sustained attention to the presentation of the faces, we focused on the sustained posterior contralateral negativity (SPCN), which emerges approximately 400 ms after stimulus onset, is maximal at occipito-parietal sites in the hemisphere contralateral to the attended stimulus, and is thought to reflect the maintenance of information in visual short-term memory (Jolicœur, Brisson, & Robitaille, 2008; Jolicœur, Sessa, Dell’Acqua, & Robitaille, 2006; Robitaille & Jolicœur, 2006). Although no study to our knowledge has yet examined the N2pc or SPCN in children, a number of studies have focused on these components in studies of attentional biases to emotional stimuli in adults (e.g., Eimer, Eimer, Kiss, & Kiss, 2007; Feldmann-Wüstefeld, Schmidt-Daffy, & Schubö, 2011; Holmes et al., 2009; Kappenman et al., 2014; 2015; Pourtois & Vuilleumier, 2006).
In addition to examining ERP indices time-locked to the onset of the face, we also examined the P3b ERP component time-locked to the onset of the probe, which provides an index of disengagement of attention from the emotional face (i.e., cognitive resources required to shift attention from the location of the emotional face to the location of the probe). The P3b is maximal at parietal sites along the midline (i.e., Pz) approximately 300 ms after stimulus onset. In a typical oddball paradigm, the P3b is larger for rare stimuli than for frequent stimuli (Polich, 2012). Within the context of the Posner spatial cueing task, where rare or “invalid” trials are those in which the probe appears on the opposite side of the screen as the emotional face (~20% of trials), the P3b is larger for invalidly-cued than validly-cued probes. A larger P3b to the probe is thought to reflect greater cognitive resources required to disengage from a previously attended location of the cue (Fichtenholtz, Hopfinger, Graham, Detwiler, & LaBar, 2007; Hugdahl & Nordby, 1994; Pollak & Tolley-Schell, 2003; Stormark, Nordby, & Hugdahl, 1995; Wright, Geffen, & Geffen, 1995). Importantly, this design also allows an examination of differences in attentional disengagement across emotional stimuli. For example, using a Posner spatial cueing task, Pollak and Tolley-Schell (2003) found that children with a history of physical abuse exhibit a larger P3b to invalidly-cued probes following angry, but not happy, faces suggesting greater difficulty disengaging from angry faces in these children. To date, however, no studies have examined the P3b as an index of attentional bias in children of depressed mothers.
Based on traditional cognitive models of depression (Clark et al., 1999; Disner et al., 2011) and the findings of two earlier studies of attentional biases in children of depressed mothers (Joormann et al., 2007; Kujawa et al., 2011), one would expect that children of depressed mothers would exhibit preferential attention toward sad facial stimuli. However, based on our earlier findings in children of depressed mothers (Gibb et al., 2009), as well as findings from the infant literature (Boyd et al., 2006; Diego et al., 2004; Field, 1984; 1995), we predicted that children of mothers with a history of depression would exhibit attentional avoidance of sad faces. Consistent with cognitive models, we predicted that this bias would be specific to sad faces and would not be observed for angry or happy faces, and would be limited to indices of sustained attention and attentional disengagement, not the initial orienting of attention. Specifically, we predicted that although children of mothers with a history of MDD during the child’s life, compared to children of never depressed mothers, would not differ in the initial allocation of attention (N2pc), they would exhibit less sustained attention to (SPCN), and difficulty disengaging from (P3b), sad faces
We followed these primary analyses with a series of additional analyses to examine the specificity and robustness of the findings. First, consistent with the hypothesis that attentional biases may be trait-like markers of risk in children of depressed mothers rather than simply correlates of mothers’ current depression, we predicted that the findings would be maintained even after statistically controlling for the influence of current depression in mothers. Second, consistent with the hypothesis that these attentional biases are linked specifically to mothers’ history of depression, we predicted that the relations would be maintained when statistically controlling for the influence of mothers’ history of anxiety disorders and current symptoms of anxiety. Finally, we predicted that the link between maternal MDD history and children’s attentional biases would be maintained even after statistically controlling for the influence of children’s current symptoms of depression and anxiety and their past histories of depressive or anxiety disorders, suggesting that the biases are not simply correlates or consequences of children’s own depression or anxiety.
Method
Participants
Participants in the present study were 133 mother-child dyads recruited for a study examining the intergenerational transmission of depression.1 To qualify for the study, mothers were required to either meet criteria for MDD during the child’s lifetime according to the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV; American Psychiatric Association, 1994) (n = 69) or have no lifetime diagnosis of any DSM-IV mood disorder and no current Axis I diagnosis (n = 64). Exclusion criteria for both groups included the presence of psychosis, a history of alcohol or substance dependence within the last six months, or lifetime history of bipolar disorder. The average age of children in our sample was 12.59 years (SD = 1.92, Range = 9–17) and 54% were male. In terms of race/ethnicity, 86% were Caucasian, 9% were biracial, 4% were African American, and the remaining 1% were from other racial/ethnic groups. Descriptive statistics for the groups are presented in Table 1.
Table 1.
Demographic and clinical characteristics of the sample
| MDD mothers |
Never depressed mothers |
reffect size | |
|---|---|---|---|
| Child Age | 12.94 (2.04) | 12.20 (1.70) | .19* |
| Child Sex (% Girls) | 42% | 50% | −.08 |
| Child Race (% Caucasian) | 77% | 95% | −.26** |
| Mother Lifetime Anxiety Disorder | 37% | 5% | .39** |
| Child Lifetime MDD | 16% | 2% | .25** |
| Child Lifetime Anxiety Disorder | 22% | 8% | .20* |
| BDI-II | 7.28 (8.49) | 3.06 (5.11) | .30** |
| BAI | 7.63 (7.55) | 2.08 (3.12) | .46** |
| CDI | 3.54 (5.14) | 2.15 (4.21) | .18* |
| MASC | 27.26 (20.08) | 19.91 (18.66) | .19** |
| VAS-Sadness | 24.52 (13.37) | 21.82 (15.47) | .09 |
| VAS-Anxiety | 27.98 (22.41) | 28.19 (22.57) | −.01 |
Note. MDD = Major depressive disorder. BDI-II = Beck Depression Inventory-II. BAS = Beck Anxiety Inventory. CDI = Children’s Depression Inventory. MASC = Multidimensional Anxiety Scale for Children. VAS = Visual Analogue Scale.
p < .05.
p < .01.
Measures
We used the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 1995) and the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997) to assess for current and past DSM-IV Axis I disorders in mothers and children, respectively. Separate interviewers administered the SCID-I and K-SADS-PL. For the K-SADS-PL, the same interviewer conducted the interview with mothers and children separately. Women were coded as having a history of MDD during the child’s lifetime (n = 69) or having no lifetime history of any mood disorder (n = 63). Two of the mothers met criteria for current MDD at the time of the assessment. Of the children, 12 met criteria for lifetime MDD (11 of whom had a mother with a history of MDD), with none of the children meeting criteria for a current depressive disorder. Inter-rater reliability was assessed for a subset of 20 SCID and 20 K-SADS interviews and reliability for diagnoses of MDD was excellent (all κs = 1.00).
We used the Beck’s Depressive Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) and the Children’s Depression Inventory (CDI; Kovacs, 1981) to assess mothers’ and children’s current levels of depressive symptoms, respectively. We used the Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988) and the Multidimensional Anxiety Scale for Children (MASC; March, Parker, Sullivan, Stallings, & Conners, 1997) to assess mothers’ and children’s current levels of anxiety, respectively. Each of these measures have demonstrated excellent reliability and validity in previous research (e.g., Beck et al., 1988; 1996; March et al., 1997; March & Sullivan, 1999; Smucker, Craighead, Craighead, & Green, 1986) and they all exhibited good internal consistency in this study (BDI-II: α = .92, CDI: α = .88, BAI: α = .89, MASC: α = .92). In addition, we used a visual analogue scale (VAS) to assess children’s levels of sadness and anxiety just prior to completing the attentional bias task. To assess current levels of sadness, children were presented with a 100 mm line anchored on the left side by “very happy” and on the right side by “very sad” and, for anxiety, the anchors were “very calm” and “very anxious.”Children were asked to indicate their current mood by placing a mark on the line between the two anchors for each scale. Levels of current sadness and anxiety were calculated by measuring the distance from the left end of the scale to the point marked by the child.
We used a spatial cueing task (cf. Posner, 1980) administered using E-Prime (Psychological Software Tools, Pittsburgh, PA) to assess children’s attentional biases for facial displays of emotion. Each trial started with a fixation cross presented in the middle of the screen for 500ms. Next, children saw one of 48 grey-scaled facial expression of emotion (angry, happy, sad, or neutral), which were cropped so that only the faces were visible (i.e., no hair or clothing). The facial stimuli were presented on either the left or right side of the computer screen drawn from a standardized stimulus set (Ekman & Friesen, 1976).The facial expression remained on the screen for 1000ms after which it disappeared and a probe (*) appeared in either the same location as the face (valid trials, 79%) or the opposite side of the screen (invalid trials, 21%). Participants were asked to indicate the location of the probe by pressing one of two buttons on a game controller. The inter-trial interval randomly varied between 750ms and 1250ms. Trials with response errors were excluded (1.87%) as were trials with response times less than 150 ms or greater than 1500 ms (3.43%) from both behavioral and ERP analyses.
During the task, continuous EEG was recorded using a custom cap and the BioSemi ActiveTwo system (Amsterdam, Netherlands). The EEG was digitized at 24-bit resolution with a sampling rate of 512 Hz. Recordings were taken from 34 scalp electrodes based on the 10/20 system. The electrooculogram was recorded from four facial electrodes. Off-line analysis was performed using the Matlab extension, EEGLAB (Delorme & Makeig, 2004) and the EEGLAB plug-in, ERPLAB (Lopez-Calderon & Luck, 2014). All data were re-referenced to the average of the left and right mastoid electrodes and band-pass filtered from 0.1 Hz to 30 Hz. EEG data were processed using both artifact rejection and correction. First, large and stereotypical ocular components were identified and removed using independent component analysis (ICA) scalp maps (e.g. eye blinks project mainly from frontal regions) (Jung et al., 2000). Artifact detection and rejection was then conducted on epoched uncorrected data files to identify and remove trials containing blinks and large eye movements within 200ms of stimulus presentation. Epochs with large artifacts (greater than 100µV) were excluded from analysis. Seven subjects were excluded from the analyses for exceeding the artifact rejection threshold (50% of trials). Of these subjects, 4 were from the MDD group and 3 were from the no MDD group. Excluded subjects did not differ significantly from included subjects on any clinical or demographic variables, including mother MDD history (lowest p = .14). For the 133 subjects included in the analyses, the number of included trials did not differ significantly between the mother MDD (M = 759.49, SD = 126.07) and no MDD (M = 786.30, SD = 116.63) groups, t(129) = −1.27, p = .21, reffect size = −.11. The interval from −200 ms to 0 ms served as the baseline for ERPs. For ERPs elicited by the facial cues, we focused on the N2pc, which reflects initial orienting of attention, and the SPCN, which reflects sustained attention. Both components are maximal at occipito-parietal sites contralateral to the side of the screen to which the participant is attending. Therefore, we focused on average activity at the following electrode sites calculated to reflect contralateral and ipsilateral activity separately: P3/P4, P7/P8, PO3/PO4, PO7/PO8, and O1/O2. The N2pc was maximal at these sites 125–225 ms following face onset and SPCN was maximal over these sites 425–1000 ms after face onset. Finally, we examined P3b amplitudes in response to probe onset. As noted above, the P3 is larger for probes presented to invalidly-cued than to validly-cued locations and likely reflects the amount of cognitive resources required to disengage from previously attended locations (Fichtenholtz et al., 2007; Hugdahl & Nordby, 1994; Pollak &Tolley-Schell, 2003; Stormark et al., 1995; Wright et al., 1995). We calculated P3b as the average activity at Pz between 250–575 ms after probe onset, separately for valid and invalid trials for each emotion type. Descriptive statistics and Guttman split-half reliability values for each ERP component are presented in Table 2. Given the increased emphasis being placed on evaluating the reliability of attentional bias measures, Table 2 also includes six-month retest reliability data (M: 6.14 months, SD = 1.01), which were available for 61 of the children.
Table 2.
Descriptive statistics and reliability data for the ERP and reaction time indices.
| MDD mothers |
Never depressed mothers |
Split-half reliability |
Retest reliability |
|
|---|---|---|---|---|
| N2pc | ||||
| Angry contralateral | 2.48 (3.63) | 1.58 (3.31) | .92 | .58 |
| Angry ipsilateral | 2.79 (3.29) | 2.33 (2.75) | .89 | .55 |
| Happy contralateral | 2.38 (3.41) | 1.41 (2.99) | .89 | .69 |
| Happy ipsilateral | 2.65 (3.34) | 2.11 (2.74) | .88 | .54 |
| Sad contralateral | 2.72 (3.21) | 1.35 (2.92) | .89 | .78 |
| Sad ipsilateral | 1.93 (2.69) | 1.72 (2.28) | .84 | .33 |
| SPCN | ||||
| Angry contralateral | 0.24 (5.04) | −0.25 (3.16) | .58 | .35 |
| Angry ipsilateral | 1.40 (4.73) | 1.30 (2.97) | .51 | .20 |
| Happy contralateral | −0.39 (4.65) | −0.60 (2.62) | .50 | .32 |
| Happy ipsilateral | 1.03 (4.60) | 1.17 (2.69) | .47 | .25 |
| Sad contralateral | −0.02 (3.12) | −0.52 (2.69) | .31 | .42 |
| Sad ipsilateral | 0.89 (3.18) | 1.05 (2.82) | .33 | .27 |
| P3b | ||||
| Angry frequent | 5.30 (3.80) | 5.15 (4.37) | 82 | .49 |
| Angry rare | 9.62 (6.15) | 9.33 (5.54) | .58 | .48 |
| Happy frequent | 5.42 (3.97) | 5.75 (4.25) | .80 | .60 |
| Happy rare | 9.00 (5.79) | 8.79 (6.33) | .69 | .36 |
| Sad frequent | 5.83 (3.67) | 6.03 (4.40) | .71 | .60 |
| Sad rare | 8.99 (4.62) | 10.54 (5.71) | .50 | .62 |
| RT bias scores | ||||
| Angry | −10.10 (45.19) | 4.91 (34.45) | −.04 | .03 |
| Happy | −1.05 (43.90) | 4.51 (39.10) | −.36 | −.06 |
| Sad | −8.41 (47.81) | 5.89 (35.14) | −.05 | .05 |
Finally, consistent with previous research in this area (e.g., Clasen, Wells, Ellis, & Beevers, 2013; Mogg, Holmes, Garner, & Bradley, 2008), we also calculated reaction time (RT) attentional bias scores for each emotion type (sad, happy, angry) using the following formula:
attentional bias = (mean RT to invalid emotional cues – mean RT to valid emotional cues) – (mean RT to invalid neutral cues – mean RT to valid neutral cues)
Positive bias scores represent preferential attention toward the emotional faces, whereas negative scores indicate attentional avoidance of the emotional faces. Descriptive and reliability statistics for these estimates are presented in Table 2. Consistent with previous research that has raised concerns about reliability of reaction time indices of attention bias, the Guttman split-half reliability for each of the bias scores in this sample was negative and the retest stability was minimal and, in one case, negative.
Procedure
Potential participants were recruited from the community through a variety of means (e.g., television, newspaper and bus ads, flyers). Mothers responding to the recruitment advertisements were initially screened over the phone to determine potential eligibility. Upon arrival at the laboratory, women were asked to provide informed consent and children were asked to provide assent to be in the study. Next, the child completed the spatial cueing task while the mother was administered the K-SADS-PL by a trained interviewer. After completing the K-SADS-PL with the mother, the same interviewer then administered the K-SADS-PL to the child. While the child was administered the K-SADS-PL, the mother was administered the SCID-I by a separate interviewer. Families were compensated a total of $50 and children received a $10 store gift card for their participation in the study.
Results
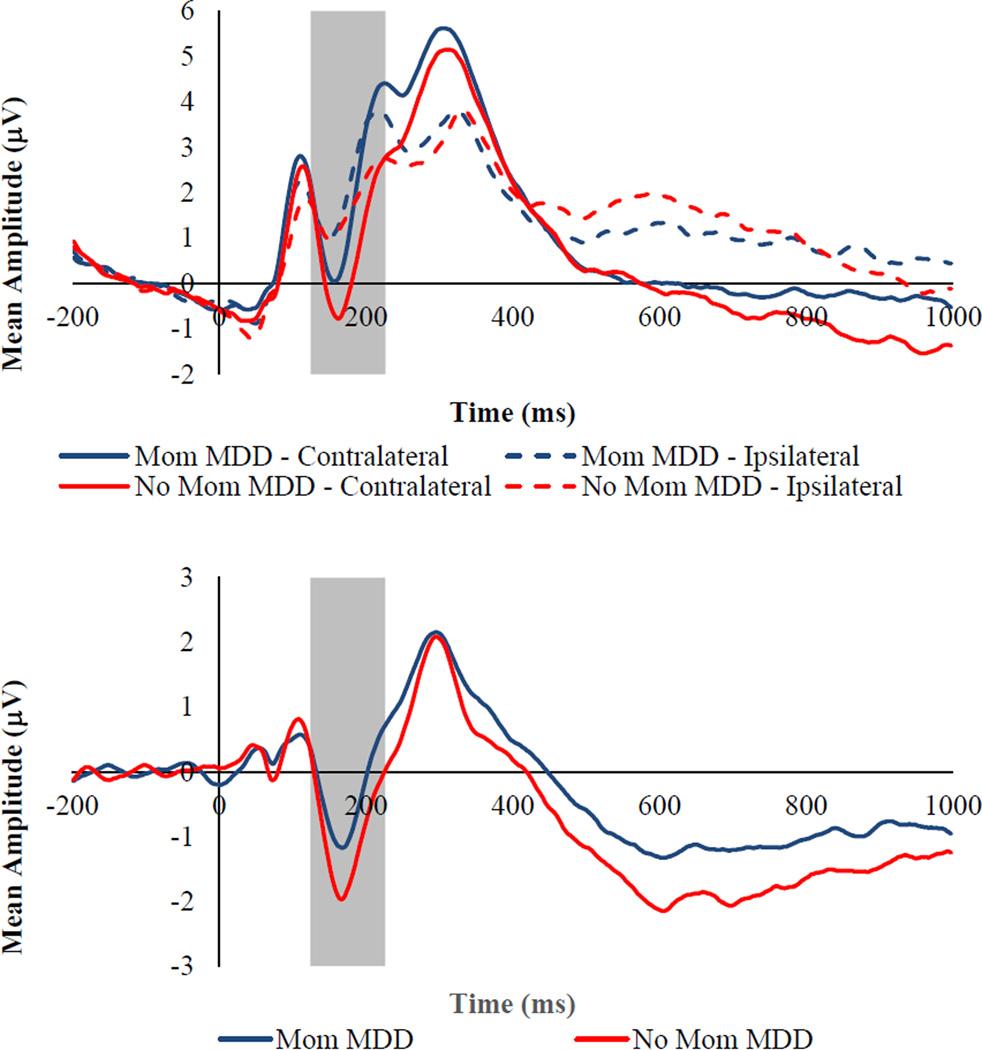
Focusing first on the ERP indices elicited by the presentation of the facial stimuli, we predicted that, although children of mothers with and without a history of MDD would not differ significantly in their initial allocation of attention (N2pc), they would differ in sustained attention (SPCN), to facial displays of sadness but not anger or happiness. Specifically, we predicted that children of depressed mothers would exhibit smaller (less negative) SPCNs to sad faces than children of never depressed mothers. To test these hypotheses, we conducted two separate 2 (Mother MDD history: yes, no) × 3 (Emotion: angry, happy, sad) × 2 (Laterality: contralateral, ipsilateral) repeated measures ANOVAs with the amplitude of either N2pc or SPCN serving as the dependent variable. For N2pc magnitude, there were significant Mother MDD × Laterality, F(1, 131) = 3.80, p = .05, ηp2 = .03, Emotion × Laterality, F(2, 262) = 13.83, p< .001, ηp2 = .10, and Mother MDD × Emotion × Laterality, F(1, 262) = 2.97, p = .03, ηp2 = .03, interactions. None of the other main or interactive effects was significant (lowest p = .06). Examining the form of the Mother MDD × Emotion × Laterality interaction, the Mother MDD × Laterality interaction was examined separately for each emotion type. The interaction was significant for the N2pc elicited by the presentation of sad faces, F(1, 131) = 7.04, p = .009, ηp2 = .05, but not angry, F(1, 131) = 1.50, p = .22, ηp2 = .01, or happy, F(1, 131) = 1.31, p = .25, ηp2 = .01, faces. To explore the nature of the significant interaction with Laterality for sad faces, we created a contralateral minus ipsilateral difference wave (Holmes et al., 2009; Luck, 2005), for which more negative numbers reflect greater neural reactivity to the presentation of the face. The main effect of Mother MDD on the N2pc difference score was significant, F(1, 131) = 7.04, p = .009, ηp2 = .05, with children of depressed mothers exhibiting a smaller N2pc to sad faces (see Figure 1).
Figure 1.
Top Panel: Grand averaged ERP waveforms time locked to face onset for sad faces presented separately by mother MDD group for contralateral and ipsilateral electrode sites. Bottom Panel: Grand averaged ERP waveforms time locked to face onset for sad faces presented separately by mother MDD group for the contralateral minus ipsilateral difference wave. The highlighted region shows the measurement windows for the N2pc (125–225ms). Waveforms are averaged across electrode pairs (P3/P4, P7/P8, PO3/PO4, PO7/PO8, O1/O2).
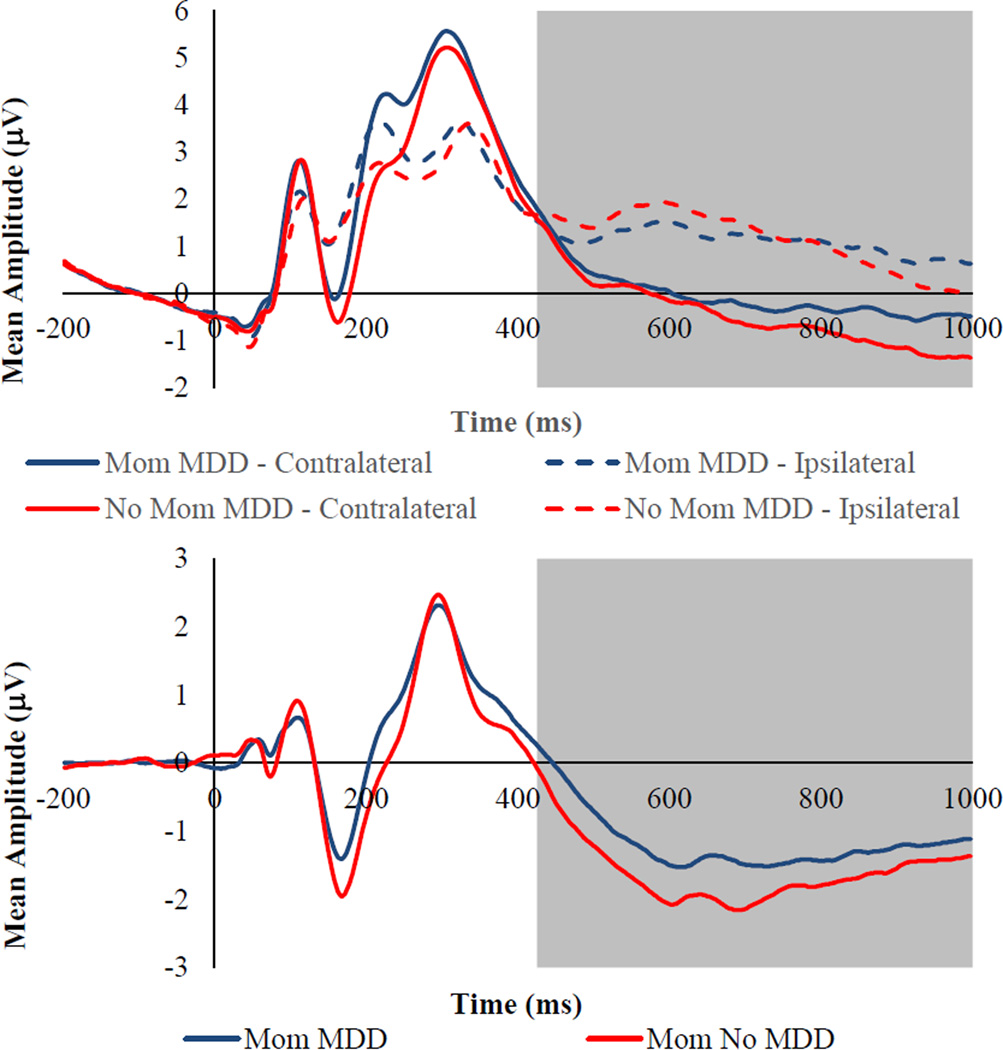
For SPCN magnitude, there was a significant main effect Laterality, F(1, 131) = 201.71, p< .001, ηp2 = .61, as well as significant Emotion × Laterality, F(1, 262) = 7.02, p= .001, ηp2 = .05, and Mother MDD × Laterality, F(1, 262) = 5.57, p = .02, ηp2 = .04, interactions. The Mother MDD × Emotion × Laterality interaction was not significant, F(1, 262) = 1.46, p = .23, ηp2 = .01. To explore the nature of the significant interactions with Laterality, we created a contralateral minus ipsilateral difference waves for each emotion, for which more negative numbers reflect greater sustained attention. The main effect of Emotion on this SPCN difference score was significant, F(2, 262) = 7.02, p = .001, ηp2 = .05, and post hoc tests revealed that children exhibited greater sustained attention to happy faces (M = −1.59, SE = .11) than to angry (M = −1.35, SE = .12, p = .01) and sad (M = −1.24, SE = .10, p = .001) faces, with the latter two not differing significantly (p = .22), which may simply reflect the fact that positively-valenced stimuli (happy faces) were more rare and therefore more salient than negatively-valenced stimuli (angry and sad faces). The main effect of Mother MDD on the SPCN difference score was also significant, F(1, 129) = 5.57, p = .02, ηp2 = .04, with children of depressed mothers exhibiting less sustained attention to all facial displays of emotion than children of never depressed mothers (see Figure 2).
Figure 2.
Top Panel: Grand averaged ERP waveforms time locked to face onset averaged across all emotional faces (angry, happy, and sad) presented separately by mother MDD group for contralateral and ipsilateral electrode sites. Bottom Panel: Grand averaged ERP waveforms time locked to face onset averaged across all emotional faces (angry, happy, and sad) presented separately by mother MDD group for the contralateral minus ipsilateral difference wave. The highlighted region shows the measurement windows for the SPCN (425–1000ms). Waveforms are averaged across electrode pairs (P3/P4, P7/P8, PO3/PO4, PO7/PO8, O1/O2).
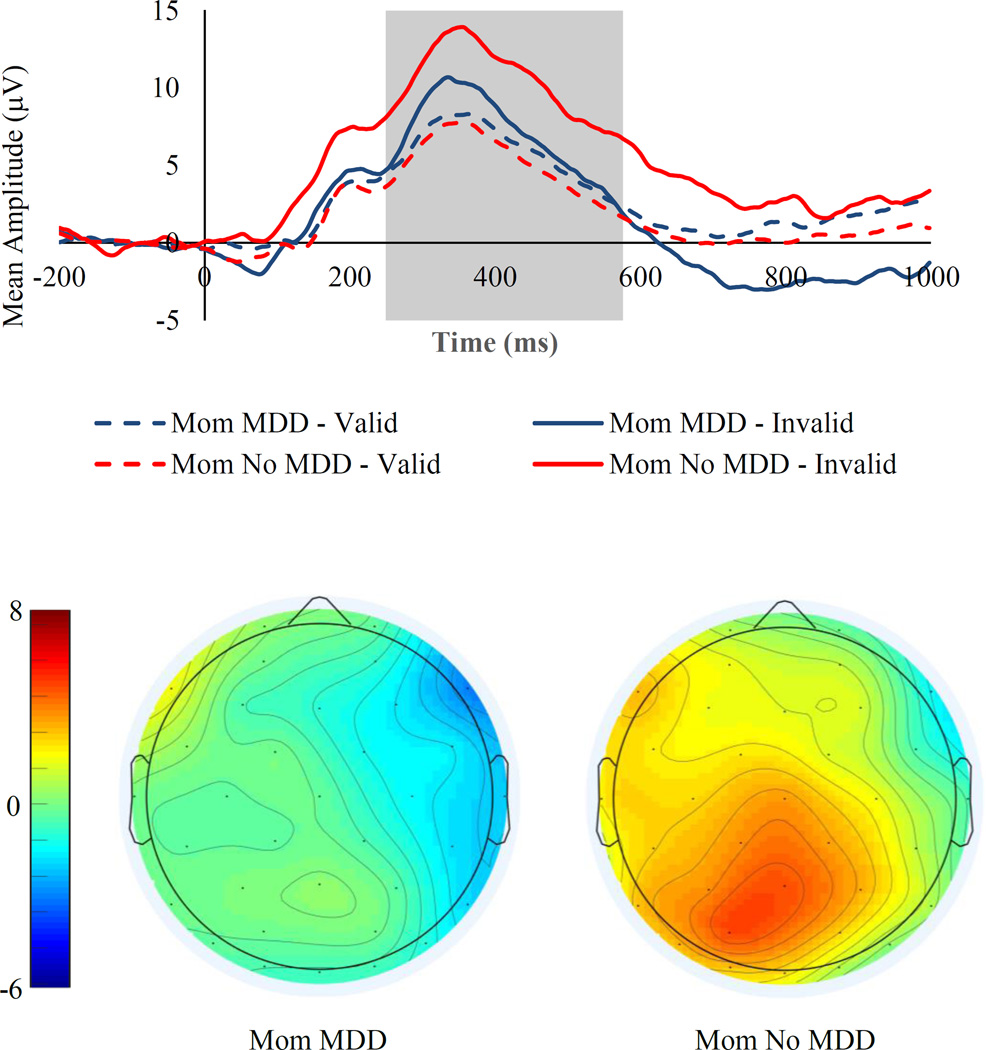
Next, for analyses of ERPs elicited by presentation of the probe, we predicted that children of depressed, compared to never depressed, mothers would exhibit smaller P3b amplitudes to invalidly-cued probes following sad faces but not angry or happy faces. To test this hypothesis, we examined the magnitude of the P3b component elicited by the presentation of the probe using a 2 (Mother MDD history: yes, no) × 3 (Emotion: angry, happy, sad) × 2 (Cue Location: left, right) × 2 (Cue Validity: valid, invalid) repeated measures ANOVA. In this analysis, there was a significant main effect of Cue Validity, F(1, 131) = 233.30, p< .001, ηp2 = .64, with children exhibiting larger a P3b for invalidly-cued probes (i.e., probes appearing on the opposite side of the screen as the face; M = 9.41, SE = 0.44) than for validly-cued probes (i.e., probes appearing on the same side of the screen as the face; M = 5.58, SE = 0.33). There was also a significant main effect of Emotion, F(2, 262) = 3.56, p = .03, ηp2 = .03, with children exhibiting larger a P3b for probes following sad faces (M = 7.86, SE = 0.37) than for probes following angry (M = 7.37, SE = 0.39) or happy (M = 7.26, SE = 0.42) faces (ps < .05). The magnitude of the P3b for probes following angry versus happy faces did not differ significantly (p = .64). Finally, there was a significant Mother MDD × Emotion × Cue Location × Cue Validity interaction, F(2, 262) = 4.91, p = .008, ηp2 = .04. To explore the nature of this interaction, we first examined the Mother MDD × Cue Location × Cue Validity interaction separately for each emotion type. The interaction was significant for probes following sad faces, F(1, 131) = 6.06, p = .02, ηp2 = .04, but not angry, F(1, 131) = 1.49, p = .22, ηp2 = .01, or happy, F(1, 131) = 0.04, p = .84, ηp2< .001, faces. Examining this further, the Mother MDD × Cue Validity interaction was significant for probes following sad faces presented on the left side of the screen, F(1, 131) = 12.38, p = .001, ηp2 = .09, but not the right side, F(1, 131) = 0.26, p = .61, ηp2 = .002. Focusing specifically on probes following sad faces that were presented on the left side of the screen, we found a significant Mother MDD group difference for invalidly-cued probes, F(1, 131) = 5.04, p = .03, ηp2 = .04, but not validly-cued probes, F(1, 131) = 0.68, p = .41, ηp2 = .005. We then created a P3b invalid minus valid difference score for probes following sad faces presented on the left side of the screen. Using this difference score, the Mother MDD group difference remained significant, F(1, 131) = 12.38, p = .001, ηp2 = .09, indicating that children of mothers with a history of MDD required fewer resources to disengage attention from the location of the sad face than did children of never depressed mothers (see Figure 3).2
Figure 3.
Top Panel: Grand averaged ERP waveforms time locked to probe onset for trials in which sad faces were on the left side of the screen presented separately by mother MDD group for validly and invalidly-cued probes. The highlighted region shows the measurement window for the P3b (250–575ms). Bottom Panel: Topographic scalp map for the P3b ERP component time locked to probe onset for trials in which sad faces were on the left side of the screen presented separately by mother MDD group for the invalid minus valid difference wave. Waveforms and maps are averaged for the Pz electrode.
Third, we examined RT bias scores. In this analysis, there was a significant main effect of Mother MDD, F(1, 131) = 3.92, p = .049, ηp2 = .03, with children of depressed mothers exhibiting less attention to all emotional faces (M = −6.52, SE = 4.07) than children of never depressed mothers M = 5.10, SE = 4.23). In contrast, the main effect of Emotion, F(2, 262) = 0.76, p = .47, ηp2 = .006, and the Mother MDD × Emotion interaction, F(2, 262) = 1.07, p = .34, ηp2 = .008, were both nonsignificant.
A series of follow-up analyses was then conducted to determine the specificity and robustness of the observed effects. First, to determine if the results were due simply to the presence of current depression in the mothers, we re-conducted all of the analyses statistically controlling for mothers’ current depressive symptom levels (BDI-2 scores) and each of the significant group differences was maintained (all ps < .05). Second, to determine whether current results would still be observed in at-risk children prior to them developing depression themselves, we re-conducted the analyses while excluding children with a lifetime history of MDD and statistically controlling for children’s current depressive symptom levels (CDI). Each of the mother MDD group differences was maintained (p< .05) with the exception of the group difference in RT biases to all emotions, F(1, 118) = 1.47, p = .23, ηp2 = .01. Third, to determine whether the results were at least partially independent of mothers’ and children’s anxiety, we re-conducted the analyses statistically controlling for mothers’ and children’s history of anxiety disorders (yes, no) and their current anxiety symptoms (BAI and MASC). Again, each of the mother MDD group differences was maintained (p< .05) with the exception of the group difference in RT biases to all emotions, F(1, 127) = 2.58, p = .11, ηp2 = .02. Fourth, to determine whether the results were at least partially independent of children’s current mood, we re-conducted the analyses statistically controlling for children’s VAS-Sadness and VAS-Anxiety ratings and, once again, each of the mother MDD group differences was maintained (p< .05) with the exception of the group difference in RT biases to all emotions, F(1, 127) = 3.62, p = .06, ηp2 = .03. Fifth, given the significant group differences in child age and race, we re-conducted the analyses statistically controlling for these variables and each of the mother MDD group differences was maintained (p< .05) with the exception of the group difference in RT biases to all emotions, F(1, 127) = 3.68, p = .06, ηp2 = .03.
We also conducted exploratory analyses to determine whether any of the attention indices were associated with characteristics of mothers’ depression (i.e., recurrent vs. single episode MDD, child age at first exposure to maternal MDD). None of these analyses was significant, suggesting the homogeneity of the impact of maternal depression on children’s attention biases. Finally, we conducted exploratory analyses to determine whether the relation between mother MDD history and children’s attentional biases was moderated by any demographic or clinical variables. Specifically, we evaluated the potential moderating role of children’s age and sex as well as their current depressive symptoms (CDI), and lifetime histories of MDD. None of these analyses was significant.3
Discussion
The goal of this study was to examine attentional biases for emotional information in children of mothers with a history of major depression. We predicted that children of depressed mothers would exhibit attentional avoidance specifically of sad faces and that this would be observed at later stages of attention (i.e., sustained attention [SPCN] and disengagement of attention [P3b]) rather than initial orienting of attention (N2pc). Our results partially supported these hypotheses. Specifically, children of mothers with a history of MDD, compared to children of mothers with no depression history, exhibited less attention to sad faces across all stages of attention including the N2pc and SPCN elicited by face onset, the P3b time locked to probe onset, and the RT index of attentional bias. Of these, the N2pc and P3b findings were specific to sad faces whereas the SPCN and RT findings were general to all facial displays of emotion. With the exception of the findings for the RT indices of attention bias, each of these results was maintained when we statistically controlled for the influence of mothers’ and children’s current depressive symptoms, children’s lifetime history of MDD, mothers and children’s current anxiety symptoms and lifetime histories of anxiety disorders, and children’s state levels of sad and anxious mood, which is consistent with our hypothesis that the relations are due specifically to mothers’ histories of MDD and not to mothers’ anxiety or current or past depression or anxiety in the child.
The strength and specificity of our findings appeared to track the reliability of the indices examined with the N2pc and P3b exhibiting the strongest split-half and retest reliability followed by the SPCN and then the RT bias scores. Indeed, the split-half reliability of the RT bias scores was negative and the retest reliability was near zero, which is consistent with prior research that has raised questions about the psychometric properties of RT indices of attentional bias (Brown et al., 2014; Kappenman et al., 2014; 2015; Price et al., 2015; Schmukle, 2005; Staugaard, 2009; Waechter et al., 2014). These findings highlight the need for studies to report the reliability of their measures, which is common practice for questionnaire-based studies but is much less common in behavioral, ERP, eye-tracking, and fMRI studies. Because reliability places an upper limit on validity, establishing the reliability of one’s measures is essential to move the field forward. This can clearly be seen in our results as the findings for the RT indices of attention bias did not survive any of the tests of robustness whereas each of the ERP indices did.
The current results are consistent with recent eye tracking studies of depression in children (e.g., Harrison & Gibb, 2014) as well as a broader body of research in infants (e.g. Bistricky et al., 2011; Boyd et al., 2006; Field, 1995; Termine & Izard, 1988) in suggesting that depressed and at-risk children may exhibit attentional avoidance of, rather than preferential attention toward, sad faces. In infants, avoidance of sad faces has been suggested as an emotion regulation strategy, which is consistent with models of emotion regulation in which attentional deployment is highlighted as one of the first emotion regulation strategies to emerge in children (e.g., Gross, 2014). In combination, these findings suggest that cognitive models of depression may need to be modified to account for developmental changes in the nature and/or function of attentional biases. Specifically, given the consistent findings of sustained attention toward depression-relevant information in adults (for reviews, see Armstrong & Olatunji, 2012; Joormann & Arditte, 2014), it may be that, at some point in the development of the person or progression of the disease, depressed individuals lose the ability to disengage their attention from sad stimuli and begin showing the characteristic pattern of attentional bias observed in depressed adults. How this transition unfolds is an important area of future research.
An unexpected finding from this study was the mother MDD group difference in children’s N2pc to sad faces. N2pc is an early emerging ERP component that reflects early attentional allocation to lateralized stimuli (Luck, 2012). We should note, however, that there is some evidence that stimulus competition (stimuli presented simultaneously in the left and right visual fields) is needed to generate an N2pc (Luck & Hillyard, 1994). Because of this, it is possible that our findings reflect differences in a more general N2 component. Either way, the current results suggest that differences in attentional allocation to sad faces in children of depressed versus nondepressed mothers occur at the earliest stages of attention. In contrast, theorists have proposed that attentional biases in depressed and at-risk individuals should be limited to later stages of attention (i.e., sustained attention or disengagement of attention; Disner et al., 2011; Gotlib & Joormann, 2010; Joormann & Arditte, 2014) and there is little evidence for early attention biases in depression from behavioral (eye tracking or reaction time) studies (for reviews, see Armstrong & Olatunji, 2012; Gibb et al., 2016). Although conclusions must remain tentative pending replication, the current results suggest that children of depressed mothers may also exhibit biases in early attention, but these differences may be limited to covert rather than overt shifts in attention.
We should also note that our P3b findings were specific to trials in which the sad face appeared on the left side of the screen. Although unexpected, this finding is consistent with research showing preferential attention to stimuli in the left compared to right visual field (Foulsham, Gray, Nasiopoulos, & Kingstone, 2013; Nuthmann & Matthias, 2014; Williams, Grealy, Kelly, Henderson, & Butler, 2016). It is also consistent with research suggesting asymmetry in neural activity associated with depression and the intergenerational transmission of depression risk (for reviews, see Goldstein & Klein, 2014; Jacobs, Orr, Gowins, & Forbes, 2015; Levin, Heller, Mohanty, Herrington, & Miller, 2007), which may differentially impact attention to stimuli presented in the left versus right visual fields. However, because this is the first study to suggest this type lateralization of attention bias in children of depressed mothers, we are hesitant draw firm conclusions about its possible significance. This said, future research should explicitly test this type of lateralization effect.
Despite the strengths of this study, important questions remain. First, although our finding of reduced attention to sad faces in children of depressed mothers is consistent with emotion regulation models that highlight shifts in attentional allocation as an early-emerging emotion regulation strategy (Gross, 2014), we did not actually measure changes in affect or arousal as a function of shifts in attention. Therefore, future research is needed to evaluate the potential emotion regulation role of attentional allocation in at-risk children. This said, we do know from previous analyses in our sample that children of depressed mothers exhibit heightened cognitive-affective reactivity specifically to the presentation of sad faces, but not other facial displays of emotion, in the form of increased pupil dilation (Burkhouse, Siegle, & Gibb, 2014), and that, among children of depressed mothers, greater pupil dilation to sad faces predicts a shorter time to depression onset during a 2-year follow-up (Burkhouse, Siegle, Woody, Kudinova, & Gibb, 2015). Therefore, we have some evidence that children of depressed mothers are more physiologically reactive to sad faces; however, future research is needed to determine if averting one’s gaze from these faces reduces this reactivity. Second, although our results are consistent with the findings of Gibb et al. (2009) and with the broader infant literature (Bistricky et al., 2011; Boyd et al., 2006; Field, 1995; Termine & Izard, 1988), they contradict those of two additional studies of children of depressed mothers both of which found evidence of preferential attention toward sad faces in these children (Joormann et al., 2007; Kujawa et al., 2011). As noted in our introduction, there are two key differences between our study and previous research. One key difference is that we utilized ERP indices of attentional allocation whereas previous research utilized reaction time indices. As noted above, there is growing concern about the reliability of reaction time indices of attention bias (for a review, see Gibb et al., 2016), a concern that is supported by the current findings. In addition, even in the presence of good reliability, reaction time indices provide only a snapshot of attentional allocation (i.e., where attention was allocated at the time of probe presentation) and do not allow one to examine dynamic changes in attention over the course of a trial. Another key difference between our studies and those of Joormann et al. (2007) and Kujawa et al. (2011) is that those studies included a negative mood induction prior to assessing attentional biases whereas we did not. It is possible that the direction of attentional bias exhibited in at-risk children varies as a function of current depressive mood. This said, our findings were maintained when we statistically controlled for children’s current levels of depression (sad mood and CDI scores) and children’s depressive symptom levels did not significantly moderate any of the relations examined. However, to provide a more conclusive test of the impact of a negative mood induction, research is needed in which attentional biases in at-risk children are assessed before and after a negative mood induction. Finally, we should also note that Kujawa et al. (2011) only found evidence for preferential attention to sad faces in daughters, but not sons, of mothers with a history of MDD and Joormann et al. (2007) only studied daughters. Although it is possible that daughters and sons exhibit different reactions to maternal depression, we found no evidence in the current study or in our earlier study (Gibb et al., 2009) that any of the relations were moderated by children’s sex.
In conclusion, the current results add to a growing body of research suggesting that the attentional biases exhibited by children of depressed mothers may be characterized by attentional avoidance of sad faces rather than preferential attention toward, or difficulty disengaging attention from, sad faces (see also Boyd et al., 2006; Field, 1995; Gibb et al., 2009). This pattern of findings has been interpreted in the context of models of emotion regulation (e.g., Gross, 2014) in which shifts in attentional deployment are recognized as one of the earliest developing methods of emotion regulation. To the extent that the attentional biases exhibited among at-risk children and currently depressed children (Harrison & Gibb, 2015) differ from those observed in depressed adults, it suggests important developmental changes in the nature and function of a person’s habitual patterns of attentional allocation to emotional stimuli. These developmental changes are not currently addressed in cognitive models of depression, which are largely based on research conducted with adults. As with many forms of psychopathology, however, a simple downward extension of the literature may not be adequate to fully explain patterns of adaptation and maladaptation in children. Future research is needed, therefore, to determine the specific points of convergence and divergence between findings in children, adolescents, and adults so that a more complete understanding of depression risk may be obtained.
Acknowledgments
This project was supported by grant number HD057066 and HD057066-03S1 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development and MH098060 from the National Institute of Mental Health awarded to B. E. Gibb. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. We would like to thank Cyma Van Petten for consultation on the project and Ashley Johnson, Lindsey Stone, Andrea Hanley, Katie Burkhouse, Mary Woody, Anastacia Kudinova, Sydney Meadows, Michael Van Wie, Devra Alper, Cope Feurer, Eric Funk, and Effua Sosoo for their help in conducting assessments for this project.
Footnotes
We should note that this is a different sample than that reported in Gibb et al. (2009).
Given the unexpected face location effect observed for P3b, we conducted exploratory analyses to determine if a similar effect would be observed for N2pc or SPCN. However, none of these analyses was significant suggesting that the N2pc and SPCN effects reported were similar in magnitude for faces presented in the left versus right visual field.
Details of these analyses can be obtained from the fist author.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Armstrong T, Olatunji BO. Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clinical Psychology Review. 2012;32(8):704–723. doi: 10.1016/j.cpr.2012.09.004. http://doi.org/10.1016/j.cpr.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. http://doi.org/10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory manual. 2nd. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Bistricky SL, Ingram RE, Atchley RA. Facial affect processing and depression susceptibility: Cognitive biases and cognitive neuroscience. Psychological Bulletin. 2011;137(6):998–1028. doi: 10.1037/a0025348. http://doi.org/10.1037/a0025348. [DOI] [PubMed] [Google Scholar]
- Boyd RC, Zayas LH, McKee MD. Mother-infant interaction, life events and prenatal and postpartum depressive symptoms among urban minority women in primary care. Maternal and Child Health Journal. 2006;10(2):139–148. doi: 10.1007/s10995-005-0042-2. http://doi.org/10.1007/s10995-005-0042-2. [DOI] [PubMed] [Google Scholar]
- Brown HM, Eley TC, Eley TC, Broeren S, MacLeod C, Rinck M, et al. Psychometric properties of reaction time based experimental paradigms measuring anxiety-related information-processing biases in children. Journal of Anxiety Disorders. 2014;28(1):97–107. doi: 10.1016/j.janxdis.2013.11.004. http://doi.org/10.1016/j.janxdis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Burkhouse KL, Siegle GJ, Gibb BE. Pupillary reactivity to emotional stimuli in children of depressed and anxious mothers. Journal of Child Psychology and Psychiatry. 2014;55(9):1009–1016. doi: 10.1111/jcpp.12225. http://doi.org/10.1111/jcpp.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Siegle GJ, Woody ML, Kudinova AY, Gibb BE. Pupillary reactivity to sad stimuli as a biomarker of depression risk: Evidence from a prospective study of children. Journal of Abnormal Psychology. 2015;124(3):498–506. doi: 10.1037/abn0000072. http://doi.org/10.1037/abn0000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, Beck AT, Alford BA. Scientific foundations of cognitive theory and therapy of depression. New York, NY: John Wiley & Sons; 1999. [Google Scholar]
- Clasen PC, Wells TT, Ellis AJ, Beevers CG. Attentional biases and the persistence of sad mood in major depressive disorder. Journal of Abnormal Psychology. 2013;122(1):74–85. doi: 10.1037/a0029211. http://doi.org/10.1037/a0029211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. http://doi.org/10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Diego MA, Field T, Jones NA, Hernandez-Reif M, Cullen C, Schanberg S, Kuhn C. EEG responses to mock facial expressions by infants of depressed mothers. Infant Behavior and Development. 2004;27(2):150–162. http://doi.org/10.1016/j.infbeh.2003.10.001. [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience. 2011;12(8):467–477. doi: 10.1038/nrn3027. http://doi.org/10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Eimer M, Eimer M, Kiss M, Kiss M. Attentional capture by task-irrelevant fearful faces is revealed by the N2pc component. Biological Psychology. 2007;74(1):108–112. doi: 10.1016/j.biopsycho.2006.06.008. http://doi.org/10.1016/j.biopsycho.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Feldmann-Wüstefeld T, Schmidt-Daffy M, Schubö A. Neural evidence for the threat detection advantage: Differential attention allocation to angry and happy faces. Psychophysiology. 2011;48(5):697–707. doi: 10.1111/j.1469-8986.2010.01130.x. http://doi.org/10.1111/j.1469-8986.2010.01130.x. [DOI] [PubMed] [Google Scholar]
- Fichtenholtz HM, Hopfinger JB, Graham R, Detwiler JM, LaBar KS. Happy and fearful emotion in cues and targets modulate event-related potential indices of gaze-directed attentional orienting. Social Cognitive and Affective Neuroscience. 2007;2(4):323–333. doi: 10.1093/scan/nsm026. http://doi.org/10.1093/scan/nsm026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T. Early interactions between infants and their postpartum depressed mothers. Infant Behavior and Development. 1984;7:517–522. [Google Scholar]
- Field T. Infants of depressed mothers. Infant Behavior and Development. 1995;18(1):1–3. doi: 10.1016/j.infbeh.2009.04.004. http://doi.org/10.1016/0163-6383(95)90003-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR axis I disorders, research version, non-patient edition. (SCID-I/NP) New York: Biometrics Research Department, NY State Psychiatric Institute; 1995. [Google Scholar]
- Foulsham T, Gray A, Nasiopoulos E, Kingstone A. Leftward biases in picture scanning and line bisection: A gaze-contingent window study. Vision Research. 2013;78:14–25. doi: 10.1016/j.visres.2012.12.001. http://doi.org/10.1016/j.visres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology: General. 2001;130(4):681–700. http://doi.org/10.1037//0096-3445.130.4.681. [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Benas JS, Grassia M, McGeary J. Children’s attentional biases and 5-HTTLPR genotype: Potential mechanisms linking mother and child depression. Journal of Clinical Child and Adolescent Psychology. 2009;38(3):415–426. doi: 10.1080/15374410902851705. http://doi.org/10.1080/15374410902851705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, McGeary JE, Beevers CG. Attentional biases to emotional stimuli: Key components of the RDoC constructs of sustained threat and loss. American Journal of Medical Genetics B. 2016;171(1):65–80. doi: 10.1002/ajmg.b.32383. http://doi.org/10.1002/ajmg.b.32383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BL, Klein DN. A review of selected candidate endophenotypes for depression. Clinical Psychology Review. 2014;34(5):417–427. doi: 10.1016/j.cpr.2014.06.003. http://doi.org/10.1016/j.cpr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH. Depression in mothers. Annual Review of Clinical Psychology. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. http://doi.org/10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106(3):458–490. doi: 10.1037/0033-295x.106.3.458. http://doi.org/10.1037/0033-295X.106.3.458. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Colich NL. Children of parents with depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 3rd. New York: Guilford; 2014. pp. 240–258. [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. http://doi.org/10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: Conceptual and empirical foundations. In: Gross JJ, editor. Handbook of emotion regulation. 2nd. New York, NY: 2014. pp. 3–20. [Google Scholar]
- Hankin BL, Gibb BE, Abela JRZ, Flory K. Selective attention to affective stimuli and clinical depression among youths: Role of anxiety and specificity of emotion. Journal of Abnormal Psychology. 2010;119(3):491–501. doi: 10.1037/a0019609. http://doi.org/10.1037/a0019609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AJ, Gibb BE. Attentional biases in currently depressed children: An eye-tracking study of biases in sustained attention to emotional stimuli. Journal of Clinical Child and Adolescent Psychology. 2014:1–7. doi: 10.1080/15374416.2014.930688. http://doi.org/10.1080/15374416.2014.930688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AJ, Gibb BE. Attentional biases in currently depressed children: An eye-tracking study of biases in sustained attention to emotional stimuli. Journal of Clinical Child and Adolescent Psychology. 2015;44(6):1008–1014. doi: 10.1080/15374416.2014.930688. http://doi.org/10.1080/15374416.2014.930688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Holmes A, Bradley BP, Kragh Nielsen M, Kragh Nielsen M, Mogg K. Attentional selectivity for emotional faces: Evidence from human electrophysiology. Psychophysiology. 2009;46(1):62–68. doi: 10.1111/j.1469-8986.2008.00750.x. http://doi.org/10.1111/j.1469-8986.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Nordby H. Electrophysiological correlates to cued attentional shifts in the visual and auditory modalities. Behavioral and Neural Biology. 1994;62(1):21–32. doi: 10.1016/s0163-1047(05)80055-x. [DOI] [PubMed] [Google Scholar]
- Jacobs RH, Orr JL, Gowins JR, Forbes EE. Biomarkers of intergenerational risk for depression: A review of mechanisms in longitudinal high-risk (LHR) studies. Journal of Affective Disorders. 2015 doi: 10.1016/j.jad.2015.01.038. http://doi.org/10.1016/j.jad.2015.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicœur P, Brisson B, Robitaille N. Dissociation of the N2pc and sustained posterior contralateral negativity in a choice response task. Brain Research. 2008;1215:160–172. doi: 10.1016/j.brainres.2008.03.059. http://doi.org/10.1016/j.brainres.2008.03.059. [DOI] [PubMed] [Google Scholar]
- Jolicœur P, Sessa P, Dell’Acqua R, Robitaille N. On the control of visual spatial attention: Evidence from human electrophysiology. Psychological Research. 2006;70(6):414–424. doi: 10.1007/s00426-005-0008-4. http://doi.org/10.1007/s00426-005-0008-4. [DOI] [PubMed] [Google Scholar]
- Joormann J, Arditte KA. Cognitive aspects of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 3rd. New York: Guilford; 2014. pp. 259–276. [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116(1):135–143. doi: 10.1037/0021-843X.116.1.135. http://doi.org/10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37(2):163–178. [PubMed] [Google Scholar]
- Kappenman ES, Farrens JL, Luck SJ, Proudfit GH. Behavioral and ERP measures of attentional bias to threat in the dot-probe task: Poor reliability and lack of correlation with anxiety. Frontiers in Psychology. 2014;5:1368. doi: 10.3389/fpsyg.2014.01368. http://doi.org/10.3389/fpsyg.2014.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappenman ES, MacNamara A, Proudfit GH. Electrocortical evidence for rapid allocation of attention to threat in the dot-probe task. Social Cognitive and Affective Neuroscience. 2015;10(4):577–583. doi: 10.1093/scan/nsu098. http://doi.org/10.1093/scan/nsu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. http://doi.org/10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatrica. 1981;46(5-6):305–315. [PubMed] [Google Scholar]
- Kujawa AJ, Torpey D, Kim J, Hajcak G, Rose S, Klein DN. Attentional biases for emotional faces in young children of mothers with chronic or recurrent depression. Journal of Abnormal Child Psychology. 2011;39(1):125–135. doi: 10.1007/s10802-010-9438-6. http://doi.org/10.1007/s10802-010-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin RL, Heller W, Mohanty A, Herrington JD, Miller GA. Cognitive deficits in depression and functional specificity of regional brain activity. Cognitive Therapy and Research. 2007;31(2):211–233. http://doi.org/10.1007/s10608-007-9128-z. [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience. 2014;8:213. doi: 10.3389/fnhum.2014.00213. http://doi.org/10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Luck SJ. Electrophysiological correlates of the focusing of attention within complex visual scenes: N2pc and related ERP components. In: Luck SJ, Kappenman ES, editors. The Oxford handbook of event-related potential components. New York: Oxford University Press; 2012. pp. 329–360. [Google Scholar]
- Luck SJ, Hillyard SA. Spatial filtering during visual search: Evidence from human electrophysiology. Journal of Experimental Psychology: Human Perception and Performance. 1994;20(5):1000–1014. doi: 10.1037//0096-1523.20.5.1000. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95(1):15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- March JS, Sullivan K. Test-retest reliability of the Multidimensional Anxiety Scale for Children. Journal of Anxiety Disorders. 1999;13(4):349–358. doi: 10.1016/s0887-6185(99)00009-2. [DOI] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(4):554–565. doi: 10.1097/00004583-199704000-00019. http://doi.org/10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Mogg K, Holmes A, Garner M, Bradley BP. Effects of threat cues on attentional shifting, disengagement and response slowing in anxious individuals. Behaviour Research and Therapy. 2008;46(5):656–667. doi: 10.1016/j.brat.2008.02.011. http://doi.org/10.1016/j.brat.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuthmann A, Matthias E. Time course of pseudoneglect in scene viewing. Cortex. 2014;52:113–119. doi: 10.1016/j.cortex.2013.11.007. http://doi.org/10.1016/j.cortex.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Polich J. Neuropsychology of P300. In: Luck SJ, Kappenman ES, editors. The Oxford handbook of event-related potential components. New York: Oxford University Press; 2012. pp. 159–188. [Google Scholar]
- Pollak SD, Tolley-Schell SA. Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology. 2003;112(3):323–338. doi: 10.1037/0021-843x.112.3.323. http://doi.org/10.1037/0021-843X.112.3.323. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32(1):3–25. doi: 10.1080/00335558008248231. http://doi.org/10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Vuilleumier P. Dynamics of emotional effects on spatial attention in the human visual cortex. Progress in Brain Research. 2006;156:67–91. doi: 10.1016/S0079-6123(06)56004-2. http://doi.org/10.1016/S0079-6123(06)56004-2. [DOI] [PubMed] [Google Scholar]
- Price RB, Kuckertz JM, Siegle GJ, Ladouceur CD, Silk JS, Ryan ND, et al. Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological Assessment. 2015;27(2):365–376. doi: 10.1037/pas0000036. http://doi.org/10.1037/pas0000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille N, Jolicœur P. Fundamental properties of the N2pc as an index of spatial attention: Effects of masking. Canadian Journal of Experimental Psychology/Revue Canadienne De Psychologie Expérimentale. 2006;60(2):101–111. doi: 10.1037/cjep2006011. http://doi.org/10.1037/cjep2006011. [DOI] [PubMed] [Google Scholar]
- Rossignol M, Philippot P, Bissot C, Rigoulot S, Campanella S. Electrophysiological correlates of enhanced perceptual processes and attentional capture by emotional faces in social anxiety. Brain Research. 2012;1460:50–62. doi: 10.1016/j.brainres.2012.04.034. http://doi.org/10.1016/j.brainres.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Schmukle SC. Unreliability of the dot probe task. European Journal of Personality. 2005;19:595–605. http://doi.org/10.1002/per.554. [Google Scholar]
- Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the Children’s Depression Inventory. Journal of Abnormal Child Psychology. 1986;14(1):25–39. doi: 10.1007/BF00917219. [DOI] [PubMed] [Google Scholar]
- Staugaard SR. Reliability of two versions of the dot-probe task using photographic faces. Psychology Science Quarterly. 2009;51:339–350. [Google Scholar]
- Stormark KM, Nordby H, Hugdahl K. Attentional shifts to emotionally charged cues: Behavioural and ERP data. Cognition and Emotion. 1995;9(5):507–523. http://doi.org/10.1080/02699939508408978. [Google Scholar]
- Termine NT, Izard CE. Infants’ responses to their mothers’ expressions of joy and sadness. Developmental Psychology. 1988 http://doi.org/10.1037/0012-1649.24.2.223. [Google Scholar]
- Waechter S, Waechter S, Nelson AL, Nelson AL, Wright C, Hyatt A, Oakman J. Measuring attentional bias to threat: Reliability of dot probe and eye movement indices. Cognitive Therapy and Research. 2014;38:313–333. http://doi.org/10.1007/s10608-013-9588-2. [Google Scholar]
- Williams LR, Grealy MA, Kelly SW, Henderson I, Butler SH. Perceptual bias, more than age, impacts on eye movements during face processing. Acta Psychologica. 2016;164:127–135. doi: 10.1016/j.actpsy.2015.12.012. http://doi.org/10.1016/j.actpsy.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Electrophysiological measurement of rapid shifts of attention during visual search. Nature. 1999;400(6747):867–869. doi: 10.1038/23698. http://doi.org/10.1038/23698. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Geffen GM, Geffen LB. Event related potentials during covert orientation of visual attention: effects of cue validity and directionality. Biological Psychology. 1995;41(2):183–202. doi: 10.1016/0301-0511(95)05128-7. [DOI] [PubMed] [Google Scholar]