Abstract
Sensorimotor abnormalities are common in individuals with autism spectrum disorder (ASD); however, the processes underlying these deficits remain unclear. This study examined force production with and without visual feedback to determine if individuals with ASD can utilize internal representations to guide sustained force. Individuals with ASD showed a faster rate of force decay in the absence of visual feedback. Comparison of force output and tests of social and verbal abilities demonstrated a link between motor memory impairment and social and verbal deficits in individuals with ASD. This finding suggests that deficits in storage or retrieval of motor memories contribute to sensorimotor deficits and implicates frontoparietal networks involved in short-term consolidation of action dynamics used to optimize ongoing motor output.
Keywords: Autism spectrum disorder, Motor, Visual feedback, Precision grip, Working memory
Introduction
Individuals with autism spectrum disorder (ASD) frequently show sensorimotor abnormalities (Fournier et al. 2010). These deficits are understudied, but their analysis holds particular promise for clarifying brain mechanisms of ASD because they are precisely quantifiable in both spatial and temporal domains, and their neural underpinnings are well understood based on human lesion, neuroimaging, and non-human primate studies (Diedrichsen et al. 2005a; Diedrichsen et al. 2005b; Grafton et al. 2008; Takagi et al. 1998, 2000; Tseng et al. 2007). Prior studies have consistently identified skeletomotor and oculomotor abnormalities in individuals with ASD (Johnson et al. 2012; Luna et al. 2007; Rosenhall et al. 1988; Schmitt et al. 2014; Takarae et al. 2007) that may emerge in infancy prior to the onset of the disorder's defining symptoms EE (Elison et al. 2014; Ozonoff et al. 2014; Lebarton and Iverson 2013; Leonard et al. 2014; Nickel et al. 2013) and are present in patients’ unaffected first-degree relatives (Mosconi et al. 2010). The latter findings suggest that sensorimotor deficits in ASD may be familial. Characterizing sensorimotor deficits in individuals with ASD thus may be important to help establish the neural mechanisms of the disorder, advance efforts to identify patients earlier in development, and determine endophenotypes useful for clarifying genetic mechanisms.
Despite this promise, sensorimotor processes affected in ASD remain understudied and poorly understood. Abnormal motor performance may reflect failures in processing sensory feedback, executing movements, or in storing and retrieving internal action representations used to guide motor output. Some studies have documented an increased reliance on visual feedback mechanisms during the execution of reaching and pointing movements (Glazebrook et al. 2009) and while maintaining postural stability (Doumas et al. 2016; Graham et al. 2015; Minshew et al. 2004; Molloy et al. 2003), suggesting a reduced reliance on learned action representations. Further, multiple studies have indicated that sensorimotor deficits may be associated with the severity of core ASD symptoms, including social-communication and cognitive deficits, suggesting that the dsymaturation of basic sensorimotor abilities may disrupt the development of these core features, or that these symptom domains may reflect common mechanisms (Travers et al. 2013). It also has been demonstrated that individuals with ASD may show an increased reliance on proprioceptive rather than visual feedback during motor learning (Haswell et al. 2009; Izawa et al. 2012; Mostofsky and Ewen 2011; Nebel et al. 2016). Such inconsistencies across studies may reflect differences in the nature of deficits for different types of movements or heterogeneity across the autism spectrum (Haswell et al. 2009; Mari et al. 2003).
In a pair of studies examining precision gripping, we reported that individuals with ASD make less accurate initial force contractions and show increased variability of sustained force output despite similar levels of overall force relative to controls (Mosconi et al. 2015; Wang et al. 2015). These findings suggest that the internal action representation used for feedforward control (i.e., the initial contraction) may not be accurate in individuals with ASD. Further, the finding that individuals with ASD produce greater variability during sustained force implicates the online visuomotor mechanisms involved in translating visual feedback into corrective motor commands. Importantly, the variability in sustained force increased further when visual feedback was reduced (Mosconi et al. 2015). Taken together, these findings demonstrate that individuals with ASD may have inaccurate internal representations to plan force output and may also be more reliant on visual feedback to make adjustments during sustained force output.
Deficits in the maintenance of sustained force also may result from difficulty forming and accessing memories of recent experience to guide motor output. In the absence of visual feedback, cutaneous and proprioceptive reflex pathways allow performance to remain relatively unaffected for 20–150 and 60–200 ms, respectively (Johansson and Cole 1992; Johansson and Westling 1984; Marsden et al. 1983). Thus, if proprioceptive and cutaneous processes were impaired in individuals with ASD, then we would expect to observe differences in force output within the first 200 ms after the removal of visual feedback. In contrast, stored motor representations remain active for up to 2 s, but performance decays over time if rehearsal is prevented (Baddeley 1986; Massaro and Loftus 1996). Previous examinations of decay in force output when visual feedback is removed have provided important insights into the control processes that contribute to sensorimotor impairments in movement disorders (Vaillancourt et al. 2001). Thus, the current work aims to provide new evidence about these processes in ASD.
Motor memory has been shown to be impaired in individuals with ASD during a test of oculomotor control in which participants were instructed to make saccades to remembered locations without sensory guidance (Minshew et al. 1999). Using functional magnetic resonance imaging (MRI) during a similar memory-guided saccade task, Luna et al. (et al. 2002) documented reduced activation in dorsolateral prefrontal and posterior cingulate cortices among individuals with ASD compared to typically developing controls. Although these studies suggest memory processes supporting motor output are disrupted in ASD, whether similar processes impact motor performance in limb movements, in which clinically evident alterations are more often reported, remains to be determined. Further, while impairments in the execution and learning of limb movements has been repeatedly shown to be associated with social-communication and cognitive symptoms of the disorder (Nebel et al. 2016) the extent to which memory of limb motor behaviors is related to core symptoms in ASD has not been examined.
In the present study, we investigated the maintenance of force output during a continuous precision gripping test in which visual feedback about performance was not available. The removal of visual feedback required that participants initially utilize proprioceptive and somatosensory feedback mechanisms to maintain a constant level of force, and subsequently rely on stored internal representations to guide force output. Participants completed a precision gripping task similar to that previously used by our group to document alterations in visually guided force control in ASD (Mosconi et al. 2015; Wang et al. 2015). They initially gripped opposing load cells with their thumb and index finger and attempted to maintain a constant level of force while viewing online visual feedback on a monitor in front of them. After 8 s of gripping, visual feedback was removed and participants were instructed to maintain their same level of force. We hypothesized that the accuracy of force output (i.e., the ratio of mean force output to the prescribed target force amplitude) would deteriorate to a greater degree in individuals with ASD than in controls after visual feedback was removed. Based on previous findings that individuals with ASD show a heightened reliance on visual information to guide ongoing motor behavior, we expected that reduced force accuracy in ASD would be more pronounced after the first 200 ms of visual feedback removal. We also hypothesized that reduced force accuracy in the absence of visual feedback would be related to more severe social-communication abnormalities and lower cognitive abilities in individuals with ASD.
Methods
Participants
Thirty-seven right-handed individuals participated in this study: 17 (2 females) with ASD and 20 (3 females) age and nonverbal IQ matched healthy controls (Table 1). Individuals with ASD were recruited through community advertisements and local specialty clinics. Diagnoses of ASD were confirmed using the autism diagnostic inventory-revised (ADI-R) (Lord et al. 1994), the Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 2000), and expert clinical opinion based on DSM-IV TR criteria (Association 1994). The ADI-R is a semi-structured parent/caregiver interview focused on individuals’ developmental history and current behavior within three core domains: social interaction, communication, and restricted, repetitive behavior. The ADOS is a semi-structured direct assessment of individuals’ behavior that is used to examine social behavior, communication abilities, and patterns of repetitive behavior. ADI-R and ADOS ratings were used to examine behaviors consistent with a diagnosis of ASD and to determine the relationships between precision force performance and diagnostic features of ASD. For all ADOS and ADI ratings, higher scores reflect greater abnormality. Participants with ASD were excluded if they had a known genetic or metabolic disorder associated with ASD (e.g., Fragile X syndrome, tuberous sclerosis). Control participants were recruited from the community and were required to have a score of 8 or lower on the Social Communication Questionnaire (SCQ) (Berument et al. 1999). Control subjects were excluded if they had current or past psychiatric or neurological disorders, family history of ASD in first-, second- or third-degree relatives, or family history in first-degree relatives of a developmental or learning disorder, psychosis, or obsessive compulsive disorder. No subjects were taking medications known to affect motor control at the time of testing, including antipsychotics, stimulants, or anticonvulsants (Reilly et al. 2008). Subjects had corrected or uncorrected far visual acuity of at least 20/40. No participant had a history of head injury, birth injury, or seizure disorder. After a complete description of the study, written informed consent was obtained for each adult participant and informed parental consent was obtained for individuals less than 18 years of age. Minors provided written assent. Study procedures were approved by the local Institutional Review Board.
Table 1.
Subject characteristics
| Subject no. | Age (Years) |
Verbal IQ |
Performance IQ |
Full-scale IQ |
||||
|---|---|---|---|---|---|---|---|---|
| ASD | Con | ASD | Con | ASD | Con | ASD | Con | |
| 1 | 7 | 8 | 97 | 106 | 78 | 108 | 83 | 7 |
| 2 | 8 | 9 | 105 | 122 | 104 | 110 | 106 | 8 |
| 3 | 8 | 10 | 85 | 120 | 104 | 95 | 97 | 8 |
| 4 | 11 | 10 | 98 | 140 | 119 | 124 | 109 | 11 |
| 5 | 12 | 11 | 102 | 81 | 102 | 89 | 102 | 12 |
| 6 | 12 | 12 | 88 | 112 | 116 | 106 | 106 | 12 |
| 7 | 15 | 12 | 89 | 131 | 86 | 114 | 88 | 15 |
| 8 | 15 | 12 | 77 | 109 | 89 | 115 | 81 | 15 |
| 9 | 15 | 13 | 107 | 91 | 96 | 105 | 100 | 15 |
| 10 | 17 | 14 | 81 | 118 | 100 | 103 | 89 | 17 |
| 11 | 18 | 14 | 118 | 113 | 119 | 114 | 120 | 18 |
| 12 | 20 | 15 | 109 | 101 | 109 | 124 | 111 | 20 |
| 13 | 22 | 16 | 117 | 108 | 109 | 106 | 115 | 22 |
| 14 | 23 | 16 | 139 | 131 | 105 | 118 | 124 | 23 |
| 15 | 23 | 16 | 95 | 121 | 59 | 94 | 76 | 23 |
| 16 | 26 | 17 | 78 | 84 | 98 | 101 | 86 | 26 |
| 17 | 34 | 20 | 78 | 133 | 100 | 99 | 87 | 34 |
| 18 | 24 | 133 | 116 | |||||
| 19 | 28 | 98 | 109 | |||||
| 20 | 32 | 125 | 103 | |||||
| Mean (SD) | 16.8 (7.2) | 15.5 (6.3) | 97.8 (17.0) | 113.9 (16.8) | 100.0 (15.2) | 107.7 (9.5) | 98.8 (14.4) | 111.3 (11.9) |
IQ intelligence quotient
All participants completed the Wechsler Abbreviated Scales of Intelligence (WASI) to assess nonverbal, verbal, and full-scale IQ (FSIQ) (Wechsler 1999). The WASI is a commonly used brief and reliable assessment of verbal and nonverbal abilities in individuals older than 6 years of age. Verbal and nonverbal abilities each are derived from two subtests including vocabulary, similarities (verbal), block design and matrices (nonverbal). The Annett (1970) and Edinburgh (Oldfield 1971) tests were administered to assess handedness for each participant. Only participants who were right-hand dominant on each scale were included in the present study.
Apparatus
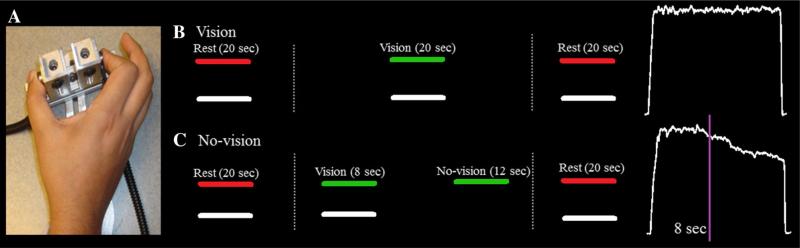
Stimuli were presented on a 102 cm (40-in.) Samsung LCD monitor with resolution 1366 × 768 and a 120 Hz refresh rate. All subjects were tested in a darkened room and seated 52 cm from the display monitor. Participants sat with their right forearm resting in a relaxed position on a custom-made arm brace that was clamped to a table 75 cm in height. Their elbow position remained stable throughout testing. Each subject's hand was pronated and lay flat on the table with their digits comfortably extended. Participants used their thumb and index finger to press against two opposing precision load cells (Entran ELFF-B4-100N, NJ, USA), 1.27 cm in diameter, secured to a custom grip device attached to the arm brace (Fig. 1a). Analog output from the load cells was amplified through a Grass Neuro-data 12 Acquisition System (Astro-Med, Inc, West Warwick, Rhode Island) amplifier at an excitation voltage of 10 V and a gain of 100. Data was digitized and a computer controlled 16-bit Data Translation (Model DT322) A/D board sampled the force output at 200 Hz. Voltages from the A/D board were transformed to Newtons using a calibration factor derived from known weights prior to the study. The voltage range was 10–10 V and the A/D board was able to detect force levels as low as 0.0016 N. Force data was analyzed using custom programs written in Lab-VIEW (National Instruments, Austin, TX) and MATLAB (The MathWorks, Inc, Natick, MA).
Fig. 1.
Experimental setup. a The precision force apparatus shown with a participant pressing on opposing load cells with their index finger and thumb. b Stimulus presentation and corresponding force output during the full vision condition. Participants were instructed to rest their fingers gently on the load cells when the target force bar was red. When the bar turned green, participants were instructed to press as quickly and accurately as possible to move the white bar up to the level of the green bar. The white bar moved upward on the screen with increasing force and downward with decreasing force. When the target force bar turned red at the end of the trial, participants again rested their fingertips on the load cells. The white force trace shows an exemplar force output during one trial of the full vision condition. c During the no-vision condition, participants followed the same instructions: rest when the target force bar is red and press when the target force bar is green. However, in this condition, the moveable white bar disappeared after 8 s. Participants were instructed to continue to press on the load cells with the same amount force for 12 s. At the conclusion of 12 s, the target force bar turned red again indicating that the participant should rest. The white force trace shows exemplar force output during one trial in the no vision condition. The purple line denotes the time at which visual feedback was removed (Color figure online)
Procedures
Prior to testing, each participant's maximum voluntary contraction (MVC) was measured using their average force output during three trials in which they pressed as hard as they could on a dynamometer using a pinch grip (Sammons Preston, Rolyan, Bolingbrook, IL). During the experimental task, participants viewed a horizontal white force bar that moved (in real-time) upwards with increased force and downwards with decreased force, and a static colored target bar (Fig. 1b). Participants were instructed to press on the load cells so that the force bar reached the same vertical level as the target bar, and then to maintain that level of force for 20 s. The target bar turned from red to green to cue participants to begin pressing at the beginning of each trial, and then from green to red to cue participants to release their grip at the end of each trial. During vision trials, the force bar remained visible for the entire duration of the trial. During no-vision trials, the force bar was removed for the last 12 s of the trial and participants were instructed to continue producing force at the target level until the trial ended (Fig. 1c). The target force level was set to 50 % of each individual's MVC for each of six trials (two vision and four no-vision trials). Trials were separated by 20 s of rest. Each participant successfully completed two practice trials of the no-vision condition, ensuring that they understood the task. The first trial was always a vision trial during the testing session so that each participant would have experience completing a trial with visual feedback prior to completing trials without visual feedback.
Data Analyses
Force output was digitally filtered using a ninth-order Butterworth filter with a 30 Hz low-pass cutoff. Each trial was visually inspected and trials in which participants stopped pressing prior to the offset cue were removed from analyses. For the last 12 s, the trials were divided into 1 s epochs and the mean force output was calculated separately for each epoch. Participants’ performance was compared across the vision and no-vision conditions using a 3-way repeated measures analysis of variance (ANOVA) including the between-subjects factor group (2: ASD, control) and two within-subjects factors, condition (2: vision, no-vision) and time (12: last 12 1 s time bins for each trial). To evaluate the rate of change of force during the no-vision period, we calculated the slope of the regression line defining mean force output as a function of time for the last 12 s of each no-vision trial. The slope values were then averaged across the four no-vision trials for each participant. Average slope values were then submitted to a two-way group (2: ASD, control) by condition (2: vision, no-vision) ANOVA. The social-affective and restricted, repetitive behavior algorithm scores from the ADOS and the current social, communication, and repetitive behavior algorithm scores from the ADI were examined in relation to force output. IQ also was examined in relation to force output.
Results
Participant Demographics
t tests demonstrated that there were no group differences in age, t(35) = –0.62, p > 0.05, or performance IQ (PIQ), t(35) = 1.96, p > 0.05. Group differences were revealed for verbal IQ (VIQ), t(35) = 2.88, p = 0.007, and full-scale IQ (FSIQ), t(35) = 2.88, p = 0.007. There was no difference between individuals with ASD and healthy controls for MVC (ASD: M = 57.41 N; SD = 24.12 N; Controls: M = 56.20 N; SD = 16.67 N; t(35) = –0.18, p = 0.86).
Force Output with and Without Visual Feedback
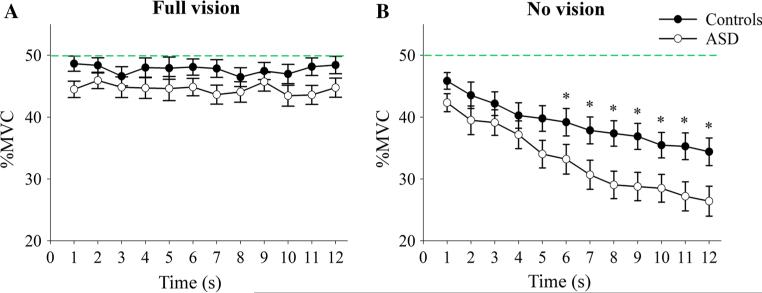
Mauchly's test of sphericity demonstrated that variances of the differences between all combinations of factors were not equal (p < 0.001), thus multivariate results are reported. The analysis of force accuracy revealed a significant group by condition by time interaction, F(11, 25) = 2.64, p < 0.02, . A vision by time interaction, F(11, 25) = 11.38, p < 0.001, , and main effects of vision, F(1, 35) = 90.39, p < 0.001, , and time, F(11, 25) = 9.18, p < 0.001, , were also observed. We decomposed the three-way interaction by conducting separate group by time ANOVAs for the vision and no-vision conditions. Within-subjects effects are reported with Huynh–Feldt correction to attenuate the violation of the assumption of sphericity. These planned comparisons were a test of the hypothesis (stated above) and followed a significant omnibus F-test. Therefore, all tests were evaluated at α = 0.05 and were not subjected to conservative corrective measures. As shown in Fig. 2, individuals with ASD did not differ from controls in their force accuracy when visual feedback was available (F(1, 35) = 3.03, p = 0.90). Further, we did not observe an effect of time in the full vision condition (F(7.6, 267.4) = 1.01, p = 0.430) or an interaction for time by group (F(7.6, 267.4) = 0.63, p = 0.801).
Fig. 2.
Individuals with ASD showed reduced force accuracy during the no-vision condition. a Mean (SE) percentage of force produced relative to each subject's maximum voluntary contraction (MVC) over the final 12 s of the vision condition for individuals with ASD (open circle) and healthy controls (closed circle). There were no differences in force accuracy between individuals with ASD and controls when visual feedback was available. b Mean force is reduced in individuals with ASD compared to controls during the no-vision condition. The mean (SE) percentage of force produced relative to the target force (green line) is shown across 1 s epochs during the last 12 s of each trial following removal of visual feedback. Asterisks indicate significant differences between groups. The green dashed line represents the target force level (Color figure online)
In the no-vision condition, a main effect of time (F(4.23, 147.95) = 40.69, p < 0.001) and a between-subjects effect of group (F(1, 35) = 4.87, p = 0.034) were observed. In addition, the interaction for group by time approached significance when using the Huynh–Feldt correction (F(4.23, 147.95) = 2.23, p = 0.065), such that individuals with ASD tended to produce less force compared to healthy controls as a function of time when visual feedback was removed. To examine the effect of decay as a function of time, independent groups t tests were conducted to determine the time point at which individuals with ASD differed from controls. The results indicated that individuals with ASD produced less force than controls for the last 6 s of the no-vision trials (all p values ≤ 0.031).
Rate of Force Decay with and Without Visual Feedback
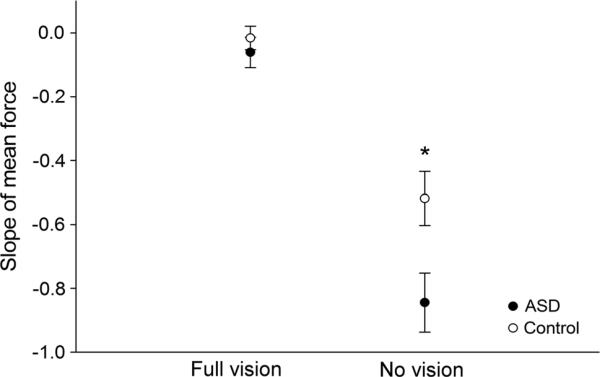
Since mean force output decreased as a function of time when visual feedback was removed, we next computed the slope of the force output as a function of time during the final 12 s of the vision and no-vision conditions. The resultant slopes were submitted to a 2 (group) by 2 (condition) repeated measures ANOVA. Within-subjects effects are reported with Huynh–Feldt correction to attenuate the violation of the assumption of sphericity. The results demonstrated an effect of vision (F(1, 35) = 116.42, p < 0.001), an effect of group (F(1, 35) = 75.90, p < 0.001), and an interaction for group by vision (F(1, 35) = 5.52, p = 0.025). As shown in Fig. 3, post hoc independent samples t tests revealed a greater rate of force decay in individuals with ASD compared to controls in the no-vision condition (ASD: M = –0.84, SD = 0.41; Controls: M = –0.52, SD = –0.38; t(35) = 2.49, p = 0.017), but not during the vision condition (ASD: M = –0.06, SD = 0.21; Controls: M = –0.02, SD = 0.16, t(35) = 0.73, p = 0.47).
Fig. 3.
Individuals with ASD showed faster force decay during the no-vision condition. The rate of force decay during the vision and no-vision conditions for individuals with ASD (closed circle) and healthy controls (open circle). Individuals with ASD showed more negative slopes relative to controls for the no-vision condition indicating greater decay in force output as a function of time. Error bars represent standard error of the mean. Asterisk indicates significant differences between groups at p < 0.001
Force Performance and Clinical Characteristics
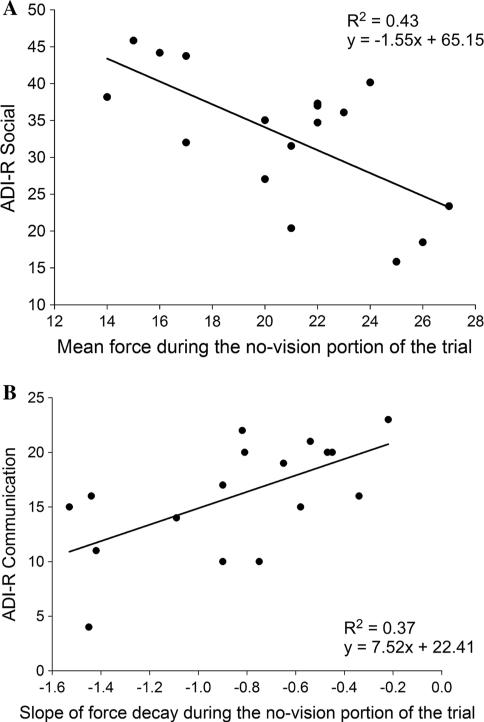
To determine the extent to which motor impairments were related to clinical symptoms in individuals with ASD, we examined correlations between clinical ratings and force measures that differed between individuals with ASD and controls. In particular, Pearson product moment correlations were conducted between verbal (VIQ), nonverbal (PIQ), and full-scale IQ (FSIQ), and the algorithms for the three primary ADI-R rated symptom domains (i.e., socialization, communication, and restricted, repetitive behaviors) as well as the ADOS domains, with force accuracy in the no-vision condition and the slope of the rate of decay in the no-vision condition. The results demonstrated that reduced force accuracy when visual feedback was not available was correlated with lower verbal IQ (VIQ), lower full-scale IQ (FSIQ), and more severe ADIR-rated social abnormalities (see Table 2). More negative slopes, indicative of a more rapid deterioration of force, were related to more severe ADI-R-rated communication abnormalities (see Table 2). No other relationships between clinical ratings and force measures reached significance (Fig. 4).
Table 2.
Correlations between clinical symptom ratings for individuals with ASD and force measures in the no-vision condition
| Standard test scores | Mean force output | Slope | ||
|---|---|---|---|---|
| VIQ | r = 0.640 | p = 0.006 | r = –0.167 | p = 0.522 |
| PIQ | r = 0.152 | p = 0.559 | r = –0.113 | p = 0.666 |
| FSIQ | r = 0.494 | p = 0.044 | r = –0.158 | p = 0.545 |
| ADI-R ratings | ||||
| Social | r = –0.653 | p = 0.004 | r = 0.287 | p = 0.264 |
| Communication | r = –0.450 | p = 0.070 | r = 0.612 | p = 0.009 |
| Restricted, repetitive behaviors | r = –0.041 | p = 0.877 | r = 0.353 | p = 0.164 |
| ADOS ratings | ||||
| Social | r = –0.436 | p = 0.080 | r = 0.364 | p = 0.151 |
| Communication | r = –0.104 | p = 0.691 | r = 0.462 | p = 0.062 |
| Restricted, repetitive behaviors | r = –0.226 | p = 0.384 | r = 0.028 | p = 0.916 |
Significant correlations are noted in bold font (p < 0.05)
Fig. 4.
In individuals with ASD, mean force output was related to social ability and slope of force decay was related to communication ability. a Relationship between mean force output during the no-vision condition and ADI-R social ratings. b Relationship between slope of force decay during the no-vision condition and ADI-R communication ratings for individuals with ASD
Discussion
The primary goal of the present study was to determine whether individuals with ASD show deficits in force control when visual feedback information is not available to guide their ongoing motor output. We also assessed the timing at which reductions in force accuracy emerged following the removal of visual feedback so that the motor memory control processes underlying reduced force accuracy in ASD could be evaluated. Results demonstrated that individuals with ASD have difficulty maintaining force output in the absence of continuous visual feedback. Further, reductions in force accuracy were not seen within the first few seconds following the removal of visual feedback suggesting that deficits in individuals with ASD reflect motor memory rather than proprioceptive or somatosensory feedback mechanisms. Reduced accuracy was associated with reduced verbal IQ, full-scale IQ, and more severe clinically rated social and communication impairments. These results indicate that motor memory alterations contribute to sensorimotor alterations commonly seen in ASD and possibly some of the core social-communication and cognitive symptoms of the disorder.
Mechanisms of Motor Impairments in ASD
In the present study, individuals with ASD showed levels of mean force comparable to those of controls when visual feedback was provided, but they were not able to maintain accurate force output without visual feedback about their performance. Individuals with ASD thus appear to be able to use visual feedback information to maintain the accuracy of their force output, but are less able to use memory representations to generate optimal motor force output.
Reduced force accuracy and increased rate of force decay in individuals with ASD following the removal of visual feedback could reflect alterations in proprioceptive, cutaneous, or motor memory systems. During the test of precision gripping studied here, proprioceptive and cutaneous feedback mechanisms are used to sustain accurate force output immediately following the removal of visual cues (Gordon et al. 1993; Johansson and Cole 1992; Johansson and Westling 1984). If proprioceptive and cutaneous processes were impaired in patients with ASD, then we would expect to observe differences in their force accuracy within the first 20–200 ms after the removal of visual feedback. However, participants with ASD and control participants produced the same amount of force for the first few seconds of the no-vision condition. This suggests that individuals with ASD are not impaired in their ability to utilize proprioceptive and cutaneous feedback cues. This finding is consistent with prior studies of ASD (Fuentes et al. 2009; Haswell et al. 2009). In contrast, however, motor memory systems involved in the short-term consolidation and retrieval of action information appear to be compromised in ASD.
Few prior studies have examined motor memory system integrity in ASD. Minshew et al. (1999) found that individuals with ASD were slower to initiate memory-guided saccades and showed reduced endpoint accuracy compared to controls, suggesting a specific deficit in retrieving or maintaining an accurate internal representation of sensorimotor plans. Our findings extend these results by showing that motor memory impairments in ASD contribute to reduced control of manual motor behaviors that show similar levels of accuracy as controls when guided by visual feedback information.
Converging literature demonstrates that motor memory operations require a distributed network of brain regions, including dorsolateral prefrontal cortex, premotor cortex, dorsal cingulate cortex, and posterior parietal cortex (Sweeney et al. 1996). Reduced activation within these regions has been seen during an fMRI study of individuals with ASD performing a memory-guided saccade task (Luna et al. 2002). Similar mechanisms seem important for memory modulation of limb control. In a grip force task similar to that studied here, Vaillancourt et al. (2003) demonstrated that participants could produce force to a learned target in the absence of visual feedback. A network of prefrontal regions, including dorsolateral prefrontal cortex, ventral prefrontal cortex, and anterior cingulate were associated with force production from memory. Thus, alterations in these integrated neocortical systems are implicated in ASD based on our laboratory findings. It also is possible that deficits reported in this study reflect reduced functional connectivity between prefrontal and pre- and primary motor cortical regions as has been suggested previously in studies of ASD (Haswell et al. 2009; Herbert et al. 2003).
Motor Memory Deficits and Clinical Features in Individuals with ASD
Reduced force accuracy in the absence of visual feedback was associated with more severe social-communication abnormalities and reduced cognitive abilities in individuals with ASD. Our findings that motor impairments are associated with core symptoms of ASD are consistent with multiple prior studies. For example, Travers et al. (2013) reported that reduced postural stability was related to increased rates of repetitive behaviors and social symptoms in individuals with ASD (Travers et al. 2013). Papadopoulos et al. (2012) demonstrated that impaired balance control was related to emotional and behavioral disturbances in individuals with ASD. On one hand, it is possible that a common neural mechanism underlies differences in sensorimotor control and ASD symptoms. On the other hand, sensorimotor deficits may have a broad impact on developmental and cognitive abilities in ASD, impacting not only intellectual achievement but sensorimotor skills and social function as well. For example, individuals who choose not to participate in group recreation or sporting activities with peers may be at a higher risk for isolation and social problems. Further, motor memory impairments may exert these influences by reducing individuals’ ability to store and subsequently respond to new information during cognitive, social, and motor operations.
Conclusions
The current work demonstrates that deficits in motor memory systems contribute to the widely reported sensorimotor impairments in individuals with ASD. These findings implicate frontostriatal and frontoparietal brain systems involved in motor memory and their functional connections with cortical and subcortical brain networks involved in controlling the accuracy of motor output. The role of sensorimotor impairments in ASD has gained increased appreciation based on findings that they are nearly ubiquitous (Green et al. 2002) and impact a broad range of developmental abilities, including social-communication and verbal skills (Dziuk et al. 2007; Schmitt et al. 2014). Our findings extend this work by showing that sensorimotor deficits in ASD are due, in part, to impairments in the consolidation or retrieval of motor memory representations used to guide motor output in the absence of visual feedback.
Acknowledgments
This study was supported by NIMH 092696 and 67631, NICHD Autism Center of Excellence 055751, NINDS 082008, NINDS 078874, NCATS TR000126, and Autism Speaks 4853.
Footnotes
Authors’ Contributions KN carried out data analyses, data interpretation, and drafting the manuscript. SM helped with statistical analyses, interpreting the data, and drafting the manuscript. LS helped with data collection, data analysis, and interpretation. ZW helped with data analysis and interpretation. JS helped with data analysis and manuscript preparation. MM provided input on conception and design of the study, assisted in data analysis and interpretation, and helped revise the manuscript for important intellectual content. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- Annett M. A classification of hand preference by association analysis. British Journal of Psychology. 1970;61(3):303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Association AP. American Psychiatric Association: Diagnostic and statistical manual of mental disorders. 4th ed. Association, A. P.; Arlington, VA: 1994. [Google Scholar]
- Baddeley A. Modularity, mass-action and memory. Quarterly Journal of Experimental Psychology A. 1986;38(4):527–533. doi: 10.1080/14640748608401613. [DOI] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. British Journal of Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. Journal of Neuroscience. 2005a;25(43):9919–9931. doi: 10.1523/JNEUROSCI.1874-05.2005. doi:10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Verstynen T, Lehman SL, Ivry RB. Cerebellar involvement in anticipating the consequences of self-produced actions during bimanual movements. Journal of Neurophysiology. 2005b;93(2):801–812. doi: 10.1152/jn.00662.2004. doi:10.1152/jn.00662.2004. [DOI] [PubMed] [Google Scholar]
- Doumas M, McKenna R, Murphy B. Postural control deficits in autism spectrum disorder: The role of sensory integration. Journal of Autism and Developmental Disorders. 2016;46(3):853–861. doi: 10.1007/s10803-015-2621-4. doi:10.1007/s10803-015-2621-4. [DOI] [PubMed] [Google Scholar]
- Dziuk MA, Gidley Larson JC, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: Association with motor, social, and communicative deficits. Developmental Medicine and Child Neurology. 2007;49(10):734–739. doi: 10.1111/j.1469-8749.2007.00734.x. doi:10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Elison JT, Wolff JJ, Reznick JS, Botteron KN, Estes AM, Gu H, et al. Repetitive behavior in 12-month-olds later classified with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(11):1216–1224. doi: 10.1016/j.jaac.2014.08.004. doi:10.1016/j.jaac.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. Journal of Autism and Developmental Disorders. 2010;40(10):1227–1240. doi: 10.1007/s10803-010-0981-3. doi:10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Fuentes CT, Mostofsky SH, Bastian AJ. Children with autism show specific handwriting impairments. Neurology. 2009;73(19):1532–1537. doi: 10.1212/WNL.0b013e3181c0d48c. doi:10.1212/WNL.0b013e3181c0d48c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook C, Gonzalez D, Hansen S, Elliott D. The role of vision for online control of manual aiming movements in persons with autism spectrum disorders. Autism. 2009;13(4):411–433. doi: 10.1177/1362361309105659. doi:10.1177/1362361309105659. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Westling G, Cole KJ, Johansson RS. Memory representations underlying motor commands used during manipulation of common and novel objects. Journal of Neurophysiology. 1993;69(6):1789–1796. doi: 10.1152/jn.1993.69.6.1789. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Schmitt P, Van Horn J, Diedrichsen J. Neural substrates of visuomotor learning based on improved feedback control and prediction. Neuroimage. 2008;39(3):1383–1395. doi: 10.1016/j.neuroimage.2007.09.062. doi:10.1016/j.neuroimage.2007.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham SA, Abbott AE, Nair A, Lincoln AJ, Muller RA, Goble DJ. The influence of task difficulty and participant age on balance control in ASD. Journal of Autism and Developmental Disorders. 2015;45(5):1419–1427. doi: 10.1007/s10803-014-2303-7. doi:10.1007/s10803-014-2303-7. [DOI] [PubMed] [Google Scholar]
- Green D, Baird G, Barnett AL, Henderson L, Huber J, Henderson SE. The severity and nature of motor impairment in Asperger's syndrome: A comparison with specific developmental disorder of motor function. Journal of Child Psychology and Psychiatry. 2002;43(5):655–668. doi: 10.1111/1469-7610.00054. [DOI] [PubMed] [Google Scholar]
- Haswell CC, Izawa J, Dowell LR, Mostofsky SH, Shadmehr R. Representation of internal models of action in the autistic brain. Nature Neuroscience. 2009;12(8):970–972. doi: 10.1038/nn.2356. doi:10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Lange N, Bakardjiev A, Caviness VS., Jr. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126(Pt 5):1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- Izawa J, Pekny SE, Marko MK, Haswell CC, Shadmehr R, Mostofsky SH. Motor learning relies on integrated sensory inputs in ADHD, but over-selectively on proprioception in autism spectrum conditions. Autism Research. 2012;5(2):124–136. doi: 10.1002/aur.1222. doi:10.1002/aur.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Cole KJ. Sensory-motor coordination during grasping and manipulative actions. Current Opinion in Neurobiology. 1992;2(6):815–823. doi: 10.1016/0959-4388(92)90139-c. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Experimental Brain Research. 1984;56(3):550–564. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- Johnson BP, Rinehart NJ, Papadopoulos N, Tonge B, Millist L, White O, Fielding J. A closer look at visually guided saccades in autism and Asperger's disorder. Frontiers in Integrative Neuroscience. 2012;6:99. doi: 10.3389/fnint.2012.00099. doi:10.3389/fnint.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBarton ES, Iverson JM. Fine motor skill predicts expressive language in infant siblings of children with autism. Developmental Science. 2013;16(6):815–827. doi: 10.1111/desc.12069. doi:10.1111/desc.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard HC, Bedford R, Charman T, Elsabbagh M, Johnson MH, Hill EL, et al. Motor development in children at risk of autism: A follow-up study of infant siblings. Autism. 2014;18(3):281–291. doi: 10.1177/1362361312470037. doi:10.1177/1362361312470037. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological Psychiatry. 2007;61(4):474–481. doi: 10.1016/j.biopsych.2006.02.030. doi:10.1016/j.biopsych. 2006.02.030. [DOI] [PubMed] [Google Scholar]
- Luna B, Minshew NJ, Garver KE, Lazar NA, Thulborn KR, Eddy WF, Sweeney JA. Neocortical system abnormalities in autism: An fMRI study of spatial working memory. Neurology. 2002;59(6):834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- Mari M, Castiello U, Marks D, Marraffa C, Prior M. The reach-to-grasp movement in children with autism spectrum disorder. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2003;358(1430):393–403. doi: 10.1098/rstb.2002.1205. doi:10. 1098/rstb.2002.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Rothwell JC, Day BL. Long-latency automatic responses to muscle stretch in man: Origin and function. Advances in Neurology. 1983;39:509–539. [PubMed] [Google Scholar]
- Massaro DW, Loftus GR. Sensory and perceptual storage. In: Bjork EL, Bjork RA, editors. Memory. Academic Press; San Diego, CA: 1996. pp. 68–96. [Google Scholar]
- Minshew NJ, Luna B, Sweeney JA. Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology. 1999;52(5):917–922. doi: 10.1212/wnl.52.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Sung K, Jones BL, Furman JM. Underdevelopment of the postural control system in autism. Neurology. 2004;63(11):2056–2061. doi: 10.1212/01.wnl.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Dietrich KN, Bhattacharya A. Postural stability in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2003;33(6):643–652. doi: 10.1023/b:jadd.0000006001.00667.4c. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Kay M, D'Cruz AM, Guter S, Kapur K, Macmillan C, et al. Neurobehavioral abnormalities in first-degree relatives of individuals with autism. Archives of General Psychiatry. 2010;67(8):830–840. doi: 10.1001/archgenpsychiatry.2010.87. doi:10.1001/archgenpsy chiatry.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Mohanty S, Greene RK, Cook EH, Vaillancourt DE, Sweeney JA. feedforward and feedback motor control abnormalities implicate cerebellar dys-functions in autism spectrum disorder. Journal of Neuroscience. 2015;35(5):2015–2025. doi: 10.1523/JNEUROSCI.2731-14.2015. doi:10.1523/Jneurosci.2731-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Ewen JB. Altered connectivity and action model formation in autism is autism. Neuroscientist. 2011;17(4):437–448. doi: 10.1177/1073858410392381. doi:10.1177/1073858410392381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel MB, Eloyan A, Nettles CA, Sweeney KL, Ament K, Ward RE, Mostofsky SH. Intrinsic visual-motor synchrony correlates with social deficits in autism. Biological Psychiatry. 2016;79(8):633–641. doi: 10.1016/j.biopsych.2015.08.029. doi:10.1016/j.biopsych.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel LR, Thatcher AR, Keller F, Wozniak RH, Iverson JM. Posture development in infants at heightened vs. low risk for autism spectrum disorders. Infancy. 2013;18(5):639–661. doi: 10.1111/infa.12025. doi:10.1111/infa.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, et al. The broader autism phenotype in infancy: When does it emerge? Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(4):398–407. e2. doi: 10.1016/j.jaac.2013.12.020. doi:10.1016/j.jaac. 2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos N, McGinley J, Tonge B, Bradshaw J, Saunders K, Murphy A, Rinehart N. Motor proficiency and emotional/behavioural disturbance in autism and Asperger's disorder: Another piece of the neurological puzzle? Autism. 2012;16(6):627–640. doi: 10.1177/1362361311418692. doi:10.1177/1362361311418692. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Lencer R, Bishop JR, Keedy S, Sweeney JA. Pharmacological treatment effects on eye movement control. Brain and Cognition. 2008;68(3):415–435. doi: 10.1016/j.bandc.2008.08.026. doi:10.1016/j.bandc.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenhall U, Johansson E, Gillberg C. Oculomotor findings in autistic children. Journal of Laryngology and Otology. 1988;102(5):435–439. doi: 10.1017/s0022215100105286. [DOI] [PubMed] [Google Scholar]
- Schmitt LM, Cook EH, Sweeney JA, Mosconi MW. Saccadic eye movement abnormalities in autism spectrum disorder indicate dysfunctions in cerebellum and brainstem. Mol Autism. 2014;5(1):47. doi: 10.1186/2040-2392-5-47. doi:10.1186/2040-2392-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology. 1996;75(1):454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. Journal of Neurophysiology. 1998;80(4):1911–1931. doi: 10.1152/jn.1998.80.4.1911. [DOI] [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor cerebellar vermis on eye movements in primate: Smooth pursuit. Journal of Neurophysiology. 2000;83(4):2047–2062. doi: 10.1152/jn.2000.83.4.2047. [DOI] [PubMed] [Google Scholar]
- Takarae Y, Minshew NJ, Luna B, Sweeney JA. Atypical involvement of frontostriatal systems during sensori-motor control in autism. Psychiatry Research. 2007;156(2):117–127. doi: 10.1016/j.pscychresns.2007.03.008. doi:10.1016/j.pscychresns.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Powell PS, Klinger LG, Klinger MR. Motor difficulties in autism spectrum disorder: Linking symptom severity and postural stability. Journal of Autism and Developmental Disorders. 2013;43(7):1568–1583. doi: 10.1007/s10803-012-1702-x. doi:10.1007/s10803-012-1702-x. [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. Journal of Neurophysiology. 2007;98(1):54–62. doi: 10.1152/jn.00266.2007. doi:10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Slifkin AB, Newell KM. Visual control of isometric force in Parkinson's disease. Neuropsychologia. 2001;39(13):1410–1418. doi: 10.1016/s0028-3932(01)00061-6. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Thulborn KR, Corcos DM. Neural basis for the processes that underlie visually guided and internally guided force control in humans. Journal of Neuro-physiology. 2003;90(5):3330–3340. doi: 10.1152/jn.00394.2003. doi:10.1152/jn.00394.2003. [DOI] [PubMed] [Google Scholar]
- Wang Z, Magnon GC, White SP, Greene RK, Vaillancourt DE, Mosconi MW. Individuals with autism spectrum disorder show abnormalities during initial and subsequent phases of precision gripping. Journal of Neurophysiology. 2015;113(7):1989–2001. doi: 10.1152/jn.00661.2014. doi:10.1152/jn.00661.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]