Abstract
This was a cohort analysis evaluating patients with pancreatic adenocarcinoma who presented with or developed ascites. Among the 180 patients analyzed, the use of serial paracenteses and indwelling catheters is a common practice to effect symptom palliation. The complication rate was higher in patients with indwelling catheters. Analyzing ascitic fluid and calculating the serum ascites albumin gradient can help attribute the etiology of the ascites and potentially identify which patients may benefit from diuretics or other intervention.
Background
Ascites develops in a subset of patients with pancreatic adenocarcinoma (PAC) at presentation or as the disease advances. Limited data exist on the prognostic importance of malignant ascites in PAC. Our hypothesis is that this information will provide an understanding of the natural history and facilitate management decisions.
Methods
We conducted a retrospective analysis of 180 patients treated at Memorial Sloan Kettering Cancer Center diagnosed between January 1, 2009 and December 31, 2014, with PAC and with ascites either at presentation or that developed during the disease course.
Results
For the 180 patients, the overall survival was 15 months. The time from diagnosis to ascites presentation was 11 months, and the survival time after ascites development was 1.8 months (range, 1.6–2.3 months; 95% confidence interval). Of 62 patients (34%) who had ascitic fluid analyzed, 36 (58%) had positive cytology. Fifty-one (82%) patients had a serum ascites albumin gradient ≥ 1, and 11 (18%) had serum ascites albumin gradient < 1. Sixty-four (36%) patients had their ascites managed solely by serial paracenteses. A total of 116 patients required a catheter; of these, 108 (93%) had a Tenckhoff catheter, 4 (3%) a Pleurx catheter, 4 (3%) a pigtail catheter, and 1 (1%) a Denver catheter. Eight (7%) patients required 2 catheters to be placed, and in 6 (5%), Tenckhoff catheters had to be removed. The main observed complications were spontaneous bacterial peritonitis in 7 (11%) managed with paracenteses versus 26 (23%) who had a catheter placed, catheter malfunction in 8 (7%), and acute renal failure in 6 (3%). After ascites development, 79 (44%) patients received active anti-cancer therapy, and 101 (56%) patients were managed with supportive care alone.
Conclusions
In patients with PAC who presented with or developed ascites, serial paracenteses and indwelling catheters are common methods used for providing symptomatic relief. The complication rate was higher with indwelling catheters, primarily related to infection (eg, bacterial peritonitis). Overall, ascites has a significantly negative prognostic import with a short median survival.
Keywords: Denver shunt, Malignant ascites, Performance status, Peritoneal catheter, Teneckoff catheter
Introduction
Background
Ascites, derived from the Greek work askites, “baglike,” is the accumulation of ascitic fluid in the abdominal cavity.1 It can either present at baseline or evolve during the disease course in patients with advanced ovarian or gastrointestinal malignancies and other non-oncologic etiologies (eg, cirrhosis, portal hypertension, Budd-Chiari syndrome).2 The etiology of ascites can be multifactorial, but typically is due to the imbalance between the production and reabsorption of fluid.2 It is known that, in cancer-related ascites, fluid accumulation is due to impaired drainage from the peritoneal cavity secondary to tumor size and increased filtration from disruption of oncotic pressures.3 Common presenting symptoms include abdominal distention, shortness of breath, diminished appetite, fatigue, and lower-extremity edema.4
Currently, most of the data in the literature is based on ovarian cancer-related ascites. The prevalence of ascites in gastrointestinal (GI) cancers is about 21%, typically from malignancies such as pancreatic, colorectal, gastric, and gallbladder cancers.5 Older literature on ascites in GI cancers report that ascites carries a poor prognosis compared with ovarian cancer, with a mean survival of 19 weeks, versus GI cancers with a mean survival of 10 weeks from the onset of ascites.6
Factors that have been associated with a poor survival include the presence of liver metastases, low serum albumin (< 3 g/dL), low serum total protein (< 6 g/dL). In addition, high levels of serum urea (> 6.5 mg/dL), creatinine (> 5.85 mg/dL), and total bilirubin (> 1.7 mg/dL) had worse overall survival in patients with malignant ascites.7 An impaired performance status with Karnofsky index score ≤ 60 or Eastern Cooperative Oncology Group (ECOG) ≥ 2 was also correlated with inferior survival.8
Management of malignant ascites is challenging given the poor prognostic import, lack of guidelines, and lack of randomized studies to guide clinical decision-making. Current treatment options are centered on the palliation of symptoms, and typically, the most effective relief of ascites is achieved by treating the underlying disease with the most success being observed in the front-line treatment setting. Available medical treatments include the use of diuretics, which are inconsistently used among physicians,9 are typically mostly used in treatment of portal hypertension-related ascites,10 and have shown not to have a significant clinical benefit when the serum albumin gradient (SAAG) is < 1.1 g/dL.11 Therapeutic paracentesis is an alternative treatment option that has been used in the clinical setting because it incurs prompt symptomatic relief in about 90% of patients12 with rare complications like infection, bowel perforation, hypotension, and hemorrhage.13 However, the need for repeated hospital visits and often rapid recurrence of symptoms is a common drawback, given that the mean interval for need of the procedure is 10.4 days.14,15
To facilitate the management of ascitic fluid recurrence without the need for serial paracentesis, permanent drains were developed. Currently 2 types are popular; one is a tunneled catheter (Pleurx catheter) and the other is a nontunneled catheter (pigtail or dialysis catheter).16 The latter was originally developed in 1968 by Henry Tenckhoff as an indwelling peritoneal dialysis catheter17 that has been shown to be effective.18
These permanent drains have been shown to provide effective palliation as an alternative to a large volume paracentesis19 and allow the patient to drain smaller amounts of ascitic fluid when appropriate and thus has improved quality of life (QoL) by reducing the number of paracenteses procedures and trips to health care facilities.20 Conversely, a major risk pertaining to an indwelling catheter is the development of peritonitis, and the literature shows that the risk is higher in untunneled compared with tunneled catheter types.21 Another type of permanent drain is a peritoneal venous shunt,22 including the LeVeen shunt, the first prototype of its kind.23 Nevertheless, due to its poor efficacy and risk of serious complications, including port occlusion, disseminated intravascular coagulation, and short life, it was later replaced by the Denver shunt.23 Given the aforementioned issues, it has not been frequently used in a cancer setting.24
Overall, the existence of malignant ascites confers a poor prognostic situation and adds a cluster of symptoms that decrease the QoL in patients with an already established advanced malignancy, including abdominal distension, dyspnea, and abdominal pain.4 Life expectancy is measured in months or less.25 Limited data exist on the exact prognostic importance of malignant ascites in pancreas adenocarcinoma and patterns of treatment algorithms and outcomes; thus, we choose to study the natural history and management interventions for ascites related to pancreas adenocarcinoma in a specialist pancreas cancer referral center.
Objectives
The purpose of this study was to evaluate patients with advanced pancreas adenocarcinoma who developed ascites either as part of their clinical presentation or during their disease course, and analyze the clinical behavior, outcomes, and therapeutic interventions and evaluate the prognostic importance of the presence of ascites in this patient population.
Methods
Setting and Study Design
The Institutional Review and Privacy Board at Memorial Sloan Kettering Cancer Center reviewed this single-center cohort study. Patients with pathologic confirmation of pancreatic adenocarcinoma and ascites from January 1, 2009 to December 31, 2014 composed the study population. Data abstraction was performed from November 2014 to January 2015.
Search Strategy and Selection Criteria
An institutional database (DARWIN) was used to retrospectively obtain medical records from patients diagnosed with pancreatic cancer that were identified either by using the International Classifications of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) with the following code: 157 pancreas ca, 157.0 pancreas ca-head,157.1 pancreas ca-body,157.2 pancreas ca-tail, 157.8 pancreas ca, 157.9 pancreas ca. Selection criteria also included patients that had any of the following ICD-9 codes, along with their pancreatic cancer diagnosis: 789.5 ascites, 789.51 malignant ascites, 789.59 ascites NEC. Additional criteria used on database query involved the placement of Denver shunt, Pleurx/aspiration catheter, catheter placement, and/or paracentesis/peritocentesis. For evaluation of the time of onset of ascites, the medical records were reviewed, and the date of diagnosis were recorded as either written by physician on the assessment and plan, physical examination section, or radiologist computed tomography (CT) scan report.
Data Extraction
One of the authors (A.M.H.) performed electronic medical record review and data abstraction, and data was stored in a secure drive. Detailed demographic and clinical information was extracted, including date of cancer diagnosis, presenting symptoms, American Joint Committee on Cancer stage at presentation, performance status (ECOG) at presentation, sites of metastasis at presentation, primary tumor location, treatment modalities (surgery, systemic therapy), types of systemic therapy received, lines of treatments, interval between diagnosis of pancreatic cancer and occurrence of ascites, presenting symptom of ascites, method of diagnosis of ascites, ECOG at diagnosis of ascites, laboratory parameters at diagnosis of ascites (cell blood count, comprehensive metabolic panel, coagulation studies), CA-19-9, ascites albumin, ascites white blood cell count, management of ascites, number of paracenteses procedure(s), catheter placement, diuretic utilization, complications of therapies used to manage ascites, and survival outcomes (Table 1).
Table 1.
Baseline Characteristics of Patients Diagnosed With Pancreatic Adenocarcinoma
| Characteristics | n = 180 | Percentage |
|---|---|---|
| Gender | ||
| Male | 105 | 58 |
| Female | 75 | 42 |
| Age, year (mean, range) | ||
| ≤60 | 52 (34–60) | 29 |
| >60 | 128 (61–91) | 71 |
| Ethnic origin | ||
| Caucasian | 138 | 77 |
| Asian | 17 | 9 |
| African-American | 13 | 7 |
| Hispanic | 12 | 7 |
| Year diagnosed | ||
| 2009–2011 | 87 | 48 |
| 2012–2014 | 93 | 52 |
| Pancreatic primary tumor location | ||
| Head | 89 | 49 |
| Body | 41 | 23 |
| Tail | 33 | 18 |
| Overlap (body and tail) | 17 | 9 |
| Surgery performed | n = 39 | 22 |
| Whipple procedure | 23 | 13 |
| Other type of resectiona | 11 | 6 |
| Exploratory laparotomy but not resected8 | 5 | 3 |
| AJCC stage at cancer diagnosis | ||
| I A + B | 3 | 2 |
| II A | 17 | 9 |
| II B | 19 | 11 |
| III | 38 | 21 |
| IV | 103 | 57 |
| CA 19-9 level at diagnosisb (range) | 1–252,427 | |
| ULN to <59,000 | 147 | 82 |
| >59 × ULN | 4 | 2 |
| Not available | 29 | 16 |
| Metastasis at diagnosis by organ site | n = 108 | 60 |
| Liver only | 39 | 22 |
| Liver and other organs | 77 | 43 |
| Abdomen/peritoneum | 23 | 13 |
| Lung and other organs | 6 | 3 |
| Other (bone) | 2 | 1 |
| First systemic chemotherapy received | n = 169 | 94 |
| Gemcitabine regimen | 91 | 51 |
| Gemcitabine monotherapy | 28 | 16 |
| Gemcitabine + oxaliplatin/cisplatin | 23 | 13 |
| Gemcitabine + Nab-paclitaxel | 4 | 2 |
| Gemcitabine + cabecitabine | 11 | 6 |
| Gemcitabine + investigational drugc | 9 | 5 |
| Gemcitabine + erlotinib | 2 | 1 |
| GTX (gemcitabine, docetaxel, capecitabine) | 6 | 3 |
| FOLFIRINOX (5-FU, irinotecan, oxaliplatin) | 59 | 33 |
| FOLFOX (5-FU, leucovorin, oxaliplatin) | 13 | 7 |
| No chemotherapy received | 11 | 6 |
| Lines of treatment received | ||
| One | 169 | 94 |
| Two | 122 | 68 |
| Three | 81 | 45 |
| Four | 30 | 17 |
| Five | 4 | 2 |
Abbreviations: AJCC = American Joint Committee on Cancer; NA = not available; ULN = upper limit of normal.
Distal pancreatectomy w/wo splenectomy, Appleby procedure.
Not all patients have the Lewis antigen and therefore cannot secrete CA19-9.
Investigational drugs: olaparib (PARP inhibitor), vismodegib (Hedgehog Pathway Inhibitor), clivatuzumab (y90 + h-PAMA4 fractionated radioimmunotherapy), nivolomab (IgG4 anti-PD-1 monoclonal antibody), BKM-120 (PI3K inhibitor), veliparib (PARP inhibitor), ipilumumab (Cytotoxic T lymphocyte antigen 4 antibody).
Statistical Analysis
The baseline patients’ characteristics, clinical findings at diagnosis of ascites, and laboratory evaluation at the time of ascites diagnosis are summarized in Tables 1–3. The Fisher exact test was used to evaluate the association between complication, number of serial paracenteses, and ascites management (patients who had only paracenteses vs. patients with paracenteses and drainage catheter placement).
Table 3.
Laboratory Analyses at Ascites Diagnosis
| n = 180 | Percentage | Range | |
|---|---|---|---|
| Serum chemistry at ascites diagnosis (median) |
|||
| BUN (mg/dL) | 13 | – | 2–102 |
| Creatinine (mg/dL) | 0.8 | – | 0.1–10 |
| Albumin (mg/dL) | 3.1 | – | 1.7–4.5 |
| Total bilirubin (mg/dL) | 0.7 | – | 0.2–24.5 |
| Total protein (mg/dL) | 5.8 | – | 3.4–8.3 |
| Hemoglobin (mg/dL) | 10.2 | – | 6.1–14 |
| Ca 19-9 | 542 | – | 0–232,496 |
| Ascitic fluid analysis | |||
| Patients with fluid analysis | |||
| Yes | 62 | 34 | – |
| No | 118 | 66 | – |
| WBC (mean) | 12,991 | – | 83–9990 |
| Albumin (mg/dL) (mean) | 1.29 | – | 0.2–3.6 |
| SAAG (mean) | 1.68 | – | 0.4–3.5 |
| <1.1 | n = 11 | 18 | |
| ≥1.1 | n = 51 | 82 | |
| Cytology ascitic fluid | n = 62 | ||
| Positive malignant cells | 36 | 58 | – |
| Negative malignant cells | 26 | 42 | – |
SAAG (gm/dL) < 1.1: Consistent with carcinomatosis, tuberculosis, pancreatitis, and nephrosis.
SAAG (gm/dL) ≥ 1.1: Consistent with portal hypertension, congestive heart failure, or Budd-Chiari syndrome.
Abbreviations: BUN = blood urea nitrogen; SAAG = serum albumin ascites gradient; WBC = white blood cell
Overall survival (OS) was calculated from date of diagnosis to date of death. Median OS and 95% confidence interval (CI) were estimated using Kaplan-Meier methods. The median time from catheter placement to death was also according to type of catheters. All P values were based on 2-tailed statistical analysis, and P < .05 was considered to indicate statistical significance. All analyses were performed with SAS version 9.3 (SAS Institute) and SPSS version 23 (SPSS Inc).
Results
Demographic and Clinical Characteristics
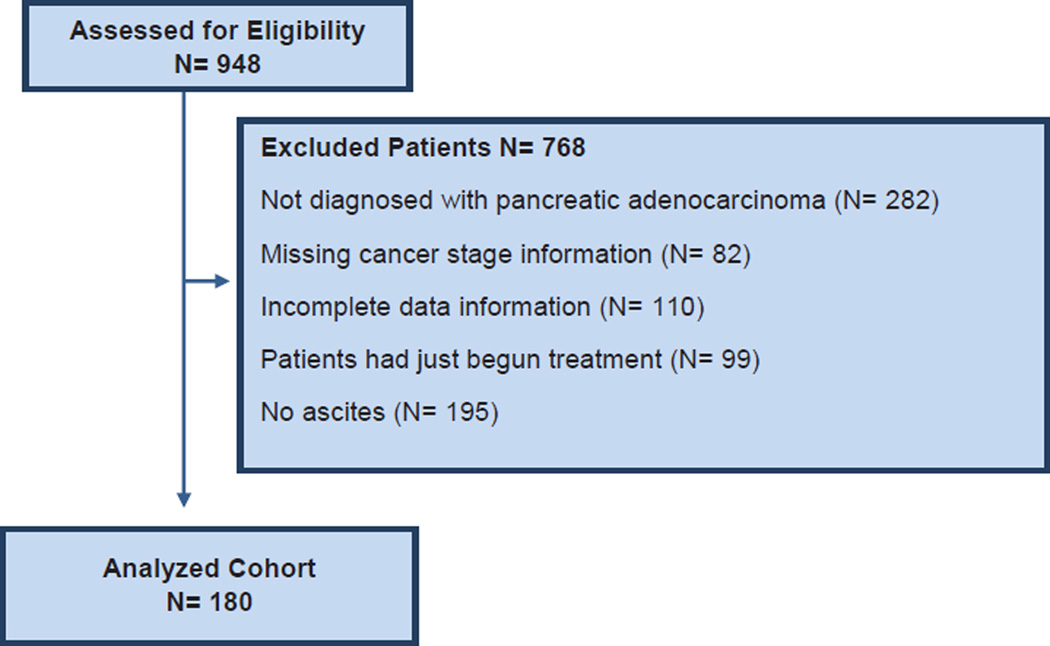
Nine hundred forty-eight patients were identified from January 1, 2009 to December 31, 2014. Figure 1 illustrates the flow of patients that composed the analyzed cohort. One hundred eighty patients fulfilled the inclusion criteria of advanced pancreas adenocarcinoma and ascites either at presentation or during the disease course, and they formed the analyzed cohort. Descriptive characteristics of the study cohort are summarized in Table 1. One hundred five (58%) were male. The median age of pancreas cancer diagnosis was 65 years (range, 34–91 years). Fifty-seven percent (n = 103) patients presented with metastatic pancreatic cancer (American Joint Committee on Cancer stage IV), 21% (n = 38) had locally advanced pancreas (stage III), and 22% (n = 39) had resectable disease at presentation (stage I–IIB). At diagnosis, assessment of performance status was adjudicated as follows: 83% (n = 149) had a performance status of ECOG 0 to 1, and 17% (n = 31) presented with performance status of ECOG ≥ 2. The primary pancreatic tumor was located in the head (61%; n = 89), body (23%; n = 41), and tail (18%; n = 33). Surgery was performed in 22% (n = 39) of patients, 13% (n = 23) had a Whipple procedure, 6% (n = 11) had other type of surgery (eg, Appleby procedure, distal pancreatectomy), and 74% (n = 133) patients had unresectable disease.
Figure 1. Study Design.
Abbreviation: N = number of patients.
For all 180 patients, complete follow-up data was available. The average follow-up time was 15 months (range, 1–47 months). For the whole cohort, the survival time after cancer diagnosis was, on average, 12 months with a range of 10 to 14 months (95% CI) and a standard deviation (SD) of 10 months. The median OS after ascites developed was 1.8 months with a range of 1.6 to 2.3 months (95% CI), and the time from diagnosis to ascites development for the whole cohort was approximately 8.8 months with a range of 0 to 45 months (SD = 9 months). One hundred eight patients (60%) presented with metastatic disease, and the time to ascites diagnosis was, on average, 7 months with a range of 0 to 31 months. Patients presenting with localized disease (n = 72; 40%) were diagnosed with ascites 15 months (range, 1–46 months) after their cancer diagnosis.
Cancer Treatment
Ninety-four percent (n = 169) of patients received at least 1 systemic therapy regimen, while 6% of patients (n = 11) did not receive any treatment at all. As shown in Table 1, gemcitabine alone or in combination (n = 91) represented about 51% of the total treatments administered. FOLFIRINOX (folinic acid, 5-FU, irinotecan, oxaliplatin) 33% (n = 59), FOLFOX (folinic acid, 5FU, oxaliplatin) 7% (n = 13), and GTX (gemcitabine, docetaxel, capecitabine) 3% (n = 6), accounted for the remainder of therapies.
Ascites Presentation
Within this cohort, our main outcomes of interest were the clinical presentation, natural history, and treatments for patients after they developed ascites. The key findings are summarized in Table 2. Ascites was diagnosed mostly by clinical examination (62%; n = 112), by CT scan in 36% (n = 64), and by sonogram/laparotomy in 2% (n = 4). Patients with ascites diagnosed by CT scan interpreted as: “moderate,” “large,” or “severe” were included in the study. Patients with ‘small’ ascites that did not have evidence of clinically significant ascites, ascites that did not progress overtime, and patients that did not require invasive interventions (paracentesis or catheter) were not included in the study. The main clinical manifestations as reported by patients were: abdominal distention and/or discomfort in 63% (n = 114), shortness of breath in 7% (n = 12), and 10% (n = 18) had complaints of weight gain, nausea, and/or vomiting; 20% (n = 36) of patients were asymptomatic. Assessment of performance status at ascites diagnosis was adjudicated as follows: 31% (n = 56) had ECOG between 0 and 1, 52% (n = 93) had ECOG of 2, and 17% (n = 31) had ECOG of 3.
Table 2.
Clinical Findings at Ascites Diagnosis
| Findings | n = 180 | Percentage |
|---|---|---|
| Ascites diagnosis | ||
| Clinical detectiona | 112 | 62 |
| CT scan (radiographically)b | 64 | 36 |
| Laparotomy/sonogram | 4 | 2 |
| Primary presenting symptom | ||
| Asymptomatic | 36 | 20 |
| Abdominal pain/discomfort/distension | 114 | 63 |
| Shortness of breath | 12 | 7 |
| Weight gain/nausea/vomiting | 18 | 10 |
| ECOG at diagnosis of ascites | ||
| 0–1 | 56 | 31 |
| 2 | 93 | 52 |
| 3 | 31 | 17 |
| Radiographic extent of disease at ascites diagnosisc |
||
| Ascites with no radiographic evidence of metastasis |
13 | 7 |
| Ascites with peritoneal carcinomatosis | 103 | 57 |
| Ascites with only liver metastases | 37 | 21 |
| Ascites with lung metastases | 15 | 8 |
| Ascites with bone metastases | 5 | 3 |
| Ascites with other organsd metastases | 7 | 4 |
| Time in months from diagnosis to ascites presentation, median, 8.8 months (range, 0–45 months) |
||
| 0–2 | 40 | 22 |
| 3–5 | 20 | 11 |
| 6–8 | 28 | 16 |
| 9–11 | 24 | 13 |
| >12 | 68 | 38 |
Abbreviations: CT = computed tomography; ECOG = Eastern Cooperative Oncology Group.
Diagnosis adjudicated by clinical documentation by the physician.
CT scan was interpreted as: “moderate,” “large,” or “severe” ascites.
Extent of disease evaluated by CT scan reports at the time ascites was diagnosed.
Other organs: adrenal gland and distant lymph nodes.
On imaging at ascites diagnosis, peritoneal carcinomatosis was observed in 57% (n = 103), liver involvement in 21% (n = 37), and lung metastases in 8% (n = 15). Thirteen (7%) patients had localized disease where ascites was attributed to a secondary manifestation of portal hypertension.
Laboratory Findings
Table 3 summarizes the median laboratory values at the time of diagnosis for the total cohort. Of particular importance is the SAAG, which is calculated by subtracting the difference between the serum albumin minus the albumin content in ascitic fluid, and has shown to be a superior discriminant than ‘transudate versus exudate’ to correctly differentiate ascites related to portal hypertension from ascites not due to portal hypertension 96.7% of the time.26 If the SAAG is a high gradient (> 1.1 g/dL), it usually indicates that ascites is due to portal hypertension, either liver-related (cirrhosis, Budd-Chiari syndrome) or nonliver-related (heart failure, portal fibrosis).27 However, if the SAAG gradient is low (< 1.1 g/dL), it points toward causes not associated with portal hypertension, like peritoneal carcinomatosis, tuberculosis, pancreatitis, or other peritoneal malignancies.11 In our cohort, the mean SAAG was 1.68 for 34% (n = 62) of patients who had their ascites fluid analyzed for cytology, albumin, and leukocyte content; in contrast, 66% (n = 118) did not have their fluid analyzed. Positive malignant cells were found in 20% (n = 36) of the patients of the total analyzed cohort, although the vast majority of patients did not have cytology sampled as the diagnosis was known.
Ascites Management
Current management of ascites is limited to the use of diuretics or fluid removal by either serial paracenteses and/or by permanent catheter placement. We evaluated the cohort by the principal treatment modality as shown in Table 4.
Table 4.
Interventions and Complications Related to Ascites Management
| Patients Who Had Only Paracenteses |
Patients with Paracenteses and Drainage Catheter Placement |
||||
|---|---|---|---|---|---|
| n = 64 | % | n = 116 | % | P Value | |
| Total number of serial paracenteses (range 1–7) | <.001 | ||||
| 0 (not clinically indicated) | 18 | 28 | 8 | 7 | |
| 1–2 | 37 | 58 | 60 | 52 | |
| ≥3 | 9 | 14 | 48 | 42 | |
| Drainage catheters placed | |||||
| Tenckhoff catheter | – | – | 108 | 93 | |
| Removeda | – | – | 6 | 5 | |
| Replaceda | – | – | 2 | 2 | |
| Denver shunt | – | – | 1 | 1 | |
| Pleurx catheter | – | – | 11 | 9 | |
| Pleurex only | – | – | 4 | 3 | |
| Pleurex and Tenckhoff | – | – | 7 | 6 | |
| Pigtail catheter | – | – | 4 | 3 | |
| Pigtail only | – | – | 3 | 3 | |
| Pigtail and Tenckhoff | – | – | 1 | 1 | |
| Patients with ≥2 catheters | – | – | 8 | 7 | |
| Patient with catheter- or paracentesis related complications | <.001 | ||||
| Yes | 11 | 17 | 46 | 56 | |
| Specific type of complications | |||||
| SBP/peritonitis | 4 | 26 | |||
| Ascites leakage | 2 | 1 | |||
| Catheter malfunction | – | 8 | |||
| Acute renal failureb | 2 | 3 | |||
| Bowel perforation | 1 | 2 | |||
| Othersc | 2 | 5 | |||
Abbreviation: SBP = spontaneous bacterial peritonitis.
Catheter removed/replaced secondary to: Tenckhoff malfunction (3), SBP/peritonitis (2), bleeding from catheter site (1), ascites leakage (2).
Consequence of increase fluid removal from serial paracentesis.
Cellulitis at catheter site (2), sepsis (2), bacteremia (1).
Our results indicate that 64 (36%) patients had their ascites managed solely by serial paracenteses, while 116 (64%) had catheter placement. For the patients that had serial paracenteses, most of the time this was addressed with 1 to 2 paracenteses procedures in 37 (58%) patients, and 9 (14%) had more than 3 procedures (range, 1–7). In patients who later needed a catheter placement, 60 (52%) had between 1 and 2 paracenteses procedures, and 49 (27%) needed more than 3.
The most common procedure was placement of a peritoneal Tenckhoff catheter (Quinton Instrument Company, Seattle, WA), in 93% (n = 108) of patients. Six patients (5%) had their catheter removed, and 2 (2%) had it replaced secondary to catheter malfunction, ascites leakage, and presence of infection (eg, bacterial peritonitis). One patient (1%) had a Denver shunt placed. This patient developed intractable ascites and hypotension such that a peritoneal catheter was not able to adequately address the ascites. Other types of catheters employed were the use of Pleurx catheter (Denver Biomedical Inc, Golden, CO) in 11 (9%) patients. Pleurx catheters were used for malignant pleural effusions that patients developed from fluid translocation from the abdominal compartment. Lastly, a small group of patients had a pigtail catheter placed (n = 3; 3%), and 8 (7%) patients had 2 catheters placed in rare circumstances when 1 catheter did not provide sufficient symptom palliation.
Complications of Interventions for Ascites
From the first group (n = 64; 36%) who had serial paracenteses, 17% (n = 11) developed complications. Encountered complications (Table 4) included bacterial peritonitis (n = 4; 6%), ascites leakage after paracentesis (n = 2; 3%), acute renal failure (n = 2; 3%), bowel perforation (n = 1; 2%), and abdominal wall cellulitis (n = 2; 1%). For patients who required a catheter placement (n = 116; 64%), 46 (40% of patients with catheter) developed complications, with 26 (23%) experiencing peritonitis, catheter malfunction (n = 8; 7%), acute renal failure (n = 3; 3%), bowel perforation (n = 2; 2%), and cellulitis and severe sepsis (n = 5; 4%) as a complication of the procedure.
Survival Outcomes
Table 5 summarizes outcomes. After ascites development, 79 (44%) patients received active therapy. One hundred one (52%) patients were managed with supportive care alone; from these, in 8 (4%) further management information was not available, and 77 (43%) were enrolled in hospice. Eleven (6%) patients died at our institution as an inpatient. The average time from the diagnosis of ascites to the start of supportive care was 1.8 months (± 2.2 months; range, 0–9 months), and to start hospice care was 2.3 months (± 2.4 months; range, 0–11 months).
Table 5.
Survival Outcomes and Management Following Ascites Diagnosis
| n = 180 | % | |
|---|---|---|
| Median overall survival, mos (95% CI) | ||
| After cancer diagnosis | 12 (10–14) | – |
| After ascites developed | 1.8 (1.6–2.3) | – |
| Systemic chemotherapy after ascites was diagnosed |
||
| Systemic chemotherapy given | 79 | 44 |
| Supportive care/expectant managementa | 101 | 56 |
| Patients on hospice | 77 | 43 |
| Lines of treatment after ascites developedb | n = 79 | |
| One | 17 | 22 |
| Two | 19 | 24 |
| Three | 23 | 29 |
| Four | 18 | 23 |
| Five | 2 | 2 |
| Survival in months by catheter typec (range) | ||
| Tenckhoff (n = 106) | 0.8 (0–8.7) | – |
| Pleurx/pigtail (n = 7) | 0.9 (0.4–8.2) | – |
Abbreviation: CI = confidence interval.
Documented in clinical note as: expectant management, supportive care only, comfort care.
Lines of treatment that patient had received by the time ascites was diagnosed.
From date of procedure to time of death.
Survival by catheter type was calculated from the date of procedure to the time of death. For a Tenckhoff catheter, the subsequent average survival was 0.8 months with a range of 0 to 8.7 months. Following placement of a Pleurx/pigtail catheter, the average survival was also 0.9 months with a range of 0.4 to 8.2 months.
Discussion
Ascites in patients with pancreatic adenocarcinoma typically develops as a late disease course manifestation and is a poor prognostic sign. It is associated with deteriorating QoL owing to symptoms such as bloating, distention, pain, early satiety, shortness of breath, and the frequent reaccumulation of ascitic fluid. For patients with untreated disease, the approach is a trial of diuresis, with the expectation of seeing certain palliation of the ascites, followed by serial paracenteses with the rationale of delaying catheter placement. Additionally, in patients with prior systemic treatment, the upfront expectation is significantly lower, and the likelihood of intervention being required is anticipated to be sooner rather than later. In our study, we were not able to derive a specific treatment algorithm owing to the absence of clear measurable parameters to assess response to treatment. We observed that 28% (n = 52) of patients were managed with diuretics; furosemide (n = 43) and/or spironolactone (n = 9). The low percentage use of diuretics is possibly attributed to the unclear evidence toward the utility of diuretics in malignant ascites, unless the presence of portal hypertension where furosemide and spironolactone have showed some utility.10
Prior studies have evaluated malignant ascites in different types of cancers,7,8,25,28–30 but very limited data exist on the prognostic importance of malignant ascites in pancreas adenocarcinoma. We present very detailed characteristics of a cohort of 180 patients, and our results correlate with smaller prior studies7,8,25,28–30 that show that ascites is a late manifestation of advanced pancreatic adenocarcinoma, and its presence carries an ominous prognosis, with observed mean OS in our cohort being 12 months (range, 10–14 months [95% CI]) from diagnosis and 1.8 months (range, 1.6–2.3 months [95%CI]) OS after ascites developed. Patients who presented with metastatic disease developed ascites 7 months following diagnosis. Most patients (93%) received chemotherapy, with the main regimens received being gemcitabine-based regimens in 91 (53%), FOLFIRINOX in 59 (33%), FOLFOX in 13 (7%), and investigational drugs in 9 (0.5%) patients. Prior studies have encountered an incidence of ascites in approximately 1 in every 8 pancreatic cancer patients.31 In our cohort, we observed that ascites developed after 11 months (± 9 months), and by that time, 57% (n = 103) of patients had advanced peritoneal carcinomatosis.
Possible variation might exist since diagnosis was based on clinical documentation in 62% (n = 112) of patients and radiographically in 36% (n = 64). Interestingly, in patients with fluid analysis (n = 62; 34%) the mean SAAG was 1.68, which associates with a possible portal hypertension etiology, perhaps related to the increased tumor burden, the increased thrombotic risk predisposing to portal vein thrombosis, or a multifactorial origin.32
Concomitantly, the existence of ascites in advanced pancreatic cancer has been challenging to treat, and oftentimes effective palliation is not achieved. Smith et al33 found that paracentesis provided palliation in 93% of patients but had a limited duration of effect, with a mean of 10.4 days and with potential risk. Therefore, to minimize repeated paracenteses and frequent hospital visits, permanent drains are frequently used.21,23,34 We observed in our cohort that 36% (n = 64) of patients underwent paracenteses only as the means of managing ascites, with 58% of this subgroup (n = 37) having 1 to 2 paracenteses, and only 14% (n = 9) having ≥ 3 paracenteses. Nearly two-thirds of the cohort required a permanent catheter (n = 116; 64% of total analyzed cohort) to obtain palliation, and we observed that 42% (n = 49) patients required ≥ 3 paracenteses to address ascites before a catheter was placed (P < .001).
The ease of placement, adequate ascites palliation, and patient tolerance has made the Tenckhoff catheter the most commonly used catheter type in 60% (n = 108) of patients.35 A Denver shunt was employed in a solitary patient with refractory ascites where Tenckhoff catheter placement was not technically feasible. In rare situations, a second catheter was placed when there was ascitic fluid translocation to the pleural compartment in 4% (n = 8) of patients.
Despite palliating symptoms and improving quality of life in end-stage pancreatic cancer, indwelling catheters incur significant complications. We found a marked difference in the complication rate of 56% (n = 46) in patients with catheters compared with 17% (n = 11) in the group where no catheters were placed (P < .001). These observations correlate with recent literature reports,18,24,36–38 although we found a higher incidence of infections (spontaneous bacterial peritonitis; SBP) with 23% (n = 26) in patients with a catheter compared with 6% (n = 4) who had a paracenteses only. The increased rate of infections could be secondary to bacterial access during procedure, or through direct contact with the abdominal cavity. Nonetheless, ascitic fluid was typically only analyzed when patients presented with signs of active infection or high suspicion for SBP, which could affect the diagnosis rate. Other not frequently observed complications in the total analyzed cohort were catheter malfunction (7%; n = 8), abdominal wall cellulitis at the catheter site (1%; n = 2), bowel perforation (2%; n = 3), and acute renal failure (3%; n = 5), the latter in part likely attributable to excessive ascitic fluid removal in patients with altered hemodynamics and impaired performance status. Ascites leakage was documented in 2% (n = 3); however, we think this complication is much underreported.
In broad observation, our total cohort had a reported complication rate of 32% (n = 57) related to catheter placement, which can be interpreted that almost 1 in 3 patients developed a complication; albeit, the most frequent observed complication was SBP in 17% (n = 30) of the total cohort. The presence of external factors may increase susceptibility to infection, including defects in host defense mechanism, cellular immune dysfunction, and presence of local factors (eg, tumor metastases, procedures, damage to mucosal surfaces).39 Our observed findings are in concordance with other reported results in the literature. Lungren et al40 reviewed a total of 188 patients with a tunneled peritoneal catheter and observed a significant higher complication rate in the pancreatic cancer population. Based on these results and the observed short median survival of less than 1 month after catheter placement, and owing to the complexity of predicting life expectancy, the decision of catheter placement should be individualized after weighting out the pros and cons in the context of the overall situation.
Finally, we observed a low incidence of cytologically confirmed malignant ascites (n = 36; 20% of total cohort), which likely reflects the known underlying malignant explanation and low need to confirm this suspicion given the clinical context. Research currently in development has placed emphasis on novel biomarkers in pancreatic cancer, like tumor exosomal proteins (CD44v6, MET, CD1014, EpCAM, Tspan8), miRNAs expression profiles (mIR-1246,4644,3976,4306),41 and telomerase activity. Exosomes are microvesicles formed by inward budding of the endosomal membrane that isolate different cell products (lipids, proteins, and nucleic acids), which subsequently are secreted into the extracellular space where they play a role in the regulation of tumor progression, tumor invasion, and neovascularization.41 Owing to the abundance and high biomarker expression in human fluids, exosomes are emerging as a more sensitive method and a valuable tool that can predict tumor progression and metastases.42 These biomarkers could ideally be an adjunct diagnostic test along with current standard cytologic analysis.43
Additionally, there is compelling evidence of catumaxomab (CATU) used as palliative treatment to delay deterioration in QoL compared with paracentesis in patients with ovarian-related ascites.44 This unique monoclonal antibody is nonhumanized, trifunctional, and bispecific (half-antibodies of mouse [IgG2a] and rat [IgG2b]) that targets the epithelial cell-adhesion molecule (EpCAM) and CD3 of most epithelial tumors.45,46 Recently, a published observational trial by Kurbacher et al concluded that, in patients with gynecologic tumors, outpatient CATU therapy can be a safe and effective method of controlling ascites.47 Therefore, we anticipate that new clinical trials should explore the role of this new therapeutic modality in patients with pancreatic-related ascites.
The results of our study should be interpreted in the context of several limitations. First, this study was a retrospective design. Second, our study was performed at a single-center specialist institution with inherent referral and other biases. Several potential confounders including patient demographics, ECOG status, stage at diagnosis, and comorbidities, along with other confounding variables from other unmeasured factors. Third, ascites diagnosis was based on radiologic documentation and physical exam performed by a physician, and thus the time point for adjudication of the development of ascites varies as this was adjudicated in retrospect. Fourth, we did not assess for ascites response after chemotherapy as this, in retrospect, would be very difficult to do; however, we believe that in treatment-naive patients, systemic chemotherapy provided palliation of ascites, but in these circumstances, it is very difficult to determine for how long paracentesis/catheter placement was avoided.
Conclusions
Our findings suggest that the presence of ascites in pancreas cancer indicates advancement of disease and a short expected survival of several months or less. Use of serial paracenteses and indwelling catheters is a common practice to alleviate the discomfort from fluid accumulation. The complication rate was significantly higher in patients who had an indwelling catheter compared with those that did not, mainly associated with infections with low rates of complications due to catheter malfunction or other issues. The complication risk may be acceptable in certain settings given the clinical benefits of fluid removal, decreased symptom burden, and convenience; however, for patients with short expected survival of a month or less, the risks likely outweigh the benefits. We encourage ascitic fluid analysis and SAAG calculation where the etiology is in question to help define which patients may benefit from diuretics versus other interventions. Future research should optimize patient selection for catheter placement and use of novel diagnostic techniques for detecting malignancy.
Clinical Practice Points.
Ascites development in pancreatic cancer is indicative of a short-term prognosis. However, patients who have not received prior therapy for their disease may fare better.
Diuretics have the most clinical benefit with portal hypertension-related ascites; therefore, we recommend analyzing ascitic fluid to identify patients with possible infection and to calculate the SAAG to potentially guide treatment and assist in determining the etiology.
Indwelling catheters (Tenckhoff, Pleurx, pigtail, Denver shunt) provide an effective way of draining ascitic fluid when serial paracenteses become too numerous and inconvenient for the patient.
Indwelling catheters offer palliation but have a high complication rate relating to infection (eg, SBP) and rarely to catheter malfunction.
Once ascites develops, integration of palliative care should be considered, and in this disease, may be a trigger time point for advanced care planning discussions.
Future research should be focused on methods of improving the sensitivity for detecting malignant cells in human fluids and patient selection optimization for catheter placement.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Gordon FD. Ascites. Clin Liver Dis. 2012;16:285–299. doi: 10.1016/j.cld.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Aslam N, Marino CR. Malignant ascites: new concepts in pathophysiology, diagnosis, and management. Arch Intern Med. 2001;161:2733–2737. doi: 10.1001/archinte.161.22.2733. [DOI] [PubMed] [Google Scholar]
- 3.Kipps E, Tan DSP, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer. 2013;13:273–282. doi: 10.1038/nrc3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Husain A, Bezjak A, Easson A. Malignant ascites symptom cluster in patients referred for paracentesis. Ann Surg Oncol. 2010;17:461–469. doi: 10.1245/s10434-009-0774-0. [DOI] [PubMed] [Google Scholar]
- 5.Cavazzoni E, Bugiantella W, Graziosi L, Franceschini MS, Donini A. Malignant ascites: pathophysiology and treatment. Int J Clin Oncol. 2012;18:1–9. doi: 10.1007/s10147-012-0396-6. [DOI] [PubMed] [Google Scholar]
- 6.Zervos EE, Osborne D, Boe BA, Luzardo G, Goldin SB, Rosemurgy AS. Prognostic significance of new onset ascites in patients with pancreatic cancer. World J Surg Oncol. 2006;4:16. doi: 10.1186/1477-7819-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayantunde AA, Parsons SL. Predictors of poor prognosis in patients with malignant ascites: a prospective study. Clin Med Diagn. 2012;2:1–6. [Google Scholar]
- 8.Ayantunde A, Parsons LS. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol. 2007;18:945–949. doi: 10.1093/annonc/mdl499. [DOI] [PubMed] [Google Scholar]
- 9.Newman G, Pudney D. A survey of current practice in the management of recurrent malignant ascites among oncologists and palliative-care physicians in the UK. Clin Oncol. 2006;18:154. doi: 10.1016/j.clon.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Parsons SL, Lang MW, Steele RJ. Malignant ascites: a 2-year review from a teaching hospital. Eur J Surg Oncol. 1996;22:237–239. doi: 10.1016/s0748-7983(96)80009-6. [DOI] [PubMed] [Google Scholar]
- 11.Khandwalla HE, Fasakin Y, El-Serag HB. The utility of evaluating low serum albumin gradient ascites in patients with cirrhosis. Am J Gastroenterol. 2009;104:1401–1405. doi: 10.1038/ajg.2009.117. [DOI] [PubMed] [Google Scholar]
- 12.Becker G. Medical and palliative management of malignant ascites. Cancer Treat Res. 2007;134:459–467. doi: 10.1007/978-0-387-48993-3_31. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SM. Palliation of malignant ascites. Gastroenterol Clin North Am. 2006;35:189–199. doi: 10.1016/j.gtc.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Becker G, Galandi D, Blum HE. Malignant ascites: systematic review and guideline for treatment. Eur J Cancer. 2006;42:589–597. doi: 10.1016/j.ejca.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Lee CW, Bociek G, Faught W. A survey of practice in management of malignant ascites. J Pain Symptom Manage. 1998;16:96–101. doi: 10.1016/s0885-3924(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 16.Chung M, Kozuch P. Treatment of malignant ascites. Curr Treat Options Oncol. 2008;9:215–233. doi: 10.1007/s11864-008-0068-y. [DOI] [PubMed] [Google Scholar]
- 17.Saif M, Siddiqui IP, Sohail M. Management of ascites due to gastrointestinal malignancy. Ann Saudi Med. 2009;29:369. doi: 10.4103/0256-4947.55167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coupe NA, Cox K, Clark K, Boyer M, Stockler M. Outcomes of permanent peritoneal ports for the management of recurrent malignant ascites. J Palliat Med. 2013;16:938–940. doi: 10.1089/jpm.2012.0535. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg S, Courtney A, Nemcek AA, Jr, Omary RA. Comparison of percutaneous management techniques for recurrent malignant ascites. J Vasc Interv Radiol. 2004;15:1129–1131. doi: 10.1097/01.RVI.0000136828.42612.B4. [DOI] [PubMed] [Google Scholar]
- 20.Walton L, Nottingham JM. Palliation of malignant ascites. J Surg Educ. 2007;64:4–9. doi: 10.1016/j.cursur.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Fleming ND, Alvarez-Secord A, Von Gruenigen V, Miller MJ, Abernethy AP. Indwelling catheters for the management of refractory malignant ascites: a systematic literature overview and retrospective chart review. J Pain Symptom Manage. 2009;38:341–349. doi: 10.1016/j.jpainsymman.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Sangisetty SL. Malignant ascites: a review of prognostic factors, pathophysiology and therapeutic measures. World J Gastrointest Surg. 2012;4:87. doi: 10.4240/wjgs.v4.i4.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White MA, Agle SC, Padia RK. Denver peritoneovenous shunts for the management of malignant ascites: a review of the literature in the post LeVeen Era. Am Surg. 2011;77:1070–1075. doi: 10.1177/000313481107700830. [DOI] [PubMed] [Google Scholar]
- 24.Tomiyama K, Takahashi M, Fujii T, et al. Improved quality of life for malignant ascites patients by Denver peritoneovenous shunts. Anticancer Res. 2006;26:2393–2395. [PubMed] [Google Scholar]
- 25.DeWitt J, Yu M, Al-Haddad MA, Sherman S, McHenry L, Leblanc JK. Survival in patients with pancreatic cancer after the diagnosis of malignant ascites or liver metastases by EUS-FNA. Gastrointest Endosc. 2010;71:260–265. doi: 10.1016/j.gie.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Lee CM, Changchien CS, Shyu WC, Liaw YF. Serum-ascites albumin concentration gradient and ascites fibronectin in the diagnosis of malignant ascites. Cancer. 1992;70:2057–2060. doi: 10.1002/1097-0142(19921015)70:8<2057::aid-cncr2820700807>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 27.Hou W, Sanyal AJ. Ascites: diagnosis and management. Med Clin North Am. 2009;93:801–817. doi: 10.1016/j.mcna.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Woopen H, Sehouli J. Current and future options in the treatment of malignant ascites in ovarian cancer. Anticancer Res. 2009;29:3353–3359. [PubMed] [Google Scholar]
- 29.Viguier J, De Muret A, Bacq Y. Ascites due to portal hypertension from breast cancer-related metastatic liver infiltration. Gastroenterol Clin Biol. 2006;30:903–905. doi: 10.1016/s0399-8320(06)73340-1. [DOI] [PubMed] [Google Scholar]
- 30.Fang N, Zhang HQ, He B, et al. Clinicopathological characteristics and prognosis of gastric cancer with malignant ascites. Tumour Biol. 2014;35:3261–3268. doi: 10.1007/s13277-013-1426-3. [DOI] [PubMed] [Google Scholar]
- 31.Thomassen I, Lemmens VE, Nienhuijs SW, Luyer MD, Klaver YL, de Hingh IH. Incidence, prognosis, and possible treatment strategies of peritoneal carcinomatosis of pancreatic origin: a population-based study. Pancreas. 2013;42:72–75. doi: 10.1097/MPA.0b013e31825abf8c. [DOI] [PubMed] [Google Scholar]
- 32.Takahara N, Isayama H, Nakai Y, et al. Pancreatic cancer with malignant ascites: clinical features and outcomes. Pancreas. 2015;44:380–385. doi: 10.1097/MPA.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 33.Smith EM, Jayson GC. The current and future management of malignant ascites. Clin Oncol. 2003;15:59–72. doi: 10.1053/clon.2002.0135. [DOI] [PubMed] [Google Scholar]
- 34.Richard HM, III, Coldwell DM, Boyd-Kranis RL, Murthy R, Van Echo DA. Pleurx tunneled catheter in the management of malignant ascites. J Vasc Interv Radiol. 2001;12:373–375. doi: 10.1016/s1051-0443(07)61919-8. [DOI] [PubMed] [Google Scholar]
- 35.Mercadante S, Intravaia G, Ferrera P, Villari P, David F. Peritoneal catheter for continuous drainage of ascites in advanced cancer patients. Support Care Cancer. 2008;16:975–978. doi: 10.1007/s00520-008-0453-x. [DOI] [PubMed] [Google Scholar]
- 36.Kuiper JJ, de Man RA, van Buuren HR. Review article: management of ascites and associated complications in patients with cirrhosis. Aliment Pharmacol Ther. 2007;26:183–193. doi: 10.1111/j.1365-2036.2007.03482.x. [DOI] [PubMed] [Google Scholar]
- 37.Schumacher DL, Saclarides TJ, Staren ED. Peritoneovenous shunts for palliation of the patient with malignant ascites. Ann Surg Oncol. 1994;1:378–381. doi: 10.1007/BF02303809. [DOI] [PubMed] [Google Scholar]
- 38.Bratby MJ, Hussain FF, Lopez AJ. Radiological insertion and management of peritoneovenous shunt. Cardiovasc Intervent Radiol. 2007;30:415–418. doi: 10.1007/s00270-006-0213-6. [DOI] [PubMed] [Google Scholar]
- 39.Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th. Hamilton, ON: BC Decker; 2003. [Google Scholar]
- 40.Lungren MP, Kim CY, Stewart JK, Smith TP, Miller MJ. Tunneled peritoneal drainage catheter placement for refractory ascites: single-center experience in 188 patients. J Vasc Interv Radiol. 2013;24:1303–1308. doi: 10.1016/j.jvir.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 41.Guo L, Guo N. Exosomes: potent regulators of tumor malignancy and potential bio-tools in clinical application. Crit Rev Oncol Hematol. 2015;95:346–358. doi: 10.1016/j.critrevonc.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Madhavan B, Yue S, Galli U, et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int J Cancer. 2014;136:2616–2627. doi: 10.1002/ijc.29324. [DOI] [PubMed] [Google Scholar]
- 43.Park ES, Lee J, Kang SY, et al. A comparative study of telomerase activity and cytologic diagnosis in malignant ascites. Anal Quant Cytopathol Histpathol. 2013;35:146–151. [PubMed] [Google Scholar]
- 44.Wimberger P, Gilet H, Gonschior AK, et al. Deterioration in quality of life (QoL) in patients with malignant ascites: results from a phase II/III study comparing paracentesis plus catumaxomab with paracentesis alone. Ann Oncol. 2012;23:1979–1985. doi: 10.1093/annonc/mds178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruf P, Gires O, Jager M, Fellinger K, Atz J. Characterisation of the new EpCAM-specific antibody HO-3: implications for trifunctional antibody immunotherapy of cancer. Br J Cancer. 2007;97:315–321. doi: 10.1038/sj.bjc.6603881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt M, Schmitt A, Reinhardt P. Opsonization with a trifunctional bispecific (αCD3 × αEpCAM) antibody results in efficient lysis in vitro and in vivo of EpCAM positive tumor cells by cytotoxic T lymphocytes. Int J Oncol. 2004;25:841–848. [PubMed] [Google Scholar]
- 47.Kurbacher CM, Horn O, Kurbacher JA, et al. Outpatient intraperitoneal catumaxomab therapy for malignant ascites related to advanced gynecologic neoplasms. Oncologist. 2015;20:1333–1341. doi: 10.1634/theoncologist.2015-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]