Abstract
The group IVA calcium-dependent cytosolic phospholipase A2 (cPLA2α) enzyme controls the release of arachidonic acid from membrane bound phospholipids and is the rate-limiting step in production of eicosanoids. A variety of different kidney injuries activate cPLA2α, therefore we hypothesized that cPLA2α activity would regulate pathologic processes in HK-2 cells, a human renal tubular epithelial cell line, by regulating cell phenotype and proliferation. In two lentiviral cPLA2α-silenced knockdowns, we observed decreased proliferation and increased apoptosis compared to control HK-2 cells. cPLA2α-silenced cells also demonstrated an altered morphology, had increased expression E-cadherin, and decreased expression of Ncadherin. Increased levels of E-cadherin were associated with increased promoter activity and decreased levels of SNAIL1, SNAIL2, and ZEB1, transcriptional repressors of E-cadherin expression. Addition of exogenous arachidonic acid, but not PGE2, reversed the phenotypic changes in cPLA2α-silenced cells. These data suggest that cPLA2α may play a key role in renal repair after injury through a PGE2-independent mechanism.
Keywords: cPLA2α, phospholipase A2, arachidonic acid, HK-2, proximal tubule, E-cadherin
Introduction
Directed damage to the renal epithelium is sufficient to cause acute kidney injury (AKI) in animal models.(1) Published data has demonstrated that during AKI renal tubular epithelial cell (RTE) damage and phenotypic change is sufficient to drive tubulointerstitial fibrosis, which is the final common histologic finding shared by all forms of progressive renal injury.(2, 3) Furthermore, while AKI in humans often resolves after withdrawal of the original insult, accumulating data has shown that many patients with AKI develop chronic kidney disease (CKD).(4, 5)
Extensive research has focused on the role of RTEs during the response to renal injury. In patients with kidney damage, the loss of epithelial markers in RTEs and re-expression of mesenchymal markers correlates closely with the level of renal dysfunction.(6, 7) These phenotypic changes by RTEs may not only be markers of injury, they may also be necessary for repair. To recover kidney function after tubular injury, RTEs must partially dedifferentiate, proliferate, and repopulate injured tubules.(8) In contrast, RTE cell cycle arrest has been shown to promote a pro-inflammatory phenotype that leads to progressive tubulointerstitial fibrosis.(9) The pathways regulating these biological processes in RTEs are still poorly understood.
Eicosanoids are biologically active lipid products of arachidonic acid metabolism. Arachidonic acid is esterified to the sn-2 position of membrane phospholipids. Hydrolysis of membrane bound phospholipids by the phospholipase A2 class of enzymes, in particular group IVA phospholipase A2 (calcium dependent cytosolic phospholipase A2α, hereafter cPLA2α), represents the rate-limiting step in eicosanoid production. Free arachidonic acid can subsequently be metabolized through three classically defined enzymatic pathways: cyclooxygenases to produce prostaglandins and thromboxane, lipoxygenases to produce leukotrienes and hydroxyl-eicosatetraenoic acids (HETEs), and cytochrome P450 to produce mostly epoxygenated fatty acids (EETs) and HETEs. Eicosanoids have long been known to regulate tubular salt and water transport, and interact with the renin-angiotensin-aldosterone system (RAAS) in the kidney.(10) Importantly, many different types of kidney injury increase activity of cPLA2α or downstream enzymes such as cyclooxygenases.(11–13) Animal CKD models, such as ureteral obstruction, glomerular disease and polycystic kidney disease (PKD), appear to also regulate cPLA2α and cyclooxygenases.(14–16) Since a wide variety of kidney insults induce cPLA2α activity, it is likely that downstream eicosanoids direct important biological processes in the renal epithelium. Furthermore, accumulating evidence supports that cPLA2α itself, independent of its canonical functions, may play fundamental roles in membrane trafficking and cellular proliferation.(17, 18)
Although cPLA2α activity is altered in renal injury, the role of cPLA2α in specific cell types has not been well studied. Since expression is ubiquitous, it is likely that regulation of cPLA2α may control different processes in different cell types. The aim of this study was to investigate the role of cPLA2α in cultured human renal proximal tubular epithelial cells (HK-2 cells). HK-2 cells are a well-established cell line that is frequently used to study renal epithelial injury and repair. We hypothesized that cPLA2α controls the epithelial phenotype of HK-2 cells, thereby changing their state of differentiation and potentially altering their response to injury.
Materials and Methods
HK-2 Epithelial Cell Culture
HK-2 cells, a human proximal tubular epithelial cell line, were purchased from ATCC (Manassas, VA). Cells were maintained in DMEM/F12 (1:1) media (Mediatech, Manassas VA) with penicillin (100 units/ml), streptomycin (100 µg/mL), bovine pituitary extract (BPE, 25 µg/mL; Life Technologies, Carlsbad, CA) and 10% fetal calf serum (Hyclone, South Logan, UT). Trypsin with EDTA (Mediatech, Manassas, VA) was used for passaging of cells. Transduced cell lines were maintained by the addition of puromycin (2 µg/mL) to this media as noted below. Arachidonic acid, prostaglandin E2, and sulindac (Sigma, St. Louis) were dissolved in dimethyl sulfoxide (DMSO, Sigma, St. Louis). Human transforming growth factor beta (TGF-β1) was purchased from R&D Systems (Minneapolis, MN). For all experiments, cells were growth-restricted in serum-free DMEM/F12 supplemented with BPE on day 2 unless otherwise indicated. Reagents were added to growth-restricted media directly via pipetting.
Lentiviral shRNA Transduction
HK-2 cells were plated in DMEM/F12 with 10% FCS and BPE on a 60mm plate. 24 hours later, 200 µL of sterile 150mM NaCl, 0.5µg of each packaging vector (PI, PII, PIII), 1 µg of lentiviral shRNA plasmid (shRNA1, shRNA2, and pLKO empty vector), and 16 µL of TurboFect (Fermentas) were mixed and incubated at room temperature for 15 minutes. The mixture was then dripped into the media of previously cultured 293T cells and incubated at 37°C overnight. The following morning, media was aspirated from the 293T cells and replaced with 4 mL of DMEM/F12 with 10% FCS and BPE. After 24 hours, target cells were treated with polybrene (final concentration 8 mcg/mL) for 60 minutes. Viral media was collected from the 293T cells and fresh media was placed on the 293T cells. Polybrene was added to the viral media and the mixture was passed through a 0.45 µm syringe filter. The media was aspirated from the target cells and the viral media was placed on the target cells and incubated for 6hr at 37°C. Thereafter, the media on the target cells was aspirated and replaced by 4 mL of DMEM/F12 with 10% FCS and BPE containing polybrene. Viral infections were repeated the following day, after which cells were placed into selection media with DMEM/F12 and 10% FCS and BPE containing 2 µg/mL puromycin. shRNAs are in lentiviral vector pGIPZ (Open biosystems) with the following hairpin sequences:
V2LHS_136016 (shRNA1): 5’-TGC TGT TGA CAG TGA GCG CCC TGA TGA ATT TGA GCG AAT ATA GTG AAG CCA CAG ATG TAT ATT CGC TCA AAT TCA TCA GGA TGC CTA CTG CCT CGG A-3’
V2LHS_271391 (shRNA2): 5’-TGC TGT TGA CAG TGA GCG CCC TTG TAT TCT CAC CCT GAT TTA GTG AAG CCA CAG ATG TAA ATC AGG GTG AGA ATA CAA GGT TGC CTA CTG CCT CGG A-3’.
Quantitative RT-PCR
Total RNA was isolated from HK-2 cells using the QIAshredder and RNeasy Plus kits (Qiagen, Valencia CA) and first strand cDNA was made using the iScript cDNA synthesis kit (BioRad, Hercules CA). qRT-PCR reaction was performed with SYBR Green PCR master mix (Applied Biosystems, Carlsbad CA). Sequence-specific primers are as follows:
β-Actin Forward 5’-AGATCAAGATCATTGCTCCTC-3’
Reverse 5’-AACAACGCATCTCATATTTGG-3’
E-cadherin Forward 5’-GGCTGGACCGAGAGAGTTTC-3’
Reverse 5’-GGTGTATACAGCCTCCCACG-3’
N-cadherin Forward 5’-GCCCGCTATTTGTCATCAGC-3’
Reverse 5’-TGCAGTTGCTAAACTTCACATTG-3’
Platelet Derived Growth Factor-B Forward 5’CCTCCAGCCTCGCTGC-3
Reverse 5’-TCTTCCTCTCCGGGGTCTC-3’
Smooth muscle α-actin Forward 5’-CAGCGACCCTAAAGCTTCCC-3’
Reverse 5’-CAGGATTCCCGTCTTAGTCCC-3’
SNAIL-1 Forward 5’ CGCGCTCTTTCTCGTCAG
Reverse 5’-TCCCAGATGAGCATTGGCAG
SNAIL-2 Forward 5’-AGACCCCCATGCCATTGAAG-3’
Reverse 5’-GGCCAGCCCAGAAAAAGTTG-3’
Transforming Growth Factor β1Forward 5’-CGTGGAGGGGAAATTGAGGG-3’
Reverse 5’-AGAAGTTGGCATGGTAGCCC-3’
Vimentin Forward 5’-GTGGACCAGCTAACCAACGA-3’
Reverse 5’-CCTGGATTTCCTCTTCGTGGA-3’
ZEB-1 Forward 5’-GGGAGGATGACACAGGAAAGG-3’
Reverse 5’-TTACACCCAGACTGCGTCAC-3’
Immunoblotting
At the indicated times, HK-2 cells were lysed with ice-cold lysis buffer (50 nM β-glycerophosphate, 100 µM Na3VO4, 2 mM MgCl2, 1mM EGTA, 0.5% Triton X-100, and 1 mM DTT) containing protease inhibitor cocktail (Sigma). Solubilized proteins were centrifuged at 14,000g in a microcentrifuge (4°C) for 10 min. Protein concentration of supernatants were determined using a Bradford assay (BioRad, Hercules CA). Protein was separated using 10% SDS-polyacrylamide gel electrophoresis and transferred to Immobilon P membranes (Millipore, Billerica MA). Membranes were blocked for 1 hour at room temperature in Tris-buffered saline (10mM Tris- HCl, pH 7.4, 140mM NaCl) containing 0.1% Tween-20 (TTBS) and 5% milk or BSA (Sigma, St. Louis MO), and then incubated with 5% milk or BSA in TTBS solution containing primary antibodies for 16 hours at 4°C. Membranes were washed in TTBS, and bound antibodies were visualized either with Horseradish Peroxidase-coupled secondary antibodies and ECL reagent (Fisher, Pittsburgh PA) or Alkaline phosphatase-coupled secondary antibodies and Lumi-Phos WB (Thermo Scientific, Rockford IL) according to the manufacturer’s directions. Antibodies used were E-cadherin and N-cadherin (BD Biosciences, San Jose, CA), cPLA2, cleaved caspase-3, total ERK 1/2, phospho-ERK, total Akt, and phospho-Akt (Cell Signaling, Beverly, MA) and β-Actin (Sigma, St. Louis MO). Anti-rabbit and anti-mouse secondary antibodies were used (Santa Cruz, Dallas TX).
Plasmids and Transient Promoter Assays
For transient transfections measuring promoter activity, a plasmid encoding −108 bp of E-cadherin promoter ligated into a luciferase vector (PA3-Luc) was used. HK-2 cells were transiently transfected with Lipofectamine 2000 (Life) in 60-mm dishes using 1 µg of the E-cadherin promoter-luciferase construct together with 1 µg of a plasmid encoding cytomegalovirus-β-galactosidase vector (Clontech, Mountain View CA) for normalization of transfection efficiency. Cells were kept in Opti-MEM (Life Technologies, Carlsbad, CA) and transfection reagents for five hours. Full DMEM/F12 media with 10% FCS was added after five hours. Cells were collected after 24 hours. Transiently transfected HK-2 cells were then washed twice with ice-cold PBS and harvested in luciferase reporter lysis buffer (Promega, Madison, WI). Cell lysates were centrifuged, and the supernatants were assayed for luciferase and β-galactosidase activities as previously described.(19)
3H-Thymidine Incorporation
HK-2 cells were passaged with trypsin-EDTA, then washed with sterile HANKS buffer, centrifuged at 300g × 5 minutes, followed by aspiration of supernatant. Cells were resuspended in DMEM/F12 media containing 10% FCS and BPE and were counted using a hemocytometer. 275,000 cells per well were then plated in a 6-well plate and incubated overnight at 37°C. On day 2, cells were serum restricted in DMEM/F12 containing BPE for an additional 24 hours. 3H-Thymidiine (1mCi/ml) was then added to the serum-free media or media containing 10% FCS and BPE and incubated for 6 hours. Cells were rinsed extensively on ice with ice-cold PBS to remove free 3H-Thymidine, and then precipitated with 10% (w/v) trichloroacetic acid (TCA, Sigma) for 30min on ice. Cells were then rinsed with 10% TCA, followed by three washes with 95% ice-cold ethanol. Ethanol was removed and samples were air-dried on ice. Precipitated material was solubilized with 300 µL of SDS/NaOH solution. 3H-Thymidine incorporation was quantified via a scintillation counter (Beckman). Protein concentration was measured with DC Protein Assay (Biorad).
Cell Counting
Passage and resuspension of HK2 cells into media containing 10% FCS was performed as noted above with the exception of removal of BPE as a supplemental growth factor. 20,000 cells per well were plated in a 24-well plate in triplicate and incubated overnight at 37°C. On day 2, baseline cell count (t = 0 hrs) was determined for each shRNA population by lifting cells with 500 µl trypsin-EDTA and washing wells with an equal volume of DMEM/F12 media containing 10% FCS. Cells were then immediately pipetted onto plastic disposable cell counting chambers and counted using an automated cell counter (Cellometer, Nexcelom, Lawrence, MA). Remaining cells were then kept in either 10% media or serum free media and allowed to proliferate longer. Cells were then collected in similar fashion at t = 24, 48, and 72 hours from baseline count.
Statistical Analysis
All data are presented as mean +/− SEM. Paired t-tests were performed to compare two groups. For multiple condition comparisons, ANOVA with Tukey’s post-test was used. P-values <0.05 were considered statistically significant.
Results
Silencing cPLA2α alters proliferation, morphology, and epithelial phenotype of HK-2 cells
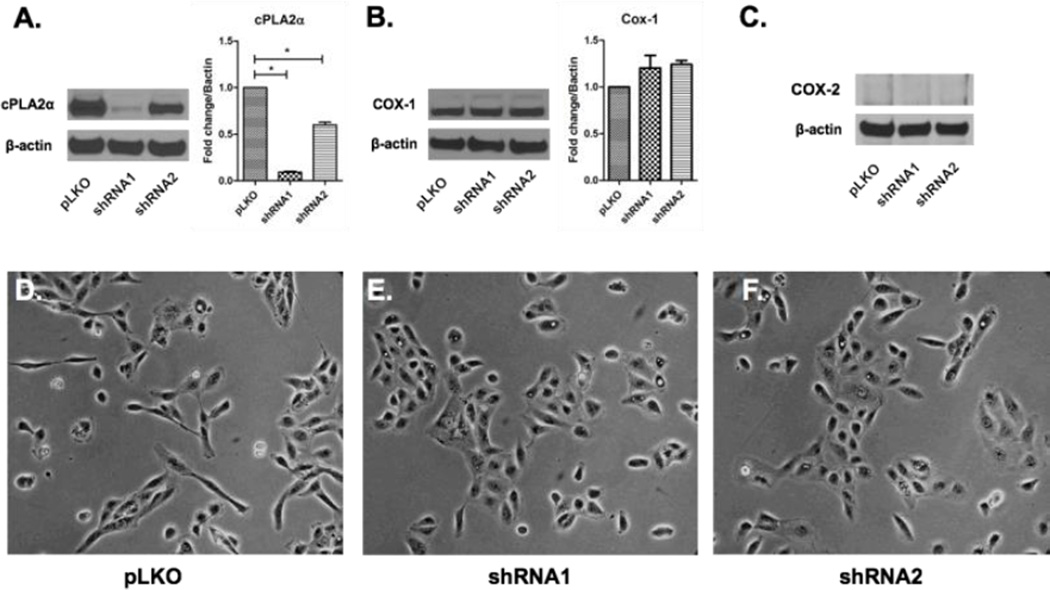
To determine the biological role of cPLA2α in renal tubular epithelial cells, we transfected HK-2 cells with two separate lentiviral short hairpin RNAs (shRNA1 and shRNA2). These two shRNA constructs target two different sequences specific to the group IVA phospholipase A2 transcript. Both cPLA2α knockdowns demonstrated significant depletion of cPLA2α although shRNA1 exhibited the most robust silencing by Western blot (Figure 1A). Successful silencing was confirmed by measuring specific enzymatic activity (Supplemental Figure 1). We next examined the effect of cPLA2α silencing on cyclooxygenase isoforms-1 and -2 expression. Cyclooxygenase 1 (COX-1) expression was unchanged in cPLA2α silenced cells assessed by immunoblotting (Figure 1B). Cyclooxygenase 2 (COX-2) was not detected in control or transfected cells (Figure 1C) by Western blotting. This is not unexpected, as COX-2 is constitutively expressed only in the distal nephron.(20)
Figure 1. Silencing cPLA2α in HK-2 cells alters epithelial phenotype.
cPLA2α-silenced cells (shRNAs -1 and -2) expressed significantly less cPLA2α compared to control cells (pLKO) by western blot (A). Expression of COX-1 (B) and COX-2 (C) did not significantly change in these cells. For cPLA2α and COX-1 blots densitometry for N=3 separate experiments is shown. Under 20× phase-contrast microscopy cells from both shRNA cells demonstrated a more cuboidal shape and more readily packed in compared to control cells under similar confluence (D–F). *p<0.05 vs pLKO controls
Under phase-contrast light microscopy, control HK-2 cells appeared elongated and demonstrated multiple spindle-like projections (Figure 1D). This phenotype has been reported with immortalized HK-2 cells and is also observed in primary cultured human proximal tubular epithelial cells possibly due to lower overall E-cadherin expression than in distal tubular epithelial cells.(21, 22) In contrast, cPLA2α silenced HK-2 cells appeared more cuboidal with less spindle-like projections and clustered more cohesively (Figure 1E,F) than control cells.
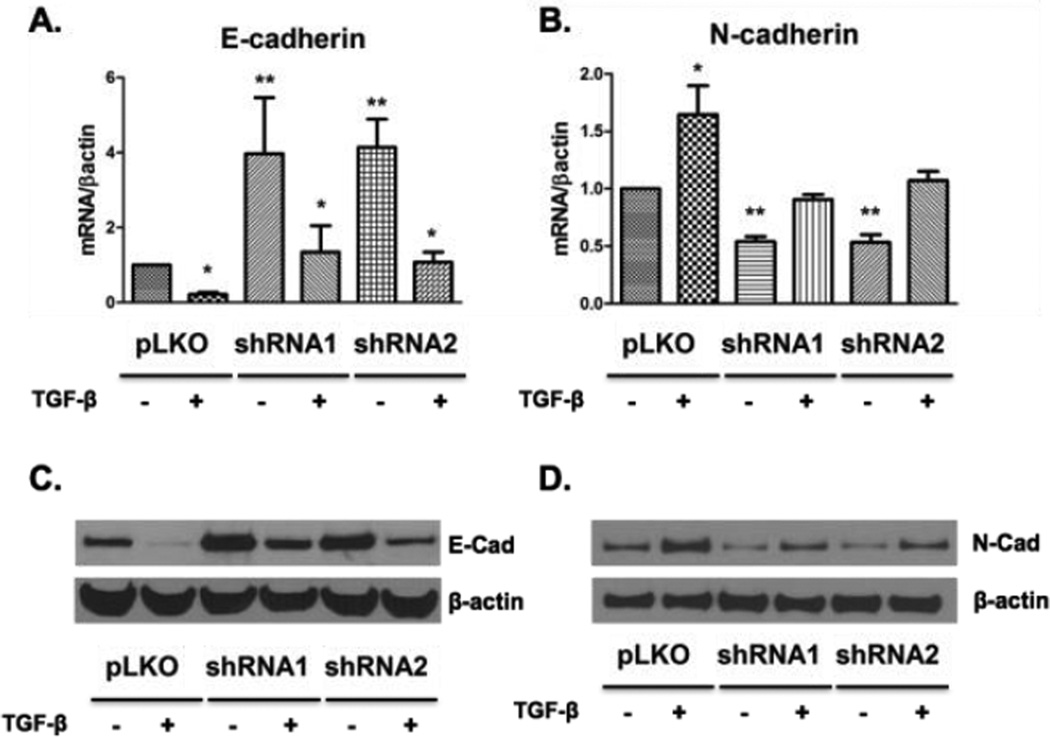
Tubular epithelial cell proliferation is critically important in renal repair after injury.(23) We sought to measure cell proliferation in cPLA2α-depleted cells using a variety of techniques. Both groups of cPLA2α knockdown cells demonstrated significantly less proliferation compared to control cells under serum free conditions using 3H-thymidine incorporation (Figure 2A). This was also observed in the presence of serum, although the difference failed to reach statistical significance. Separately, cell counts were obtained and as compared to pLKO controls we appreciated significantly decreased cell numbers while growing in serum-supplemented conditions at 48 hours (p<0.001 and p<0.05 for shRNA1 and shRNA2 vs pLKO controls, respectively) and 72 hours (p<0.001 for both shRNAs vs pLKO controls). pLKO control cells also proliferated at a faster rate in serum starved conditions at 72 hours (p<0.001 and p<0.01 for shRNA1 and shRNA2 vs pLKO controls, respectively) (Figure 2B,D). We next investigated potential downstream signaling pathways. cPLA2α-silenced cells exhibited no significant difference in basal or serum-stimulated ERK 1/2 and Akt activation (Supp. Figure 2A,B), implicating additional pathways are responsible for the changes in proliferation. To determine if cPLA2α regulates apoptosis, we examined changes in cleaved caspase-3. In both populations of silenced cells we observed higher levels of cleaved caspase-3 by western blot (Figure 2B), suggesting increased levels of apoptosis may contribute to altered proliferation. To determine potential phenotypic changes at the molecular level, we examined mRNA and protein expression of E-cadherin, an epithelial marker, and N-cadherin, a mesenchymal marker, under basal serum free conditions or in the presence of TGF-β (Figure 3A,B). TGF-β is a growth factor that induces an epithelial to mesenchymal transformation (EMT) in many different cell types, including renal epithelial cells.(24) Under basal conditions, both cPLA2α silenced cell populations expressed significantly more E-cadherin (Figure 3A,C) and less N-cadherin (Figure 3B,D) compared with control HK-2 cells, correlating with the observed phenotype under microscopy. TGF-β significantly decreased E-cadherin expression in all cells; however, levels of E-cadherin remained higher in the cPLA2α- depleted cells (Figure 3A,C).
Figure 2. Silencing cPLA2α alters cellular proliferation and apoptosis in HK-2 cells.
Cells were growth restricted for 24 hours before addition of 3H-thymidine and were either kept in serum-free media for 6 hours or treated with 10% fetal calf serum (FCS) (A). Automated cell counts from the three populations of HK-2 cells proliferating under serum (B) and serum free (D) conditions are shown. Lysates from serum-restricted cells were analyzed for cleaved caspase-3 expression and normalized to β-actin (C). Densitometry for N=3 separate experiments is shown. * p<0.05 vs. pLKO controls
Figure 3. cPLA2α in HK-2 cells alters expression of E-cadherin and N-cadherin.
pLKO controls and cPLA2α-silenced cells were treated with TGF-β (10 ng/ml) or vehicle (DMSO) in serum free media for 24 hours before collection. Results in upper panels demonstrate mRNA levels assessed by qPCR for E-cadherin (A) and N-cadherin (B) normalized to β-actin; as well as protein levels assessed by western blotting (C and D, respectively). * p<0.05 with TGF-β verses vehicle treated pLKO controls, ** p<0.05 without TGF-β versus vehicle treated pLKO controls
AKI leads to a temporary re-induction of mesenchymal markers such as vimentin and smooth muscle alpha actin (α-SMA).(6, 25) Expression of platelet-derived growth factor subunit B (PDGF-B) is also seen in injured epithelial cells and may promote renal fibrosis.(26) Expression of α-SMA, vimentin, and PDGF-B were significantly downregulated with cPLA2α silencing (Figure 4), consistent with the acquisition of a more differentiated phenotype in these cells. We also examined production of connective tissue growth factor (CTGF) and TGF-β; both were unchanged. These results show that cPLA2α promotes a more mesenchymal phenotype and also increase production of growth factors associated with mesenchymal transformation in epithelial cells.
Figure 4. cPLA2α silencing alters expression of proximal tubular epithelial mesenchymal markers and growth factors.
qPCR was used to assess message levels of the mesenchymal markers vimentin and αSMA, along with growth factors such as PDGF-B, TGF-β, and CTGF. Results displayed are mRNA/βactin expression relative to values obtained by pLKO controls (dotted line). * p<0.05 vs. control.
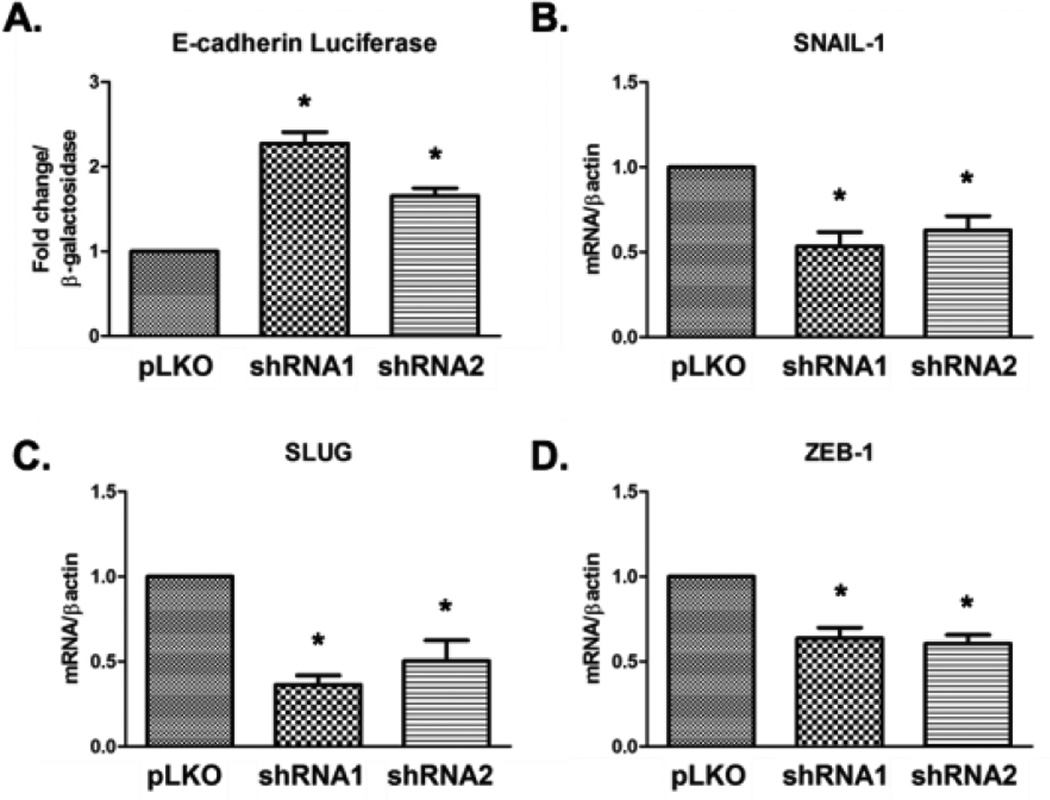
cPLA2α directly regulates E-cadherin promoter activity
E-cadherin expression is regulated at many levels, including transcriptionally. To determine if cPLA2α regulated transcription of E-cadherin, we measured E-cadherin promoter activity using a luciferase reporter construct. Both cPLA2α-silenced cells demonstrated increased E-cadherin promoter activity as compared with control HK-2 cells (Figure 5A). We then measured expression of known transcriptional repressors of E-cadherin including SNAIL-1, SNAIL-2 (SLUG), and ZEB-1;(27) all demonstrated lower baseline levels compared to HK-2 controls (Figure 5B–D). Therefore, cPLA2α increases levels of numerous transcriptional repressors of E-cadherin and promotes epithelial de-differentiation.
Figure 5. cPLA2α regulates the E-cadherin promoter.
Control and shRNA cells were transfected with an E-cadherin promoter luciferase construct and a CMV β-galactosidase construct and collected after 48 hours. Luciferase activity was measured using a luminometer and is normalized to β -galactosidase levels (A). mRNA levels for SNAIL-1 (B), SNAIL-2 (SLUG) (C), and ZEB-1 (D) were measured by qPCR. * p<0.05 vs. control.
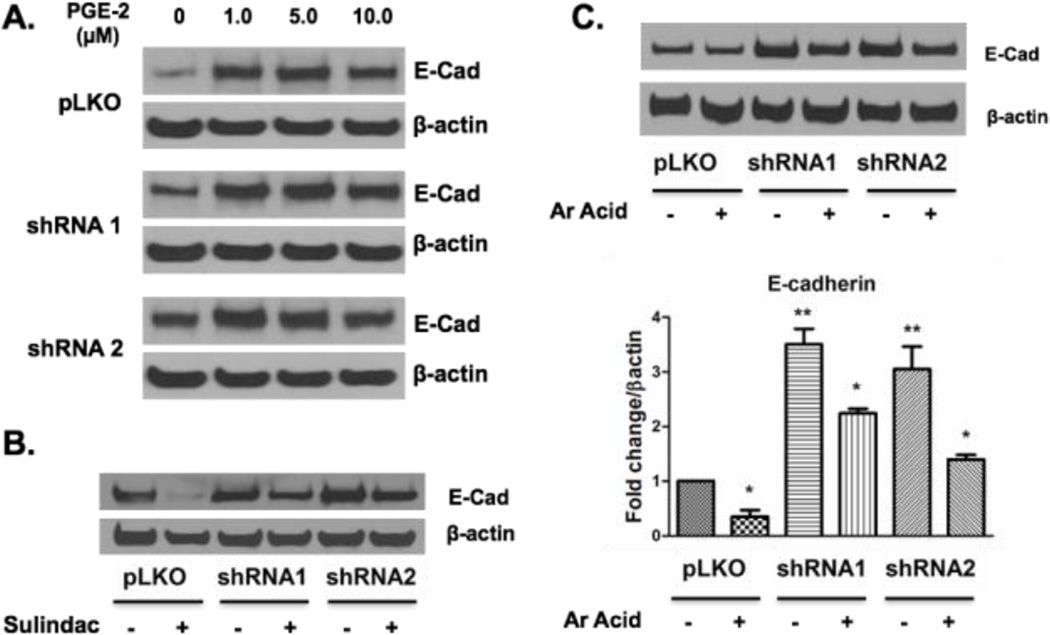
Exogenous Arachidonic Acid reverses the phenotype of cPLA2α silenced HK-2 cells
We hypothesized that the effects of cPLA2α on HK-2 cells were mediated through altered production of specific eicosanoid products. Shunting of arachidonic acid through the COX pathway resulting in production of PGE2 is known to stimulate cell proliferation.(28) Since proximal tubular epithelial cells express cyclooxygenase enzymes responsible for PGE2 generation,(20) we sought to determine if addition of exogenous PGE2 would reverse the effect of cPLA2α depletion. We treated cells with increasing physiologic concentrations of PGE2, which produced a dose dependent increase in E-cadherin expression in both control cells and cells silenced for cPLA2α (Figure 6A). These data suggest that a decrease in PGE2 is not responsible for epithelial phenotype in cPLA2α- silenced cells. We also administered sulindac sulfide, a pan-COX antagonist, to all three cell lines. Sulindac (5 µM) decreased E-cadherin levels in all cell lines (Figure 6B), but to a greater extent in control cells. This result is consistent with increases in E-cadherin seen with PGE2 treatment. Treatment with NS398 (10 µM), a selective COX-2 antagonist, did not result in changes in E-cadherin expression (Supplemental Figure 3). Since the direct action of cPLA2α is to increase free arachidonic acid, we examined the effect of adding exogenous arachidonic acid to these cells. Arachidonic acid (5 µM) decreased E-cadherin expression in control and cPLA2α-silenced cells (Figure 6C). This data shows that cPLA2α regulates epithelial phenotype through an arachidonic acid-dependent but prostaglandin E2-independent mechanism.
Figure 6. Arachidonic Acid, not PGE2, reverses the phenotype of cPLA2α silenced epithelial cells.
PGE2 was given at physiologic (1.0, 5.0 µM) and supra physiologic (10.0 µM) concentrations to all three cell lines (A) under serum restricted conditions 72 hours before collection. Similarly, vehicle solution (DMSO) or sulindac (5.0 µM) (B), and vehicle (DMSO) or arachidonic acid (5 µM) (C) were given to all three cell lines. Respective densitometry for 3 parallel arachidonic acid experiments is displayed with results normalized to β-actin levels with fold change values normalized to vehicle treated control cells. Protein levels of E-cadherin and β-actin were measured by western blot. PGE2 = prostaglandin E2. Ar Acid = Arachidonic Acid * p<0.05 with arachidonic acid versus vehicle treated pLKO cells, ** p<0.05 without arachidonic acid vs vehicle treated pLKO cells.
Discussion
In this study, we tested the hypothesis that cPLA2α, the rate-limiting enzyme in the production of arachidonic acid derived eicosanoids, is important in controlling the basal epithelial phenotype of proximal tubular epithelial cells. We found that loss of cPLA2α promotes a quiescent, more differentiated epithelial phenotype. To our knowledge, this is the first report to establish a connection between cPLA2α activity and epithelial phenotypic changes in renal tubular epithelial cells. PGE2 would seem a logical mediator of increasing E-cadherin expression in renal tubular epithelial cells. Silencing of cPLA2α has been shown to upregulate COX-1 and PGE2 production, and downregulate lipoxygenase products, in a prostate cancer cell line.(29) Additionally, Zhang et al. showed that addition of exogenous PGE2 blunts TGF-β induced epithelial to mesenchymal transformation in Madin-Darby canine kidney cells (MDCK).(30) These data are consistent with our own observations that physiologically relevant concentrations of PGE2 increase E-cadherin expression. However, as compared with distal tubular epithelial cells, proximal tubular epithelial cells express lower concentrations of COX-1, prostaglandin synthases, and much less COX-2 expression.(20, 31) These are consistent with our own observations. We also did not detect a significant change in COX-1 expression among our cPLA2α-silenced cells in contrast to data published by Niknami et al. which demonstrated that cPLA2α silencing increased COX-1 expression in prostate cancer cells.(29) Furthermore, we did not detect PGE2 production in any of our HK-2 populations by ELISA using whole cell homogenates (data not shown).
PGE2 is only one of many potential downstream products of cPLA2α. Depending on the cell type, cPLA2α will catalyze the production of arachidonic acid-dependent prostaglandins, thromboxanes, leukotrienes, HETEs and EETs. Since cPLA2α is regulated by diverse stimuli, it is important to investigate the net effects of cPLA2α activity on tubular epithelial cells. Proximal tubular epithelial cells produce other metabolites of the COX pathway such as PGD2; however, treatment with a pan COX inhibitor (Figure 6B) and a selective COX-2 inibitor (Supplemental Figure 3) did not replicate the phenotype of cPLA2α-silenced cells. Renal tubular epithelial cells are not known to express 5-lipoxygenase or other lipoxygenases, which are mostly found in myeloid-derived cells.
Cytochrome P450 epoxygenases and ω-hydroxylases are mainly expressed in the vasculature however these enzymes have been detected in the rat proximal tubule.(32) Given that some P450-derived products have been shown to induce proliferation in renal tubular epithelial cells(33, 34) it remains possible that cPLA2α silencing suppresses the production of these potential mitogens. However, most of the available data has studied tubular epithelial cells as targets and not effectors of P450-derived EET and HETE products, and there is scant data that the human proximal tubule is a major site of P450-derived EET and HETE synthesis. Additionally, we have analyzed eicosanoid production using an unbiased liquid chromatography, tandem mass-spectrometry (LC/MS/MS) approach(35) in order to identify any alteration in the pool of downstream eicosanoids produced by the cPLA2α silenced cells. We detected very low levels of arachidonic acid from each population of cells but no downstream eicosanoid products, in particular no derivatives of cyclooxygenase or P450 enzymatic action (data not shown). LC/MS/MS was performed on serum restricted cells as with other experiments but not performed using a calcium ionophore nor were nuclear or cytosolic fractions obtained from the cells. The limit of detection for eicosanoids using this method is around 100pg of total protein and experiments were plated in triplicate with > 1.0×106 cells yielding approximately 1–2mg of total protein.
Our data suggest that arachidonic acid itself may promote EMT in epithelial cells. Prior published data from our lab has also shown that arachidonic acid was protective to rat proximal tubules in a model of AKI due to hypoxia.(36) There is a limited literature that arachidonic acid has biological actions independent of its metabolites. Some studies suggest that arachidonic acid increases caspase-driven apoptosis directly.(37) Contrary to this data we appreciated increased levels of cleaved caspase-3 expression in our cPLA2α-silenced cells, though we did not analyze changes in apoptosis and proliferation in arachidonic acid-treated cells. We did appreciate a reduction in E-cadherin expression in our AA-treated pLKO cells, suggesting that loss of cPLA2α wasn’t required for the effects of AA-treatment. Ultimately, we cannot be certain that a particular downstream eicosanoid product wasn’t responsible for these effects since we did not attempt to measure eicosanoid production after the addition of arachidonic acid.
Actions of cPLA2α itself, independent of its downstream mediators, might be responsible for the phenotypic changes observed in our shRNA knockdown cells. Published data has demonstrated that cPLA2α directly regulates mitotic entry through the G2/M checkpoint through its interaction with the tumor suppressor gene SIRT2.(18) Additionally, cPLA2α may play a role in intracellular membrane trafficking.(17) Unfortunately, there is limited data regarding the role of cPLA2α in non-transformed epithelial cells. cPLA2α is highly expressed in human and murine cancer cells, and the level of activity is closely linked with proliferation(28). We have appreciated that HK-2 cells have significantly lower cPLA2α levels than transformed cancer cell lines, such as Lewis Lung Carcinoma (LLC) cells via Western blot (data not shown). Detection of cPLA2α in HK-2 cells required a 10-fold increase in protein loading compared to proliferating LLCs. We believe this lower basal expression of cPLA2α, or alterations in phosphorylation status, might be responsible for the similar biological activity observed among our two cPLA2α knockdown cells despite a difference in expression by Western blotting (Figure 1A). In cells with lower basal cPLA2α expression a greater than 50% degree of knockdown might be all that is required to elucidate similar biologic changes as compared with cells with relatively higher cPLA2α expression. Our findings cannot be generalized to non-immortalized proximal tubular epithelial cells, or other tubular epithelial cells. We focused on proximal tubular epithelial cells due to their importance in renal injury, such as in ischemia and toxin-related injury. Furthermore, HK-2 cells are one of the most well studied proximal tubular epithelial cell line.
We believe that our findings may have important implications for kidney disease research. Recent data has documented the significance of RTE phenotype in models of progressive renal injury. Some investigators propose that these changes in epithelial cells may be the earliest events that dictate the complex downstream pro-inflammatory, pro-growth signals culminating with pathologic renal tubulointerstitial fibrosis.(38, 39) It is possible that early cPLA2α activation in renal epithelium may promote renal repair by initiating partial dedifferentiation, proliferation, and migration, which are associated with repair. In contrast, cPLA2α activation in other cell types, including inflammatory cells results in eicosanoid production, which promotes injury. Thus, the net response will likely be a balance between cell-specific pathways that have opposing effects on progression of renal injury. Further research is needed to define the production of specific eicosanoids by different cell populations in diverse models of renal injury. Interventional studies could then test whether the manipulation of cPLA2α in specific cell types modifies renal recovery and repair.
Supplementary Material
Highlights.
Silencing cPLA2α expression in HK-2 cells alters epithelial morphology and proliferation
cPLA2α alpha controls E-cadherin promoter activity directly in HK-2 cells
These effects are mediated by arachidonic acid or cPLA2α itself, and are not dependent on PGE2
Acknowledgments
This research was supported by NIH/NIDDK T32 DK-7135 and F32DK104475 (to JRM) and NIH/NHBI K08 HL103774 (to SBF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grgic I, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82(2):172–183. doi: 10.1038/ki.2012.20. 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lovisa S, et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nature medicine. 2015;21(9):998–1009. doi: 10.1038/nm.3902. 10.1038/nm.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grande MT, et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nature medicine. 2015;21(9):989–997. doi: 10.1038/nm.3901. 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]
- 4.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82(5):516–524. doi: 10.1038/ki.2012.208. 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 5.Wald R, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302(11):1179–1185. doi: 10.1001/jama.2009.1322. 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 6.Witzgall R, et al. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93(5):2175–2188. doi: 10.1172/JCI117214. 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rastaldi MP, et al. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002;62(1):137–146. doi: 10.1046/j.1523-1755.2002.00430.x. 10.1046/j.1523-1755.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 8.Humphreys BD, et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A. 2011;108(22):9226–9231. doi: 10.1073/pnas.1100629108. 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nature medicine. 2010;16(5):535–543. doi: 10.1038/nm.2144. 531p following 143. 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao CM, Breyer MD. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 2007;71(11):1105–1115. doi: 10.1038/sj.ki.5002192. 10.1038/sj.ki.5002192. [DOI] [PubMed] [Google Scholar]
- 11.Cui XL, et al. Oxidative signaling in renal epithelium: Critical role of cytosolic phospholipase A2 and p38(SAPK) Free Radic Biol Med. 2006;41(2):213–221. doi: 10.1016/j.freeradbiomed.2006.02.004. 10.1016/j.freeradbiomed.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terao Y, et al. Phospholipase A2 is activated in the kidney, but not in the liver during ischemia-reperfusion. Res Commun Mol Pathol Pharmacol. 1997;96(3):277–289. [PubMed] [Google Scholar]
- 13.Khan NS, et al. Cytosolic Phospholipase A2alpha Is Essential for Renal Dysfunction and End-Organ Damage Associated With Angiotensin II-Induced Hypertension. Am J Hypertens. 2015 doi: 10.1093/ajh/hpv083. 10.1093/ajh/hpv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa N, et al. The intrinsic prostaglandin E2-EP4 system of the renal tubular epithelium limits the development of tubulointerstitial fibrosis in mice. Kidney Int. 2012;82(2):158–171. doi: 10.1038/ki.2012.115. 10.1038/ki.2012.115. [DOI] [PubMed] [Google Scholar]
- 15.Takano T, Cybulsky AV. Complement C5b-9-mediated arachidonic acid metabolism in glomerular epithelial cells : role of cyclooxygenase-1 and -2. Am J Pathol. 2000;156(6):2091–2101. doi: 10.1016/S0002-9440(10)65080-8. 10.1016/S0002-9440(10)65080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aukema HM, et al. Alterations in renal cytosolic phospholipase A2 and cyclooxygenases in polycystic kidney disease. FASEB J. 2003;17(2):298–300. doi: 10.1096/fj.02-0460fje. 10.1096/fj.02-0460fje. [DOI] [PubMed] [Google Scholar]
- 17.Leslie CC. Cytosolic phospholipase A2: physiological function and role in disease. J Lipid Res. 2015;56(8):1386–1402. doi: 10.1194/jlr.R057588. 10.1194/jlr.R057588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Movahedi Naini S, et al. Group IVA Cytosolic Phospholipase A2 Regulates the G2-to-M Transition by Modulating the Activity of Tumor Suppressor SIRT2. Mol Cell Biol. 2015;35(21):3768–3784. doi: 10.1128/MCB.00184-15. 10.1128/mcb.00184-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehman AM, et al. Activation of the retinoid X receptor modulates angiotensin II-induced smooth muscle gene expression and inflammation in vascular smooth muscle cells. Mol Pharmacol. 2014;86(5):570–579. doi: 10.1124/mol.114.092163. 10.1124/mol.114.092163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitzthum H, et al. Gene expression of prostanoid forming enzymes along the rat nephron. Kidney Int. 2002;62(5):1570–1581. doi: 10.1046/j.1523-1755.2002.00615.x. 10.1046/j.1523-1755.2002.00615.x. [DOI] [PubMed] [Google Scholar]
- 21.Keller C, et al. Distinct mesenchymal alterations in N-cadherin and E-cadherin positive primary renal epithelial cells. PLoS One. 2012;7(8):e43584. doi: 10.1371/journal.pone.0043584. 10.1371/journal.pone.0043584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan MJ, et al. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45(1):48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- 23.Kusaba T, et al. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci U S A. 2014;111(4):1527–1532. doi: 10.1073/pnas.1310653110. 10.1073/pnas.1310653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu CF, et al. Transforming growth factor beta-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol. 2013;182(1):118–131. doi: 10.1016/j.ajpath.2012.09.009. 10.1016/j.ajpath.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villanueva S, Cespedes C, Vio CP. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R861–R870. doi: 10.1152/ajpregu.00384.2005. 10.1152/ajpregu.00384.2005. [DOI] [PubMed] [Google Scholar]
- 26.Frank J, et al. Human renal tubular cells as a cytokine source: PDGF-B, GM-CSF and IL-6 mRNA expression in vitro. Exp Nephrol. 1993;1(1):26–35. [PubMed] [Google Scholar]
- 27.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7(6):415–428. doi: 10.1038/nrc2131. 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi M, Rosenberg DW. Roles of cPLA2alpha and arachidonic acid in cancer. Biochim Biophys Acta. 2006;1761(11):1335–1343. doi: 10.1016/j.bbalip.2006.09.005. 10.1016/j.bbalip.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niknami M, et al. Decrease in expression or activity of cytosolic phospholipase A2alpha increases cyclooxygenase-1 action: A cross-talk between key enzymes in arachidonic acid pathway in prostate cancer cells. Biochim Biophys Acta. 2010;1801(7):731–737. doi: 10.1016/j.bbalip.2010.03.003. 10.1016/j.bbalip.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Zhang A, et al. Prostaglandin E2 is a potent inhibitor of epithelial-to-mesenchymal transition: interaction with hepatocyte growth factor. Am J Physiol Renal Physiol. 2006;291(6):F1323–F1331. doi: 10.1152/ajprenal.00480.2005. 10.1152/ajprenal.00480.2005. [DOI] [PubMed] [Google Scholar]
- 31.Sakurai M, Oishi K, Watanabe K. Localization of cyclooxygenases-1 and-2, and prostaglandin F synthase in human kidney and renal cell carcinoma. Biochem Biophys Res Commun. 2005;338(1):82–86. doi: 10.1016/j.bbrc.2005.08.194. 10.1016/j.bbrc.2005.08.194. [DOI] [PubMed] [Google Scholar]
- 32.Zhao X, Imig JD. Kidney CYP450 enzymes: biological actions beyond drug metabolism. Curr Drug Metab. 2003;4(1):73–84. doi: 10.2174/1389200033336892. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, et al. Arachidonic acid metabolites inhibit the stimulatory effect of angiotensin II in renal proximal tubules. Hypertens Res. 2008;31(12):2155–2164. doi: 10.1291/hypres.31.2155. 10.1291/hypres.31.2155. [DOI] [PubMed] [Google Scholar]
- 34.Chen JK, et al. Epoxyeicosatrienoic acids and their sulfonimide derivatives stimulate tyrosine phosphorylation and induce mitogenesis in renal epithelial cells. J Biol Chem. 1998;273(44):29254–29261. doi: 10.1074/jbc.273.44.29254. [DOI] [PubMed] [Google Scholar]
- 35.Murphy RC, et al. Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal Biochem. 2005;346(1):1–42. doi: 10.1016/j.ab.2005.04.042. 10.1016/j.ab.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 36.Alkhunaizi AM, et al. Arachidonic acid protects against hypoxic injury in rat proximal tubules. Kidney Int. 1996;49(3):620–625. doi: 10.1038/ki.1996.89. [DOI] [PubMed] [Google Scholar]
- 37.Pompeia C, Lima T, Curi R. Arachidonic acid cytotoxicity: can arachidonic acid be a physiological mediator of cell death? Cell Biochem Funct. 2003;21(2):97–104. doi: 10.1002/cbf.1012. 10.1002/cbf.1012. [DOI] [PubMed] [Google Scholar]
- 38.Venkatachalam MA, et al. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015010006. 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015 doi: 10.1038/nrneph.2015.3. 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.