Abstract
Homer proteins are integral components of the postsynaptic density that are necessary for alcohol-induced neuroplasticity within the nucleus accumbens (NAC). In this report, we describe the effects of chronic alcohol consumption upon NAC Homer expression and investigate the functional consequences of mimicking the alcohol-induced changes in Homer expression vis-à-vis alcohol-induced changes in NAC neurochemistry and behavior. Chronic alcohol consumption under continuous access (3 months; daily intake ≈ 11.2 ± 1.5 g/kg/day) produced a robust increase in NAC Homer2 protein levels that was apparent at 2 days, 2 weeks and 2 months following withdrawal from alcohol drinking. The increased Homer2 expression was accompanied by a less enduring elevation in total mGluR1 and NR2b levels that were evident at 2 days and 2 weeks but not at the 2-month time-point. Mimicking the alcohol-induced increase in Homer2 levels by viral transfection of NAC neurons in alcohol-preferring C57BL6/J inbred mice enhanced behavioral output for alcohol reinforcement and increased alcohol intake under both pre-prandial and post-prandial conditions. Moreover, NAC Homer2 over-expression facilitated the expression of an alcohol-conditioned place-preference, as well as the development of motor tolerance. Finally, NAC Homer2 over-expression facilitated NAC glutamate and dopamine release following an acute alcohol injection and augmented alcohol-induced dopamine and glutamate sensitization, but did not affect NAC GABA levels. Thus, an up-regulation in NAC mGluR-Homer2-NMDA receptor signaling appears to be an important molecular adaptation to alcohol that promotes neuroplasticity facilitating motivational drive for alcohol and the development of alcoholism-related behaviors.
Keywords: Homer proteins, ethanol, glutamate receptors, neuroplasticity, glutamate, dopamine
Introduction
The transition from recreational alcohol consumption to compulsive drinking (alcoholism) involves neuroadaptations in both pre- and postsynaptic aspects of mesocorticolimbic glutamate transmission (for reviews, Koob 2003; Krystal et al. 2003; Siggins et al. 2005), implicating proteins regulating mesocorticolimbic glutamate transmission as molecular candidates in the etiology of addiction. The Homer family of proteins are encoded by 3 genes (Homer1-3) (Soloviev et al. 2000) and act to coordinate synaptic proteins for a variety of cellular functions, including calcium signaling and activity-dependent synaptic remodeling (for reviews, de Bartolomeis and Iasevoli 2003; Duncan et al. 2005; Fagni et al. 2002; Xiao et al. 2000). Homer proteins regulate signaling through, and the trafficking of, Group1 metabotropic glutamate receptors and NMDA receptors (e.g., Naisbitt et al. 1999; Shiraishi et al. 2003; Smothers et al. 2005; Szumlinski et al. 2004; Szumlinski et al. 2005b; Tu et al. 1998; Tu et al. 1999; Xiao et al. 1998). Such regulation is of potential relevance to the neurobiology of alcoholism as these glutamate receptors are two sites of action for alcohol in the brain (e.g., Lovinger 1993; Minami et al. 1998). Moreover, in vivo neural genetic studies revealed a critical role for constitutive Homer protein expression in regulating NAC NMDA and Group1 mGluR function or expression (Szumlinski et al. 2004; 2005b), maintaining and modulating drug-induced changes in extracellular glutamate levels within the corticoaccumbens pathway (Lominac et al. 2005; Swanson et al. 2001; Szumlinski et al. 2004; 2005a; 2005b; 2006a).
Alternative splicing of the Homer2 generates four gene products (Soloviev et al. 2000), of which, Homer2a and Homer2b are localized in brain (e.g., Shiraishi et al. 1999). Homer2a/b are structurally and functionally similar proteins that differ from each other by 11 amino acids (Soloviev et al. 2000). Like other constitutively expressed Homer proteins, Homer2a/b contain ~175 amino acid residue long regions in their C-termini, which adopt a coiled-coiled structure that enables Homer2a/b to multimerize and mediate interactions between their EVH1-bound partners (for reviews, de Bartolomeis and Iasevoli 2003; Duncan et al. 2005; Fagni et al. 2003; Xiao et al. 2000). Studies of Homer2 knock-out (KO) mice revealed an important role for Homer2 isoforms in regulating alcohol-induced neuroplasticity; KO mice do not develop alcohol-induced dopamine or glutamate sensitization within the NAC and exhibit an alcohol-avoiding and behaviorally intolerant phenotype (Szumlinski et al. 2005b). Viral transfection of NAC neurons with Homer2b cDNA “rescues” the alcohol behavioral and neurochemical phenotype of Homer2 KO mice and shifts the alcohol preference function up and to the left in alcohol-preferring C57BL/6J (B6) inbred mice, supporting an active role for this Homer2 isoform in regulating alcohol-induced neuroplasticity (Szumlinski et al. 2005b). This collection of data has lead to the over-arching hypothesis in our laboratory that alcohol-induced changes in NAC Homer expression may be an important mediator of the neuroplasticity relevant to alcoholism.
To test this hypothesis, we first examined the effects of alcohol consumption upon Homer levels in the NAC and then employed behavioral genetic and neurochemical approaches to ascertain the functional consequences of alcohol-mediated changes in NAC Homer expression. As Homer2 proteins regulate the trafficking and expression of Group1 mGluRs and NR2 subunits of the NDMA receptors in vivo (Szumlinski et al. 2004; Szumlinski et al. 2005b), the co-regulation of Homers with these proteins was also assessed. Cocaine co-regulates the NAC expression of Homer1b/c and mGluR5 in a time-dependent manner (Swanson et al. 2001). Thus, water- and alcohol-drinking mice were sacrificed at 2 days, 2 weeks or 2 months withdrawal to examine for the time-dependency of alcohol’s effects upon protein expression. Our data provide novel evidence that alcohol produces a large and persistent increase in NAC Homer2a/b expression that is accompanied by more transient changes in mGluR1 and NR2b levels. Our earlier behavioral data demonstrated an active and necessary role for NAC Homer2b expression in regulating various aspects of alcohol reward in mice (Szumlinski et al. 2005a). Thus, to determine whether or not an increase in NAC Homer2b expression is sufficient to alter alcohol-induced neuroplasticity, AAV-mediated delivery of Homer2b to the NAC of B6 was used to mimic the alcohol-induced rise in Homer2b levels. Our data demonstrate that an up-regulation in NAC Homer2b expression promotes alcohol-induced neurochemical plasticity that enhances or facilitates the development of alcoholism-related behaviors.
MATERIALS AND METHODS
Subjects
Adult male (8 weeks of age) C57BL/6J (B6) mice were obtained from Jackson Laboratories (Bar Harbor, ME). All mice were individually housed and maintained in polyethylene cages in a colony room, controlled for temperature (25°C) and humidity (71%), under a 12 h day-12 h night cycle (lights off: 18:00 h). All experimental protocols were consistent with the guidelines of the NIH Guide for Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996).
Immunoblotting
B6 mice were permitted 24-hr free-access to water or alcohol (4 bottle-choice with 0, 3, 6 and 12% alcohol; Lominac et al. 2006) for a period of 3 months. At 2 days, 2 weeks and 2 months withdrawal from end of the 3-month period of drinking, the entire NAC (shell and core) was dissected out over ice and homogenized in a medium consisting of RIPA buffer [65 mM Tris, 150 mM NaCl, 1.8 mM Na-deoxycholic acid, 1.3 mM EDTA, 0.01% NP-40, 1% SDS, Complete Mini-tab Protease Inhibitor Cocktail tablet (Roche Diagnostics GmbH, Mannheim, Germany)]. Protein determinations were performed using the BCA Protein Assay Kit (Pierce, Rockford, IL). The procedures employed to quantify Homer1b/c and Homer2a/b were identical to those employed previously (Ary et al. 2007), originally adapted from those described in Shin et al. (2003) and Swanson et al. (2001). Samples (30 μg) were subjected to SDS-polyacrylamide gel electrophoresis using Bis-Tris gradient gels (4–12%) (Invitrogen, Carlsbad, CA) and proteins were transferred to PVDF membranes, preblocked with phosphate-buffered saline containing 0.1% (v/v) Tween-20 and 5% (w/v) nonfat dried milk powder for 1 hr before overnight incubation with the following rabbit primary antibodies: anti-Homer1b/c and anti-Homer2a/b primary antibodies (a generous gift from Dr. Paul F. Worley, Johns Hopkins University School of Medicine; 1:1000 dilution), anti-mGluR1a and anti-mGluR5 (Upstate Cell Signaling Solutions, Lake Placid, NY; 1:1000 dilution), anti-NR2a and anti-NR2b (Calbiochem, San Diego CA; 1:1000 dilution). A rabbit anti-calnexin primary antibody (Stressgen Biotechnologies, Ann Arbor, MI; 1:1000 dilution) was used to verify even loading and protein transfer. Membranes were washed, incubated with horseradish-peroxidase-conjugated goat anti-rabbit secondary antibody (Upstate; 1:20,000–1:40,000 dilution) for 90 min, and immunoreactive bands were detected by enhanced chemiluminescence (ECL Plus; Amersham Biosciences Inc., Piscataway, NJ). Immunoreactive levels were quantified by integrating band density X area using computer-assisted densitometry (Image J, NIH). As statistical analysis of each individual gel failed to detect differences across samples, the density X area measurements were averaged over control samples within each gel and all bands were normalized as percent of the control values for each gel.
Surgery, Construction and Infusion of viral vectors
The procedures employed to construct and infuse recombinant adeno-associated virus (rAAV) into the NAC of mice were identical to those described previously in mice (Lominac et al. 2005; Szumlinski et al. 2004; 2005b). Under isoflurane anesthesia, mice were implanted unilaterally with a 26-gauge stainless steel guide cannula (20 mm long) aimed 3 mm above the NAC (AP: +0.5 mm; ML: ± 0.6 mm; DV: −2.0 mm, relative to bregma) (Franklin & Paxinos, 1997). Following at least 7 days recovery, AAVs carrying equal ratios of AAV1 and AAV2 capsid proteins were used to express either HA-tagged Homer2b or enhanced green fluorescent protein (GFP) under the control of the chicken-beta actin promoter. A total volume of 0.25 μl (1011 viral genomes/ml) was infused unilaterally intra-NAC via injector cannulae (33 gauge, 22 mm in length; fitted into 20 mm of 30 gauge tubing). As in our earlier AAV studies (Klugmann et al. 2005; Lominac et al. 2005; Szumlinski et al. 2004; 2005b; 2006a), a period of 3 weeks was allowed for maximal and persistent transgene expression.
Instrumental responding for alcohol
The apparatus employed to assess instrumental responding for alcohol and alcohol intake under response-contingent conditions consisted of standard mouse operant conditioning chambers (MedAssociates, St. Albans, VT) located within ventilated sound-attenuated chambers (Middaugh and Kelley 1999). The procedures employed to train mice to lever-press for alcohol were similar to those described previously by Middaugh and Kelley (1999). In brief, training sessions were conducted during daily 21-min sessions during the light cycle beginning at 14:00 hr. For these sessions, 12% ethanol (v/v) was delivered via an infusion pump (MedAssociates) that was connected to a fountain located directly below a nose-poke hole located 15 cm from the lever. Mice were first trained to press the lever for ethanol delivery (0.06 ml/infusion) on an fixed ratio 1 (FR1) schedule of reinforcement with a 20-sec timeout period. To expedite the acquisition of self-administration, mice were restricted to 90% of their ad libitum body weight throughout testing and mice were tested under post-prandial conditions (low hunger/high thirst), 1 hr following the receipt of their daily food ration. Water was available ad libitum with the exception of the 1 hr prior to self-administration training. Mice continued on the FR1 schedule until a response criterion of 3 consecutive days of greater than 10 reinforced lever presses/session with at least 100 contacts with the ethanol delivery fountain. Once initial self-administration criterion for 12% alcohol was met on an FR1 schedule, the FR schedule was increased to an FR2. Following stabilization of responding on the FR2 schedule, a dose-response function for alcohol was established by substituting the 12% alcohol training solution with 1 of 4 alcohol concentrations (0, 3, 6 or 12% v/v). The order of testing was randomized across AAV treatment groups. Testing occurred every 3–5 days, upon re-establishment of stable responding for 12% alcohol.
Upon completion of the dose-response function for alcohol self-administration under post-prandial conditions, the dose-response function was established under pre-prandial conditions (high hunger/low thirst) (Middaugh and Kelley 1999). In this condition, mice were not fluid restricted and received their daily food ration after the self-administration sessions. Once responding for 12% alcohol stabilized, the 12% alcohol training solution was substituted with 1 of 4 alcohol concentrations (0, 3, 6 and 12% v/v). Again, testing occurred every 3–5 days upon re-establishment of stable responding for 12% alcohol. At the completion of the pre-prandial dose-response function, mice were allowed to respond again for 12% alcohol. Following 3 days of stable responding, blood was sampled from the infraorbital sinus immediately upon completion of the 21-min session. Blood was analyzed for alcohol concentrations using gas-chromatography procedures described previously (Middaugh et al. 2003; Szumlinski et al. 2005b).
Alcohol-conditioned place preference
An unbiased place conditioning procedure was employed using an apparatus with two distinct compartments differing in wall pattern and floor texture and digital video-tracking automatically recorded the time spent in each of the two compartments (Stoelting Company, Wood Dale, IL). The procedures to induce an alcohol-conditioned place-preference were similar to those described previously for B6 mice (Lominac et al. 2006). To verify that the apparatus was unbiased prior to conditioning, a pre-conditioning test was conducted in which mice had free-access to both compartments for 15 min. Alcohol-induced place-conditioning was produced by 8 repeated pairings, on alternating days, of interperitoneal (IP) injections of 2 g/kg alcohol with one of the compartments and saline (vol=0.02 ml/g body weight) with the opposite compartment of the place conditioning apparatus. This alcohol injection regimen was selected as it produces a significant increase in the time spent in the alcohol-conditioned compartment when animals are tested using a biased place-conditioning procedure (Lominac et al. 2006; Szumlinski et al 2005a). Paired and unpaired compartments were counterbalanced across the groups. A post-conditioning test followed the last alcohol conditioning session in which animals again had free access to both compartments. The difference in the amount of time spent in the alcohol-paired versus unpaired environment on the post-conditioning test served to index the magnitude of place conditioning.
Tolerance to the motor-inhibitory and sedative effects of alcohol
To assess the effects of NAC Homer2 over-expression upon the acute motor-inhibitory effect of alcohol and the development of tolerance upon repeated alcohol administration, groups of AAV-treated mice were injected repeatedly with either saline or 2 g/kg alcohol, every other day, and the locomotor activity of the mice was assessed on injections 1 and injection 8 of repeated treatment. For this experiment, mice were placed in opaque Plexiglas activity chambers (23 × 24 × 22 cm) equipped with photocells and locomotor activity was monitored for 15 min following injection.
In vivo microdialysis and HPLC for monoamines and amino acids
To relate the behavioral effects of NAC Homer2 over-expression to alcohol-induced changes in NAC neurochemistry, in vivo microdialysis was performed in groups of AAV-GFP and AAV-Homer2b mice following repeated treatment with either saline or alcohol (8 × 2 g/kg alcohol, every other day). The procedures for microdialysis probe construction and dialysate collection were identical to those described previously (e.g., Lominac et al. 2006; Szumlinski et al. 2005b; 2007). A microdialysis probe (24 gauge, 23 mm in length, including 0.7–1.0 mm active membrane) was inserted unilaterally into the NAC and perfused at a rate of 2.0 μl/min with artificial cerebrospinal fluid. Three to four hours later, when baseline neurotransmitter levels were stable (see Figure 6), baseline dialysate was then collected in 20-min fractions into 10 μl of preservative (0.075 μM NaH2PO4, 25 μM EDTA, 0.0017 μM 1-octansulfonic acid, 10% acetonitrile (v/v), pH= 3.0) for 1 h. Two microdialysis sessions were conducted per animal. Forty-eight hrs following the last alcohol/saline injection, a no net-flux study was conducted in which mice were infused, through the microdialysis probe, with increasing concentrations of glutamate (0, 2.5, 5 and 10 μM) as described previously (Szumlinski et al. 2004; 2005a; 2006a). Three to four days following the no-net flux study, a conventional microdialysis experiment was performed using the opposite side to assess for the effects of AAV infusion upon the neurochemical response to a challenge injection of alcohol. For this experiment, dialysate was collected every 20 min for 1 hr prior to, and then for 3 hrs following, an IP injection of 2 g/kg alcohol, in a manner similar to that conducted previously in Homer2 KO and B6 mice (Lominac et al. 2006; Szumlinski et al. 2005b; Szumlinski et al. 2007).
Figure 6. NAC Homer2 over-expression facilitates alcohol-mediated glutamate and dopamine release.
a, An acute injection of 2 g/kg alcohol elevated NAC glutamate levels only in mice infused with AAV-Homer2 [AAV X Time: F(11,143)=1.96, p=0.04]. b, NAC Homer2 over-expression augmented the sensitized glutamate response to alcohol following repeated alcohol treatment (8 × 2 g/kg) [AAV X Time: F(11,143)=2.39, p=0.009]. c, An acute alcohol injection elicited a slightly larger rise in NAC dopamine levels in mice infused with AAV-Homer2 [AAV X Time: F(11,143)=2.06, p=0.03]. d, NAC Homer2 over-expression augmented the sensitized dopamine response to alcohol following repeated alcohol treatment [AAV X Time: F(11,165)=1.95, p=0.05]. e & f, NAC GABA levels were unaffected by either AAV infusion or alcohol administration (3-way ANOVA, p>0.05 for all main effects and interactions). The data represent the mean percent change from baseline values per 20-min fraction ± SEM of the number of animals indicated in parentheses. *p<0.05 vs. baseline; +p<0.05 vs. AAV-GFP.
The high pressure liquid chromatography (HPLC) system and the procedures for the electrochemical detection of dopamine, glutamate and GABA in the dialysate of mice were identical to those described previously (Lominac et al. 2006; Szumlinski et al. 2007). The neurotransmitter content in each sample was analyzed by peak height and was compared with external standard curves (one for each neurotransmitter examined) for quantification using ESA Coularray for Windows software.
Verification of AAV transduction
Animals were transcardially perfused with phosphate-buffered saline, followed by a 4% paraformaldehyde solution. Brains were removed and sliced along the coronal plane in 50 μm sections at the level of the NAC. AAV transfection was verified by immunostaining for the HA tag and for GFP as described previously (Lominac et al. 2005; Szumlinski et al. 2004; Szumlinski et al. 2005b; Szumlinski et al. 2006a).
Statistical analyses
All data were analyzed using analyses of variance (ANOVAs). If significant interactions were found, the data were decomposed for main effects, followed by LSD post-hoc comparisons.
RESULTS
Alcohol consumption increases accumbens Homer2 expression
Earlier behavioral genetic data supported an important role for Homer2 isoforms in alcohol-induced neuroplasticity (Szumlinski et al. 2005b) and constitutive Homer1b/c protein expression is down-regulated in the NAC during withdrawal from repeated cocaine in a time-dependent fashion (Swanson et al. 2001). Accordingly, we examined for the regulation of Homer and glutamate receptor expression during the course of protracted alcohol withdrawal by immunoblotting for the constitutively expressed Homer isoforms Homer1b/c and Homer2a/b, the mGluR1 and mGluR5 subtypes of Group 1 mGluRs, as well as the NR2a and NR2b subunits of the NMDA receptor on whole NAC tissue derived from B6 mice. For this, mice were allowed free-access to 4 bottles containing 0, 3, 6 and 12% alcohol for a period of 3 months (Lominac et al. 2006) and then mice were sacrificed at 2 days, 2 weeks and 2 months after cessation of alcohol drinking (n=7–9/time-point). As per our earlier study in B6 mice (Lominac et al. 2006), mean daily alcohol intake was consistently high and averaged 11.2 ± 1.5 g/kg/day during the last week of the 3-month drinking period. The data for immunoblotting for these mice at the various withdrawal time-points are presented in Fig. 1. Relative to water-drinking controls, chronic alcohol consumption produced a robust, 2.5-fold, increase in NAC Homer2 levels when assessed at 2 days withdrawal, and the magnitude of this increase was unchanged at 2 weeks and 2 months withdrawal. The rise in Homer2 observed was accompanied by a similar rise in NR2b levels and an approximately 50% elevation in mGluR1 expression. However, in contrast to Homer2 expression, the alcohol withdrawal-induced elevations in NR2b and mGluR1 levels were no longer apparent at 2 months withdrawal. Alcohol consumption did not consistently affect the expression of Homer1b/c, mGluR5 or NR2a. Thus, withdrawal from chronic alcohol drinking produces an enduring up-regulation of the expression of specific components of the mGluR-Homer-NMDA signaling cascade and in the case of Homer2, this up-regulation persists for up to 2 months following drinking cessation.
Figure 1. Chronic voluntary alcohol intake produces enduring Homer2, but transient NR2b and mGluR1, elevations in the NAC.
a, Representative immunoblots for the total protein levels of Homer2a/b, Homer1b/c, NR2a, NR2b, mGluR1, mGluR5 and calnexin (loading control) in the NAC of groups of mice sacrificed at 2 days, 2 weeks and 2 months withdrawal from 3 months of water (W) or alcohol consumption (mean daily intake = 11.2 ± 1.5 g/kg). b, Summary of the change in protein expression following withdrawal from 3 months of continuous alcohol consumption. Compared to water-drinking mice, chronic alcohol consumption elevated NAC Homer2a/b levels at all withdrawal time-points [F(3,33)=14.0, p<0.0001], but did not affect significantly NAC Homer1b/c levels (p=0.25). Alcohol withdrawal did not affect NR2a levels (p=0.24) or the levels of mGluR5 (p=0.35), but elicited a rise in NR2b and mGluR1a that persisted for at least 2 weeks [for NR2b: F(3,34)=2.7, p=0.06; for mGluR1a: F(3,34)=8.1, p<0.0001]. Data in b represent the mean ± SEM of 7–9 animals/time-point. *p<0.05 vs. water control.
Homer2 over-expression enhances instrumental responding for alcohol
Next, we examined the consequences of NAC Homer2 over-expression by assessing for alcohol-induced changes in behavior and neurochemistry in the alcohol-preferring B6 mouse following transfection of NAC neurons with an AAV carrying Homer2b cDNA (Homer2) or green fluorescent protein (GFP) control. As in our earlier AAV studies (Lominac et al. 2005; Szumlinski et al. 2004; Szumlinski et al. 2005b; Szumlinski et al. 2006a), fluorescent immunocytochemical labeling of the HA tag revealed neuronal transfection within the NAC that was restricted to 1–1.5 mm of the injection site (Fig 2a–a″).
Figure 2. AAV transfection of NAC GABAergic neurons in B6 mice.
a, Cellular transfection by AAV-GFP at 4.5 weeks after infusion into the NAC. ac=anterior commisure. a’ and a”, High-power micrographs showing cellular transfection within 0.5–1.5 mm from the injection site (arrow in a). b, Schematic of the placements of the active membrane of the microdialysis probes within the NAC of the mice in the neurochemical study. The probes were localized mostly, but not exclusively, within the shell region of the NAC. Solid symbols denote placements of AAV-Homer2 animals, open symbols denote placements of AAV-GFP animals.
To assess the functional importance of the alcohol drinking-induced rise in NAC Homer2 levels vis-à-vis the motivational drive for alcohol, we employed an operant self-administration paradigm to compare the alcohol dose-effect functions for lever-pressing and intake between B6 mice infused intra-NAC with an adeno-associated virus (AAV) carrying GFP or Homer2b. As alcohol consumption depends upon internal drive states, alcohol dose-effect functions were established under both post-prandial (high thirst/low hunger) and pre-prandial (low thirst/high hunger) conditions (Middaugh and Kelley 1999; Middaugh et al. 1999). The data from this experiment are summarized in Fig. 3. NAC Homer2 over-expression in B6 mice increased both appetitive and consummatory aspects of alcohol reinforcement under response-contingent conditions. The effect of Homer2 over-expression was independent of internal drive state, as the increase in lever-pressing for and intake of alcohol was apparent under both pre- and post-prandial conditions. Moreover, the facilitation of instrumental responding and alcohol intake by Homer2 over-expression was observed only during responding for the two highest concentrations tested. Consistent with the large differences in alcohol intake between Homer2- and GFP-infused mice, the blood alcohol levels attained following the last pre-prandial session in which animals were responding for 12% alcohol revealed an almost 2-fold increase in alcohol concentrations in mice infused with AAV-Homer2 (Homer2: 181.9 ± 9.3 mg%; GFP: 108.5 ± 7.1 mg%; t25=6.26, p<0.0001). Thus, elevating NAC Homer2 levels enhances the reinforcing properties of higher alcohol concentrations in mice genetically predisposed to high alcohol intake.
Figure 3. NAC Homer2 over-expression increases appetitive behavior for and the consumption of higher concentrations of alcohol.
An intra-NAC infusion of AAV-Homer2b increased both consummatory and appetitive aspects of alcohol reward, independent of internal drive state (for both lever pressing and intake: AAV effect: p<0.002; AAV X Condition: p>0.05). Moreover, the facilitatory effect of Homer2 over-expression was most apparent at the highest alcohol concentrations tested (for both variables: AAV X Concentration: p<0.007; AAV X Concentration X Condition, p>0.05). AAV-Homer2b infusion increased lever-pressing for alcohol when assessed under both (a) post-prandial conditions [AAV effect: F(1,28)=4.14, p=0.05] and (b) pre-prandial conditions [Concentration X AAV: F(3,75)=2.8, p=0.05]. AAV-Homer2b infusion also increased alcohol consumption under both (c) post-prandial conditions [Concentration X AAV: F(2,56)=12.0, p<0.00001] and (d) pre-prandial conditions [Concentration X AAV: F(2,50)=7.6, p=0.001]. Data represent the mean ± SEM of the number of animals indicated in parentheses. *p<0.05 vs. AAV-GFP.
Homer2 over-expression facilitates the development of an alcohol-induced place preference
NAC Homer2 actively regulates the development of an alcohol-conditioned place-aversion (Szumlinski et al. 2005b). Given our above data for alcohol reinforcement, we next assessed the effects of NAC Homer2 over-expression upon alcohol-conditioned reward in B6 mice. An unbiased place-conditioning procedure was employed in which 2 g/kg alcohol was paired 8 times with a distinct compartment of a 2-compartment apparatus (Lominac et al. 2006). The data are summarized in Fig. 4. Prior to conditioning, the time spent in the alcohol-paired and -unpaired sides did not differ for either AAV treatment groups [Side effect: p=0.68; AAV X Side: p=0.33; data not shown]. While the repeated pairing of 2 g/kg alcohol was insufficient to alter the motivational valence of either compartment in GFP controls, AAV-Homer2b mice exhibited a significant increase in the time spent in the alcohol-paired side, relative to the -unpaired side when the mice were tested in an alcohol-free state. Thus, NAC Homer2 over-expression also facilitates the development of alcohol-conditioned reward in B6 mice.
Figure 4. NAC Homer2 over-expression facilitates the development of an alcohol-conditioned place-preference.
Eight repeated pairings of 2 g/kg with a distinct compartment in a two-compartment place-conditioning apparatus resulted in a conditioned place-preference in mice over-expressing Homer2b in the NAC, but in GFP controls [Side effect: F(1,16)=2.39, p=0.14; AAV X Side: F(1,16)=3.96, p=0.05]. Data represent the mean time spent in the alcohol-paired and –unpaired compartments when the mice were tested in an alcohol-free state ± SEM of the number of animals indicated in parentheses. *p<0.05 vs. Unpaired side; +p<0.05 vs. AAV-GFP.
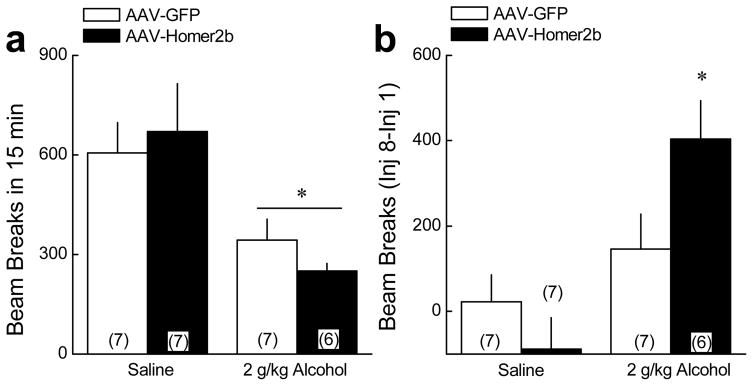
Homer2 over-expression facilitates the development of tolerance to alcohol-induced motor inhibition
NAC Homer2 actively regulates the development of tolerance to the motor-inhibitory effects of higher alcohol doses (Szumlinski et al. 2005b). Thus, we next examined the effects of NAC Homer2 over-expression upon the changes in locomotor activity produced by the repeated administration of 2 g/kg alcohol. The data summarizing the effects of NAC Homer2 over-expression upon alcohol-induced locomotion are summarized in Fig. 5. Consistent with the findings of earlier AAV studies (Szumlinski et al. 2004; Szumlinski et al. 2005b), an acute injection of 2 g/kg alcohol lowered the locomotor activity of AAV-GFP and AAV-Homer2b mice to a similar extent, compared to saline-injected controls (Fig. 5a). Thus, NAC Homer2 over-expression did not affect the locomotor inhibition produced by an acute injection of 2 g/kg alcohol. However, an examination of the change in locomotion from injection 1 to 8 of repeated treatment revealed a significant increase in the locomotor response to 2 g/kg alcohol only in Homer2-infused mice but no group differences were observed for the change in locomotion produced by repearted saline administration (Fig. 5b). Thus, NAC Homer2 over-expression facilitated the development of tolerance to the locomotor-inhibitory effects of a moderate dose of alcohol.
Figure 5. NAC Homer2 over-expression facilitates the development of tolerance to alcohol-induced motor impairment.
a, NAC Homer2 over-expression did not alter the acute locomotor-inhibitory effect of 2 g/kg alcohol [Dose effect: F(1,26)=16.44, p<0.0001; AAV X Dose: p=0.15]. b, NAC Homer2 over-expression facilitated the development of tolerance to alcohol’s locomotor-inhibitory effects upon repeated alcohol administration (8 × 2 g/kg) [Dose effect: F(1,26)=10.57, p=0.004; Dose X AAV: F(1,26)=4.80, p=0.05]. Data represent the mean ± SEM of the number of animals indicated in parentheses. *p<0.05 vs. saline.
Homer2 over-expression facilitates the development of alcohol-induced neurochemical sensitization
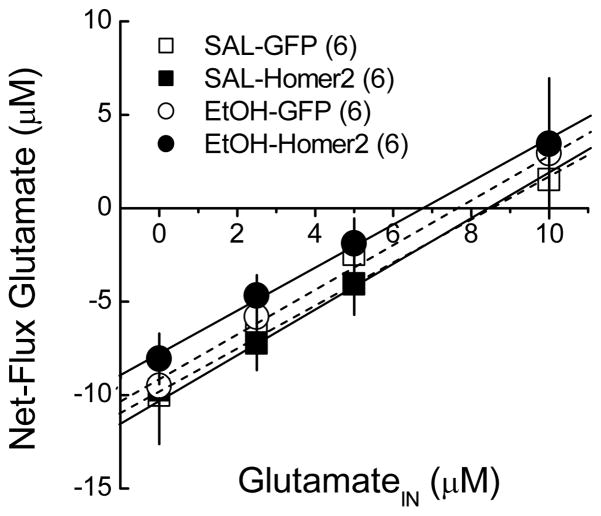
Homer2 expression both maintains NAC basal extracellular glutamate levels (Szumlinski et al. 2004; Szumlinski et al. 2005b; Szumlinski et al. 2006a) and regulates the increase in extracellular glutamate and dopamine produced by repeated alcohol administration (Szumlinski et al. 2005b). Thus, in vivo microdialysis was conducted in the NAC to assess the neurochemical correlates of the facilitated alcohol-induced behavioral adaptation produced by NAC Homer2 over-expression and these data are summarized in Fig. 6 and Table 1. Neither NAC Homer2 over-expression nor repeated alcohol administration (8 × 2 g/kg, IP, every other day) affected the basal extracellular levels of any of the neurotransmitters examined (Table 1) (no main effects of, or interactions with the AAV factor, p>0.05). As illustrated in Fig. 7, the lack of effects of alcohol and AAV infusion were supported by the results of the no-net flux in vivo microdialysis study, which indicated equivalent basal glutamate content (y=0) and probe recovery (slope of the linear regression plots) between the four treatment groups (p>0.05). NAC Homer2 over-expression facilitated a rise in NAC glutamate following an acute injection of 2 g/kg alcohol, which resembled that of repeated alcohol-injected GFP control mice (compare Fig 6a & 6b). Moreover, NAC Homer2 over-expression augmented the alcohol-sensitized glutamate response in repeated alcohol-injected animals (Fig. 6b). While not as robust as the effects upon NAC glutamate, NAC Homer2 over-expression also elicited a moderate rise in NAC dopamine following acute alcohol, which was less robust in GFP controls (Fig 6c) and augmented the alcohol-sensitized dopamine response in repeated alcohol-injected mice (Fig. 6d). In contrast to both glutamate and dopamine, NAC GABA levels were unaffected by either alcohol administration or AAV infusion (Fig. 6e & 6f).
Table 1.
Comparison of the average basal extracellular levels of glutamate, GABA and dopamine in the nucleus accumbens (± SEM) of AAV-infused mice treated repeatedly with saline or 2 g/kg alcohol, as determined using conventional in vivo microdialysis techniques. Statistical analysis of the data failed to indicate significant effects of AAV infusion, repeated treatment or an interaction between these factors (p>0.05) for any of the neurotransmitters examined. Sample sizes are indicated in parentheses.
| Repeated Treatment | Glutamate (ng/sample) | GABA (pg/sample) | Dopamine (pg/sample) | |||
|---|---|---|---|---|---|---|
| GFP | Homer2 | GFP | Homer2 | GFP | Homer2 | |
| Saline | 1.3 ± 0.4 (6) | 1.9 ± 0.2 (9) | 2.4 ± 0.06 (6) | 2.9 ± 0.1 (9) | 2.7 ± 0.5 (7) | 2.0 ± 0.3 (8) |
| Alcohol | 1.7 ± 0.1 (7) | 1.6 ± 0.1 (8) | 2.1 ± 0.4 (7) | 2.3 ± 0.08 (8) | 2.5 ± 0.4 (10) | 2.8 ± 0.3 (7) |
Figure 7. NAC Homer2 over-expression or repeated alcohol administration does not alter NAC basal glutamate content.
Forty-eight hours following repeated saline/alcohol administration (8 × 2 g/kg), we failed to detect significant group differences in y=0 or for the slopes of the linear regressions of the plots [no main effects of, or interaction between the AAV and EtOH factors, p>0.05]. The data represent the mean ± SEM of 6 animals/group.
Discussion
The present report provides the first evidence that chronic alcohol consumption induces a large and persistent increase in NAC Homer2a/b protein expression that was accompanied by an enduring, albeit less persistent, up-regulation in mGluR1a and NR2b (Fig. 1). Consistent with a critical role for Homer2 isoforms in alcohol-induced neuroplasticity (Szumlinski et al. 2005b), mimicking the alcohol-induced rise in NAC Homer2b expression via virus-mediated gene delivery to NAC neurons of C57BL/6J (B6) mice (Fig. 2) increased both the appetitive and consummatory aspects of alcohol reward (Fig. 3, Fig. 4) and facilitated the development of tolerance to alcohol’s sedative effects following repeated alcohol administration (Fig. 5). Alcohol-induced alterations in NAC dopamine and glutamate neurotransmission are highly implicated in the development of alcohol dependence and addiction (for reviews, Carpenter-Hyland and Chandler 2007; Chandler 2003; Chandler et al. 1998; Murphy et al. 2002; Krystal et al. 2003; Koob 2003; Tupula and Tiihonen 2004). Consistent with this, the “pro-alcoholic” behavioral phenotype induced by NAC Homer2 over-expression was accompanied by an augmentation in the NAC dopamine and glutamate responses to acute and repeated alcohol (Fig. 6), but no effect upon basal neurotransmitter content (Table 1; Fig. 7). Thus, an up-regulation in NAC mGluR-Homer2b-NMDA signaling is an important cellular adaptation to alcohol that promotes the neuroplasticity underlying motivational drive for alcohol and the development of other alcoholism-related behaviors.
Alcohol exposure augments Homer2b and glutamate receptor expression in the NAC
Alcohol acts as an allosteric inhibitor of a number of glutamate receptors, including NMDA receptors and mGluRs (Lovinger 1996; Minami et al. 1998). Moreover, the hyper-excitable state observed during early withdrawal from alcohol is attributed to a rebound up-regulation in glutamate receptor expression and their trafficking to the postsynaptic density (Carpenter-Hyland and Chandler 2006; Carpenter-Hyland et al. 2004; Chandler 2003; Chandler et al. 1999; Chandler et al. 2006; Hendricson et al. 2007; Trevisan et al. 1994). Our data are consistent with other reports for alcohol demonstrating an up-regulation in the expression of Group1 mGluRs and NMDA receptors during either short- or longer-term withdrawal from various alcohol treatment regimens (e.g., Carpenter-Hyland and Chandler 2006; Carpenter-Hyland et al. 2004; Chandler et al. 1999; Hendricson et al. 2007; Qiang and Ticku 2000; Shella Rani and Ticku 2006; Simonyi et al. 1996; Simonyi et al. 2004; Sircar and Sircar 2006; Trevison et al. 1994). Moreover, our present data are consistent with the preliminary results of other immunoblotting studies in our laboratory demonstrating an up-regulation in NAC Homer2a/b/NR2 expression following repeated bouts of binge alcohol drinking (Szumlinski et al. 2006b) and an injection number-dependent increase in NAC Homer2a/b/Group1 mGluR/NR2 expression at 24 hrs following intraperitoneal alcohol injections (Szumlinski 2006). Further study is required to determine whether or not the parallel up-regulation of Homer2a/b, mGluR1 and NR2b by alcohol results directly from drug-induced inhibition of these receptors. However, in support of this suggestion, the application of the selective NMDA antagonist 2-amino-5-phosphonate (AP-V) to developing hippocampal neuronal cultures increases significantly the amount of Homer-glutamate receptor co-clustering within the postsynaptic density (Shiraishi et al. 2003). Thus, glutamate receptor inhibition by alcohol during chronic alcohol consumption may serve as a pharmacological trigger to increase the formation of, and signaling through, Homer-containing multi-protein complexes within the postsynaptic density (Chandler et al. 2006; Szumlinski et al. 2006c).
Converging behavioral genetics data derived from both human and animal studies implicate Homer gene products in addiction vulnerability to a variety of substances (for review, Szumlinski et al. 2006c). Yet, our understanding of how drugs of abuse regulate the expression of different Homer proteins and their interacting partners in brain and the functional consequences of this regulation is very limited. While Homer1a mRNA is up-regulated within the NAC following acute cocaine (Brakeman et al. 1997), withdrawal from repeated cocaine injections produces a time-dependent down-regulation in NAC Homer1b/c and mGluR5 protein expression, that coincides with a reduction in Group1 mGluR function (Swanson et al. 2001). Supporting earlier evidence that Homer gene products regulate the expression of glutamate receptors in vivo (Ghasemzadeh et al. 2003; Szumlinski et al. 2004; Szumlinski et al. 2005b), an alcohol-induced increase in NAC Homer2a/b expression was paralleled by elevations in changes in mGluR1 and NR2b levels. Whether or not the opposite effects of repeated cocaine and repeated alcohol upon constitutive Homer protein expression are due to pharmacological properties of the drugs or relate to a number of procedural differences between the studies, including: the species employed (rat vs. mouse), control over drug administration (experimenter-administered vs. self-controlled), as well as, the duration and timing of drug exposure (once daily for 1 week vs. continuous access for 3 months), cannot be discerned at the present time.
Accumbens Homer2b up-regulation promotes alcohol reward
Converging behavioral genetic and pharmacological evidence implicates signaling through mGluR-Homer-NDMA complexes in the development of alcoholism-related behaviors (Smothers et al. 2005; Szumlinski et al. 2005b; Szumlinski et al. 2006a; Szumlinski et al. 2006b; Urizar et al. 2007; present data). Deletion of Homer2 (Szumlinski et al. 2005b) or pharmacological blockade of either Group1 mGluRs (e.g., Backstrom et al. 2004; Backstrom and Hyytia 2007; Hodge et al. 2006; Lominac et al. 2006; Schroeder et al. 2005) or NMDA receptors (e.g., Boyce-Rustay et al. 2004; for reviews, Chandler 2003; Chandler et al. 1998; Hoffman 2003; Krystal et al. 2003) reduces various aspects of alcohol reward in laboratory animals. Furthering an active and important role for Homer2 proteins in the behavioral effects of alcohol, an intra-NAC infusion of AAV-Homer2b reverses the alcohol-avoiding phenotype of Homer2 KO mice (Szumlinski et al. 2005b). Moreover, mimicking alcohol’s effect upon NAC Homer2a/b expression in B6 mice via an intra-NAC infusion of AAV-Homer2b shifts the dose-response functions for alcohol preference, for alcohol reinforcement and for alcohol intake under response-contingent conditions up and to the left of controls (Szumlinski et al. 2005b; present study). These data indicate that NAC Homer2b over-expression is sufficient to increase alcohol’s potency and efficacy to elicit reward, a finding supported by our observation that a moderate dose of alcohol elicited a significant conditioned place-preference only in mice over-expressing Homer2b (Fig. 4). This facilitation of alcohol-induced changes in reward-related behavior is consistent with the results of numerous studies demonstrating an increase in the rewarding or reinforcing effects of alcohol following withdrawal from repeated or chronic alcohol administration (e.g., Lopez and Becker 2005; Melendez et al. 2006; Roberts et al. 2000; Valdez et al. 2002; for reviews, Rodd et al. 2004; Spanagel 2000) and indicate that an alcohol-induced increase in NAC Homer2b expression is sufficient to “pre-sensitize” B6 mice to alcohol’s rewarding and reinforcing properties.
The effects of manipulating Homer expression upon alcohol reward do not likely reflect changes in general reward mechanisms as little evidence supports a role for Homer2 isoforms in regulating food or water reward. Homer2 deletion does not affect sucrose or water reinforcement (Szumlinski et al. 2004; Fig. 3), nor does it affect intake of food, body weight regulation, water or a saccharin solution under response-independent conditions (Szumlinski et al. 2004; 2005b). Homer2 deletion also does not affect the magnitude of a food-conditioned place-preference induced under pre-prandial conditions (Szumlinski et al. 2005a). Thus, while the effects of NAC Homer2b over-expression upon alcohol reinforcement appeared to be greater when the animals were tested under pre-prandial conditions (Fig. 3), this interaction does not appear to depend on direct effects of NAC Homer2b manipulation upon the neural mechanisms mediating homeostatic motivation (i.e., food/thirst regulation).
An inverse relationship exists between the rewarding and motor-impairing effects of alcohol in both humans and laboratory animals and the development of tolerance to alcohol’s aversive, motor-impairing effects is theorized to underlie enhanced alcohol intake upon repeated alcohol exposure (c.f., Gauvin et al. 2000; Schukit and Smith 2000; Tabakoff and Hoffman 1988). Consistent with this theory, the alcohol aversion exhibited by Homer2 KO mice is accompanied by alcohol-intolerance - a phenotype that can also be “rescued” by an intra-NAC AAV-Homer2b infusion (Szumlinski et al. 2005b). This latter finding for tolerance in Homer2 KO mice is very much consistent with recent observations that Drosophila with null mutations in D. Homer, a gene encoding a single Homer protein that is highly homologous in both structure and function to mammalian Homer1b/c and Homer2a/b proteins (Diagana et al. 2002), exhibit increased sensitivity to the sedative effect of acute alcohol and fail to exhibit rapid tolerance upon a subsequent alcohol exposure (Urizar et al. 2007). Moreover, the alcohol phenotype of D. Homer mutants can be reversed by both pan-neuronal expression of WT Homer and by selective expression of WT Homer within a subset of neurons that include the ellipsoid body (Urizar et al. 2007). Thus, in both mammalian and non-mammalian species, Homers regulate acute behavioral sensitivity to alcohol and are required for the development of tolerance.
Furthering the notion that NAC Homer2b over-expression elicits a behavioral phenotype similar to that produced by repeated alcohol experience (e.g., Crabbe et al. 1981; Crabbe et al. 1982; Kalant et al. 1978; LeBlanc et al. 1969; Phillips et al. 1991; Tabakoff and Culp 1984; Tabakoff et al. 1980), tolerance developed to alcohol’s motor-impairing effects following the repeated administration of a moderate dose of alcohol only in mice over-expressing Homer2b (Fig. 5). While increases and decreases, respectively in NAC Homer2b over-expression appears to be sufficient to promote and prevent tolerance to alcohol’s motor-impairing effects (Szumlinski et al. 2005b; Fig. 5), recent evidence implicates both mGluR1 and NMDA receptors in regulating alcohol-induced sedation and motor sensitization (e.g., Kotlinska et al. 2006; Lominac et al. 2006). While we have yet to assess the effects of an acute bout of alcohol drinking upon mGluR/Homer2/NMDA expression, an acute injection of 3 g/kg alcohol is sufficient to elevate NAC Homer2a/b, Group1 mGluR and NR2 levels (Szumlinski 2006) and the repeated administration of this dose, which supports place-conditioning and induces tolerance to alcohol-induced motor-inhibition (e.g., Lominac et al. 2006; Szumlinski et al. 2005b), enhances alcohol’s effect upon Homer2a/b expression. Thus, we propose that a sensitization of signaling through mGluR-Homer-NMDA complexes is a neuroadaptation to alcohol that promotes the development of tolerance and heightens the rewarding properties of this drug. As the effect of chronic alcohol consumption upon mGluR1/Homer2a/b/NR2b expression is enduring (Fig. 1), this neuroadaptation may underlie the development of excessive alcohol intake, a defining feature of alcoholism, as well as contribute to the chronic, relapsing nature of this disease.
Accumbens Homer2b up-regulation increases NAC neurochemical responsiveness to alcohol
Alcohol consumption, under either continuous or scheduled access conditions, enhances NAC levels of dopamine (e.g., De Montis et al. 2004; Doyon et al. 2003; Doyon et al. 2004; Doyon et al. 2006; Gonzales and Weiss 1998; Melendez et al. 2002; Middaugh et al. 2003; Szumlinski et al. 2007; Weiss et al. 1993) and sensitizes the capacity of alcohol to elevate NAC glutamate (Szumlinski et al. 2007). While neither Homer1 nor Homer2 proteins appear to be necessary for the regulation of NAC basal dopamine content, deletion of Homer1 or Homer2 produces a number of glutamatergic abnormalities within this region that have been implicated in regulating sensitivity to several drugs of abuse (Szumlinski et al. 2006c). Earlier phenotyping of the Homer2 KO mouse revealed a necessary role for Homer2 gene products in regulating NAC alcohol-induced dopamine and glutamate sensitization (Szumlinski et al. 2005b). Moreover, viral transfection of the NAC with Homer2b rescues the neurochemical hypo-responsiveness of Homer2 KO mice, demonstrating an active role for this Homer2 gene product in regulating these alcohol-induced neurochemical adaptations within the NAC. Furthering this role, NAC Homer2b over-expression in B6 mice is sufficient to enhance the dopamine and glutamate response toacute alcohol and to augment alcohol-induced neurochemical sensitization (Fig. 6). Thus, as observed for behavior, NAC Homer2b over-expression “pre-sensitizes” the capacity of alcohol to elevate NAC levels of dopamine and glutamate in B6 mice – a neurochemical phenotype akin to that produced by repeated alcohol experience (e.g., Doyon et al. 2005; Melendez et al. 2002; Middaugh et al. 2003; Szumlinski et al. 2006a; Szumlinski et al. 2007; Weiss et al. 1993; but see Zapata et al. 2006).
Whether or not the alcohol “pre-sensitized” neurochemical phenotype produced by NAC Homer2b over-expression relates to an up-regulation in mGluR-Homer2-NMDA signaling cannot be discerned from the present study. However, little data exists to support the regulation of alcohol-induced changes in NAC neurotransmitter levels by NMDA receptors (Ericson et al. 2003; Gonzales and Roper 1993) and both mGluR1 and mGluR5 are necessary for the rise in NAC dopamine and glutamate producecd by an acute alcohol injection (Lominac et al. 2006). As Group1 mGluR antagonists are effective at blocking various aspects of alcohol reward in rodents (e.g., Backstrom et al. 2004; Backstrom and Hyytia 2007; Hodge et al. 2006; Lominac et al. 2006; McMillen et al. 2005; Olive et al. 2005; Schroeder et al. 2005) and can alter sensitivity to the motor effects of alcohol (Lominac et al. 2006), we propose alcohol-induced increases in Group1 mGluR signaling through Homer2 as a key substrate mediating alcohol-induced neurochemical sensitization within the NAC that underlies the development of alcohol-induced behavioral plasticity relevant to alcoholism.
Conclusions
Mounting preclinical evidence indicate members of the Homer protein family of postsynaptic scaffolding proteins as important cellular regulators of vulnerability to addiction-related neuroplasticity within the mesolimbic motive circuit (Szumlinski et al. 2006c). The present study demonstrates that chronic alcohol consumption in alcohol-preferring B6 mice produces a large and persistent increase in Homer2 expression within the NAC that was accompanied by shorter-lasting increases in mGluR1 and NR2b expression. Mimicking the alcohol-induced rise in NAC Homer2 levels enhanced or facilitated behavioral responsiveness to alcohol in a variety of paradigms and augmented the capacity of acute and repeated alcohol to elevate NAC levels of dopamine and glutamate. Thus, an increase in mGluR-Homer2-NMDA signaling may be a cellular adaptation to alcohol that promotes dopamine and glutamate neurotransmission within the NAC and drives a “pro-alcoholic” behavioral phenotype.
Table 2.
Comparison of the effects of manipulating Homer2 expression upon measures related to alcohol-induced neuroplasticity. The data provided in this table summarizes the results of the present study and those reported in Szumlinski et al. (2005b).
| Measure | Homer2 KO | NAC Homer2b over-expression in B6-129 hybrid mice | NAC Homer2b over-expression in inbred B6 mice |
|---|---|---|---|
| Alcohol preference | ↓* | ↑ | ↑ |
| Alcohol intake in home cage | ↓* | — | — |
| Alcohol reinforcement | NA | NA | ↑ |
| Place-preference upon repeated alcohol injection | ↓* | — | ↑ |
| Acute alcohol-induced sedation or motor-impairment | ↑ | — | — |
| Locomotor tolerance/sensitization upon repeated alcohol injection | ↓* | — | ↑ |
| Acute alcohol-induced rise in NAC dopamine and Glutamate | — | — | ↑ |
| NAC dopamine and glutamate sensitization upon repeated alcohol injection | ↓* | — | ↑ |
↑ indicates increase relative to control condition;
↓ indicates decrease relative to control condition;
— indicates no difference from control condition;
indicates reversed by intra-NAC AAV-Homer2b infusion; NA indicates not assessed.
Acknowledgments
The authors would like to thank Ms. Valerie Aguilar for her technical assistance with the immunostaining. The authors would also like to thank Dr. Paul F. Worley and his laboratory (Johns Hopkins University School of Medicine) for the Homer primary antibodies and Dr. Lawrence D. Middaugh and his laboratory (Medical University of South Carolina) for the BEL assays. This work was supported by NIAAA grants AA-015351, AA-0135017 (INIA West) and AA-016650 (INIA West) to KKS and by an AMBRF grant to TEK.
Footnotes
Disclosure/Conflict of Interest
- Karen K. Szumlinski: Department of Psychology and The Neuroscience Research Institute, University of California at Santa Barbara
- Alexis W. Ary: Department of Psychology and The Neuroscience Research Institute, University of California at Santa Barbara
- Kevin D. Lominac: Department of Psychology and The Neuroscience Research Institute, University of California at Santa Barbara
- Matthias Klugmann: Interdisciplinary Center for Neurosciences (IZN), University of Heidelberg
- Tod E. Kippin: Department of Psychology and The Neuroscience Research Institute, University of California at Santa Barbara
References
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2007 doi: 10.1007/s00213-007-0753-8. Epub ahead of press. [DOI] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:221–223. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol Biochem Behav. 2007;86:200–208. doi: 10.1016/j.pbb.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Homeostatic plasticity during alcohol exposure promotes enlargement of dendritic spines. Eur J Neurosci. 2006;24:3496–3506. doi: 10.1111/j.1460-9568.2006.05247.x. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ. Ethanol and brain plasticity: receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol Ther. 2003;99(3):311–326. doi: 10.1016/s0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Carpenter-Hyland E, Hendricson AW, Maldve RE, Morrisett RA, Zhou FC, Sari Y, Bell R, Szumlinski KK. Structural and functional modifications in glutamateric synapses following prolonged ethanol exposure. Alcohol Clin Exp Res. 2006;30(2):368–376. doi: 10.1097/01.ALC.0000167959.84516.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ, Harris RA, Crews FT. Ethanol tolerance and synaptic plasticity. Trends Pharmacol Sci. 1998;19:491–495. doi: 10.1016/s0165-6147(98)01268-1. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Norwood D, Sutton G. Chronic ethanol upregulates NMDA and AMPA, but not kainate receptor subunit proteins in rat primary cortical cultures. Alcohol Clin Exp Res. 1999;23:363–370. [PubMed] [Google Scholar]
- Crabbe JC, Gray DK, Young ER, Janowsky JS, Rigter H. Initial sensitivity and tolerance to ethanol in mice: correlations among open field activity, hypothermia, and loss of righting reflex. Behav Neural Biol. 1981;33:188–203. doi: 10.1016/s0163-1047(81)91625-3. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Jr, Johnson NA, Gray DK, Kosobud A, Young ER. Biphasic effects of ethanol on open-field activity: sensitivity and tolerance in C57BL/6N and DBA/2N mice. J Comp Physiol Psychol. 1982;96:440–451. doi: 10.1037/h0077898. [DOI] [PubMed] [Google Scholar]
- de Bartolomeis A, Iasevoli F. The Homer family and the signal transduction system at glutamatergic postsynaptic density: potential role in behavior and pharmacotherapy. Psychopharmacol Bull. 2003;37:51–83. [PubMed] [Google Scholar]
- De Montis MG, Grappi S, Gambarana C, Leggio B, Nanni G, Scheggi S, Tagliamonte A. Sardinian alcohol-preferring rats show low 5-HT extraneuronal levels in the mPFC and no habituation in monoaminergic response to repeated ethanol consumption in the NAcS. Brain Res. 2004;1006:18–27. doi: 10.1016/j.brainres.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem. 2005;93:1469–1481. doi: 10.1111/j.1471-4159.2005.03137.x. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Howard EC, Shippenberg TS, Gonzales RA. Kappa-opioid receptor modulation of accumbal dopamine concentration during operant ethanol self-administration. Neuropharmacology. 2006;51:487–496. doi: 10.1016/j.neuropharm.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, Ramachandra V, Samson HH, Czachowski CL, Gonzales RA. Accumbal dopamine concentration during operant self-administration of a sucrose or a novel sucrose with ethanol solution. Alcohol. 2004;34:261–271. doi: 10.1016/j.alcohol.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral alcohol self-administration. Alcohol Clin Exp Res. 2003;27:1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Duncan RS, Hwang SY, Koulen P. Effects of Vesl/Homer proteins on intracellular signaling. Exp Biol Med. 2005;230:527–535. doi: 10.1177/153537020523000803. [DOI] [PubMed] [Google Scholar]
- Ericson M, Molander A, Lof E, Engel JA, Soderpalm B. Ethanol elevates accumbal dopamine levels via indirect activation of ventral tegmental nicotinic acetylcholine receptors. Eur J Pharmacol. 2003;467(1–3):85–93. doi: 10.1016/s0014-2999(03)01564-4. [DOI] [PubMed] [Google Scholar]
- Fagni L, Worley PF, Ango F. Homer as both a scaffold and transduction molecule. Science STKE. 2002;137:RE8. doi: 10.1126/stke.2002.137.re8. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Gauvin DV, Baird TJ, Briscoe RJ. Differential development of behavioral tolerance and the subsequent hedonic effects of alcohol in AA and ANA rats. Psychopharmacology. 2000;151:335–343. doi: 10.1007/s002130000477. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Permenter LK, Lake R, Worley PF, Kalivas PW. Homer1 proteins and AMPA receptors modulate cocaine-induced behavioural plasticity. Eur J Neurosci. 2003;18:1645–1651. doi: 10.1046/j.1460-9568.2003.02880.x. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Roper LC. Ethanol effects of NMDA-stimulated levels of extracellular neurotransmitters by in vivo microdialysis. Alcohol Alcohol Suppl. 1993;2:371–376. [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricson AW, Maldve RE, Salinas AG, Theile JW, Zhang TA, Diaz LM, Morrisett RA. Aberrant synaptic activation of NMDA receptors underlies ethanol withdrawal hyperexcitability. J Pharmacol Exp Ther. 2007 doi: 10.1124/jpet.106.111419. print copy in press (originally published online Jan 17, 2007, at jpet.aspetjournals.org/cgi/content/abstract/jpet.106.111419v1) [DOI] [PubMed]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology. 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalant H, LeBlanc AE, Gibbins RJ, Wilson A. Accelerated development of tolerance during repeated cycles of ethanol exposure. Psychopharmacology. 1978;60:59–65. doi: 10.1007/BF00429180. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27(2):232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Bochenski M, Danysz W. N-methyl-D-aspartate and group I metabotropic glutamate receptors are involved in the expression of ethanol-induced sensitization in mice. Behav Pharmacol. 2006;17(1):1–8. doi: 10.1097/01.fbp.0000181600.95405.c7. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Krupitsky E, Schutz C, Trevisan L, D’Souza DC. NMDA receptor antagonism and the ethanol intoxication signal: from alcoholism risk to pharmacotherapy. Ann N Y Acad Sci. 2003;1003:176–184. doi: 10.1196/annals.1300.010. [DOI] [PubMed] [Google Scholar]
- LeBlanc AE, Kalant H, Gibbins RJ, Berman ND. Acquisition and loss of tolerance to ethanol by the rat. J Pharmacol Exp Ther. 1969;168:244–250. [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Oleson EB, Pava M, Klugmann M, Schwarz MK, Seeburg PH, During MJ, Worley PF, Kalivas PW, Szumlinski KK. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J Neurosci. 2005;25:11586–11594. doi: 10.1523/JNEUROSCI.3764-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181:688–96. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Interactions between ethanol and agents that act on the NMDA-type glutamate receptor. Alcohol Clin Exp Res. 1996;20(Suppl):187A–191A. doi: 10.1111/j.1530-0277.1996.tb01773.x. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Crawford MS, Kulers CM, Williams HL. Effects of a metabotropic, mglu5, glutamate receptor antagonist on ethanol consumption by genetic drinking rats. Alcohol Alcohol. 2005;40(6):494–497. doi: 10.1093/alcalc/agh200. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW. Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:2017–2025. doi: 10.1111/j.1530-0277.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li TK, McBride WJ, Murphy JM. Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res. 2002;26:318–325. [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM. Operant ethanol reward in C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17(3):185–194. doi: 10.1016/s0741-8329(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Cuison ER, Jr, Groseclose CH. Naltrexone effects on ethanol reward and discrimination in C57BL/6 mice. Alcohol Clin Exp Res. 1999;23:456–464. [PubMed] [Google Scholar]
- Middaugh LD, Szumlinski KK, van Patten Y, Marlow A-L, Kalivas PW. Chronic ethanol consumption by C57BL/6 mice alters the behavioral and neurochemical effects of ethanol: blockade by naltrexone. Alcohol Clin Exp Res. 2003;27:1892–1900. doi: 10.1097/01.ALC.0000099264.36220.48. [DOI] [PubMed] [Google Scholar]
- Minami K, Gereau RW, 4, Minami M, Heinemann SF, Harris RA. Effects of ethanol and anesthetics on type 1 and 5 metabotropic glutamate receptors expressed in Xenopus oocytes. Mol Pharmacol. 1998;53:148–156. doi: 10.1124/mol.53.1.148. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32(5):363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Burkhart-Kasch S, Crabbe JC. Locomotor activity response to chronic ethanol treatment in selectively bred FAST and SLOW mice. Alcohol Alcohol. 1991;(Suppl 1):109–113. [PubMed] [Google Scholar]
- Qiang M, Ticku MK. Role of AP-1 in ethanol-induced N-methyl-D-aspartate receptor 2B subunit gene up-regulation in mouse cortical neurons. J Neurochem. 2005;95(5):1332–1341. doi: 10.1111/j.1471-4159.2005.03464.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology. 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. J Stud Alcohol. 2000;61:827–835. doi: 10.15288/jsa.2000.61.827. [DOI] [PubMed] [Google Scholar]
- Sheela Rani CS, Ticku MK. Comparison of chronic ethanol and chronic intermittent ethanol treatments on the expression of GABA(A) and NMDA receptor subunits. Alcohol. 2006;38(2):89–97. doi: 10.1016/j.alcohol.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Shin DM, Dehoff M, Luo X, Kang SH, Tu J, Nayak SK, Ross EM, Worley PF, Muallem S. Homer 2 tunes G protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCbeta GAP activities. J Cell Biol. 2003;162(2):293–303. doi: 10.1083/jcb.200210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi Y, Mizutani A, Bito H, Fujisawa K, Narumiya S, Mikoshiba K, Furuichi T. Cupidin, an isoform of Homer/Vesl, interacts with the actin cytoskeleton and activated rho family small GTPases and is expressed in developing mouse cerebellar granule cells. J Neurosci. 1999;19:8389–8400. doi: 10.1523/JNEUROSCI.19-19-08389.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi Y, Mizutani A, Mikoshiba K, Furuichi T. Coincidence in dendritic clustering and synaptic targeting of homer proteins and NMDA receptor complex proteins NR2B and PSD95 during development of cultured hippocampal neurons. Mol Cell Neurosci. 2003;22(2):188–201. doi: 10.1016/s1044-7431(03)00037-x. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107(1):80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Zhang JP, Sun AY, Sun GY. Chronic ethanol on mRNA levels of IP3R1, IP3 3-kinase and mGluR1 in mouse Purkinje neurons. Neuroreport. 1996;7:2115–2118. doi: 10.1097/00001756-199609020-00010. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Christian MR, Sun AY, Sun GY. Chronic ethanol-induced subtype- and subregion-specific decrease in the mRNA expression of metabotropic glutamate receptors in rat hippocampus. Alcohol Clin Exp Res. 2004;28:1419–1423. doi: 10.1097/01.alc.0000139825.35438.a4. [DOI] [PubMed] [Google Scholar]
- Sircar R, Sircar D. Repeated ethanol treatment in adolescent rats alters cortical NMDA receptor. Alcohol. 2006;39(1):51–58. doi: 10.1016/j.alcohol.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Smothers CT, Szumlinski KK, Worley PF, Kalivas PW, Woodward JJ. Synaptic function in mice lacking the Homer2 gene. Alcohol Clin Exp Res. 2005;29(Suppl):141A. [Google Scholar]
- Soloviev MM, Ciruela F, Chan WY, McIlhinney RA. Mouse brain and muscle tissues constitutively express high levels of Homer proteins. Eur J Biochem. 2000;267(3):634–639. doi: 10.1046/j.1432-1327.2000.01078.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Recent animal models of alcoholism. Alcohol Res Health. 2000;24:124–131. [PMC free article] [PubMed] [Google Scholar]
- Swanson C, Baker D, Carson D, Worley P, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: A potential role for Homer 1b/c. J Neuroscience. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK. Up, Up and Away! Facilitation of alcohol-induced neural plasticity by Homer over expression. Alcohol Clin Exp Ther. 2006;30(Suppl):60A. [Google Scholar]
- Szumlinski KK, Abernathy KE, Oleson EB, Klugmann M, Lominac KD, He D-Y, Ron D, During MT, Kalivas PW. Homer isoforms differentially regulate cocaine-induced neuroplasticity. Neuropsychopharmacology. 2006a;31:768–777. doi: 10.1038/sj.npp.1300890. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Rohrer J, Griffin W, III, Klugmann M, Toda S, Champtiaux NP, Berry T, Shealy S, Tu JC, During MT, Middaugh LD, Worley PF, Kalivas PW. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology. 2007;190:415–431. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Friedman J, Rahn A, Cozzoli D, Ary AW. Excessive alcohol consumption sensitizes glutamate transmission: Link to Homer proteins and kinase activation. Neuropsychopharmacology. 2006b;31:S140. [Google Scholar]
- Szumlinski KK, Kalivas PW, Worley PF. A possible role of Homer proteins in neuropsychiatric disorders. Curr Opin Neurobiol. 2006c;16:251–257. doi: 10.1016/j.conb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Kleschen M, Oleson EB, Dehoff M, Schwartz M, Seeberg P, Worley PF, Kalivas PW. Behavioural and neurochemical phenotyping of Homer1 mutant mice: Possible implications for schizophrenia. Genes Brain Behav. 2005a;4:273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During MT, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for ethanol-induced neuroplasticity. J Neurosci. 2005b;25:7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Culp SG. Studies on tolerance development in inbred and heterogeneous stock National Institutes of Health rats. Alcohol Clin Exp Res. 1984;8:495–499. doi: 10.1111/j.1530-0277.1984.tb05711.x. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. Tolerance and the etiology of alcoholism: hypothesis and mechanism. Alcohol Clin Exp Res. 1998;12:184–186. doi: 10.1111/j.1530-0277.1988.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Ritzmann RF, Raju TS, Deitrich RA. Characterization of acute and chronic tolerance in mice selected for inherent differences in sensitivity to ethanol. Alcohol Clin Exp Res. 1980;4:70–73. doi: 10.1111/j.1530-0277.1980.tb04794.x. [DOI] [PubMed] [Google Scholar]
- Trevisan L, Fitzgerald LW, Brose N, Gasic GP, Heinemann SF, Duman RS, Nestler EJ. Chronic ingestion of ethanol up-regulates NMDAR1 receptor subunit immunoreactivity in rat hippocampus. J Neurochem. 1994;62:1635–1638. doi: 10.1046/j.1471-4159.1994.62041635.x. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Urizar NL, Yang Z, Edenberg HJ, Davis RL. Drosophila homer is required in a small set of neurons including the ellipsoid body for normal ethanol sensitivity and tolerance. J Neurosci. 2007;27:4541–4551. doi: 10.1523/JNEUROSCI.0305-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, et al. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10(3):370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]