Abstract
Tubulointerstitial nephritis (TIN) is a frequent cause of acute kidney injury (AKI) that can lead to chronic kidney disease (CKD). TIN is associated with an immune-mediated infiltration of the kidney interstitium by inflammatory cells, which may progress to fibrosis. Patients often present with non-specific symptoms, which can lead to delayed diagnosis and treatment of the disease. Etiology of TIN can be drug-induced, infectious, idiopathic, genetic, or related to a systemic inflammatory condition such as tubulointerstitial nephritis and uveitis (TINU) syndrome, inflammatory bowel disease, or IgG4-associated immune complex multiorgan autoimmune disease (MAD). It is imperative to have a high clinical suspicion for TIN in order to remove potential offending agents and treat any associated systemic diseases. Treatment is ultimately dependent on underlying etiology. While there are no randomized controlled clinical trials to assess treatment choice and efficacy in TIN, corticosteroids have been a mainstay of therapy and recent studies have suggested a possible role for mycophenolate mofetil. Urinary biomarkers such as alpha1-microglobulin and beta2-microglobulin may help diagnose and monitor disease activity in TIN. Screening for TIN should be implemented in children with inflammatory bowel disease, uveitis, or IgG4-associated MAD.
Keywords: Tubulointerstitial Nephritis, Acute Kidney Injury, Chronic Kidney Disease, TINU Syndrome, Inflammatory Bowel Disease, Treatment, Monitoring
Introduction
Tubulointerstitial nephritis (TIN) is a well-described entity, although often has a delayed diagnosis given non-specific presenting signs and symptoms. TIN can be categorized based on underlying etiology, histology, or duration (acute versus chronic). This review will focus on common etiologies of TIN as well as developments in genetic discoveries and novel biomarkers to aid in the diagnosis, prognosis, and treatment of the disease.
Definition
TIN is characterized by an immune-mediated infiltration of the kidney interstitium by inflammatory cells, leading to non-oliguric or oliguric acute kidney injury (AKI) [1-4]. Less frequently, the interstitial inflammation can lead to chronic changes with subsequent development of chronic kidney disease (CKD) [5]. Numerous genetic and environmental factors can cause or contribute to development of TIN. Particular aspects of histologic diagnosis (i.e. granulomas) or associated systemic disease can aid in identification of underlying etiology. TIN accounts for 2 % of native renal biopsies [6] and up to 27 % of cases of unexplained kidney disease in adult patients [3]. In children, TIN (both acute and chronic) accounts for 1-7 % of the histological diagnoses in renal biopsies [7, 8].
Etiology
TIN has multiple etiologies, including drug-related, infectious, systemic, autoimmune, genetic, and idiopathic (Table 1). The most common cause of TIN is related to a medication or drug exposure [2-4, 9]. Many medications have been implicated, with beta-lactam antibiotics and non-steroidal anti-inflammatory (NSAID) drugs being the most common, and presenting with classic TIN (Table 2). The presentation related to rifampin use is unique and can be accompanied by sudden onset of symptoms and renal biopsy findings ranging from classic acute TIN to acute tubular necrosis [10-12]. Overall, drug-induced TIN has been noted in 7-27 % of adult patients with unexplained non-oliguric or oliguric AKI [13]. Infectious causes of TIN include viral, bacterial, fungal or parasitic [14-16]. TIN has been reported as the third leading cause of graft dysfunction in renal transplant patients [17]. TIN in immunosuppressed renal transplant recipients is primarily related to infectious causes, including polyoma virus or cytomegalovirus, and unfortunately can lead to increased risk of subsequent rejection [17-20]. TIN associated with polyoma virus infection has been reported in association with primary immunodeficiency [21]. Bone marrow transplant recipients are at risk for necrotizing TIN caused by adenovirus [22-24] and patients with HIV-associated nephropathy can have a component of TIN [25]. Epstein-Barr infections have been associated with TIN with uveitis (TINU) syndrome in children and adults [26, 27]. Among other infections associated with TIN are Mycoplasma pneumoniae, Yersinia pseudotuberculosis and Leptospira shermani [28-31]. TIN has been described in association with systemic inflammatory conditions such as inflammatory bowel disease, TINU syndrome, sarcoidosis, systemic lupus erythematosus (SLE), and Sjögren's disease. IgG4-associated immune complex multiorgan autoimmune disease (MAD) has also been linked with development of TIN with IgG4 positive plasma cell interstitial infiltrates and C3 deposition [32]. Autoimmune pancreatitis is in the spectrum of IgG4-associated MAD where TIN is part of the disease manifestation [33-35]. In the IgG4-associated conditions, hypocomplementemia and C3 interstitial deposition are frequently observed in addition to elevated serum levels of IgG and IgE. By contrast, hypocomplementemic immune-complex-mediated TIN described in the setting of advanced renal failure, eosinophilia, eosinophiluria and lymphopenia and characterized by near-pure plasma cell interstitial infiltrates was unaccompanied by any extrarenal manifestations [36]. Despite severe hypocomplementemia, C3, C4 or C1q complement was absent from tubulointerstitial immune complex deposits [36]. TIN has also been described in patients with anti-tubular basement membrane antibodies [37]. Often, TIN is under-recognized in these inflammatory conditions and diagnosed later in the disease course.
Table 1.
| Drug-Related |
| Antimicrobials |
| NSAID's |
| Other |
| Infectious |
| Viral: |
| Cytomegalovirus, Hepatitis, HIV, Epstein - Barr virus, Hantavirus, Polyomavirus |
| Bacterial: |
| Salmonella, Streptococcus, Yersinia, Brucella, Leptospirosis |
| Fungal: |
| Histoplasmosis |
| Parasitic: |
| Leishmania, Toxoplasma |
| Localized TIN with acute pyelonephritis |
| Immune-Mediated |
| Sarcoidosis |
| Systemic Lupus Erythematosus |
| Sjögren's Disease |
| Inflammatory Bowel Disease |
| Idiopathic |
| TINU |
| Granulomatous TIN |
| Medications |
| Sarcoidosis |
| Tuberculosis |
| Bacterial/Fungal infections |
| TINU |
| Granulomatosis with polyangiitis |
*NSAID's: Non-steroidal anti-inflammatories, TIN: Tubulointerstitial Nephritis, TINU: Tubulointerstitial Nephritis and Uveitis Syndrome
Table 2.
| Antimicrobials | NSAIDs | Diuretics | Neuropsychiatric | Other |
|---|---|---|---|---|
| Beta-lactams | Ibuprofen | Furosemide | Carbamezepine | Allopurinol |
| Cephalosporins | Ketorolac | Thiazide diuretics | Lamotrigine | Azathioprine |
| Sulfonamides | Levetiracetam | Antiepileptics | ||
| Macrolides | Triamterene | Phenytoin | Proton-pump inhibitors | |
| Gentamicin | Amiloride | Lithium | ||
| Nitrofurantoin | Tienlinic acid | Alendronate | ||
| Clotrimazole | Chlorpropamide | |||
| Doxycycline | Captopril | |||
| Rifampin | Sulfasalazine | |||
| Ethambutol | ||||
| Isoniazid | ||||
| Vancomycin | ||||
| Ciprofloxacin | ||||
| Acyclovir | ||||
| Indinavir | ||||
*NSAID's: Non-steroidal anti-inflammatories
Several genetic factors have been associated with development of TIN. TIN antigen (TIN-ag) is an extracellular matrix basement membrane protein and a target antigen in anti-tubular basement membrane antibody-mediated TIN [38]. Deletion of the TIN-ag gene hTIN-ag localized on chromosome 6 leads to disruption of the structure and function of tubulointerstitial epithelium and basement membrane [39]. Additionally, Kidney Disease Improving Global Outcomes (KDIGO) released a consensus report describing autosomal dominant tubulointerstitial kidney disease [14]. Thus far, four causal genes, Uromodulin, Renin, Hepatocyte nuclear factor 1B, and Mucin-1 have been identified [40]. These are a group of disorders that lead to progressive tubulointerstitial fibrosis, a chronic form of TIN which inevitably leads to end-stage renal disease.
Pathophysiology
Acute interstitial inflammatory reactions are associated with damage to the tubulointerstitium, leading to AKI associated with TIN [4]. The high metabolic demand of the tubulointerstitium makes it particularly susceptible to injury because the inflammation and associated edema compromise renal blood flow, causing a decrease in glomerular filtration rate (GFR) [5]. In some situations, damage may lead to fibrosis (see below).
Interstitial edema and infiltration of lymphocytes and plasma cells, as well as poor tubular function in acute TIN, causes a decrease in GFR. In chronic TIN, fibrosis of the interstitium (as opposed to edema) causes the decrease in GFR [5, 41]. If prolonged, acute interstitial inflammatory reactions can lead to accumulation of extracellular matrix that causes irreversible impairment of renal function with interstitial fibrosis and tubular atrophy [4, 13]. Initially macrophages may help repair acute injury, but eventually can contribute to inflammation and production of fibrogenic cytokines [5]. Studies have shown that the cytokine transforming growth factor-beta (TGF-β) may mediate profibrotic responses in the tubulointerstitium [5, 42]. Tubular damage can decrease the number of functional nephrons, eventually resulting in hyperfiltration and burnout of the remaining nephrons leading to CKD [5].
The pathophysiology of drug-induced TIN is thought to be immune-mediated and related to an allergic reaction. There are five concepts that support this view: (1) TIN only occurs in a small proportion of individuals taking a certain medication; (2) There is no dose-dependence; (3) Patients develop systemic manifestations of a hypersensitivity reaction; (4) TIN can recur after re-exposure to the drug; and (5) Eosinophils are often present on renal biopsy [4, 9]. This process likely involves cellular immunity, as there are seldom immune deposits noted by immunofluorescence on renal biopsies in patients with TIN [4].
Pathology
Regardless of underlying etiology, TIN is characterized histopathologically by tubulointerstitial inflammatory cell infiltrate (primarily lymphocytic and eosinophilic) and interstitial edema [6] (Fig. 1). When a significant number of eosinophils are present, drug-induced TIN needs to be considered, but neither the presence nor absence of eosinophils is absolutely diagnostic [13]. NSAID-induced TIN is less likely to be associated with eosinophils on renal biopsy, likely due to the anti-inflammatory properties of NSAIDs. A higher density of neutrophils and plasma cells are suggestive of bacterial etiology [13].
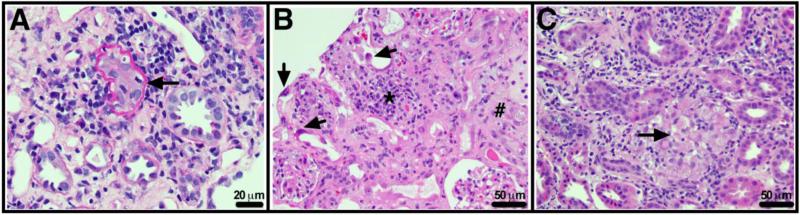
Figure 1. Renal Histopathology in tubulointerstitial nephritis (TIN).
A. TIN with predominantly lymphocytic infiltrate associated with tubular damage and tubulitis (arrow). Periodic acid Schiff stain, original magnification × 400. B. Acute drug-induced tubular injury, in this case secondary to cidofovir. There is interstitial infiltrate (*), edema (#) and marked tubular regenerative changes (arrows). Glomeruli show little change. Hematoxylin and eosin stain, original magnification × 200. C. Granulomatous tubulointerstitial nephritis (arrow), in this case likely secondary to lamotrigine. Hematoxylin and eosin stain, original magnification × 200.
Granulomatous TIN
Inflammatory cells infiltrating the tubulointerstitium can form granulomas, which are usually scarce, and non-necrotic with few multinucleate giant cells [4, 43] (Fig. 1c). By contrast, necrotic granulomas are commonly seen in TIN associated with bacterial (tuberculosis) or fungal infections [44]. The presence of granulomas on renal biopsy defines granulomatous TIN, which is relatively rare, with renal biopsy incidence of 0.5 % [9]. With time, the granulomas are often replaced by fibrosis, but despite varying histopathology, a diagnosis of granulomatous TIN does not necessarily correlate with poor prognosis [43, 45]. The underlying etiology for granulomatous TIN is similar to TIN, although sarcoidosis, TINU syndrome, and certain drug-related cases are more common (Table 1). Systemic diseases such as Crohn's disease have also been implicated, though rare [46]. One study reported that the underlying etiology of granulomatous TIN is not found in 50 % of patients [45]. Interestingly, granulomatous TIN has been described in renal transplant recipients and was hypothesized to result from infections, which are more common in this patient population because of the use of immunosuppressive agents [44, 47].
Clinical presentation
A challenging feature of TIN is the non-specific symptomatic presentation, which often leads to delayed diagnosis that may portend worse outcomes. Classically, presentation is thought to be associated with a hypersensitivity reaction including rash, arthralgia and fever, but as few as 5-10 % of patients present with all of these findings [3, 9]. In a comprehensive study of acute TIN, at presentation 15 % of patients had rash, 27.3 % had fever, 23 % had eosinophilia and only 10 % had all three findings [3]. The tubulointerstitial infiltrate of inflammatory cells can cause edema and painful stretching of the renal capsule, leading to abdominal, flank or loin pain [6]. Thus, the kidneys in TIN are typically found to be normal sized or enlarged with increased cortical echogenicity by ultrasound. If drug-related, TIN can manifest between 1 and 3 weeks after exposure to the medication in most cases [44], with an average presentation of about 10 days after exposure [4], except for rifampin exposure when the presentation may be much faster, as described above. Presence of extrarenal manifestations may be helpful in identifying a risk for TIN. A renal biopsy is the only definitive diagnostic modality that can confirm TIN suspected on clinical grounds. A renal biopsy should be considered with severe renal dysfunction, lack of an identifiable offending agent, lack of renal recovery, uncommon features of TIN or prior to initiation of treatment [1], but otherwise TIN remains a clinical diagnosis. Tubulointerstitial dysfunction should be suspected in patients who develop hyperkalemic, hyperchloremic metabolic acidosis that is out of proportion to renal dysfunction [9]. Most patients are initially noted to have AKI (elevated BUN and/or creatinine) with further workup revealing TIN. Other presenting signs and symptoms are noted in Table 3. Tubular dysfunction can manifest as Fanconi's syndrome, so patients may present with electrolyte abnormalities (as above), metabolic acidosis, elevated fractional excretion of sodium, glycosuria, and aminoaciduria. Additionally, eosinophilia, pyuria, hematuria, eosinophiluria, and mild proteinuria are present in a variable number of cases [2, 6].
Table 3.
| Symptoms |
| Fatigue |
| Anorexia, Weight Loss |
| Headache |
| Flank pain |
| Arthralgias |
| Myalgias |
| Signs |
| Fever |
| Skin rash |
| Costovertebral Angle tenderness |
| Laboratory Findings |
| Blood studies: Renal Failure, Anemia, Eosinophilia |
| Urine studies: Sterile Pyuria, Proteinuria, Eosinophiluria, White Blood Cell Casts, Micro/Macroscopic Hematuria (rare) |
The differential diagnosis of both acute and chronic TIN is broad. Chronic TIN may manifest similarly to obstructive nephropathy, chronic pyelonephritis, papillary necrosis, tubulopathies including Fanconi syndrome, progressive interstitial fibrosis or Balkan endemic nephropathy (BEN), Chinese herb nephropathy, and radiation nephritis [41, 48, 49]. Acute TIN has a differential diagnosis that could include acute glomerulonephritis, pyelonephritis, atheroembolic disease, or any cause of AKI (acute tubular necrosis, prerenal azotemia, urinary obstruction or drug-induced AKI).
While eosinophiluria can be helpful in the diagnosis of TIN, it is neither sensitive nor specific. Eosinophiluria can also be seen with cystitis, prostatitis, pyelonephritis, atheroembolic renal disease, acute tubular necrosis and rapidly progressive glomerulonephritis [9]. In a study evaluating drug-induced TIN, of the patients with biopsy-confirmed TIN, 67 % had eosinophiluria whereas 33 % did not. Additionally, 13 % of patients without TIN had eosinophiluria [4].
Tubulointerstitial Nephritis and Uveitis Syndrome (TINU) Syndrome
TINU is a rare disorder with only 133 cases reported in the literature by 2001 [50]. TINU accounts for less than 2 % of cases of uveitis [1, 51, 52]. The median age at presentation is 15 years and the female to male ratio is 3:1 [52, 53]. Diagnosis requires the presence of both TIN and uveitis, and is suggested by abnormal renal function, abnormal urinalysis, photophobia, eye pain and redness, eyelid edema, rapidly progressive loss of vision, as well as symptoms of systemic illness, including weight loss, fever, and fatigue [3, 50, 52]. In addition to the aforementioned eye symptoms, conjunctival and perilimbal injection are present and pupils can be small with sluggish or no light reaction. The ophthalmologic examination reveals anterior chamber cells and flare, hypopyon, keratic nongranulomatous precipitates, vitreous humor cells, intraretinal hemorrhages or retinal vascular sheathing, cotton wool spots, dilated retinal vessels and retinal edema (Fig. 2). If there is a prolonged inflammatory process, anterior (iridocorneal) or posterior (irido-lenticular) synechiae may develop. Anterior uveitis (Fig. 2a) is present in 80 % of cases of TINU while posterior and panuveitis (Fig. 2b and 2c) are less common [50, 54]. The ocular changes are bilateral in about 80 % of cases [55]. Uveitis generally occurs after onset of TIN (60 % of cases), but may be present between one month before and three months after the onset of TIN [3, 52]. In general the course and severity of uveitis does not correlate with that of TIN [52, 53, 56-59].
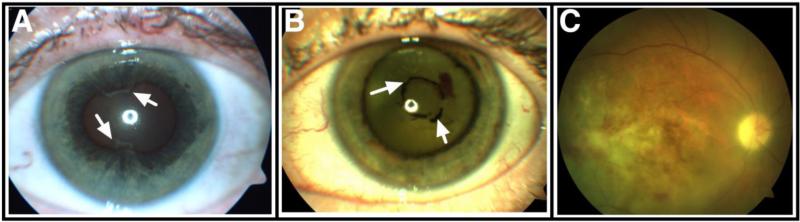
Figure 2. Ophthalmologic findings in tubulointerstitial nephritis with uveitis (TINU).
A. Anterior uveitis complicated by posterior (irido-lenticular) synechiae (arrows). B. Panuveitis with endothelial precipitates and chronic anterior synechiae (arrows). C. Fundus photograph of a patient with panuveitis demonstrating retinal infiltrates.
Recurrence of uveitis occurs in about 40 % of patients with TINU and relapses tend to be more severe than the initial episode. Younger patients are more likely to develop a chronic course of uveitis lasting more than 3 months [52, 53, 57, 58, 60, 61]. Intraocular complications occur in about 20 % of TINU patients and include posterior synechiae, optic disc swelling, cataracts, elevated intraocular pressure or chorioretinal scarring (Fig. 2). Importantly some complications, particularly cataracts and elevated intraocular pressure, are strongly associated with the use of systemic corticosteroids (see below). TINU syndrome remains a diagnosis of exclusion [53, 60, 62, 63]. Sarcoidosis and Sjögren's disease are in the differential diagnosis of TINU, although, the median age and the type of nephritis and uveitis differ.
In general, the renal prognosis is good in the majority of treated patients with TINU [50]. While the uveitis is more difficult to control, it carries a fairly good prognosis for visual acuity that rarely decreases below 20/40, with no reported cases of permanent vision loss [3, 54, 64]. Up to 50 % of TINU patients present with no ocular symptoms [54], emphasizing the critical need for uveitis screening in patients with TIN. This is particularly important in those patients who do not have medication-induced or systemic disease-associated TIN. Conversely, TINU may be underdiagnosed in patients presenting with idiopathic uveitis [50], also highlighting the importance of screening uveitis patients for TIN. Recently, HLA-DR and –DQ alleles have been identified and associated with TINU and are considered “risk alleles” [64]. DNA typing for these alleles may be particularly useful in screening pediatric patients with idiopathic panuveitis (as opposed to anterior uveitis), which can aid in the diagnosis of TINU [64].
TIN Associated with Inflammatory Bowel Disease (IBD)
IBD has been associated with a variety of renal and urologic complications that occur in up to 23 % of patients [46]. TIN has a strong association with IBD [65]. Other renal conditions in IBD patients include nephrolithiasis/urolithiasis, fistulas, glomerulonephritis, and renal amyloidosis [46, 66]. These manifestations may be secondary to systemic inflammation, susceptibility to autoimmunity, nutritional deficits, medication use, genetic predisposition, and infectious agents [65, 67]. One study reviewing renal biopsies in adults with IBD showed that TIN was present in 19 % of kidney biopsies [65]. Of those, 44 % were acute TIN, 25 % were chronic TIN, and 31 % were granulomatous TIN [65]. Mesalamine, used in the treatment of IBD, is a well-known medication associated with TIN [9, 46, 66, 67], but it has been clearly demonstrated that TIN can occur independently of medication use in IBD.
Inflammation and disease activity in IBD has been associated with low-molecular-weight proteinuria [46, 66], adding to the utility of urinary biomarkers for monitoring disease activity and screening for TIN in IBD patients. Studies have shown an association between IBD activity and elevated urinary beta2-microglobulin (B2M) [68], alpha1-microglobulin (A1M) [69], or N-acetyl-beta-D-glucosaminidase (NAG) [70]. By contrast, other studies have found no such correlation [71, 72]. One study showed that elevated urinary A1M was associated with TIN and tubular damage, but this was independent of IBD activity [73]. A possible explanation for such discrepancy in correlating urinary biomarkers with disease activity is the timing of TIN diagnosis. For example, in those IBD patients who do not have routine urinary studies TIN may be diagnosed late when irreversible renal damage might have already occurred leading to CKD [67, 74]. The above studies indicate that routine screening for TIN should be implemented in patients with IBD. This may be particularly important in patients receiving mesalamine, given that associated TIN may be severe, chronic, and progressive if not detected early [65].
Treatment
Treatment for TIN remains influenced by clinicians’ prior experience with the disease and is only supported by several small studies and case reports with conflicting results. There are no randomized, controlled, prospective studies, and corticosteroids are the mainstay of treatment although no consensus has been established regarding therapy duration or dose. It has been theorized that early steroid treatment could prevent fibrosis by decreasing inflammatory infiltrates [75], but has not yet been proven. Treatment is primarily guided by underlying pathophysiology, if it can be determined. For example, drug-induced TIN may recover spontaneously with cessation of the offending medication, particularly if identified early [2]. Aside from treating obvious sources of infection, other treatment options for infection-related TIN have not been well-described, although in transplant patients immunosuppressive medication can be decreased [14] or Cidofovir used in polyoma virus-associated infections [76]. Since medication-related acute TIN usually resolves after discontinuation of the offending drug, we recommend that the first line of treatment for antibiotic-related acute TIN is its discontinuation while the infection is treated with an alternative agent. The need for additional medications such as corticosteroids should be assessed based on the subsequent clinical course. On the opposite end of the spectrum, systemic rheumatologic and inflammatory conditions associated with TIN (including TINU) are more often treated with corticosteroids or with other agents based on the systemic disease [34, 36, 77].
A retrospective study of 60 adults with acute TIN with a variety of underlying etiologies showed no difference in outcome when comparing treatment with corticosteroids to supportive care alone when assessing serum creatinine at 1, 6, and 12 months follow-up or dialysis independence [6]. Conversely, a more recent prospective study assessing pediatric patients with idiopathic TIN or TINU showed that corticosteroids sped up recovery of TIN, particularly in patients with more severe disease [78]. Notably though, the renal function did not differ significantly at 6-month follow-up. The study suggested that because TIN may be self-limited, treatment may be delayed by two weeks in uncomplicated cases [78]. One multicenter retrospective study in adult patients with drug-induced TIN showed that steroid treatment, particularly if started early, may decrease the risk of incomplete renal recovery [75]. Results demonstrated that patients not treated with corticosteroids were more likely to have a higher final serum creatinine and had a higher likelihood of needing chronic dialysis. The most prominent difference between patients who regained renal function and those who didn't was the time between removal of the offending medication and initiation of corticosteroid treatment [75].
In granulomatous TIN, one small retrospective study suggested that treatment with corticosteroids is associated with a better prognosis, irrespective of degree of tubulointerstitial fibrosis or inflammation on biopsy [45]. Another report stated that findings of mild tubulointerstitial fibrosis were associated with a better response to steroid therapy in granulomatous TIN [44]. The IgG4-associated TIN is characterized by good response to corticosteroids [34].
Aside from steroid therapy, mycophenolate mofetil has been proposed as a possible treatment option in TIN. A retrospective chart review assessing a small group of adult patients with acute TIN showed that mycophenolate mofetil was well-tolerated and may be a useful therapy for steroid-resistant TIN or in patients with contraindications to steroid therapy [77].
In TINU, the treatment of anterior uveitis includes topical corticosteroids and cycloplegic agents and is effective in about 50 % of patients [53, 61, 78, 79]. However, most patients (80 %) are treated with systemic corticosteroids because of TIN. In patients who do not respond to systemic corticosteroids or who demonstrate ocular or systemic toxicity from these medications, immunomodulatory agents such as methotrexate, cyclosporine A, azathioprine, and mycophenolate mofetil have been used to treat the uveitis [53, 58, 61]. While the interstitial nephritis in TINU may resolve, uveitis requires long-term ophthalmologic care.
Monitoring
Aside from following renal function and electrolytes, clinicians often have a difficult time monitoring TIN, particularly in chronic cases. Serum C3 and C4 complement, IgG isotypes and IgE levels can help identify patients with IgG4-associated immune complex mediated TIN variants. Urinary biomarkers have been proposed as a way of identifying and prognosticating TIN. BEN provides an example of a chronic, progressive TIN that predominantly affects the proximal tubule and serves as a useful model for testing biomarkers [80]. Low-molecular-weight (LMW) proteinuria is suggestive of tubulointerstitial disease and possible fibrosis [78]. Beta-2 microglobulin (B2M) and alpha-1 microglobulin (A1M) are both LMW proteins that are normally freely filtered through the glomerulus and reabsorbed by cells in the proximal tubule [78, 81]. When renal tubules are damaged or dysfunctional, there is increased urinary excretion of LMW proteins. One study of urinary biomarkers in patients with BEN concluded that B2M had higher sensitivity and specificity than A1M in differentiating healthy controls from patients with BEN [81]. A study assessing the utility of A1M as a marker for chronic TIN showed that increased urinary ratios of A1M/albumin or A1M/protein in a 24-hour urine collection showed an appropriate relationship with chronic TIN, and helped to differentiate it from healthy control subjects and those with glomerulonephritis [41]. Another study analyzed 61 urinary proteins present in patients with BEN, and found that A1M and B2M were consistently found in larger amounts in patients with BEN compared to healthy controls and patients with pre-renal AKI [80]. Additionally, in comparing BEN with glomerulonephritis, elevated B2M was the most accurate biomarker for identifying BEN as opposed to glomerulonephritis [80]. Urinary B2M has also been proposed as a screening measure for individuals with uveitis to help detect TINU syndrome [50]. One study revealed that when using both serum creatinine and urinary B2M levels in patients with uveitis, there is a positive predictive value of 100 % and negative predictive value of 97 % when assessing for associated TIN [82]. At this time, compared to other urinary biomarkers, B2M and A1M are most commonly used for testing for tubular damage. B2M is degraded in urine when pH falls below 6, while A1M remains stable [81]. A Finnish study of pediatric patients with TIN showed that patients with elevated and prolonged urinary LMW protein excretion (B2M and A1M) had an associated decrease in measured GFR when compared to those with normal urinary LMW protein excretion [78]. Another group studying urinary biomarkers concluded that urinary monocyte chemotactic peptide-1 (MCP-1) levels showed a close correlation with interstitial inflammation and edema in patients with drug-induced TIN [83]. Eventually, MCP-1 may be used to help differentiate TIN from ATN, but is not yet commercially available. Taken together, the above studies demonstrate that measurement of urinary LMW protein excretion may be a feasible tool to monitor progression of tubulointerstitial disease in patients with TIN.
Chronic TIN
While some episodes of acute TIN are reversible (particularly if an offending medication is discontinued), others may progress into chronic TIN. The likelihood is increased with systemic inflammatory or rheumatologic diseases as well as delayed removal of the medication in drug-induced TIN [9], including analgesic and lithium nephropathy [84]. In one retrospective study of biopsies from adult patients with TIN, the median percentage of interstitial fibrosis was 30 % and median glomerulosclerosis was 8 % [6], which indicate chronic change. In an Italian registry of renal biopsies, children with CKD most frequently had chronic interstitial diseases which included juvenile nephronophthisis, chronic TIN or reflux nephropathy [7]. Rarer causes of chronic TIN in children include heavy metal exposure [85] and neoplasia [86, 87]. As mentioned previously, TIN-ag is an integral component of the renal tubular basement membrane. Defects in the tubular basement membrane observed in juvenile nephronophthisis have been associated with abnormalities in TIN-ag synthesis which can eventually lead to renal failure [39]. Ongoing detection of LMW proteins in the urine may be signs of ongoing renal tubulointerstitial inflammation or fibrosis, or both, which again supports the use of these biomarkers in follow-up of patients with TIN [78]. Chronic TIN rarely results from bacterial infections alone [88].
Prognosis
Prognosis primarily depends upon the cause of TIN, in combination with therapy for systemic diseases, timing of therapy, previous renal function, and removal of any known offending agents. Chronicity portends a worse outcome, and detection of fibrosis on renal biopsy is a marker of irreversible change. Early identification of TIN can often improve renal outcomes. Prolonged LMW proteinuria is a marker for poorer prognosis and decreased GFR [78]. In a review of adults with TIN, 64 % made a full recovery, while 23 % had partial recovery and 13 % remained on renal replacement therapy [3].
Conclusion
In summary, tubulointerstitial nephritis is an under-recognized disease that often presents with non-specific symptoms. A high clinical suspicion and particular attention to extrarenal manifestations and thorough review of potential risk factors are needed for accurate identification and diagnosis. It is most important to remove any potential offending agent and treat associated systemic disease to help preserve or recover renal function. Monitoring for TIN in patients with uveitis or IBD could be a useful tool for early diagnosis and treatment. While there are promising urinary biomarkers to diagnose and prognosticate TIN, A1M and B2M are most promising for clinical use. Treatment is based on underlying pathophysiology, and use of corticosteroids remains poorly supported by clinical trials. Randomized controlled prospective trials are needed to best assess prognosis and therapy.
Key Summary Points.
Tubulointerstitial nephritis is often diagnosed late, so clinical suspicion is necessary for early identification and possible intervention (or removal of the offending agent)
Presenting signs and symptoms of TIN can include non-specific systemic symptoms (fatigue, weight loss, headache, flank pain), fever, rash, eosinophilia/eosinophiluria and evidence of elevated creatinine and Fanconi's syndrome (glucosuria, aminoaciduria, acidosis)
Etiology of TIN can be drug-induced, infectious, idiopathic, genetic, or related to a systemic inflammatory condition such as tubulointerstitial nephritis and uveitis (TINU) syndrome or inflammatory bowel disease (IDB)
Treatment is based on etiology; aside from removal of offending agents, the mainstay of therapy is corticosteroids, and less often mycophenolate mofetil
Urinary biomarkers such as alpha1-microglobulin (A1M) and beta2-microglobulin (B2M) may help diagnose and monitor disease activity in TIN
Questions (answers appear following the reference list)
- Tubulointerstitial dysfunction is often accompanied by electrolyte abnormalities that include:
- Hyperkalemia, hyperchloremia, and metabolic acidosis
- Hyperkalemia, hyponatremia, and metabolic acidosis
- Hypokalemia, hypernatremia, and metabolic alkalosis
- Hypokalemia, hyperchloremia, and metabolic acidosis
- What is the most common type of uveitis present in patients with TINU Syndrome?
- Anterior uveitis
- Posterior uveitis
- Intermediate uveitis
- Panuveitis
- The most common underlying etiology of TIN is:
- TINU Syndrome
- Inflammatory Bowel Disease
- Drug-Induced
- Infection
- Regarding drug-induced TIN, which of the following is FALSE?
- TIN can recur after re-exposure to the drug
- Eosinophils are a predominant finding on renal biopsy
- NSAIDs are a common cause for drug-induced TIN
- The risk for drug-induced TIN increases with increasing dose of the drug
- Which of the following are low-molecular-weight proteins that can be used as urinary biomarkers for diagnosis and monitoring in TIN?
- Beta2-microglobulin (B2M)
- Monocyte chemotactic peptide-1 (MCP-1)
- TIN antigen
- Mucin-1
Answers
A
A
C
D
A
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ulinski T, Sellier-Leclerc A-L, Tudorache E, Bensman A, Aoun B. Acute tubulointerstitial nephritis. Pediatr Nephrol. 2011;27:1051–1057. doi: 10.1007/s00467-011-1915-9. [DOI] [PubMed] [Google Scholar]
- 2.Andreoli SP. Acute kidney injury in children. Pediatr Nephrol. 2009;24:253–263. doi: 10.1007/s00467-008-1074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker RJ, Pusey CD. The changing profile of acute tubulointerstitial nephritis. Nephrol Dial Transplant. 2004;19:8–11. doi: 10.1093/ndt/gfg464. [DOI] [PubMed] [Google Scholar]
- 4.Rossert J. Drug-induced acute interstitial nephritis. Kidney Int. 2001;60:804–817. doi: 10.1046/j.1523-1755.2001.060002804.x. [DOI] [PubMed] [Google Scholar]
- 5.Hodgkins KS, Schnaper HW. Tubulointerstitial injury and the progression of chronic kidney disease. Pediatr Nephrol. 2011;27:901–909. doi: 10.1007/s00467-011-1992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarkson MR. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant. 2004;19:2778–2783. doi: 10.1093/ndt/gfh485. [DOI] [PubMed] [Google Scholar]
- 7.Coppo R, Gianoglio B, Porcellini MG. Frequency of renal diseases and clinical indications for renal biopsy in children (report of the Italian National Registry of Renal Biopsies in Children). Nephrol Dial transplant. 1998;13:293–297. doi: 10.1093/oxfordjournals.ndt.a027821. [DOI] [PubMed] [Google Scholar]
- 8.Greising J, Trachtman H, Gauthier B, Valderrama E. Acute interstitial nephritis in adolescents and young adults. Child Nephrol Urol. 1990;10:189–195. [PubMed] [Google Scholar]
- 9.Perazella MA, Markowitz GS. Drug-induced acute interstitial nephritis. Nat Rev Nephrol. 2010;6:461–470. doi: 10.1038/nrneph.2010.71. [DOI] [PubMed] [Google Scholar]
- 10.Schubert C, Bates WD, Moosa MR. Acute tubulointerstitial nephritis related to antituberculous drug therapy. Clin Nephrol. 2010;73:413–419. doi: 10.5414/cnp73413. [DOI] [PubMed] [Google Scholar]
- 11.Nessi R, Bonoldi GL, Redaelli B, di Filippo G. Acute renal failure after rifampicin: a case report and survey of the literature. Nephron. 1976;16:148–159. doi: 10.1159/000180596. [DOI] [PubMed] [Google Scholar]
- 12.Flynn CT, Rainford DJ, Hope E. Acute renal failure and rifampicin: danger of unsuspected intermittent dosage. Br Med J. 1974;2:482. doi: 10.1136/bmj.2.5917.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perazella MA. Drug use and nephrotoxicity in the intensive care unit. Kidney Int. 2010;81:1172–1178. doi: 10.1038/ki.2010.475. [DOI] [PubMed] [Google Scholar]
- 14.Storsley L, Gibson IW. Adenovirus interstitial nephritis and rejection in an allograft. J Am Soc Nephrol. 2011;22:1423–1427. doi: 10.1681/ASN.2010090941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baksh FK, Finkelstein SD, Swalsky PA, Stoner GL, Ryschkewitsch CF, Randhawa P. Molecular genotyping of BK and JC viruses in human polyomavirus-associated interstitial nephritis after renal transplantation. Am J Kidney Dis. 2001;38:354–365. doi: 10.1053/ajkd.2001.26101. [DOI] [PubMed] [Google Scholar]
- 16.Chang J-F, Peng Y-S, Tsai C-C, Hsu M-S, Lai C-F. A possible rare cause of renal failure in streptococcal infection. Nephrol Dial Transplant. 2010;26:368–371. doi: 10.1093/ndt/gfq569. [DOI] [PubMed] [Google Scholar]
- 17.Ozdemir BH, Sar A, Uyar P, Suren D, Demirhan B, Haberal M. Posttransplant tubulointerstitial nephritis: clinicopathological correlation. Transplant Proc. 2006;38:466–469. doi: 10.1016/j.transproceed.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 18.Richardson WP, Colvin RB, Cheeseman SH, Tolkoff-Rubin NE, Herrin JT, Cosimi AB, Collins AB, Hirsch MS, McCluskey RT, Russel PS, Rubin RH. Glomerulopathy associated with cytomegalovirus viremia in renal allografts. N Engl J Med. 1981;305:57–63. doi: 10.1056/NEJM198107093050201. [DOI] [PubMed] [Google Scholar]
- 19.Howell DN, Smith SR, Butterly DW, Klassen PS, Krigman HR, Burchette JL, Jr, Miller SE. Diagnosis and management of BK polyomavirus interstitial nephritis in renal transplant recipients. Transplantation. 1999;68:1279–1288. doi: 10.1097/00007890-199911150-00011. [DOI] [PubMed] [Google Scholar]
- 20.Platt JL, Sibley RK, Michael AF. Interstitial nephritis associated with cytomegalovirus infection. Kidney Int. 1985;28:550–552. doi: 10.1038/ki.1985.163. [DOI] [PubMed] [Google Scholar]
- 21.Rosen S, Harmon W, Krensky AM, Edelson PJ, Padgett BL, Grinnell BW, Rubino MJ, Walker DL. Tubulo-interstitial nephritis associated with polyomavirus (BK type) infection. N Engl J Med. 1983;308:1192–1196. doi: 10.1056/NEJM198305193082004. [DOI] [PubMed] [Google Scholar]
- 22.Asim M, Chong-Lopez A, Nickeleit V. Adenovirus infection of a renal allograft. Am J Kidney Dis. 2003;41:696–701. doi: 10.1053/ajkd.2003.50133. [DOI] [PubMed] [Google Scholar]
- 23.Ito M, Hirabayashi N, Uno Y, Nakayama A, Asai J. Necrotizing tubulointerstitial nephritis associated with adenovirus infection. Hum Pathol. 1991;22:1225–1231. doi: 10.1016/0046-8177(91)90104-w. [DOI] [PubMed] [Google Scholar]
- 24.Mori K, Yoshihara T, Nishimura Y, Uchida M, Katsura K, Kawase Y, Hatano I, Ishida H, Chiyonobu T, Kasubuchi Y, Morimoto A, Teramura T, Imashuku S. Acute renal failure due to adenovirus-associated obstructive uropathy and necrotizing tubulointerstitial nephritis in a bone marrow transplant recipient. Bone Marrow Transplant. 2003;31:1173–1176. doi: 10.1038/sj.bmt.1704077. [DOI] [PubMed] [Google Scholar]
- 25.Cohen AH, Nast CC. HIV-associated nephropathy. A unique combined glomerular, tubular, and interstitial lesion. Mod Pathol. 1988;1:87–97. [PubMed] [Google Scholar]
- 26.Cigni A, Soro G, Faedda R, Caucci F, Amadori F, Manca A, Tanda F, Satta AE. A case of adult-onset tubulointerstitial nephritis and uveitis (“TINU syndrome”) associated with sacroileitis and Epstein-Barr virus infection with good spontaneous outcome. Am J Kidney Dis. 2003;42:E4–10. doi: 10.1016/s0272-6386(03)00795-9. [DOI] [PubMed] [Google Scholar]
- 27.Grefer J, Santer R, Ankermann T, Faul S, Nolle B, Eggert P. Tubulointerstitial nephritis and uveitis in association with Epstein-Barr virus infection. Pediatr Nephrol. 1999;13:336–339. doi: 10.1007/s004670050621. [DOI] [PubMed] [Google Scholar]
- 28.Yang CW, Wu MS, Pan MJ. Leptospirosis renal disease. Nephrol Dial Transplant. 2001;16(Suppl 5):73–77. doi: 10.1093/ndt/16.suppl_5.73. [DOI] [PubMed] [Google Scholar]
- 29.Pasternack A, Helin H, Vänttinen T, Jarventie G, Vesikari T. Acute tubulointerstitial nephritis in a patient with Mycoplasma pneumoniae infection. Scand J Infect Dis. 1979;11:85–87. doi: 10.3109/inf.1979.11.issue-1.15. [DOI] [PubMed] [Google Scholar]
- 30.Saïd MH, Layani MP, Colon S, Faraj G, Glastre C, Cochat P. Mycoplasma pneumoniae-associated nephritis in children. Pediatr Nephrol. 1999;13:39–44. doi: 10.1007/s004670050559. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi Y, Honda M, Yoshikawa N, Ito H. Acute tubulointerstitial nephritis in 21 Japanese children. Clin Nephrol. 2000;54:191–197. [PubMed] [Google Scholar]
- 32.Yamaguchi Y, Kanetsuna Y, Honda K, Yamanaka N, Kawano M, Nagata M, Japanese study group on IgG4-related nephropathy Characteristic tubulointerstitial nephritis in IgG4-related disease. Hum Pathol. 2011;43:536–549. doi: 10.1016/j.humpath.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Cornell LD, Chicano SL, Deshpande V, Collins AB, Selig MK, Lauwers GY, Barisoni L, Colvin RB. Pseudotumors due to IgG4 immune-complex tubulointerstitial nephritis associated with autoimmune pancreatocentric disease. Am J Surg Pathol. 2007;31:1586–1597. doi: 10.1097/PAS.0b013e318059b87c. [DOI] [PubMed] [Google Scholar]
- 34.Saeki T, Nishi S, Imai N, Ito T, Yamazaki H, Kawano M, Yamamoto M, Takahashi H, Matsui S, Nakada S, Origuchi T, Hirabayashi A, Homma N, Tsubata Y, Takata T, Wada Y, Saito A, Fukase S, Ishioka K, Miyazaki K, Masaki Y, Umehara H, Sugai S, Narita I. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int. 2010;78:1016–1023. doi: 10.1038/ki.2010.271. [DOI] [PubMed] [Google Scholar]
- 35.Uchiyama-Tanaka Y, Mori Y, Kimura T, Sonomura K, Umemura S, Kishimoto N, Nose A, Tokoro T, Kijima Y, Yamahara H, Nagata T, Masaki H, Umeda Y, Okazaki K, Iwasaka T. Acute tubulointerstitial nephritis associated with autoimmune-related pancreatitis. Am J Kidney Dis. 2004;43:e18–25. doi: 10.1053/j.ajkd.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Vaseemuddin M, Schwartz MM, Dunea G, Kraus MA. Idiopathic hypocomplementemic immune-complex-mediated tubulointerstitial nephritis. Nat Clin Pract Nephrol. 2006;3:50–58. doi: 10.1038/ncpneph0347. [DOI] [PubMed] [Google Scholar]
- 37.Clayman MD, Michaud L, Brentjens J, Andres GA, Kefalides NA, Neilson EG. Isolation of the target antigen of human anti-tubular basement membrane antibody-associated interstitial nephritis. J Clin Invest. 1986;77:1143–1147. doi: 10.1172/JCI112414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikeda M, Takemura T, Hino S, Yoshioka K. Molecular cloning, expression, and chromosomal localization of a human tubulointerstitial nephritis antigen. Biochem Biophys Res Commun. 2000;268:225–230. doi: 10.1006/bbrc.2000.2103. [DOI] [PubMed] [Google Scholar]
- 39.Takemura Y, Koshimichi M, Sugimoto K, Yanagida H, Fujita S, Miyazawa T, Miyazaki K, Okada M, Takemura T. A tubulointerstitial nephritis antigen gene defect causes childhood-onset chronic renal failure. Pediatr Nephrol. 2010;25:1349–1353. doi: 10.1007/s00467-010-1463-8. [DOI] [PubMed] [Google Scholar]
- 40.Eckardt K-U, Alper SL, Antignac C, Bleyer AJ, Chauveau D, Dahan K, Deltas C, Hosking A, Kmoch S, Rampoldi L, Wiesener M, Wolf MT, Devuyst O. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management—A KDIGO consensus report. Kidney Int. 2015;88:676–683. doi: 10.1038/ki.2015.28. [DOI] [PubMed] [Google Scholar]
- 41.Robles NR, Lopez-Gomez J, Garcia-Pino G, Ferreira F, Alvarado R, Sanchez-Casado E, Cubero JJ. Use of α1-microglobulin for diagnosing chronic interstitial nephropathy. Clin Exp Med. 2013;14:315–320. doi: 10.1007/s10238-013-0242-9. [DOI] [PubMed] [Google Scholar]
- 42.García-Sánchez O, López-Hernández FJ, López-Novoa JM. An integrative view on the role of TGF-beta in the progressive tubular deletion associated with chronic kidney disease. Kidney Int. 2010;77:950–955. doi: 10.1038/ki.2010.88. [DOI] [PubMed] [Google Scholar]
- 43.Tong JE, Howell DN, Foreman JW. Drug-induced granulomatous interstitial nephritis in a pediatric patient. Pediatr Nephrol. 2006;22:306–309. doi: 10.1007/s00467-006-0318-9. [DOI] [PubMed] [Google Scholar]
- 44.Shah S, Carter-Monroe N, Atta MG. Granulomatous interstitial nephritis. Clin Kidney J. 2015;8:516–523. doi: 10.1093/ckj/sfv053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joss N, Morris S, Young B, Geddes C. Granulomatous Interstitial Nephritis. Clin J Am Soc Nephrol. 2007;2:222–230. doi: 10.2215/CJN.01790506. [DOI] [PubMed] [Google Scholar]
- 46.Oikonomou K, Kapsoritakis A, Eleftheriadis T, Stefanidis I, Potamianos S. Renal Manifestations and Complications of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2011;17:1034–1045. doi: 10.1002/ibd.21468. [DOI] [PubMed] [Google Scholar]
- 47.Meehan SM, Josephson MA, Haas M. Granulomatous tubulointerstitial nephritis in the renal allograft. Am J Kidney Dis. 2000;36:E27. doi: 10.1053/ajkd.2000.17735. [DOI] [PubMed] [Google Scholar]
- 48.Cosyns JP, Jadoul M, Squifflet JP, De Plaen FJ, Ferluga D, van Ypersele de Strihou C. Chinese herbs nephropathy: a clue to Balkan endemic nephropathy? Kidney Int. 1994;45:1680–1688. doi: 10.1038/ki.1994.220. [DOI] [PubMed] [Google Scholar]
- 49.Tatu CA, Orem WH, Finkelman RB, Feder GL. The etiology of Balkan endemic nephropathy: still more questions than answers. Environ Health Perspect. 1998;106:689–700. doi: 10.1289/ehp.106-1533478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackensen F, Smith JR, Rosenbaum JT. Enhanced Recognition, Treatment, and Prognosis of Tubulointerstitial Nephritis and Uveitis Syndrome. Ophthalmology. 2007;114:995–999. doi: 10.1016/j.ophtha.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Levinson RD, Mandeville J, Holland GN, Rosenbaum JT. Tubulointerstitial nephritis and uveitis syndrome: recognizing the importance of an uncommon disease. Am J Ophthalmol. 2000;129:798–799. doi: 10.1016/s0002-9394(00)00483-9. [DOI] [PubMed] [Google Scholar]
- 52.Thomassen VH, Ring T, Thaarup J, Baggesen K. Tubulointerstitial nephritis and uveitis (TINU) syndrome: a case report and review of the literature. Acta Ophthalmol. 2009;87:676–679. doi: 10.1111/j.1755-3768.2008.01302.x. [DOI] [PubMed] [Google Scholar]
- 53.Mandeville J, Levinson RD, Holland GN. The tubulointerstitial nephritis and uveitis syndrome. Surv Ophthalmol. 2001;46:195–208. doi: 10.1016/s0039-6257(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 54.Saarela V, Nuutinen M, Ala-Houhala M, Arikoski P, Ronnholm K, Jahnukainen T. Tubulointerstitial Nephritis and Uveitis Syndrome in Children: A Prospective Multicenter Study. Ophthalmology. 2013;120:1476–1481. doi: 10.1016/j.ophtha.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 55.Hettinga YM, Scheerlinck LME, Lilien MR, Rothova A, de Boer JH. The value of measuring urinary β2-microglobulin and serum creatinine for detecting tubulointerstitial nephritis and uveitis syndrome in young patients with uveitis. JAMA Ophthalmol. 2014;133:140–145. doi: 10.1001/jamaophthalmol.2014.4301. [DOI] [PubMed] [Google Scholar]
- 56.Nussenblatt RB. Investigation of anterior uveitis. Can J Ophthalmol. 2008;43:630–633. doi: 10.1139/i08-156. [DOI] [PubMed] [Google Scholar]
- 57.Murray N, Wakefield D. Primary tubulointerstitial nephritis and uveitis syndrome. Aust N Z J Ophthalmol. 1993;21:121–122. doi: 10.1111/j.1442-9071.1993.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 58.Janda J, Rambousek V, Kolský A, Stejskal J. Acute interstitial nephritis with uveitis in children and adolescents. Cesk Pediatr. 1990;45:7–11. [PubMed] [Google Scholar]
- 59.Sanchez-Burson J, Garcia-Porrua C, Montero-Granados R, Gonzalez-Escribano F, Gonzalez-Gay MA. Tubulointerstitial nephritis and uveitis syndrome in Southern Spain. Semin Arthritis Rheum. 2002;32:125–129. doi: 10.1053/sarh.2002.33718. [DOI] [PubMed] [Google Scholar]
- 60.Kanski JJ, Bowling B. Clinical ophthalmology: a synopsis. Elselvier; Philadelphia: 2009. [Google Scholar]
- 61.Gafter U, Kalechman Y, Zevin D, Korzets A. Tubulointerstitial nephritis and uveitis: association with suppressed cellular immunity. Nephrol Dial Transplant. 1993;8:821–826. [PubMed] [Google Scholar]
- 62.Auclin F, Bodard E. Interstitial tubulo-nephritis and uveitis (Nitu syndrome). Apropos of a case. J Fr Ophtalmol. 1988;12:307–311. [PubMed] [Google Scholar]
- 63.Gion N, Stavrou P, Foster CS. Immunomodulatory therapy for chronic tubulointerstitial nephritis–associated uveitis. A J Ophthalmol. 2000;129:764–768. doi: 10.1016/s0002-9394(00)00482-7. [DOI] [PubMed] [Google Scholar]
- 64.Reddy AK, Hwang Y-S, Mandelcorn ED, Davis JL. HLA-DR, DQ Class II DNA Typing in Pediatric Panuveitis and Tubulointerstitial Nephritis and Uveitis. A J Ophthalmol. 2014;157:678–686. doi: 10.1016/j.ajo.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 65.Ambruzs JM, Walker PD, Larsen CP. The histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease. Clin J Am Soc Nephrol. 2013;9:265–270. doi: 10.2215/CJN.04660513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tokuyama H, Wakino S, Konishi K, Hashiguchi A, Hayashi K, Itoh H. Acute interstitial nephritis associated with ulcerative colitis. Clin Exp Nephrol. 2010;14:483–486. doi: 10.1007/s10157-010-0294-z. [DOI] [PubMed] [Google Scholar]
- 67.Marcus SB, Brown JB, Melin-Aldana H, Strople JA. Tubulointerstitial nephritis: an extraintestinal manifestation of Crohn disease in children. J Pediatr Gastroenterol Nutr. 2008;46:338–341. doi: 10.1097/MPG.0b013e31806dc2c4. [DOI] [PubMed] [Google Scholar]
- 68.Poulou AC, Goumas KE, Dandakis DC, Tyrmpas I, Panagiotaki M, Gerogouli A, Soutos DC, Archimandritis A. Microproteinuria in patients with inflammatory bowel disease: is it associated with the disease activity or the treatment with 5-aminosalicylic acid? World J Gastroenterol. 2006;12:739–746. doi: 10.3748/wjg.v12.i5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herrlinger KR, Noftz MK, Fellermann K, Schmidt K, Steinhoff J, Stange EF. Minimal renal dysfunction in inflammatory bowel disease is related to disease activity but not to 5-ASA use. Aliment Pharmacol Ther. 2001;15:363–369. doi: 10.1046/j.1365-2036.2001.00940.x. [DOI] [PubMed] [Google Scholar]
- 70.Kreisel W, Wolf LM, Grotz W, Grieshaber M. Renal tubular damage: an extraintestinal manifestation of chronic inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1996;8:461–468. [PubMed] [Google Scholar]
- 71.Schreiber S, Hämling J, Zehnter E, Howaldt S, Daerr W, Raedler A, Kruis W. Renal tubular dysfunction in patients with inflammatory bowel disease treated with aminosalicylate. Gut. 1997;40:761–766. doi: 10.1136/gut.40.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riley SA, Lloyd DR, Mani V. Tests of renal function in patients with quiescent colitis: effects of drug treatment. Gut. 1992;33:1348–1352. doi: 10.1136/gut.33.10.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fraser JS, Muller AF, Smith DJ, Newman DJ, Lamb EJ. Renal tubular injury is present in acute inflammatory bowel disease prior to the introduction of drug therapy. Aliment Pharmacol Ther. 2001;15:1131–1137. doi: 10.1046/j.1365-2036.2001.01041.x. [DOI] [PubMed] [Google Scholar]
- 74.Pardi DS, Tremaine WJ, Sandborn WJ, McCarthy JT. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol. 1998;93:504–514. doi: 10.1111/j.1572-0241.1998.156_b.x. [DOI] [PubMed] [Google Scholar]
- 75.González E, Gutiérrez E, Galeano C, Chevia C, de Sequera P, Bernis C, Parra EG, Delgado R, Sanz M, Ortiz M, Goicoechea M, Quereda C, Olea T, Bouarich H, Hernandez Y, Segovia B, Praga M, Grupo Madrileno De Nefritis Intersticiales Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int. 2008;73:940–946. doi: 10.1038/sj.ki.5002776. [DOI] [PubMed] [Google Scholar]
- 76.Araya CE, Lew JF, Fennell RS, Neiberger RE, Dharnidharka VR. Intermediate-dose cidofovir without probenecid in the treatment of BK virus allograft nephropathy. Pediatr Transplant. 2006;10:32–37. doi: 10.1111/j.1399-3046.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 77.Preddie DC, Markowitz GS, Radhakrishnan J, Nickolas TL, D'Agati VD, Schwimmer JA, Gardenswartz M, Rosen R, Appel GB. Mycophenolate Mofetil for the Treatment of Interstitial Nephritis. Clin J Am Soc Nephrol. 2006;1:718–722. doi: 10.2215/CJN.01711105. [DOI] [PubMed] [Google Scholar]
- 78.Jahnukainen T, Saarela V, Arikoski P, Ylinen E, Ronnholm K, Ala-Houhala M, Nuutinen M. Prednisone in the treatment of tubulointerstitial nephritis in children. Pediat Nephrol. 2013;28:1253–1260. doi: 10.1007/s00467-013-2476-x. [DOI] [PubMed] [Google Scholar]
- 79.Kenouch S, Belghiti D, Di Costanzo P. A new syndrome: acute interstitial nephropathy with uveitis. Apropos of a case. Ann Med Interne (Paris) 1988;140:169–172. [PubMed] [Google Scholar]
- 80.Pešić I, Stefanović V, Müller GA, Müller CA, Cukuranovic R, Jahn O, Bojanic V, Koziolek M, Dihazi H. Identification and validation of six proteins as marker for endemic nephropathy. J Proteomics. 2011;74:1994–2007. doi: 10.1016/j.jprot.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 81.Stefanović V, Djukanović L, Cukuranović R, Bukvic D, Lezaic V, Maric I, Ogrizovic SS, Jovanovic I, Vlahovic P, Pesic I, Djordjevic V. Beta2-microglobulin and alpha1-microglobulin as markers of Balkan endemic nephropathy, a worldwide disease. Ren Fail. 2011;33:176–183. doi: 10.3109/0886022X.2011.552152. [DOI] [PubMed] [Google Scholar]
- 82.Hettinga YM, Scheerlinck LME, Lilien MR, Rothova A, de Boer JH. The Value of Measuring Urinary β2-Microglobulin and Serum Creatinine for Detecting Tubulointerstitial Nephritis and Uveitis Syndrome in Young Patients With Uveitis. JAMA Ophthalmol. 2015;133:140–145. doi: 10.1001/jamaophthalmol.2014.4301. [DOI] [PubMed] [Google Scholar]
- 83.Wu Y, Yang L, Su T, Wang C, Liu G, Li XM. Pathological significance of a panel of urinary biomarkers in patients with drug-induced tubulointerstitial nephritis. Clin J Am Soc Nephrol. 2010;5:1954–1959. doi: 10.2215/CJN.02370310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nolin TD, Himmelfarb J. Mechanisms of drug-induced nephrotoxicity. Handb Exp Pharmacol. 2009:111–130. doi: 10.1007/978-3-642-00663-0_5. [DOI] [PubMed] [Google Scholar]
- 85.Perazella MA. Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol. 2009;4:1275–1283. doi: 10.2215/CJN.02050309. [DOI] [PubMed] [Google Scholar]
- 86.Akil I, Ozguven A, Canda E, Yilmaz O, Nese N, Ozkol M, May S, Franke A, Cirak S. Co-existence of chronic renal failure, renal clear cell carcinoma, and Blau syndrome. Pediatr Nephrol. 2010;25:977–981. doi: 10.1007/s00467-009-1413-5. [DOI] [PubMed] [Google Scholar]
- 87.Nakajima T, Tsujimoto I, Ujihira N, Sezaki R. Tubulointerstitial nephritis caused by chronic lymphocytic leukemia. Intern Med. 2015;54:685–686. doi: 10.2169/internalmedicine.54.3174. [DOI] [PubMed] [Google Scholar]
- 88.Murray T, Goldberg M. Chronic interstitial nephritis: etiologic factors. Ann Intern Med. 1975;82:453–459. doi: 10.7326/0003-4819-82-4-453. [DOI] [PubMed] [Google Scholar]
- 89.Jahnukainen T, Ala-Houhala M, Karikoski R, Kataja J, Saarela V, Nuutinen M. Clinical outcome and occurrence of uveitis in children with idiopathic tubulointerstitial nephritis. Pediatr Nephrol. 2010;26:291–299. doi: 10.1007/s00467-010-1698-4. [DOI] [PubMed] [Google Scholar]