Abstract
Background
Effective methods for the treatment of reproductive dysfunction are limited. Previous studies have reported that acupuncture can modulate female hormone levels, improve menstrual disorders, alleviate depression and improve pregnancy rates. However, studies of acupuncture for diminished ovarian reserve (DOR) are lacking.
Objective
To carry out a prospective observational study aimed at assessing the effect of EA on the reproductive hormone levels of patients with DOR seeking fertility support and consider its safety.
Methods
Eligible patients with DOR received EA for 12 weeks: five times a week for 4 weeks followed by three times a week for 8 weeks. The primary outcome was the change in mean follicle-stimulating hormone (FSH) level at week 12. Mean luteinising hormone (LH) and serum oestradiol (E2) levels, FSH/LH ratios and symptom scale scores were simultaneously observed.
Results
Twenty-one patients with DOR were included in the final analysis. Mean FSH levels fell from 19.33±9.47 mIU/mL at baseline to 10.58±6.34 mIU/mL at week 12 and 11.25±6.68 mIU/mL at week 24. Change in mean FSH from baseline was −8.75±11.13 mIU/mL at week 12 (p=0.002) and −8.08±9.56 mIU/mL at week 24 (p=0.001). Mean E2 and LH levels, FSH/LH ratios and irritability scores were improved at weeks 12 and/or 24. Approximately 30% patients reported subjective increases in menstrual volume after treatment.
Conclusions
EA may modulate reproductive hormone levels and the effects seem to persist for at least 12 weeks after treatment with no significant side effects. EA may improve the ovarian reserve of patients with DOR, though further research is needed.
Trial registration number
NCT02229604; Results.
Keywords: COMPLEMENTARY MEDICINE, GYNAECOLOGY
Introduction
Diminished ovarian reserve (DOR) is a manifestation of ovarian ageing. As women increasingly delay childbearing, DOR is becoming a greater challenge for providers of assisted reproductive technology.1 Studies have shown that DOR is diagnosed in 10% of women undergoing investigation for subfertility.2 DOR may also have adverse implications for a woman's wellbeing beyond her reproductive concerns. Changes in ovarian hormones, concomitant with DOR, can cause accelerated bone turnover, low bone mineral density, sexual dissatisfaction and disturbed sleep.3–5 Involuntary childlessness can also cause significant distress.6
In Western countries, DOR is usually diagnosed in reproductive medicine centres. Effective methods for improving ovarian reserve are limited. Various treatments have been reported to improve ovarian response and pregnancy rates during in vitro fertilisation (IVF), but none is clearly better than the others.7 8 Dehydroepiandrosterone (DHEA) supplementation may improve pregnancy rates in women with DOR, but more evidence is required.9 In China, patients with DOR often choose alternative treatments, such as herbs and acupuncture. Acupuncture may help patients regain regular menses, alleviate depression and improve pregnancy rates and quality of life.10
To our knowledge, however, there are no published reports on the potential role of acupuncture specifically for DOR. There are, nonetheless, three published case series studies and one low-quality RCT of acupuncture for the related condition of primary ovarian insufficiency (POI). Two case studies found that EA decreased serum follicle-stimulating hormone (FSH) and luteinising hormone (LH) levels, increased serum oestradiol (E2) levels, relieved anxiety, reduced mental stress and improved menopausal symptoms,11 12 while the third simply reported that hormone levels were improved in 9 out of 15 patients without providing specific data.13 The single RCT14 randomised 151 patients to receive acupuncture (n=76) or clomiphene and diethylstilboestrol (n=75) for a total of 6 months, without explicitly disclosing the inclusion/exclusion criteria or methods of randomisation and allocation concealment. Menstrual symptoms, hot flushes and levels of FSH and E2 were improved at 6, 7 and 9 months. Although the conclusions of these studies of POI are encouraging, they are limited by poor design and cannot be directly extrapolated to DOR, which differs from POI in several respects.15
The aim of this prospective observational study was to investigate the effects of EA on markers of ovarian reserve in patients with DOR seeking fertility support and to consider its safety.
Methods
Study design
This was a prospective observational study conducted from January 2014 to August 2015 in the acupuncture department of Guang'anmen Hospital. The study was conducted in accordance with the Declaration of Helsinki and was approved by the review board and ethics committee of Guang'anmen Hospital (reference no. 2014EC097). It was also retrospectively registered in the National Institutes of Health clinical trials registry at https://clinicaltrials.gov (reference no. NCT02229604).
Participants
Patients with DOR were recruited by posters at our hospital and advertisements on the hospital website. The main criterion for inclusion was a diagnosis of DOR, defined as a twofold rise in FSH level (≥10 mIU/mL but <40 mIU/mL) in a woman under the age of 40.16 Patients were excluded if they (1) had undergone oopherectomy; (2) had a past history of cytotoxic chemotherapy or radiotherapy; (3) had a current infection or tumour of the genital tract; (4) had an autoimmune disease or (5) were amenorrhoeic due to reproductive abnormality or pregnancy. Patients were also excluded if they had taken any immunosuppressive agents in the past 6 months, had accepted hormone therapy/herbs in the preceding month or could not adhere to treatment for personal reasons. Written informed consent was provided by all participants.
Electroacupuncture
Standardised acupuncture treatment was provided using two alternating formulae: A (BL33) and B (ST25, Zigong and CV4). In formula A, stainless steel Huatuo brand needles (diameter 0.30 mm, length 40–75 mm; Suzhou Medical Appliances, Suzhou, Jiangsu Province, China) were inserted bilaterally into the third posterior sacral foramina (at BL33) and angled inwards and downwards at 30–45° to a depth of 50–60 mm. An EA stimulator (model GB6805-2, Huayi Medical Supply & Equipment Co Ltd, Shanghai, China) was used to deliver a dilatational wave (pulse width 0.5 ms) at 2/15 Hz frequency (alternating at 1.5 s intervals) and 0.1–1.0 mA intensity. In formula B, needles (same brand) were quickly inserted vertically through the skin bilaterally at ST25, Zigong and CV4. They were then slowly advanced vertically through the layer of fatty tissue into the muscles of the abdominal wall. The needle was stopped when there was resistance on its tip and the participant felt a sting. Needles at left and right Zigong and ST25, respectively, were paired for EA (using the same parameters). The two formulae were alternated. Each EA session lasted for 20 min and patients underwent five sessions a week for the first 4 weeks and three sessions a week thereafter (44 sessions in total over 12 weeks). If participants did not want to be needled during menstruation, treatment and outcome assessment were postponed slightly.
Outcome measures
The primary outcome was the change from baseline in the mean FSH level at week 12. Secondary outcomes included changes in FSH/LH ratio, mean LH, E2 and FSH levels, and a four-point symptom scale (measuring irritability and depression) at weeks 4, 8, 12 and 24 (0=not at all, 1=mild, 2=moderate, 3=severe). Patients scored themselves according to their own feelings.
The proportion of participants with a subjective improvement in menses at weeks 12 and 24 was simultaneously recorded. Patients reported both menstrual cycle length and blood loss, calculated based on the number of sanitary pads used and the estimated volume of blood on the pads. A native-brand sanitary pad was used, for which about 5 mL blood dyed half of the back side of the pad.
Participants underwent an initial 2-week baseline assessment of menstruation and measurement of symptom scale and serum hormone levels—namely, FSH, LH and E2, using ARCHITECT chemiluminescent microparticle immunoassay reagent kits (Abbott Diagnostics, Abbott Park, Illinois, USA). This was repeated immediately after EA treatment at 12 weeks. A further follow-up visit was performed in the clinic or by email/telephone (for those unable to reattend) at 24 weeks. As reproductive hormone levels vary throughout the menstrual cycle, hormone tests were carried out on day 2 or 3 of the cycle.
During treatment and follow-up, any adverse events and acupuncture-related side effects, such as fainting, haematoma, severe pain and local infection, were recorded.
Statistical analysis
SPSS V.13.0 (SPSS Inc, Chicago, Illinois, USA) was used for statistical analysis. The paired t test and Wilcoxon's signed rank test were used for continuous and categorical data, respectively, in order to compare outcomes before and after acupuncture treatment. All analyses were two-sided and the level of significance was set at p<0.05.
Results
From January 2014 to August 2015, 40 subfertile patients with DOR attending our clinic were screened. After excluding patients who were taking hormone therapy or DHEA, or who could not undergo acupuncture treatments on time, 31 eligible patients signed the informed consent form. Of these, six patients did not attend clinical visits on time and were contacted by phone. All six patients failed to undergo blood tests when scheduled (two in the treatment period and four in the follow-up period, with >1 month's delay) for personal reasons. An additional four patients withdrew from the study because they became pregnant (two by natural conception and two by IVF). Therefore, a total of 21 patients were included in the final analysis (figure 1).
Figure 1.
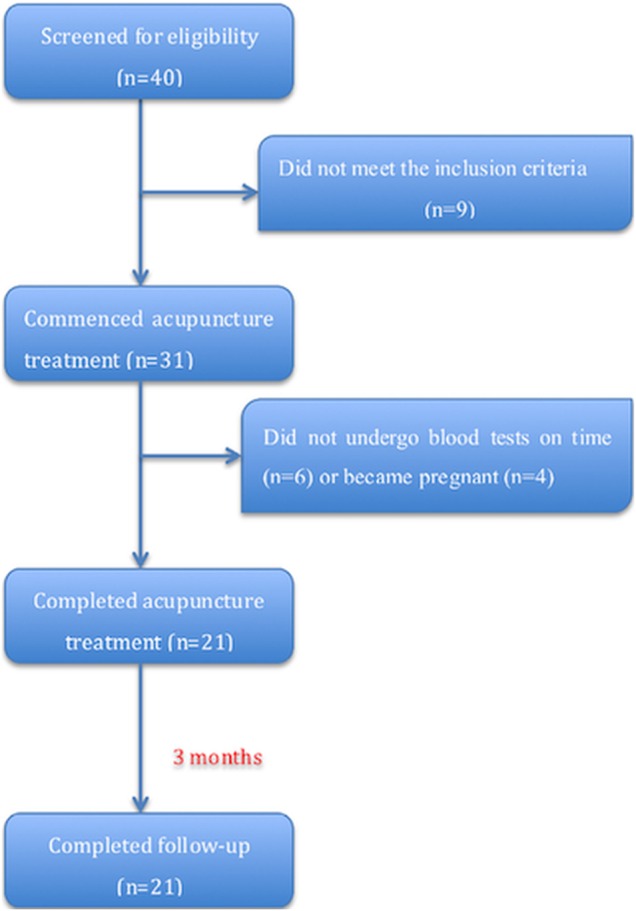
Flow chart of the study.
Baseline characteristics
The baseline characteristics of the 21 patients are shown in table 1. Seven patients had used hormone therapy or DHEA in the past and seven had tried herbs; however, none had previously used acupuncture for DOR. One patient had undergone IVF, which had failed, and all 21 were planning a future pregnancy. All patients met the inclusion criteria (with no exclusion criteria) at the time they signed the informed consent form.
Table 1.
Baseline characteristics of the 21 patients
| Characteristics | Mean±SD or N (%) |
|---|---|
| Age (years) | 37.05±5.40 |
| Duration of DOR (years) | 3.28±3.27 |
| Married | 21 (100) |
| Previous pregnancy | 10 (48) |
| Previous early embryonic demise | 9 (43) |
| Previous IVF | 1 (5) |
| Previous delivery | 1 (25) |
DOR, diminished ovarian reserve; IVF, in vitro fertilisation.
Reproductive hormones
Mean FSH levels fell over the course of the study from 19.33±9.47 mIU/mL at baseline to 10.58±6.34 mIU/mL at week 12 and 11.25±6.68 mIU/mL at week 24 (figure 2). The change in FSH compared with baseline (primary outcome) was −8.75±11.13 mIU/mL at week 12 (p=0.002) and −8.08±9.65 mIU/mL at week 24 (p=0.001), respectively. Meanwhile, mean LH levels decreased more gradually from 6.35±3.40 mIU/mL at baseline to 4.55±3.47 mIU/mL at week 12 (p=0.062) and 4.33±2.04 mIU/mL at week 24 (p=0.004). Furthermore, we found that the FSH/LH ratio was significantly reduced compared with baseline (3.43±0.32) at week 24 (2.70±0.42, p=0.017) but not at week 12 (3.26±2.84, p=0.274). In addition, mean E2 levels were significantly higher than at baseline (39.16±7.47) at week 12 (96.73±30.72, p=0.016) but not at week 24 (63.16±92.50 mIU/mL, p=0.052).
Figure 2.

Serial measurements of serum follicle-stimulating hormone (FSH) and luteinising hormone (LH) obtained by chemiluminescent microparticle immunoassay, over 24 weeks in 21 women with diminished ovarian reserve receiving a 12-week course of EA treatment. **p<0.01 (compared with baseline).
Symptom scale
Table 2 shows serial changes in the symptom scale, which consisted of irritability and depression. For the total score and irritability subscores, significant differences were shown at weeks 12 and 24 compared with baseline. By contrast, no difference was found for the depression subscore at any time point compared with baseline. There were no significant differences in total score or irritability subscore between weeks 12 and 24 (p>0.05).
Table 2.
Symptom scale
| Baseline | Week 4 | Week 8 | Week 12 | Week 24 | ||
|---|---|---|---|---|---|---|
| Irritability score | Mean±SD | 1.06±0.93 | 1.13±0.96 | 0.88±1.03 | 0.56±0.81 | 0.63±0.72 |
| p Value | – | 0.564 | 0.334 | 0.046 | 0.053 | |
| Depression score | Mean±SD | 0.75±0.93 | 0.75±1.00 | 0.75±1.00 | 0.56±0.63 | 0.37±0.62 |
| p Value | – | 1.00 | 1.00 | 0.414 | 0.161 | |
| Total score | Mean±SD | 1.81±1.52 | 1.87±1.63 | 1.63±1.78 | 1.13±1.31 | 1.00±1.21 |
| p Value | – | 0.655 | 0.034 | 0.041 | 0.527 |
p Values indicate comparison with baseline values for each score (Wilcoxon's rank sum test).
Menstruation
None of the 21 patients had amenorrhoea at baseline. Before treatment, mean cycle length and blood loss were 27.2±3.45 days and 34.38±16.21 mL, respectively. The corresponding values at weeks 12 (29.53±5.19 days and 36.25±9.03 mL) and 24 (28.25±3.75 days and 35.63±9.29 mL) were not significantly different compared with baseline. Seven patients (33.3%) and six patients (28.6%), respectively, reported a subjective increase in the volume of menstrual blood loss at 12 weeks and 24 weeks.
Safety
In this study, abdominal subcutaneous haematomas occurred after treatment in four cases, all of which resolved spontaneously within 2 weeks. Mild abdominal pain occurred after treatment in two cases and disappeared within 12 h. Two patients caught a cold during the treatment period and this was deemed unlikely to have been related to acupuncture. There were no significant or major adverse events.
Discussion
Low-frequency EA has been used to improve reproductive dysfunction,17–19 although the exact underlying mechanism of action remains unknown. Acupuncture has been shown to modulate the sympathetic nervous system as well as the endocrine and neuroendocrine systems.20 21 Acupuncture is believed to affect the hypothalamic–pituitary–gonadal axis by modulating central β-endorphin production and secretion, thereby influencing the release of hypothalamic gonadotrophin-releasing hormone and pituitary secretion of gonadotrophins, in polycystic ovary syndrome (PCOS).22 A similar mechanism may be responsible for the apparent effects of acupuncture in DOR. However, further research is required to elucidate the precise effects and mechanisms of acupuncture for reproductive dysfunction.
The aim of the study was to examine whether acupuncture could improve ovarian reserve and thereby increase the chances of pregnancy for patients with DOR planning a family. The results showed that mean FSH levels, which are inversely related to the ovarian reserve, decreased significantly at weeks 4, 8, 12 and 24. Differences were also demonstrated in E2 and LH levels at weeks 12 and 24, respectively. Our results are comparable to those of previous studies in POI.11–14 Similar influences of acupuncture on FSH, LH and E2 levels have also been seen in studies of PCOS,23 menopausal symptoms and other gynaecological conditions,24 indicating that acupuncture can modulate reproductive hormone levels and improve ovarian reserve. In addition to FSH, LH and E2 levels, we examined for changes in the FSH/LH ratio, an important reproductive marker that is used in some diagnostic criteria,25 albeit no longer part of the Rotterdam criteria for the diagnosis of PCOS.26 The FSH/LH ratio showed a similar downward trend to that of the individual FSH and LH levels, culminating in a significant difference compared with baseline at week 24. Based on the observation of differences in FSH and LH levels, FSH/LH ratio and irritability score at week 24 (ie, 12 weeks after completion of treatment), acupuncture may have a long-term effect on hormone levels and emotion. Such long-term effects have also been seen in other studies.11 12 For menstruation, no significant differences were demonstrated in cycle length or blood volume, although this might represent the fact that menstrual symptoms were mild in the sample we observed.
Unlike other studies, measurements were also taken after 4 and 8 weeks of treatment, at which point FSH (but no other outcome measure) differed significantly. It was unclear whether this effect was related to the small sample size or the limited duration of treatment. In support of the latter assumption, it has been reported that 10–14 weeks of treatment are needed before changes in ovulation frequency are seen in patients with PCOS.26 27 Interestingly, the FSH level seemed to be more easily affected by acupuncture than the other parameters.
In order to fully interpret our results, it must first be acknowledged that there is variability in the methods used to diagnose DOR across clinics, states and regions. In China, the FSH cut-off point for the diagnosis of DOR is usually ≥10 and <40 mIU/mL.14 However, Barad et al have suggested that FSH thresholds for the diagnosis of DOR should be age specific: >7.0 mIU/mL for patients <33 years of age; >7.9 mIU/mL for those aged 33–37; and >8.4 mIU/mL for those aged 38–40.28 In this study, we used the Chinese FSH cut-off point because it is stricter, thus all patients diagnosed using a >10 threshold also met the criteria proposed by Barad et al. For the symptom scale, as most patients with DOR in our clinic have emotional symptoms rather than somatic symptoms (sweating, flushing, etc), we used a two-item emotional scale instead of a more heterogeneous measure such as the Menopause Rating Scale. Most previous trials on ovarian reserve in DOR have been conducted by reproductive centres and outcome measures have included pregnancy rates, antral follicle count and levels of inhibin B, anti-Müllerian hormone (AMH) and/or FSH.10 29 30 Although AMH appears to be better than FSH as a marker of ovarian reserve, especially among older women,31 we did not use it owing to funding considerations. We chose instead the (change in) FSH level as our primary outcome as this is still considered to be a robust marker of ovarian reserve. However, we have started a controlled trial using AMH as an outcome measure, which is now at follow-up stage.
This study has some further limitations that must be acknowledged. First, as a single group observational study, the lack of a control group unfortunately means that we are unable to quantify the contribution of potential placebo effects and thereby confirm any specific effects of EA for DOR. Randomised controlled clinical trials are needed to determine the efficacy (relative to sham) and effectiveness (compared with the best available treatment) of acupuncture for this condition.22 Cooperation between hospitals practising traditional Chinese medicine and reproductive centres is likely to be beneficial for planning such a trial in China. Second, patients receiving EA were seeking an increased chance of pregnancy through IVF or natural conception. Although we demonstrated a significant effect on hormone levels, these are only surrogate markers. By completion of 24 weeks’ follow-up, four of 25 patients (16%) had become pregnant and were therefore not included in the final analysis. We did not extend the follow-up further to observe the pregnancy rate among the remaining patients, therefore the effect of EA on conception remains unknown and requires further investigation.
Conclusion
EA may decrease FSH and LH levels and FSH/LH ratios, increase E2 level and improve emotional symptoms in patients with DOR without significant side effects. These effects appear to last for at least 3 months after completion of treatment.
Footnotes
Contributors: ZL and YW conceived the study. RC, XC and YL participated in trial communication. JY performed the statistical analysis. YW drafted the manuscript and ZL revised it. All authors read and approved the final version of the manuscript.
Funding: This work was supported by the Fundamental Research Funds for the Central Public Welfare Research Institutes (grant no. ZZ07080801).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The review board and ethics committee of Guang'anmen Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Levi AJ, Raynault MF, Bergh PA, et al. Reproductive outcome in patients with diminished ovarian reserve. Fertil Steril 2001;76:666–9. 10.1016/S0015-0282(01)02017-9 [DOI] [PubMed] [Google Scholar]
- 2.Scott RT Jr, Hofmann GE. Prognostic assessment of ovarian reserve. Fertil Steril 1995;63:1–11. [PubMed] [Google Scholar]
- 3.Pal L, Beviacqua K, Zeitlian G, et al. Implications of diminished ovarian reserve (DOR) extend well beyond reproductive concerns. Menopause 2008;15:1086–94. 10.1097/gme.0b013e3181728467 [DOI] [PubMed] [Google Scholar]
- 4.Lixia C. The clinical and empirical research on the effect of the Zi Gui Huo Xue Yi Jing recipe on the peri-POF period. Guangzhou: Guangzhou University of Chinese Medicine, 2007. [Google Scholar]
- 5.Lixia C, Yanhua L, Xiaoyun L, et al. Influence of Jianpi Yishen method on the ovarian reserve. Chin J Clin Res 2013;26:73–4. [Google Scholar]
- 6.Cizmeli C, Lobel M, Franasiak J, et al. Levels and associations among self-esteem, fertility distress, coping and reaction to potentially being a genetic carrier in women with diminished ovarian reserve. Fertil Steril 2013;99:2037–44. 10.1016/j.fertnstert.2013.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loutradis D, Drakakis P, Vomvolaki E, et al. Different ovarian stimulation protocols for women with diminished ovarian reserve. J Assist Reprod Genet 2007;24:597–611. 10.1007/s10815-007-9181-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narkwichean A, Maalouf W, Campbell BK, et al. Efficacy of dehydroepiandrosterone to improve ovarian response in women with diminished ovarian reserve: a meta-analysis. Reprod Bio Endocrinol 2013;11:44 10.1186/1477-7827-11-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR). Reprod Biol Endocrinol 2011;9:67 10.1186/1477-7827-9-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yingchun Z, Fangyuan L, Lanrong L, et al. Influence of acupuncture and herbs for ovarian reserve. J Sichuan Tradit Chin Med 2010;28:103–4. [Google Scholar]
- 11.Zhou K, Jiang J, Wu J, et al. Electroacupuncture modulates reproductive hormone levels in patients with primary ovarian insufficiency: results from a prospective observational study. Evid Based Complement Alternat Med 2013;2013;657234 10.1155/2013/657234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Fang Y, Yang J, et al. Effect of acupuncture on premature ovarian failure: a pilot study. Evid Based Complement Alternat Med 2014;2014;718675 10.1155/2014/718675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen-yang HE. Acupuncture for 15 premature ovarian insufficiency patients. Zhongguo Zhenjiu 2000;7:399. [Google Scholar]
- 14.Gui-e SHA, Wen-min ZHAO, Ren-hai MA. Observation of acupuncture for 76 premature ovarian insufficiency patients. Zhonguo Zhenjiu 1999;34:653–6. [Google Scholar]
- 15.Cohen J, Chabbert-Buffet N Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder-a plea for universal definitions. J Assist Reprod Genet 2015;32:1709–12. 10.1007/s10815-015-0595-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao-feng XU, Chun FAN, Guang-qin BAO. , et al. Investigation of the factors related to premature ovarian failure and diminished ovarian reserve. Chin J Evid Based Med 2011;11:400–3. [Google Scholar]
- 17.Stener-Victorin E, Waldenström U, Tägnfors U, et al. Effects of electro-acupuncture on anovulation in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand 2000;79:180–8. 10.1080/j.1600-0412.2000.079003180.x [DOI] [PubMed] [Google Scholar]
- 18.Gerhard I, Postneek F. Auricular acupuncture in the treatment of female infertility. Gynecol Endocrinol 1992;6:171–81. 10.3109/09513599209015552 [DOI] [PubMed] [Google Scholar]
- 19.Carr D. Somatosensory stimulation and assisted reproduction. Acupunct Med 2015;33:2–6. 10.1136/acupmed-2014-010739 [DOI] [PubMed] [Google Scholar]
- 20.He J, Yang L, Qing Y, et al. Effects of electroacupuncture on bone mineral density, oestradiol level and osteoprotegerin ligand expression in ovariectomised rabbits. Acupunct Med 2014;32:37–42. 10.1136/acupmed-2012-010271 [DOI] [PubMed] [Google Scholar]
- 21.Stener-Victorin E, Wu X. Effects and mechanisms of acupuncture in the reproductive system. Auton Neurosci 2010;157:46–51. 10.1016/j.autneu.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 22.Stener-Victorin E, Jedel E, Mannerås L. Acupuncture in polycystic ovary syndrome: current experimental and clinical evidence. J Neuroendocrinol 2008;20:290–8. 10.1111/j.1365-2826.2007.01634.x [DOI] [PubMed] [Google Scholar]
- 23.Johansson J, Redman L, Veldhuis PP. et al. Acupuncture for ovulation induction in polycystic ovary syndrome: a randomized controlled trial. Am J Physiol Endocrinol Metab 2013;304:E934–43. 10.1152/ajpendo.00039.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sunay D, Ozdiken M, Arslan H, et al. The effect of acupuncture on postmenopausal symptoms and reproductive hormones: a sham controlled clinical trial. Acupunct Med 2011;29:27–31. 10.1136/aim.2010.003285 [DOI] [PubMed] [Google Scholar]
- 25.Lejie. Gynecology. Beijing: People's Medical Publishing House, 2008:308. [Google Scholar]
- 26.Johansson J, Redman L, Veldhuis PP. et al. Acupuncture for ovulation induction in polycystic ovary syndrome: a randomized controlled trial. Am J Physiol Endocrinol Metab 2013;304:E934–43. 10.1152/ajpendo.00039.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jedel E, Labrie F, Odén A, et al. Impact of electro-acupuncture and physical exercise on hyperandrogenism and oligo/amenorrhea in women with polycystic ovary syndrome: a randomized controlled trial. Am J Physiol Endocrinol Metab 2011;300:E37–45. 10.1152/ajpendo.00495.2010 [DOI] [PubMed] [Google Scholar]
- 28.Barad DH, Weghofer A. Gleicher N. Age-apecific levels of basal follicle stimulating hormone assessment of ovarian function. Obstet Gynecol 2007;109;1404–10. 10.1097/01.AOG.0000264065.37661.a0 [DOI] [PubMed] [Google Scholar]
- 29.Gurtcheff SE, Klein NA. . Diminished ovarian reserve and infertility. Clin Obstet Gynecol 2011;54: 4666–67. [DOI] [PubMed] [Google Scholar]
- 30.Barad D, Brill H, Gleicher N. Update on the use of dehydroepiandrosterone supplementation among women with diminished ovarian function. J Assist Reprod Genet 2007;24:629–34. 10.1007/s10815-007-9178-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barad DH, Weghofer A, Gleicher N. Comparing anti-Mullerian hormone (AMH) and follicle-stimulating hormone (FSH) as predictors of ovarian function. Fertil Steril 2009;91(4 Suppl):1553–5. 10.1016/j.fertnstert.2008.09.069 [DOI] [PubMed] [Google Scholar]


