Abstract
Aim
To analyze the effect of radiotherapy (RT) in patients with metastatic spinal cord compression (MSCC) and poor prognosis in our center.
Background
RT is an effective treatment for MSCC.
Materials and methods
Prospective evaluation on patients with MSCC and limited survival (according to Rades’ scale), and treated with single-dose 8 Gy RT (February 2013–August 2014). Pain, ambulatory status and sphincter control were recorded. Pain relief was evaluated following the International Bone Metastases Consensus Working Party Guidelines. Ambulatory status was evaluated with Frankel's scale. Spinal fracture and instability were recorded. Health aspects were evaluated via a short survey and measuring the time spent on RT.
Results
35 patients were included. 51% had unfavorable histologies; 60% bone fracture and 17% spinal instability. Median Karnofsky score was 60; 100% were on high doses of opioids. Median survival was 1.5 months. 49% had a partial pain response at 2 weeks post-radiation, and 47% at one month. Significant reductions in pain intensity were present at 2 weeks (Visual analog scale, VAS score, from 8 ± 1.5 to 5 ± 1.9). Negligible effects were observed on motor and bladder function, along with side effects. KPS score was maintained during follow-up. 80% of patients spent ≤5% of their remaining lifetime on RT. A survey comparison between clinical judgment and the results according to treatment decision consider that these patients merit treatment evaluation.
Conclusions
A moderate pain response tailored to life expectancy can be obtained in patients treated with radiation. 8-Gy single-dose is an option for patients with limited survival.
Keywords: Limited survival, Metastatic spinal cord compression, Radiotherapy, Poor prognosis
1. Background
MSCC is considered an oncologic emergency occurring in 5–10% of all cancer patients during their disease.1 The majority of patients have a short survival of only a few months.2, 3 Short-course radiotherapy (RT) administered in a week or less is associated with less discomfort and considered appropriate for patients with short life expectancy. Single fraction RT with 8 Gy results in similar pain relief and improvement of motor deficits in patients who receive radiation for MSCC4, 5, 6, 7, 8.
The capacity of physicians to predict survival is controversial. This is reflected in a study evaluating the adequacy of palliative radiation treatment in end-stage cancer patients, where median survival was 15 days and survival estimates by the treating physician were correct in only 16% of patients.9 Objective prognostic systems can improve prognostic accuracy. A scoring system validated to estimate survival of MSCC patients, based on 6 prognostic factors that can be easily recorded, separates well those patients with higher 6-month survival expectancy from the rest.10 A modified version of this score, adding ECOG performance status to the prognostic factors, has been proposed to identify patients with MSCC and an extraordinarily limited life expectancy.11
Little information is known regarding radiation use at the end of life. A study showed that 6–7% of patients receiving palliative radiation at the end of life died within 30 days post treatment.12
The first sign of MSCC is generally back pain.13, 14 Common manifestations include weakness, sensory changes, and autonomic dysfunction. Although RT plays an important role in the management of MSCC, it is well documented that the symptomatic effect of radiation is lower in cases of vertebral fracture and spinal instability.
2. Aim
This study aimed to prospectively evaluate the potential effect of single dose RT in patients with MSCC and low survival expectancy.
3. Methods
3.1. Patients
Data from all patients diagnosed with MSCC from February 2013 to August 2014 at Vall d’Hebron University Hospital were analyzed. The eligibility criteria were: known history of cancer and estimated life expectancy less than 6 months; that the patient had not been treated previously at the compression level; and signed informed consent.
Diagnosis of MSCC was conducted with an MRI/CT of the entire spine in clinically suspicious patients, i.e. presenting with pain, weakness, sensory disturbance, and/or sphincter dysfunction. MSCC was defined as compression of the dural sac and its contents by an extradural tumor mass. The minimum radiologic evidence for cord compression is indentation of the theca at the level of clinical features.15 Radiology also allowed the identification of vertebral fractures and spinal instability. Spinal instability was evaluated using the Spinal Instability Neoplastic Score (SINS), where higher SINS scores denote spine instability.16
Prognostic factors assessed for life expectancy include the type of primary tumor, presence of bone or visceral metastases at the time of RT, the interval from tumor diagnosis to MSCC, ambulatory status and time of developing motor deficits before RT. A score was assigned to each patient and three groups were established, as defined by Rades.10 Patients in groups I–II have a short life expectancy, and form this study's cohort.
This study was approved by the Ethical Review Committee at Vall d’Hebron University Hospital.
3.2. Treatment
Parenteral dexamethasone (16 mg/d) was administered from MSCC diagnosis until 5 days post-RT, and then progressively diminished. Non-responders continued taking corticosteroids as needed. Patients with fields covering the upper abdomen received parenteral 5-hydroxitriptamine-3 receptor antagonist 60 min before radiation treatment. The antiemetic was maintained as needed after radiation treatment. Emergency RT was planned to start within 24–72 h of the diagnosis and delivered from a 6-MV linear accelerator. One vertebral body above and below the involved vertebrae and paravertebral mass were included in the treatment portal.
3.3. Assessment
The response to treatment was evaluated according to the patients’ back pain, walking capacity, and bladder function before and after RT. The pain intensity score and need of analgesics were recorded. On the basis of physical examination, motor performance was graded according to Frankel's scale, a system that considers a patient ambulatory if any useful motor function is present. Bladder function was defined by the need of a urinary catheter. Treatment toxicity was evaluated by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0). Analysis of response was performed 2 weeks after the end of RT and the follow-up examination was continued monthly until death. Clinical information was recorded by the treating physician at regular visits or with telephone interviews.
3.4. Response definition
Pain response was calculated using the International Bone Metastases Consensus Working Party Guidelines (IBCWPG) which take into account changes in pain intensity and the administration of analgesics.17 A complete response was defined as a pain score of 0 without analgesic increase. A partial response was defined as (1) a decrease in the initial pain intensity ≥2 points on the Visual Analogic Scale, without analgesic increase; or (2) an analgesic decrease (≥25%) without increase in pain. Progression of pain was defined as (1) an increase in pain ≥2 points above baseline, without analgesic increase; or (2) an analgesic increase (≥25%), irrespective of the pain score. Patients who became ambulatory and those who recovered sphincter function were considered responders.
3.5. Outcome measures
Percentage of response and duration of improvement, survival and toxicity were evaluated. Duration of response was calculated as time from improvement to regression of improvement or death. We used the duration of therapy compared with the rest of life (i.e. days of RT divided by total remaining lifetime)9 as a quality indicator of treatment burden. To evaluate general health aspects, three independent and confidential estimates regarding RT indication were requested before the analyses were done. These estimates were provided by staff physicians and the chief resident (n = 9) through an electronic survey. Aspects evaluated include: what was clinically considered short survival expectancy, the clinically expected benefit from RT, and the pain control judged relevant to indicate the RT palliation. Results from this survey were compared with those of the study and comments were made.
3.6. Statistical analysis
Overall survival was measured from the date of treatment to the date of death from any cause. A descriptive analysis has been carried out for each variable. The Skillings–Mack test was used to test changes over time for quantitative variables and the symmetry test for qualitative ones. For survival analysis a Kaplan–Meier curve has been estimated. A p-value <0.05 was considered statistically significant. All of the analyses were conducted using STATA software version 14.0.
4. Results
4.1. Baseline characteristics
Of the 56 patients diagnosed with MSCC in the study period, 35 were classified in the Rades prognostic groups I–II and treated with 8 Gy single fraction. Patient characteristics are listed in Table 1. The primary tumor was considered of unfavorable histology in 32 patients, 14 of them in the lung. In 32 cases the Karnofsky score (KPS) was between 60 and 70. All were on opioids. As expected, 24 of MSCC were located in the thoracic segment and 11 in the lumbosacral. While in 16 cases only one vertebra was involved, there were ≥3 vertebrae involved in 11. In addition, 21 had a vertebral fracture and 6 had spinal instability at the compression level.
Table 1.
Patient characteristics (n = 35).
| Characteristic | Number of patients (%) |
|---|---|
| Sex | |
| Male | 23 (65.7%) |
| Female | 12 (34.3%) |
| Age (y) | |
| Median | 63.3 |
| Interquartile range | 34–86 |
| Karnofsky performance status | |
| 40–50 | 10 (28.6%) |
| 60–70 | 23 (65.7%) |
| >80 | 2 (5.7%) |
| Primary tumor | |
| Lung cancer | 14 (40%) |
| Prostate cancer | 2 (5.7%) |
| Breast cancer | 1 (2.9%) |
| Unfavorable histologya | 18 (51.4%) |
| Back pain, basal | |
| Yes | 35 (100%) |
| No | 0 (0%) |
| Motor function, basal | |
| Ambulatory | 15 (42.9%) |
| Non-ambulatory | 20 (57.1%) |
| Sphincter control, basal | |
| Yes | 11 (31.4%) |
| No | 24 (68.6%) |
| Other bone metastases | |
| Yes | 31 (88.6%) |
| No | 4 (11.4%) |
| Visceral metastases | |
| Yes | 32 (91.4%) |
| No | 3 (8.6%) |
| Interval from tumor diagnosis to MSCC (m) | |
| ≤15 | 22 (62.9%) |
| >15 | 13 (37.1%) |
| Time to develop motor deficits before RT (d) | |
| 1–7 | 12 (34.3%) |
| 8–14 | 2 (5.7%) |
| >14 | 21 (60%) |
| Rades group | |
| I | 26 (74.3%) |
| II | 9 (25.7%) |
| Spine instability | |
| Yes | 6 (17.15%) |
| No | 23 (65.7%) |
| Undetermined | 6 (17.15%) |
| Spinal fracture | |
| Yes | 21 (60%) |
| No | 14 (40%) |
| Antiemetic use | |
| Yes | 15 (43%) |
| No | 20 (57%) |
Head and neck, n = 4; melanoma, n = 3; kidney/gynaecologic/thyroid, n = 2; pleura/gastrointestinal/unknown origin, n = 1.
4.2. Response to treatment
The median time from MSCC diagnosis to radiation treatment was 1 day. Only 5 patients were treated >7 days after MSCC diagnosis due to their medical condition. Antiemetics were used in 43% of patients. In all but 5 patients, pain was controlled during radiation treatment. Response to treatment could be calculated in 33 patients.
4.3. Survival
Median survival time was 1.5 months. The proportion of patients alive at 1, 3 and 6 months post-radiation was 65.7, 14.3, and 8.6%, respectively (Fig. 1). No significant associations were identified between the primary tumor (p = 0.89), sex (p = 0.62), age (p = 0.90), presence of visceral metastases (p = 0.45), presence of other bone metastases (p = 0.11), spine level of the cord compression (p = 0.16), vertebra fracture (p = 0.07), or instability (p = 0.57), and survival in the univariate analysis. KPS was the only parameter significantly associated with survival (p = 0.003). In addition, we identified important differences in survival in those patients presenting complications post-radiation (nausea, vomiting, esophagitis, paralytic ileum). Patients without complications had longer survival (p = 0.003) (median survival: 6.29 months vs. 1.9 months).
Fig. 1.
Overall survival.
4.4. Pain response
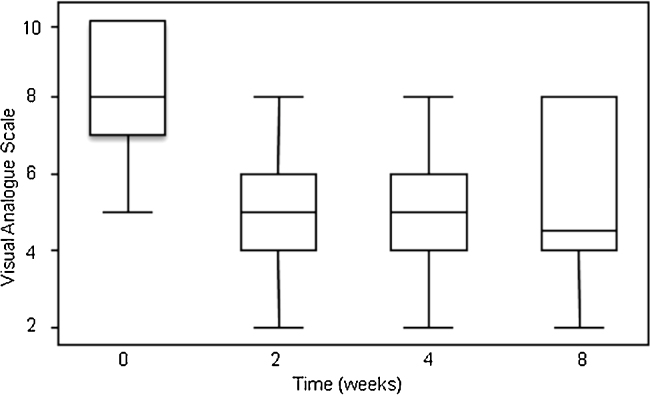
A mild reduction in the median dose of opioids needed 2 weeks after radiation was identified (oral morphine equivalent dose, 120–90 mg/d), although it did not show any significance. However, a significant reduction in pain intensity was present at 2 weeks. The initial pain intensity decreased from 8 ± 1.5 (mean ± sd) to 5 ± 1.9 two weeks post-radiation and remained stable at 1 month (p < 0.0001, Fig. 2). Following IBCWPG, a partial pain response was obtained in 49% and 47% of patients at 2 weeks and 1 month post-radiation, respectively. There were no complete responses.
Fig. 2.
Box plot of pain intensity score evolution over time (p < 0.0001). Time 0: RT treatment.
4.5. Motor and bladder function
15 patients were ambulatory and 11 had bladder function preserved at baseline. As patients approached the end of life, their motor function worsened although it was not significant. No significant differences were observed in preserved bladder function over time. When evaluating improvement or worsening of function after radiation, most patients remained unchanged.
4.6. Complications
Gastrointestinal and pharyngeal toxicities post-radiation were evaluated by CTCAE v4.0. Grade 1–2 nausea (12.5%) and vomiting (10%) were the main toxicities registered in these patients. Other toxicities were grade 1–2 esophagitis or paralytic ileus in 7.5% of patients.
4.7. Palliative radiotherapy use at end of life
Because these were patients with a short survival expectancy, we decided to include some general health measures in the overall evaluation. It has been recently described that the ratio between duration of therapy and remaining lifetime may be a useful quality indicator. In this cohort, 80% of patients remained in treatment for ≤5% of their remaining lifetime, and overall 97% for ≤25% (Table 2).
Table 2.
Time on treatment during remaining lifetime.
| Time on treatment | Number of patients (%) |
|---|---|
| 0–5% | 28 (80%) |
| 6–10% | 4 (11.4%) |
| 11–25% | 2 (5.7%) |
| >25% | 1 (2.9%) |
In a survey conducted at our department, two thirds of treating radiation oncologists considered 3 months as a low survival expectancy, and 1 month as being reasonable to indicate only the best supportive care. All considered pain control as a relevant endpoint when indicating radiation. Two thirds considered a pain response of 50% after radiation as meaningful.
5. Discussion
In this study we have evaluated a potential role of 8 Gy single dose of radiation in MSCC patients with low survival expectancy. Median survival was 1.5 months, with a partial pain response of 49% and 47% at 2 weeks and 1 month post-radiation, respectively. Treatment toxicities were mild but, in this severely compromised population, may alter prognosis. KPS score was maintained in most patients. Finally, a single dose radiation treatment represented ≤5% of the remaining lifetime in the majority patients.
Because physicians’ ability to predict survival in metastatic patients remains difficult, it is desirable to use objective scales that can assist in decision-making. We wished to test a score proposed by Rades10 in a population with MSCC and presumed low survival expectancy. Results in this series were probably influenced by unfavorable histologies and assignment of the vast majority of cases to group I. With a median survival of 1.5 months and only 8.6% of patients alive at 6 months, we can accept that this scale performs well in this population and can help clinicians to establish prognosis in these patients. We attempted to test the performance of a modified scale by Rades that incorporates the ECOG performance status in the final score calculation,11 but it did not work in our sample.
Multiple different time points have been used to evaluate treatment response after radiation treatment of bone metastases and MSCC. It is well established that 3 months post-radiation allows for a stable estimation of treatment effects.6 The Dutch Bone Trial reported that median time to response was three weeks and some effects could be seen as soon as 2 weeks post-radiation.4 A recent study evaluating pain response of bone metastases treated with different radiation schedules used 2 weeks post-treatment as the first time point for evaluation and reported a response rate that ranged between 49 and 55%,18 suggesting that it might be of clinical value. In the study by Maranzano,19 pain response of MSCC treated with radiation, was evaluated 1 month post-RT and then monthly. Patients were included in this study on the basis of low survival expectancy, and for that reason we decided to use 2 weeks post-radiation as the first time point for response evaluation.
The evaluation of patients with MSCC is handicapped because several different conditions can influence treatment outcomes. In that regard, objective parameters such as changes in the ambulatory status or bladder function and IBCWPG for pain response evaluation are routinely used. The IBCWPG criteria rely on analgesics needed (objective) and pain intensity (subjective). Most changes were seen in the pain intensity score with mild reduction in opioid consumption, which can be explained by the extended and severe bone disease in these patients. No relevant changes were seen in motor or bladder function, probably because more time would have been needed to see them beyond patient survival.
Treatment was well tolerated with mild toxicities in a minority of patients. Nonetheless, we observed that it could influence prognosis. Possible explanations include patient fragility and treatment of very complicated cases like those affecting 3 or more vertebrae or with spinal fracture or instability. We tried to manage toxicities by adding an antiemetic to radiation treatment, with good pain control during radiation treatment. However, only 43% of patients received an antiemetic. Because the main toxicities were nausea and vomiting, we will be prescribing antiemetics to all patients and pursue a more intensive control of emesis.
In this study, we observed an association between higher KPS scores and survival and we observed that Karnofsky score was stably maintained during patient follow-up. It is well described that the Karnofsky Performance Status is a useful tool in predicting survival.17, 20 In fact, there is a weak evidence to support clinicians’ estimates alone for survival prediction. Karnofsky Performance Status can complement clinical estimates and help to establish survival prediction.
Finally, quality indicators should be routinely used in palliative radiotherapy. Two opposed situations happen with regard to palliative radiotherapy and short survival. Some investigators have reported an infrequent use of radiotherapy for palliation, while others consider that palliative radiotherapy may have little clinical benefit for patients near the end of life.21 The concept of when palliative radiotherapy may be of value is currently being explored.22 Several recent studies describing patterns of radiotherapy use at the end of life provide a set of baseline characteristics that may help identify patients who could benefit from radiotherapy. Further, palliative radiotherapy use within the last 30 days of life has not been defined as a specific quality measure, meaning that an optimal rate of palliative radiotherapy use near the end of life is unknown.21 Although, in this study, pain response to 8 Gy single dose of radiation in MSCC patients with low survival expectancy was moderate and of short duration, it had mild toxicity and was tailored to life expectancy. In a great majority of patients, the duration of treatment represented ≤5% of their life expectancy. This could represent an acceptable option in this context.
Even after using objective criteria for establishing prognosis and evaluating treatment effects, it became difficult to decide which was considered the best option for these patients. Survival in cancer patients improves and the incidence of metastatic sites suitable for palliation increases and so does the demand for additional treatments. In addition, many patients still maintain an acceptable Karnofsky score, making decisions difficult. It is now recognized that this situation can only be expected to increase in the coming years. We believe that a culture of care that recognizes and responds to this demand properly will be needed. As a first step, we thought that it might be helpful to complement the objective data of this study with our own opinions in an effort to reach a stable internal consensus to serve as a baseline for future discussions. In a brief survey conducted before data analysis it was concluded that less than 1 month of survival might be a reasonable option for best supportive care, and that an acceptable pain response in these patients was 50%. Based on our results, this situation represents a boundary in patient care but still merits treatment evaluation.
Limitations of the study are the small sample size, the variety of primary tumors, being observational and difficulties in making comparisons with other studies.
6. Conclusions
Evaluation of patients referred for palliative radiotherapy is becoming increasingly complex. An extreme situation may be that of patients with MSCC and short survival expectancy. We have presented our data of 1.5 years for such patients and conclude that they still merit treatment evaluation based on the moderate pain response observed. It is recommended to use 8 Gy single-dose when indicating radiation in patients with MSCC and low survival expectancy. We recommend using quality indicators in palliative RT.
Conflicts of interest
None declared.
Financial disclosure
None declared.
References
- 1.Prasad D., Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6:15–24. doi: 10.1016/S1470-2045(04)01709-7. [DOI] [PubMed] [Google Scholar]
- 2.Rades D., Fehlauer F., Schulte R. Prognostic factors for local control and survival after radiotherapy of metastático spinal cord compression. J Clin Oncol. 2006;24:3388–3393. doi: 10.1200/JCO.2005.05.0542. [DOI] [PubMed] [Google Scholar]
- 3.Maranzano E., Latini P. Effectiveness of radiation therapy without surgery in metastático spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959–967. doi: 10.1016/0360-3016(95)00572-g. [DOI] [PubMed] [Google Scholar]
- 4.Steenland E., Leer J.W., van Houwelingen H. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52:101–109. doi: 10.1016/s0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 5.8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Bone Pain Trial Working Party Radiother Oncol. 1999;52:111–121. [PubMed] [Google Scholar]
- 6.Chow E., Harris K., Fan G., Tsao M., Sze W.M. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25:1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 7.Rades D., Lange M., Veninga T. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2011;79:524–530. doi: 10.1016/j.ijrobp.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 8.Bayard L.G., Buzón M.D.C.S., Paín E.A., de Ingunza Barón L. Radiation therapy for the management of painful bone metastases: results from a randomized trial. Rep Pract Oncol Radiother. 2014;19:405–411. doi: 10.1016/j.rpor.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gripp S., Mjartan S., Boelke E., Willers R. Palliative radiotherapy tailored to life expectancy in end-stage cancer patients: reality or myth? Cancer. 2010;116:3251–3256. doi: 10.1002/cncr.25112. [DOI] [PubMed] [Google Scholar]
- 10.Rades D., Douglas S., Veninga T. Validation and simplification of a score predicting survival in patients irradiated for metastatic spinal cord compression. Cancer. 2010;116:3670–3673. doi: 10.1002/cncr.25223. [DOI] [PubMed] [Google Scholar]
- 11.Rades D., Hueppe M., Schild S.E. A score to identify patients with metastático spinal cord compression who may be candidates for best supportive care. Cancer. 2013;119:897–903. doi: 10.1002/cncr.27849. [DOI] [PubMed] [Google Scholar]
- 12.Patel A., Dunmore-Griffith J., Lutz S., Johnstone P.A. Radiation therapy in the last month of life. Rep Pract Oncol Radiother. 2014;19:191–194. doi: 10.1016/j.rpor.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiff D. Spinal cord compression. Neurol Clin. 2003;21:67–86. doi: 10.1016/s0733-8619(02)00033-6. viii. [DOI] [PubMed] [Google Scholar]
- 14.Abrahm J.L., Banffy M.B., Harris M.B. Spinal cord compression in patients with advanced metastatic cancer: all I care about is walking and living my life. JAMA. 2008;299:937–946. doi: 10.1001/jama.299.8.937. [DOI] [PubMed] [Google Scholar]
- 15.Loblaw D.A., Laperriere N.J. Emergency treatment of malignant extradural spinal cord compression: an evidence-based guideline. J Clin Oncol. 1998;16:1613–1624. doi: 10.1200/JCO.1998.16.4.1613. [DOI] [PubMed] [Google Scholar]
- 16.Fourney D.R., Frangou E.M., Ryken T.C. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011;29:3072–3077. doi: 10.1200/JCO.2010.34.3897. [DOI] [PubMed] [Google Scholar]
- 17.Chow E., Wu J.S.Y., Hoskin P., Coia L.R., Bentzen S.M., Blitzer P.H. International consensos on palliative radiotherapy endpoints for future clinical trials in bone metastases. Radiother Oncol. 2002;64:275–280. doi: 10.1016/s0167-8140(02)00170-6. [DOI] [PubMed] [Google Scholar]
- 18.Franco P., Migliaccio F., Angelini V. Palliative radiotherapy for painful bone metastases from solid tumors delivered with static ports of tomotherapy (TomoDirect): feasibility and clinical results. Cancer Invest. 2014;32:458–463. doi: 10.3109/07357907.2014.958495. [DOI] [PubMed] [Google Scholar]
- 19.Maranzano E., Trippa F., Casale M. 8 Gy single-dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicentre Italian trial. Radiother Oncol. 2009;93:174–179. doi: 10.1016/j.radonc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Chow E., Harth T., Hruby G., Finkelstein J., Wu J., Danjoux C. How accurate are physicians’ clinical predictions of survival and the available prognostic tools in estimating survival times in terminally ill cancer patients? A systematic review. Clin Oncol. 2001;13:209–218. doi: 10.1053/clon.2001.9256. [DOI] [PubMed] [Google Scholar]
- 21.Jones J.A., Lutz S.T., Chow E., Johnstone P.A. Palliative radiotherapy at the end of life: a critical review: palliative radiotherapy. CA Cancer J Clin. 2014;64:295–310. doi: 10.3322/caac.21242. [DOI] [PubMed] [Google Scholar]
- 22.Lutz S., Korytko T., Nguyen J., Khan L., Chow E., Corn B. Palliative radiotherapy when is it worth it and when is it not? Cancer J. 2010;16:473–482. doi: 10.1097/PPO.0b013e3181f28b4d. [DOI] [PubMed] [Google Scholar]